Abstract
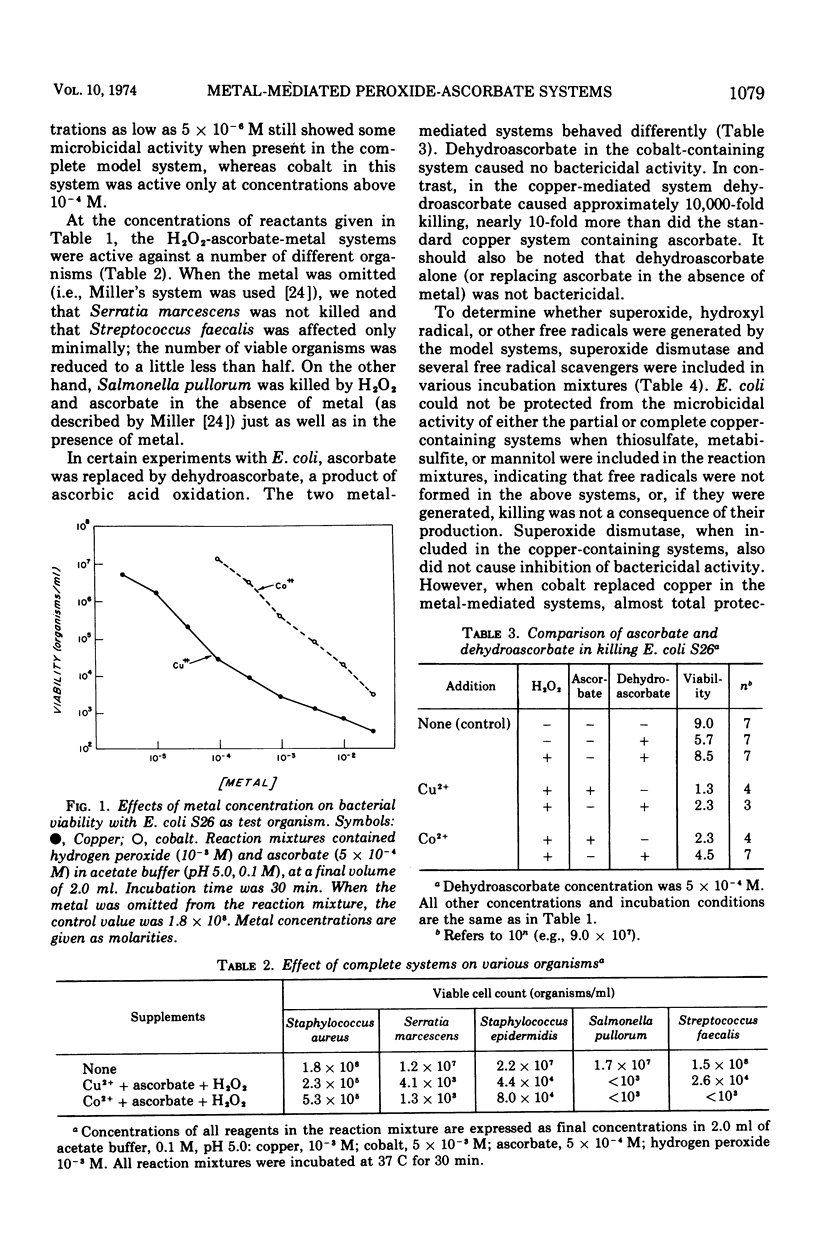
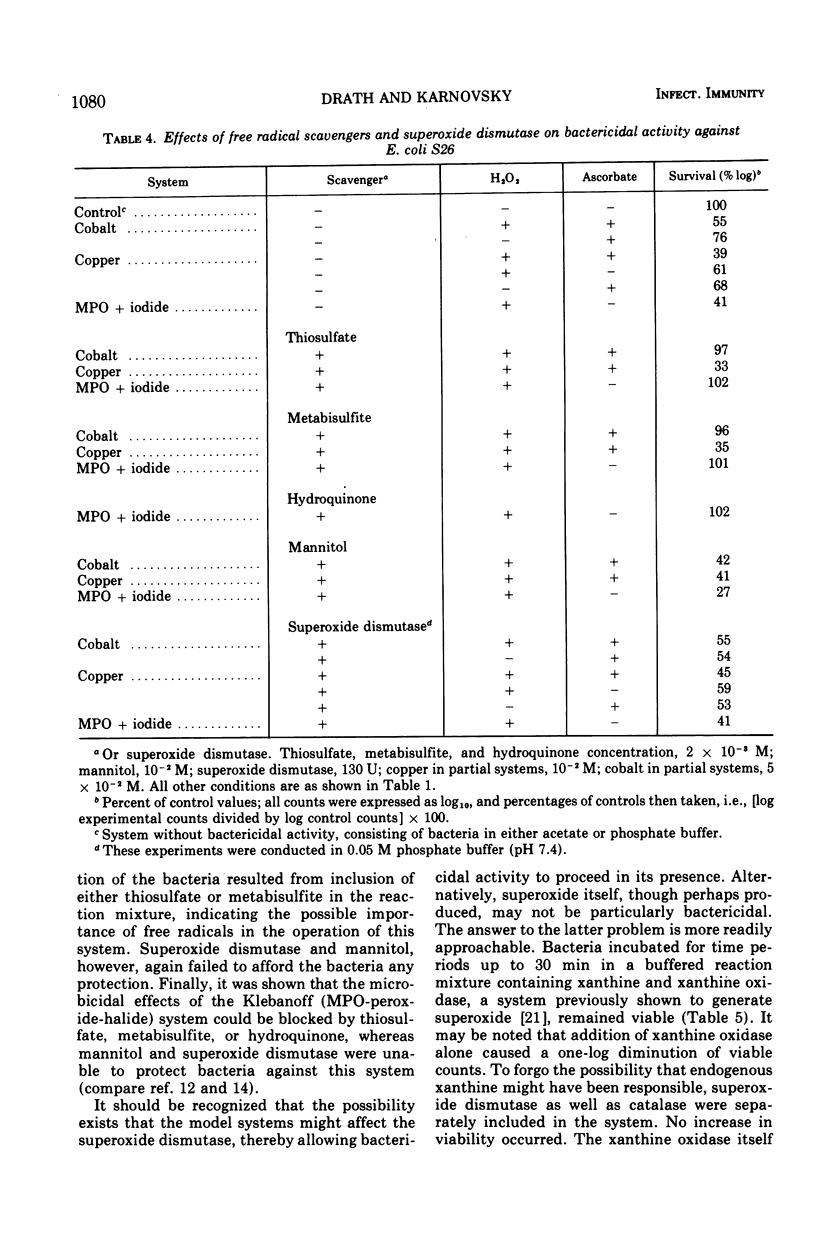
Model systems containing ascorbate, hydrogen peroxide, and divalent copper or cobalt have been shown to possess marked bactericidal activity. At equivalent concentrations, copper-containing systems were more bactericidal than the corresponding mixtures containing cobalt. Cobalt at concentrations below 10−4 M did not appreciably augment microbicidal activity, whereas systems containing copper at concentrations as low as 5 × 10−6 M were still capable of causing some bacterial death. Manganese was inactive. None of these systems was as potent as the well known myeloperoxidase-peroxide-halide system. The mechanisms of action of these systems are not as yet clear. The possibility that they function through the generation of superoxide (O2−), hydroxyl radical (OH·), or other free radicals was explored through the use of superoxide dismutase and several free radical scavengers. It seems likely at present that the two active metal-mediated systems function via separate mechanisms. The copper system acts with dehydroascorbate, whereas the cobalt system does not. Activity in the cobalt system appears to depend upon the generation of free radicals.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belding M. E., Klebanoff S. J., Ray C. G. Peroxidase-mediated virucidal systems. Science. 1970 Jan 9;167(3915):195–196. doi: 10.1126/science.167.3915.195. [DOI] [PubMed] [Google Scholar]
- Curnutte J. T., Whitten D. M., Babior B. M. Defective superoxide production by granulocytes from patients with chronic granulomatous disease. N Engl J Med. 1974 Mar 14;290(11):593–597. doi: 10.1056/NEJM197403142901104. [DOI] [PubMed] [Google Scholar]
- DAMRON C. M., MONIER M. M., ROE J. H. Metabolism of l-ascorbic acid, dehydro-l'ascorbic acid, and diketo-l-gulonic acid in the guinea pig. J Biol Chem. 1952 Apr;195(2):599–606. [PubMed] [Google Scholar]
- DeChatelet L. R., McCall C. E., Cooper M. R., Shirley P. S. Ascorbic acid levels in phagocytic cells. Proc Soc Exp Biol Med. 1974 Apr;145(4):1170–1173. doi: 10.3181/00379727-145-37974. [DOI] [PubMed] [Google Scholar]
- ERICSSON Y., LUNDBECK H. Antimicrobial effect in vitro of the ascorbic acid oxidation. I. Effect on bacteria, fungi and viruses in pure cultures. Acta Pathol Microbiol Scand. 1955;37(6):493–506. doi: 10.1111/j.1699-0463.1955.tb00975.x. [DOI] [PubMed] [Google Scholar]
- ERICSSON Y., LUNDBECK H. Antimicrobial effect in vitro of the ascorbic acid oxidation. II. Influence of various chemical and physical factors. Acta Pathol Microbiol Scand. 1955;37(6):507–527. doi: 10.1111/j.1699-0463.1955.tb00976.x. [DOI] [PubMed] [Google Scholar]
- Himmelhoch S. R., Evans W. H., Mage M. G., Peterson E. A. Purification of myeloperoxidases from the bone marrow of the guinea pig. Biochemistry. 1969 Mar;8(3):914–921. doi: 10.1021/bi00831a022. [DOI] [PubMed] [Google Scholar]
- Karnovsky M. L. Chronic granulomatous disease--pieces of a cellular and molecular puzzle. Fed Proc. 1973 Apr;32(4):1527–1533. [PubMed] [Google Scholar]
- Klebanoff S. J. Iodination of bacteria: a bactericidal mechanism. J Exp Med. 1967 Dec 1;126(6):1063–1078. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J Bacteriol. 1968 Jun;95(6):2131–2138. doi: 10.1128/jb.95.6.2131-2138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Role of the superoxide anion in the myeloperoxidase-mediated antimicrobial system. J Biol Chem. 1974 Jun 25;249(12):3724–3728. [PubMed] [Google Scholar]
- LAHEY M. E., GUBLER C. J., CARTWRIGHT G. E., WINTROBE M. M. Studies on copper metabolism. VII. Blood copper in pregnancy and various pathologic states. J Clin Invest. 1953 Apr;32(4):329–339. doi: 10.1172/JCI102743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I. Antifungal effects of peroxidase systems. J Bacteriol. 1969 Aug;99(2):361–365. doi: 10.1128/jb.99.2.361-365.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infection. J Clin Invest. 1969 Aug;48(8):1478–1488. doi: 10.1172/JCI106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh H. S. The effects of vitamin C on the relationship between leucocyte and plasma ascorbic acid concentrations and the blood glutathione concentration. Int J Vitam Nutr Res. 1973;43(3):363–369. [PubMed] [Google Scholar]
- MYRVIK Q., LEAKE E. S., FARISS B. Studies on pulmonary alveolar macrophages from the normal rabbit: a technique to procure them in a high state of purity. J Immunol. 1961 Feb;86:128–132. [PubMed] [Google Scholar]
- Mandell G. L. Intraphagosomal pH of human polymorphonuclear neutrophils. Proc Soc Exp Biol Med. 1970 Jun;134(2):447–449. doi: 10.3181/00379727-134-34810. [DOI] [PubMed] [Google Scholar]
- McCall C. E., DeChatelet L. R., Cooper M. R., Ashburn P. The effects of ascorbic acid on bactericidal mechanisms of neutrophils. J Infect Dis. 1971 Aug;124(2):194–198. doi: 10.1093/infdis/124.2.194. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. The reduction of cytochrome c by milk xanthine oxidase. J Biol Chem. 1968 Nov 10;243(21):5753–5760. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. The utility of superoxide dismutase in studying free radical reactions. I. Radicals generated by the interaction of sulfite, dimethyl sulfoxide, and oxygen. J Biol Chem. 1969 Nov 25;244(22):6056–6063. [PubMed] [Google Scholar]
- Miller T. E. Killing and lysis of gram-negative bacteria through the synergistic effect of hydrogen peroxide, ascorbic acid, and lysozyme. J Bacteriol. 1969 Jun;98(3):949–955. doi: 10.1128/jb.98.3.949-955.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekarek R. S., Powanda M. C., Wannemacher R. W., Jr The effect of leukocytic endogenous mediator (LEM) on serum copper and ceruloplasmin concentrations in the rat. Proc Soc Exp Biol Med. 1972 Dec;141(3):1029–1031. doi: 10.3181/00379727-141-36926. [DOI] [PubMed] [Google Scholar]
- Simmons S. R., Karnovsky M. L. Iodinating ability of various leukocytes and their bactericidal activity. J Exp Med. 1973 Jul 1;138(1):44–63. doi: 10.1084/jem.138.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., Weber T. The vitamin B12-binding protein in human leukocytes. Biochim Biophys Acta. 1966 Mar 28;117(1):201–208. doi: 10.1016/0304-4165(66)90167-x. [DOI] [PubMed] [Google Scholar]
- Zeya H. I., Spitznagel J. K. Cationic proteins of polymorphonuclear leukocyte lysosomes. II. Composition, properties, and mechanism of antibacterial action. J Bacteriol. 1966 Feb;91(2):755–762. doi: 10.1128/jb.91.2.755-762.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


