Abstract
BACKGROUND
Immunofluorescence testing is an important tool for diagnosing blistering diseases.
OBJECTIVE
To characterize the immunofluorescence findings in patients diagnosed with autoimmune blistering skin diseases.
METHODS
We retrospectively analyzed immunofluorescence results encompassing a 10-year period.
RESULTS
421 patients were included and divided into 2 groups: group 1- intraepidermal blistering diseases (n=277) and 2- subepidermal blistering diseases (n=144). For group 1, positive DIF findings demonstrated: predominance of IgG intercellular staining (ICS) and C3 for pemphigus foliaceus-PF (94% and 73% respectively), pemphigus vulgaris-PV (91.5%-79.5%) and paraneoplastic pemphigus-PNP (66%-33%); ICS IgA in 100% of IgA pemphigus cases, and IgG deposits in the basement membrane zone (BMZ) along with ICS in one Hailey-Hailey patient. The IIF findings revealed mean titers of 1:2.560 for PV and 1:1.280 for PF. For paraneoplastic pemphigus, IIF was positive in 2 out of 3 cases with rat bladder substrate. In group 2, positive DIF findings included multiple deposits at basement membrane zone for epidermolysis bullosa acquisita-EBA (C3-89%,IgG-79%,IgA-47%,IgM-21%) mucous membrane pemphigoid-MMP (C3,IgG,IgA,IgM-80%) and bullous pemphigoid-BP (C3-91%,IgG-39%,IgA-11%,IgM-6%), and IgA at basement membrane zone for IgA linear disease (99%) and dermatitis herpetiformis-DH (dermal papillae in 84.6%). For lichen planus pemphigoides, there was C3 (100%) and IgG (50%) deposition at basement membrane zone. indirect immunofluorescence positive findings revealed basement membrane zone IgG deposits in 46% of BP patients, 50% for EBA, 15% for IgA linear dermatosis and 50% for LPP. Indirect immunofluorescence positive results were higher for BP and EBA with Salt-Split skin substrate.
CONCLUSION
Our results confirmed the importance of immunofluorescence assays in diagnosing autoimmune blistering diseases, and higher sensitivity for indirect immunofluorescence when Salt-split skin technique is performed.
Keywords: Autoimmune diseases; Epidermolysis bullosa acquisita; Linear IgA bullous dermatosis; Pemphigoid, benign mucous membrane; Pemphigoid, bullous; Pemphigoid gestationis; Skin Diseases; Skin diseases, vesiculobullous
INTRODUCTION
Immunofluorescence assays (IF) are an important tool for diagnosing acquired auto-immune blistering diseases, since they detect "in vivo" autoantibodies. There are two main subtypes: direct immunofluorescence (DIF), which is performed on perilesional skin or mucous membranes to detect tissue-bound autoantibodies; and indirect immunofluorescence (IIF), that quantifies a patient's circulating autoantibodies, utilizing human foreskin or monkey esophagus as substrates.1 Other sources of epithelial tissues, such as rat bladder (rich in desmoplaquin), are used to diagnose paraneoplastic pemphigus (PNP).2 Furthermore, there are additional IF assays such as Salt-Split Skin (SS), a higher sensitivity diagnostic tool for detecting antibasement membrane zone (BMZ) antibodies detection in subepidermal blistering dermatoses.3
IF techniques have emerged as useful methods for diagnosing autoimmune blistering conditions since the early 1960s, and are still considered efficient for this purpose, due to their role in differential diagnosis of indistinguishable clinical bullous diseases and their therapeutical implications.4,5 Furthermore, IF techniques are helpful in follow-ups and clarifying suspected cases of epitope spreading.6
Our study aimed to characterize retrospectively IF findings from a University hospital encompassing a 10-year period, concerning Brazilian patients diagnosed with autoimmune blistering conditions.
MATERIALS AND METHODS
We retrospectively analyzed the histopathological and immunofluorescence records of patients diagnosed with autoimmune bullous dermatoses, from the Autoimmune Blistering Disease Clinic, University of São Paulo Medical School, São Paulo, Brazil, between 01/01/ 2002 and 01/01/2012.
Inclusion criteria were: a histopathologic exam suggesting bullous dermatosis and a simultaneous DIF during admission. Patients were divided into two groups, according to the level of blistering formation: 1) intraepidermal blistering diseases (pemphigus foliaceus-PF, pemphigus vulgaris-PV, IgA pemphigus, paraneoplastic pemphigus-PNP and Hailey-Hailey disease); and 2) subepidermal blistering diseases (bullous pemphigoid-BP, epidermolysis bullosa acquisita-EBA, IgA linear dermatosis-LAD, dermatitis herpetiformis-DH, mucous membrane pemphigoid-MMP and lichen planus pemphigoid-LPP). DIF results were analyzed, according to autoantibody deposition (IgA, IgM, IgG and C3), and circulating IIF titers (IgG and IgA) were recorded, including SS, when performed.
RESULTS
Six hundred and six records from patients evaluated at the autoimmune blistering unit within the period of a decade were analyzed, and 421 were included, according to the aforementioned criteria.
In group 1 (intraepidermal blistering diseases), 277 individuals were included, and the most frequent diseases were: PV (51.2%- 142/277) and PF (40.8%- 117/277). As displayed in table 1, the positive DIF findings for group 1 were as follows:
TABLE 1.
Group 1: Direct and indirect immunofluorescence assays in intraepidermal blistering diseases
| Bullous disease (group 1) | n (patients) | n (positive DIF) | DIF: intercellular deposits (conjugate=n) | DIF: BMZ deposits (conjugate=n) | DIF: cytoid bodies (conjugate=n) | Positive IIF/Performed IIF |
|---|---|---|---|---|---|---|
| PF | 117 | 115 | IgG=110 | IgM=14 | IgM=6 | 104/115 |
| C3=85 | ||||||
| PV | 142 | 135 | IgG=130; | IgG-4;C3-8 | IgM-4 | 109/115 |
| C3=113 | ||||||
| IgM=11 | ||||||
| PNP | 3 | 2 | IgG=2 | C3=1 | - | 2/3 |
| C3=1 | ||||||
| IgA | 8 | 8 | IgG=1 | IgM=1 | - | 1/8 |
| IgA=8 | ||||||
| Hailey-Hailey disease | 7 | 1 | IgG=1 | IgG=1 | - | 0/5 |
Legend: DIF: direct immunofluorescence; IIF: indirect immunofluorescence; PF: pemphigus foliaceus, PV: pemphigus vulgaris, PNP: paraneoplastic pemphigus, IgA: IgA pemphigus.
PV (135/142): intercellular, intraepidermal IgG: 91.5% (130/142), and intercellular, intraepidermal C3 in 79.5% (113/142). Interestingly, 51% (73/142) of the patients showed C3 deposits in the lower levels of the epidermis;
PF (115/117): intercellular, intraepidermal IgG in 94% (110/117), and intercellular, intraepidermal C3 in 73% (85/117) (Figure 1);
PNP (3/3): intercellular, intraepidermal IgG in two patients (2/3), intercellular, intraepidermal C3 in one patient (1/3), and linear C3 deposits at the BMZ in another individual (1/3);
IgA pemphigus (8/8): intercellular, intraepidermal IgA deposits in 100% (8/8) with intercellular, intraepidermal IgG in 12.5% (1/8) and IgM deposits at the BMZ in 12.5% (1/8);
Hailey-Hailey disease (1/7): intercellular, intraepidermal and BMZ IgG deposits in one patient (1/7).
FIGURE 1.

Direct immunofluorescence staining patterns: A: intercellular intraepidermal staining; B: granular staining at dermal papillae; C: linear continuous basement membrane zone staining; D: homogeneous continuous basement membrane zone staining
IIF analysis was performed in 246 out of 277 patients from group 1, as shown in table 1:
PV: intercellular, intraepidermal IgG in 95% (109/115), titers above 1:2560 in 43.5% (50/115), and mean titer of 1/1280.
PF: intercellular, intraepidermal IgG in 90% (104/115), titers above 1:2560 in 49% (56/115), and mean titer of 1/2560.
PNP: intercellular, intraepidermal IgG in rat bladder epithelium in 66% (2/3).
IgA pemphigus: IgA in 12.5% (1/8).
Hailey-Hailey disease: negative in all cases performed (0/5).
In group 2 (subepidermal blistering diseases), 144 individuals were included. The most frequent diseases were BP (62.5%- 90/144) and EBA (13%-19/144).
As shown in table 2, positive DIF findings for group 2 were as follows:
TABLE 2.
Group 2: Direct and indirect immunofluorescence assays, including salt-split skin test in subepidermal blistering diseases
| Bullous disease (group 2) | n (patients) | n (positive DIF) | DIF: intercellular deposits (conjugate=n) | DIF: cytoid bodies (conjugate=n) | Positive IIF/Performed IIF | Salt-Split Skin Technique (conjugate=n/total) |
|---|---|---|---|---|---|---|
| BP | 90 | 88 | IgG=35 C3=82 | IgM=5 | 33/72 | Epidermal: |
| IgA=10 | IgG=25/33; | |||||
| IgM=6 | IgG/IgA=2/33 | |||||
| Both sides: | ||||||
| IgG=1/33, IgA-1/33 | ||||||
| Dermal side: | ||||||
| IgG/C3=1/33 | ||||||
| EBA | 19 | 19 | IgG=15 | IgM=1 | 9/18 | Dermal side: |
| C3=17 | IgG=5/13, | |||||
| IgM=4 | IgA/IgG=3/13 | |||||
| IgA=9 | ||||||
| MMP | 5 | 4 | IgG=4 | NA | 0/2 | NA |
| C3=4 | ||||||
| IgM=4 | ||||||
| IgA=4 | ||||||
| IgA linear dermatosis | 15 | 15 | IgG=2 | IgM=1 | 2/13 | Epidermal: IgA=4/5 |
| C3=2 | ||||||
| IgM=1 | ||||||
| IgA=15 | ||||||
| DH | 13 | 12 | IgG=1 | NA | 0/1 | NA |
| C3=2 | ||||||
| IgA=12 | ||||||
| LPP | 2 | 2 | IgG=1 | IgM=2 | 1/2 | IgG-epidermal=2/2; |
| C3=2 | focal epidermal | |||||
| IgA=1/2 |
Legend: DIF: direct immunofluorescence; IIF: indirect immunofluorescence; SS=salt-split skin technique; EBA: epidermolysis bullosa acquisita; MMP: mucous membrane pemphigoid; DH: dermatitis herpetiformis; LPP: lichen planus pemphigoides. NA: not available
BP (88/90): linear or homogeneous C3 deposits in 91% (82/90), IgG in 39% (35/90), IgA in 11% (10/90) and IgM in 6% (6/90) at the BMZ (Figure 1);
EBA (19/19): linear or homogeneous C3 in 89% (17/19), IgG in 79% (15/19), IgA in 47% (9/19) and IgM in 21% (4/19) at the BMZ;
Mucous membrane pemphigoid (MMP) (4/5): linear or homogeneous IgG, IgA, IgM and C3 in 80% (4/5) at the BMZ;
IgA linear dermatosis (15/15): linear IgA deposition in 66% (10/15) and homogeneous IgA deposition in 33% (5/15) at BMZ; linear C3 deposition in 13% (2/15) and homogeneous IgG deposition in 13% (2/15) at BMZ, as well as granulous IgM deposition at BMZ in 6.6% (1/15);
Dermatitis herpetiformis-DH (12/13): granular IgA deposition in the upper dermal papillae in 84.6% (11/13) and granular segmental IgA deposition at the BMZ in 7.6% (1/13). There were also IgG granulous deposits in 7.6% (1/13) and C3 in 15.4% (2/13) at the BMZ (Figure 1);
LPP: linear C3 deposits in 100% (2/2) and IgG deposits in 50% (1/2) at the BMZ; cytoid bodies at dermal papillae in 100% (2/2).
In group 2, 108 out of 144 individuals underwent IIF and 54 indirect SS.
Table 1 displays positive IIF findings for group 2 as follows:
BP: IgG in 46% (33/72) at BMZ (Figure 2). SS: epidermal side: IgG in 75.7% (25/33), IgG and IgA in 6% (2/33); epidermal and dermal sides: IgG in 3% (1/33) and IgA in 3% (1/33); only 1 patient out of 33 showed dermal IgG and C3 deposits.
EBA: IgG in 50% (9/18) at BMZ. SS: dermal side: IgG in 38.5% (5/13), IgA and IgG in 23% (3/13) (Figure 3).
MMP: IIF negative in the 2 cases performed. SS: none performed.
IgA linear dermatosis: IgA in 15% (2/13) at BMZ. SS: IgA on epidermal side in 80% (4/5).
DH: negative in one performed case.
LPP: IgG in 50% (1/2) at BMZ. SS: IgG on epidermal side in 100% (2/2) and focal IgA on epidermal side in 50% (1/2).
FIGURE 2.

Indirect immunofluorescence. Bullous pemphigoid. Linear IgG at basement membrane zone
FIGURE 3.
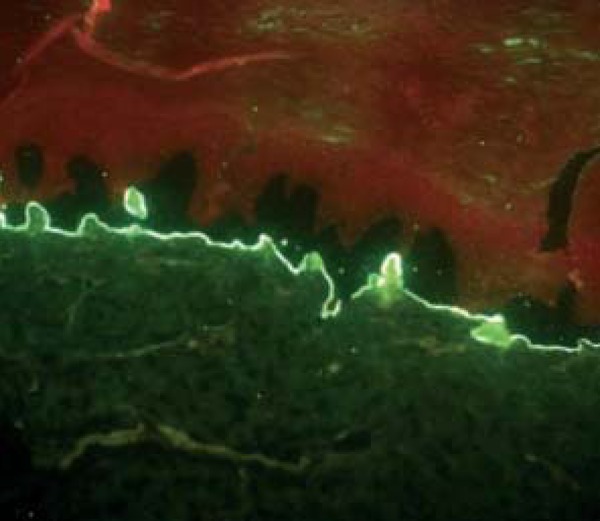
Salt-split skin. Epidermolysis bullosa acquisita. IgG on dermal side
DISCUSSION
Immunofluorescence microscopy is the gold standard for diagnosing autoimmune blistering diseases.7 The most common DIF findings for each autoimmune blistering dermatosis are summarized in table 3.1,5,7-9
TABLE 3.
Common findings in direct immunofluorescence for autoimmune blistering diseases
| DERMATOSIS | DIF FINDINGS | IIF FINDINGS |
| Pemphigus foliaceus/vulgaris | Intercellular IgG and C3. | Intercellular IgG |
| Paraneoplastic pemphigus | Weak intercellular IgG and linear C3; granulous BMZ deposits. | Intercellular IgG +/- linear BMZ. Epithelial surface staining on rat bladder |
| IgA pemphigus | Intercellular IgA | Intercellular IgA |
| Hailey-Hailey disease | Negative | Negative |
| Bullous pemphigoid | Linear IgG and C3 at BMZ. | Linear IgG at BMZ. SS: epidermal |
| Epidermolysis Bullosa Acquisita | Linear IgG and C3 at BMZ +/-IgA , IgM | Linear IgG. SS: dermal |
| Mucous membrane pemphigoid | Linear IgG, C3 and IgA at BMZ. | Linear IgG at BMZ |
| IgA linear dermatosis | Linear IgA at BMZ. | Linear IgA at BMZ. SS: epidermal, dermal (both) |
| Dermatitis herpetiformis | Granular IgA deposits in the dermal papillae +/- C3 | IgA class endomysial antibody |
| Lichen planus pemphigoid | Linear IgG and C3 at BMZ. Cytoid bodies. | Linear IgG at BMZ. SS: epidermal |
Our results were similar to those reported in the literature concerning intraepidermal blistering diseases (group 1): We found elevated positive DIF in more than 90% of the biopsied PV and PF patients, confirming the high sensitivity of this technique. In serology, elevated titers reported for PV and PF may correlate with earlier stages of disease or active disease.10 In PNP, positive IIF utilizing rat bladder epithelium as substrate obtained similar results (75% -86%) to those reported in previous studies.11,12
Notably, there was a predominance of C3 deposition in lower layers of the epidermis in 50% of our PV patients by DIF. Sanches Jr had already reported this finding and detected C3 deposits in epidermal basal layers in 73% of PV patients, reflecting preferential autoantibody deposition in the acantholysis zone, and stressing the amplifying role of complement in the pathogenesis of pemphigus.13
Uncommon results in our analysis included: 1-intercellular IgG deposition at DIF in one Hailey-Hailey patient with negative IIF, suggesting a possible complementary role for autoimmunity in this condition; 2-IgM deposits at the BMZ at DIF in 1 IgA pemphigus patient; 3- IIF positive in 12.5% in IgA pemphigus patients, differing from other reports that document up to 50% of circulating IgA antibodies.1,5
With respect to group 2, DIF results were similar to those already reported: predominant C3 and IgG deposition at BMZ for BP and multiple deposits at BMZ for EBA (Table 2).1,5 For dermatitis herpetiformis, we found IgA deposition at BMZ in 92% of cases, similar to the 92.4% described in a Mayo Clinic study. Equally, for LPP, our results were similar to those reported by Zaraa.14,15
Regarding IIF, our findings in group 2 were consonant with others for EBA, linear IgA dermatosis and MMP; however, for BP, we detected lower values than those reported.1,5 In contrast, LPP sera showed higher positive rates than those reported (100% versus 47%).15 This discrepancy may be due to technical issues, especially the nature of the substrate utilized. Salt-split skin technique increased IIF sensibility both in BP and EBA, and confirmed the predominance of epidermal deposits for BP.16
CONCLUSION
Our results confirmed the relevance of direct and indirect immunofluorescence assays as a complementary diagnostic tool in autoimmune blistering diseases. The Salt-split skin technique is a useful laboratory tool to improve test sensibility and establish a better diagnosis for these dermatoses.
Footnotes
Conflict of interests: None
Financial support: None
How to cite this article: Arbache ST, Nogueira TG, Delgado L, Miyamoto D, Aoki V. Immunofluorescence for the diagnosis of autoimmune blistering diseases: a 10-year Brazilian overview. An Bras Dermatol. 2014;89(6):885-9.
Work performed at the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (HC-FMUSP) - São Paulo (SP), Brasil.
REFERENCES
- 1.Aoki V, Sousa JX, Jr, Fukumori LM, Périgo AM, Freitas EL, Oliveira ZN. Direct and indirect immunofluorescence. An Bras Dermatol. 2010;85:490–500. doi: 10.1590/s0365-05962010000400010. [DOI] [PubMed] [Google Scholar]
- 2.Ortolan DG, Souza DP, Aoki V, Santi CG, Gabbi TV, Ichimura LM, et al. Clinics. Vol. 66. Sao Paulo: 2011. Analysis of the reactivity of indirect immunofluorescence in patients with pemphigus foliaceus and pemphigus vulgaris using rat bladder epithelium as a substrate; pp. 2019–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woodley DT. Immunofluorescence on salt-split skin for the diagnosis of epidermolysis bullosa acquisita. Arch Dermatol. 1990;126:229–231. [PubMed] [Google Scholar]
- 4.Beutner EH, Jordon RE. Demonstration of Skin Antibodies in Sera of Pemphigus Vulgaris Patients by Indirect Immunofluorescent Staining. Proc Soc Exp Biol Med. 1964;117:505–510. doi: 10.3181/00379727-117-29622. [DOI] [PubMed] [Google Scholar]
- 5.Mutasim DF, Adams BB. Immunofluorescence in dermatology. J Am Acad Dermatol. 2001;45:803–822. doi: 10.1067/mjd.2001.117518. [DOI] [PubMed] [Google Scholar]
- 6.Aoki V, Lago F, Yamazaki MH, Santi CG, Maruta CW. The Cooperative Grupo on Fogo Selvagem Research. Significance of epitope spreading in the pathogenesis of pemphigus vulgaris and foliaceus. An Bras Dermatol. 2008;83:5. [Google Scholar]
- 7.Jukić IL, Marinović B. Significance of immunofluorescence in the diagnosis of autoimmune bullous dermatoses. Clin Dermatol. 2011;29:389–397. doi: 10.1016/j.clindermatol.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Kneisel A, Hertl M. Autoimmune bullous skin diseases. Part 2: diagnosis and therapy. J Dtsch Dermatol Ges. 2011;9:927–947. doi: 10.1111/j.1610-0387.2011.07809.x. [DOI] [PubMed] [Google Scholar]
- 9.Benmously-Mlika R, Bchetnia M, Deghais S, Ben Brick SA, Charfeddine C, Debbiche A, et al. Hailey-Hailey disease in Tunisia. Int J Dermatol. 2010;49:396–401. doi: 10.1111/j.1365-4632.2010.04403.x. [DOI] [PubMed] [Google Scholar]
- 10.Aksu D, Peksari Y, Arica IE, Gurgey E. Assessing the autoantibody levels in relation to disease severity and therapy response in pemphigus patients. Indian J Dermatol. 2010;55:342–347. doi: 10.4103/0019-5154.74536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helou J, Allbritton J, Anhalt GJ. Accuracy of indirect immunofluorescence testing in the diagnosis of paraneoplastic pemphigus. J Am Acad Dermatol. 1995;32:441–447. doi: 10.1016/0190-9622(95)90066-7. [DOI] [PubMed] [Google Scholar]
- 12.Joly P, Richard C, Gilbert D, Courville P, Chosidow O, Roujeau JC, et al. Sensitivity and specificity of clinical, histologic, and immunologic features in the diagnosis of paraneoplastic pemphigus. J Am Acad Dermatol. 2000;43:619–626. doi: 10.1067/mjd.2000.107488. [DOI] [PubMed] [Google Scholar]
- 13.Sanches Junior JA. Imunofluorescência direta nos pênfigos vulgar e foliáceo endêmico (Fogo selvagem) - Estudo comparativo e revisão da literatura. São Paulo, SP: Universidade de São Paulo; 1995. [dissertação] [Google Scholar]
- 14.Alonso-Llamazares J, Gibson LE, Rogers RS., 3rd Clinical, pathologic, and immunopathologic features of dermatitis herpetiformis: review of the Mayo Clinic experience. Int J Dermatol. 2007;46:910–919. doi: 10.1111/j.1365-4632.2007.03214.x. [DOI] [PubMed] [Google Scholar]
- 15.Zaraa I, Mahfoudh A, Sellami MK, Chelly I, El Euch D, Zitouna M, et al. Lichen planus pemphigoides: four new cases and a review of the literature. Int J Dermatol. 2013;52:406–412. doi: 10.1111/j.1365-4632.2012.05693.x. [DOI] [PubMed] [Google Scholar]
- 16.Ghohestani RF, Nicolas JF, Rousselle P, Claudy AL. Diagnostic value of indirect immunofluorescence on sodium chloride-split skin in IFDferential diagnosis of subepidermal autoimmune bullous dermatoses. Arch Dermatol. 1997;133:1102–1107. [PubMed] [Google Scholar]


