Abstract
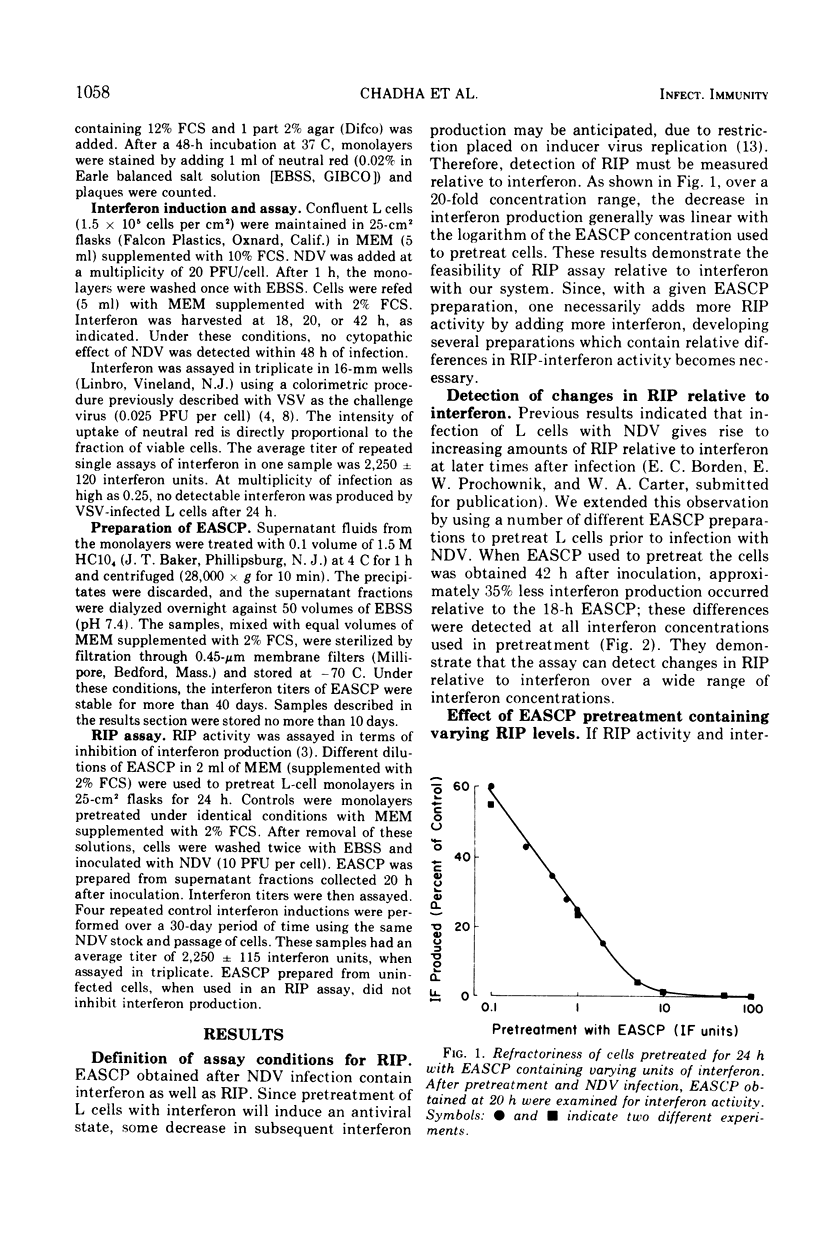
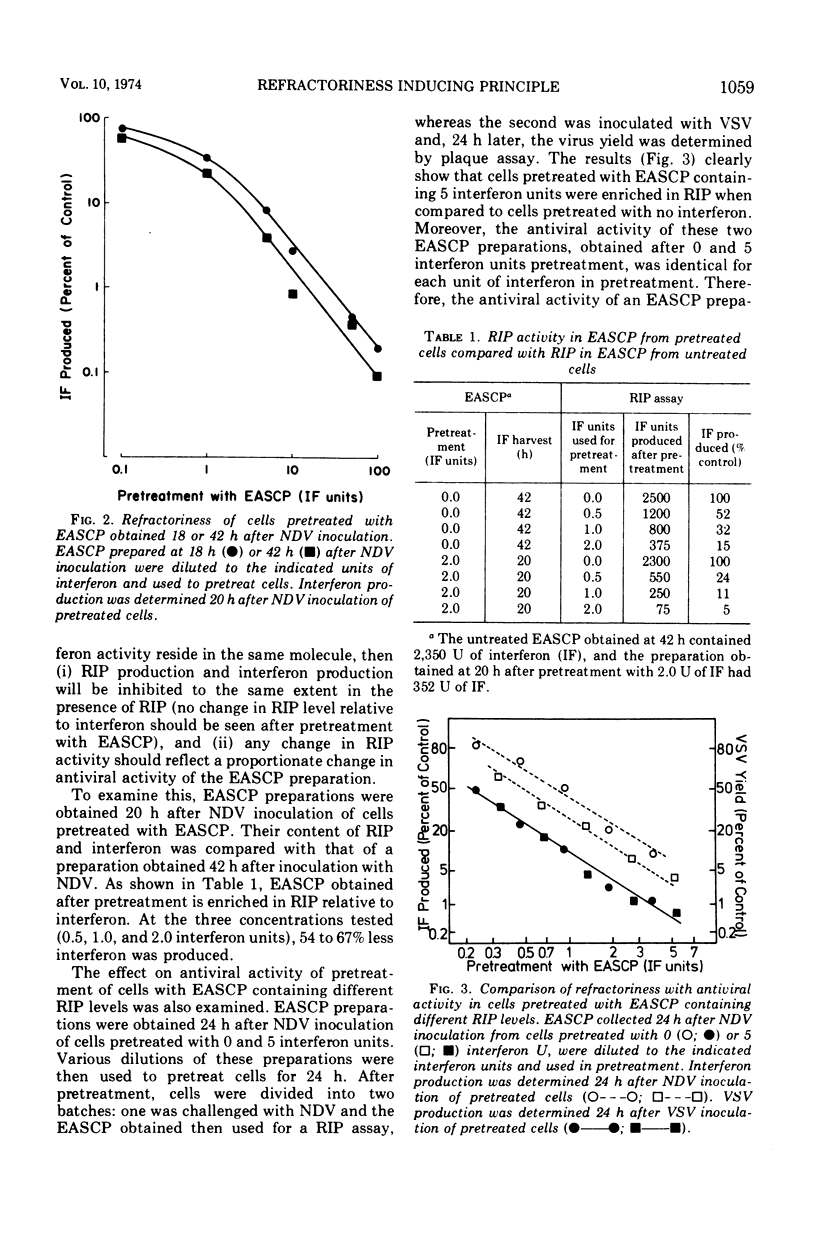
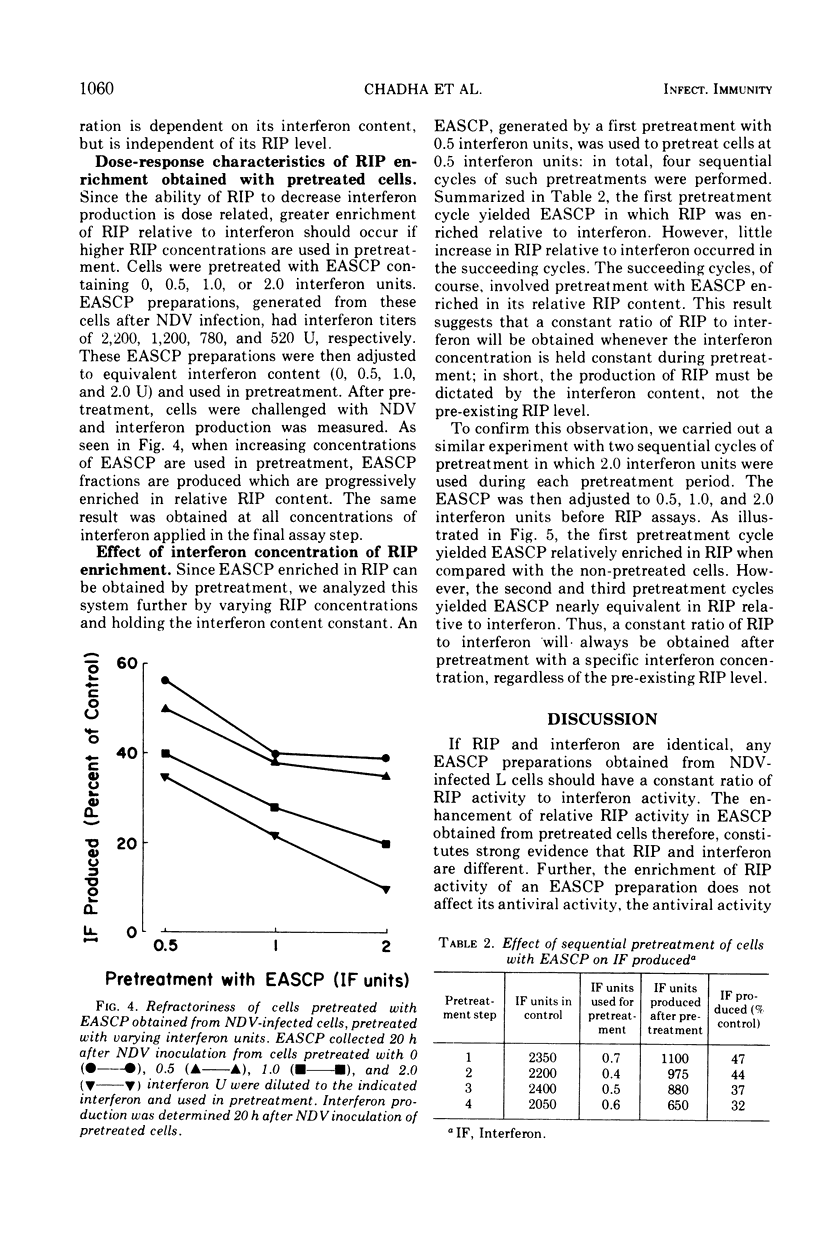
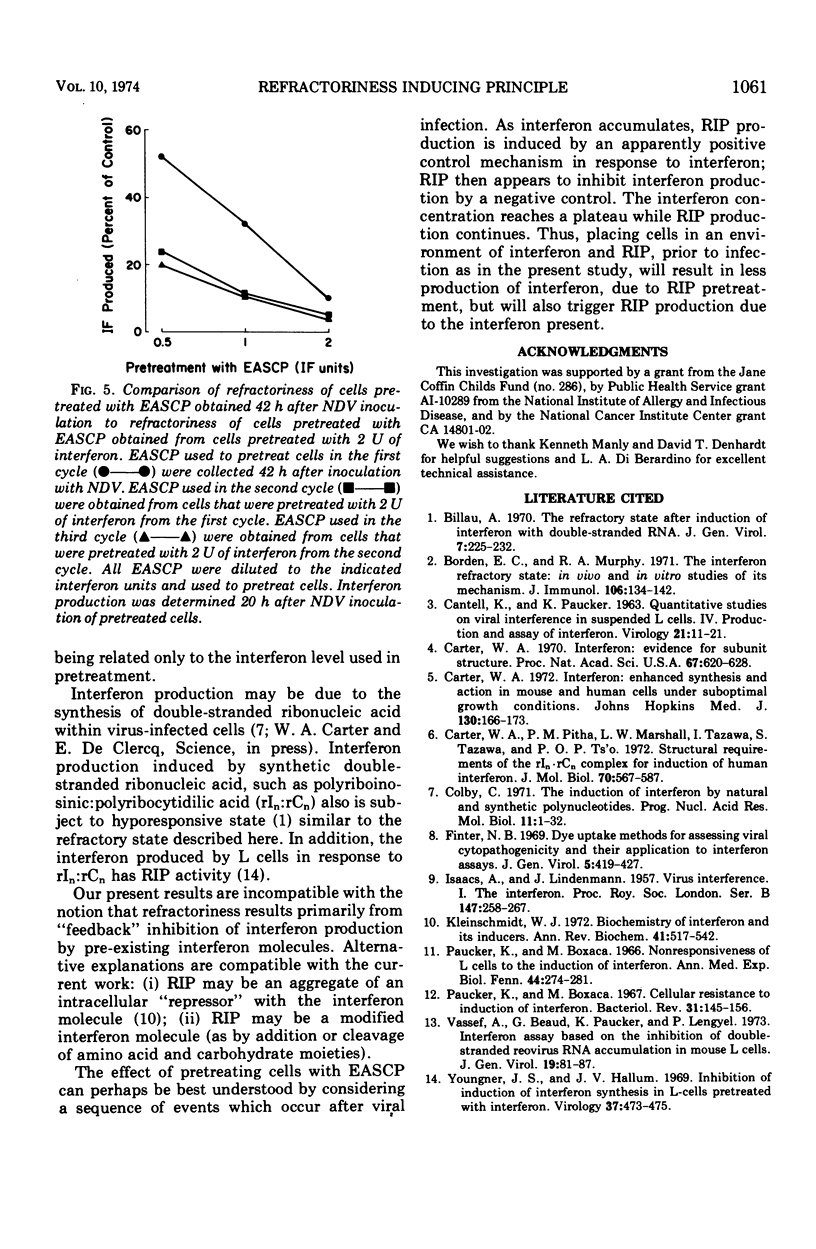
The extracellular, acid-soluble cell products (EASCP) from Newcastle disease virus-infected L929 cells contain both interferon, defined as antiviral activity, and refractoriness inducing principle, defined as an activity that inhibits interferon production. L cells pretreated with EASCP and then infected with Newcastle disease virus give rise to EASCP with decreased amounts of interferon but an increased ratio of refractoriness inducing principle activity to interferon activity in a dose related manner. The antiviral activity of an EASCP preparation is not dependent upon its refractoriness inducing principle level, but is entirely dependent on its interferon content. Our results provide additional evidence that interferon and refractoriness inducing principle are different biological entities and not polymorphic functions of the interferon molecule.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billiau A. The refractory state after induction of interferon with double-stranded RNA. J Gen Virol. 1970 Jun;7(3):225–232. doi: 10.1099/0022-1317-7-3-225. [DOI] [PubMed] [Google Scholar]
- Borden E. C., Murphy F. A. The interferon refractory state: in vivo and in vitro studies of its mechanism. J Immunol. 1971 Jan;106(1):134–142. [PubMed] [Google Scholar]
- CANTELL K., PAUCKER K. QUANTITATIVE STUDIES ON VIRAL INTERFERENCE IN SUSPENDED L CELLS. IV. PRODUCTION AND ASSAY OF INTERFERON. Virology. 1963 Sep;21:11–21. doi: 10.1016/0042-6822(63)90298-8. [DOI] [PubMed] [Google Scholar]
- Carter W. A. Interferon: enhanced synthesis and action in mouse and human cells under suboptimal growth conditions. Johns Hopkins Med J. 1972 Mar;130(3):166–173. [PubMed] [Google Scholar]
- Carter W. A. Interferon: evidence for subunit structure. Proc Natl Acad Sci U S A. 1970 Oct;67(2):620–628. doi: 10.1073/pnas.67.2.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter W. A., Pitha P. M., Marshall L. W., Tazawa I., Tazawa S., Ts'o P. O. Structural requirements of the rI n -rC n complex for induction of human interferon. J Mol Biol. 1972 Oct 14;70(3):567–587. doi: 10.1016/0022-2836(72)90560-8. [DOI] [PubMed] [Google Scholar]
- Colby C., Jr The induction of interferon by natural and synthetic polynucleotides.hs. Prog Nucleic Acid Res Mol Biol. 1971;11:1–32. doi: 10.1016/s0079-6603(08)60324-4. [DOI] [PubMed] [Google Scholar]
- ISAACS A., LINDENMANN J. Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci. 1957 Sep 12;147(927):258–267. doi: 10.1098/rspb.1957.0048. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt W. J. Biochemistry of interferon and its inducers. Annu Rev Biochem. 1972;41(10):517–542. doi: 10.1146/annurev.bi.41.070172.002505. [DOI] [PubMed] [Google Scholar]
- Paucker K., Boxaca M. Cellular resistance to induction of interferon. Bacteriol Rev. 1967 Jun;31(2):145–156. doi: 10.1128/br.31.2.145-156.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paucker K., Boxaca M. Nonresponsiveness of L cells to the induction of interferon. Ann Med Exp Biol Fenn. 1966;44(2):274–281. [PubMed] [Google Scholar]
- Vassef A., Beaud G., Paucker K., Lengyel P. Interferon assay based on the inhibition of double-stranded reovirus RNA accumulation in mouse L cells. J Gen Virol. 1973 Apr;19(1):81–87. doi: 10.1099/0022-1317-19-1-81. [DOI] [PubMed] [Google Scholar]
- Youngner J. S., Hallum J. V. Inhibition of induction of interferon synthesis in L-cells pretreated with interferon. Virology. 1969 Mar;37(3):473–475. doi: 10.1016/0042-6822(69)90231-1. [DOI] [PubMed] [Google Scholar]


