Abstract
BACKGROUND
Trichoscopy is becoming increasingly popular in diagnosing hair and scalp diseases. Scalp involvement in pemphigus is common. The scalp may be the first or only site of clinical manifestation of the disease.
OBJECTIVE
The aim of this study was to analyze whether trichoscopy may be useful in aiding differential diagnosis of scalp lesions in patients with pemphigus vulgaris and pemphigus foliaceus.
METHODS
Trichoscopy was performed in 19 patients with scalp lesions in the course of pemphigus (9 patients with pemphigus vulgaris and 10 with pemphigus foliaceus). In all patients, the diagnosis of scalp pemphigus was confirmed by histopathology. The working magnification was 20-fold and 70-fold.
RESULTS
The most frequently observed trichoscopy features of pemphigus lesions were: extravasations (18/19; 94.7%) and yellow hemorrhagic crusts (11/19; 57.9%). Yellow dots with whitish halo were observed in 6/19 (31.6%) patients with pemphigus. White polygonal structures were observed in pemphigus foliaceus (6/10; 60%), but not in pemphigus vulgaris. Vascular abnormalities were more frequent in pemphigus vulgaris, when compared to pemphigus foliaceus, and were associated with a severe course of disease. Linear serpentine vessels were the most frequent vascular abnormality in patients with pemphigus vulgaris and pemphigus foliaceus (77.8% and 30%, respectively).
CONCLUSION
Trichoscopy may serve as a useful supplementary method in the differential diagnosis of pemphigus, especially in cases of desquamative or exudative lesions limited to the scalp. Extravasations, yellow hemorrhagic crusts, yellow dots with whitish halo, white polygonal structures and linear serpentine vessels are trichoscopy features which may suggest the diagnosis of pemphigus.
Keywords: Dermoscopy, Desmoglein 1, Desmoglein 3, Hair, Hair follicle, Pemphigus
INTRODUCTION
Pemphigus is a rare, potentially life-threatening autoimmune vesiculobullous disease affecting the skin and mucosa. It is characterized by the presence of circulating and in vivo-bound autoantibodies directed against desmogleins.1,2 Pemphigus vulgaris (PV) is characterized by blisters and erosions of the skin and/or mucous membranes, and circulating antibodies directed against desmoglein 3 and 1.1,2 In pemphigus foliaceus (PF), there is only skin involvement, without mucosal lesions, and antibodies are exclusively directed against desmoglein 1.3
Scalp involvement is often observed in patients with pemphigus vulgaris and pemphigus foliaceus.4,5 Frequently, the scalp is the first site of clinical manifestation of the disease.4,6 Isolated pemphigus lesions of the scalp have been reported in the literature.7-9 Occasionally, scalp lesions may be extremely resistant to treatment.10,11 Scalp lesions in the course of pemphigus can lead to several types of non-cicatricial and cicatricial alopecia.6,12-14 Thus, differential diagnosis of isolated scalp lesions and hair loss in the course of pemphigus
Trichoscopy (hair and scalp dermoscopy) is a useful tool in the differential diagnosis of hair loss and inflammatory scalp diseases.15 It is a non-invasive technique in which either a handheld dermoscope or a digital videodermoscope can be used to visualize hair and scalp structures.16 The method has well-established position as an ancillary tool in the diagnosis of such disorders as tinea capitis, alopecia areata, androgenetic alopecia, discoid lupus erythematosus, lichen planopilaris, folliculitis decalvans and other hair and scalp diseases.17-27 However, according to our literature search, there is only one case report on dermoscopy of pemphigus.28 The aim of our study was to evaluate trichoscopy features of scalp lesions in patients with pemphigus vulgaris and pemphigus foliaceus, and to establish the potential value of this method in the differential diagnosis of pemphigus.
MATERIAL AND METHODS
A total of 19 patients with scalp involvement in the course of pemphigus were included into the study (9 patients with pemphigus vulgaris and 10 with pemphigus foliaceus). The diagnosis of pemphigus was based on physical examination, histopathology of skin lesions, direct and indirect immunofluorescence results, and presence of anti-desmoglein 1 or 3 antibodies in enzyme-linked immunosorbent test (ELISA). In all patients the diagnosis of scalp pemphigus was confirmed by histopathology and direct immunofluorescence.
Patients with paraneoplastic pemphigus were not included into the study.29 The group of patients with PV consisted of 4 women and 5 men, aged 18-89, with pemphigus lasting 4 months to 19 years, and scalp involvement lasting from 4 months to 6 years. The group of patients with PF consisted of 3 women and 7 men, aged 44-88 years, with pemphigus lasting from 4 months to 16 years, and scalp involvement lasting from 2 months to 11 years. The extent of scalp lesions was determined according to the Pemphigus Disease Area Index for the scalp (Table 1).30
TABLE 1.
Pemphigus Disease Area Index for the scalp
| Score | Erosion/Blisters or new erythema |
|---|---|
| 0 | Absent |
| 1 | In one quadrant |
| 2 | In two quadrants |
| 3 | In three quadrants |
| 4 | Affects whole skull |
| 10 | At least one lesion > 6 cm |
At the time of the scalp examination, skin lesions were observed in 4/9 (44,4%) patients with pemphigus vulgaris, whereas mucosal lesions were present in 6/9 (66,7%) patients. Skin lesions were present in 8/10 (80%) patients with pemphigus foliaceus. Mucosal lesions were not present in these patients.
A total of 5/9 (55,6%) patients with PV and 5/10 (50%) patients with PF were newly diagnosed with no previous therapy. In other cases trichoscopy was performed during a clinical and immunological recurrence in the course of immunosuppressive therapy that consisted of prednisone and azathioprine in PV and of prednisone in monotherapy in PF.
Trichoscopy was performed with the use of the Fotofinder 2 Videodermoscopy System, with a 20-fold and 70-fold working magnification. Trichoscopy abnormalities in patients with pemphigus were examined according to the standard protocol and definitions.20
RESULTS
In all patients with pemphigus vulgaris trichoscopy showed extravasations within the scalp lesions (9/9; 100%) (Figure 1). Yellow hemorrhagic crusts were observed in 5/9 (55.6%) patients. Linear serpentine vessels were observed in 7/9 (77.8%) patients. Lace-like vessels were observed in 5/9 (55.6%) patients. Linear helical vessels, as well as glomerular vessels and dotted vessels with whitish halo were observed less frequently, but corresponded to the highest PDAI for the scalp. Additionally, in one patient we observed an area of glomerular vessels aligned in rings, forming "a string of pearls"-like arrangement. Detailed data are presented in Table 2.
FIGURE 1.
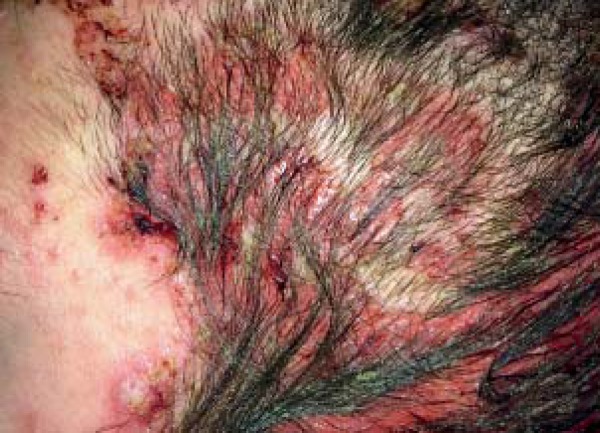
Scalp involvement in pemphigus vulgaris
TABLE 2.
Trichoscopy features of scalp lesions in patients with pemphigus vulgaris (PV) and pemphigus foliaceus (PF)
| PV | PF | |
|---|---|---|
| Sex (F/M) | 4/5 | 3/7 |
| Age | 58.8 +/-19.7 | 64.2 +/- 16.4 |
| Duration of the disease [years] | 7.3+/-10.6 | 3.8+/- 5.3 |
| Duration of the scalp involvement [years] | 2.1+/-2.4 | 2.9 +/- 4.0 |
| PDAI - scalp | 4.2+/-4.4 | 3.4 +/- 3.6 |
| PDAI - skin | 5.6+/-14.1 | 15.3+/-24.9 |
| PDAI - mucous membranes | 6.9+/-14.6 | 0/0 |
| Dotted vessels | 1/9 (11.1%) | 0 |
| Dotted vessels with whitish halo | 2/9 (22.2%) | 0 |
| Thin arborizing vessels | 2/9 (22.2%) | 2/10 (20%) |
| Thick arborizing vessels | 1/9 (11.1%) | 0 |
| Yellow hemorrhagic crusts | 5/9 (55.6%) | 6/10 (60%) |
| Extravasations | 9/9 (100%) | 9/10 (90%) |
| Linear helical vessels | 2/9 (22.2%) | 1/10 (10%) |
| Lace-like vessels | 5/9 (55.6%) | 2/10 (20%) |
| Glomerular vessels | 2/9 (22.2%) | 1/10 (10%) |
| Linear serpentine vessels | 7/9 (77.8%) | 3/10 (30%) |
| White diffuse scaling | 4/9 (44.4%) | 10/10 (100%) |
| Yellow diffuse scaling | 2/9 (22.2%) | 3/10 (30%) |
| Tubular scaling | 0 | 2/10 (20%) |
| White perifollicular areas "white veil" | 3/9 (33.3%) | 0 |
| Pink areas | 2/9 (22.2%) | 0 |
| Perifollicular yellow areas | 3/9 (33.3%) | 2/10 (20%) |
| Black dots | 1/9 (11.1%) | 0 |
| Yellow dots | 7/8 (77.8%) | 3/30 (30%) |
| White dots | 2/9 (22.2%) | 0 |
| Yellow dots with whitish halo, 'fried egg sign' | 4/9 (44.4%) | 2/10 (20%) |
| White dots with yellow halo 'reversed fried egg' | 0 | 2/20 (20%) |
| Hair casts | 1/9 (11.1%) | 2/10 (20%) |
| White polygonal structures | 0 | 6/10 (60%) |
PDAI = Pemphigus Disease Area Index
Extravasations were observed in 9/10 (90 %) patients with pemphigus foliaceus (Figure 2). Yellow hemorrhagic crusts were observed in 6/10 (60%) patients. The most common type of vessels were linear serpentine vessels, observed in 3/10 (30%) patients. Linear helical vessels and glomerular vessels were present in 2 patients with a PDAI score for the scalp equal to 10.
FIGURE 2.
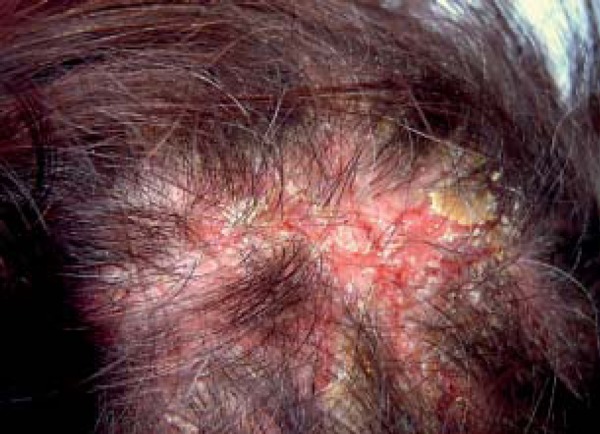
Scalp involvement in pemphigus foliaceus
Various types of scaling were observed in patients with pemphigus foliaceus, namely: white diffuse scaling (in all patients), white polygonal structures (60% of patients), yellow diffuse scaling (30%), tubular perifollicular scaling and hair casts (20%). Yellow dots with whitish halo were observed in 2/10 (20%) patients. The most characteristic trichoscopy findings are illustrated in Figure 3. Detailed data are presented in table 2.
FIGURE 3.
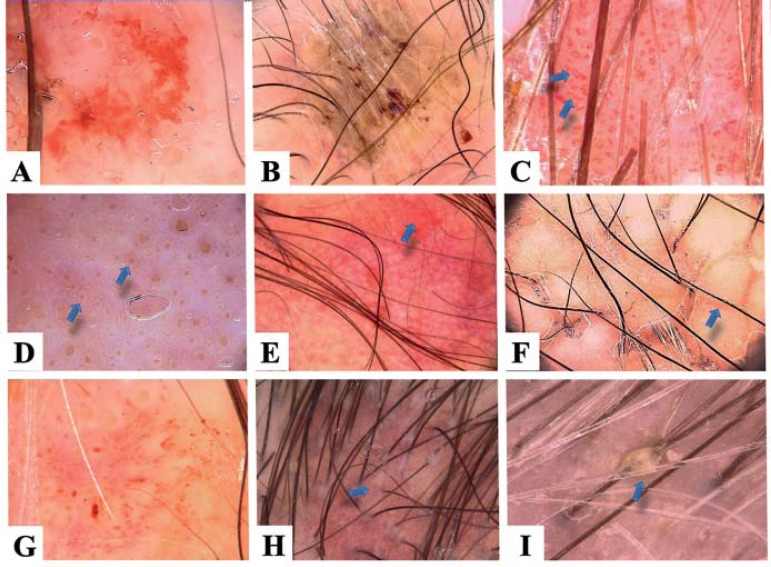
Trichoscopy features of pemphigus: extravasations (A), yellow hemorrhagic crusts (B), serpentine vessels (C), yellow dots with whitish halo – the ‘fried egg sign’ (D), circular alignment of vessels (E), white polygonal structures (F), irregularly distributed blood vessels (G), tubular scaling (H) and hair casts (I)
DISCUSSION
Differential diagnosis of scalp lesions in the course of pemphigus vulgaris and pemphigus foliaceus may be a diagnostic challenge, especially when they are the first or sole cutaneous manifestation of disease. Pemphigus of the scalp is clinically manifested by cutaneous inflammation with crusts and intense scaling.4 These changes may be accompanied by erosions, but blisters are usually not present.4 Less common manifestations of pemphigus of the scalp are verrucous, vegetating plaques and tufted folliculitis.10,31 These inflammatory changes are commonly associated with cicatrical or non-cicatricial alopecia.32,33 Thus, it seems apparent that trichoscopy may potentially serve as a rapid and easy method for the initial differential diagnosis of scalp lesions in such cases.
There are currently no literature data addressing the usefulness of trichoscopy (or dermoscopy in general) in the differential diagnosis of pemphigus, except a case report of hair casts in a 57-year-old patient with pemphigus vulgaris.28 Hair casts are 3-7-mm-long, whitish or yellowish tubular structures which envelop the hair shafts.16,34 In this first description, Pirmez suggested that hair casts develop in pemphigus vulgaris in a mechanism associated with acantholysis within the outer root sheet.28 As the hair grows, the outer root sheath keratinocytes move up through the follicular openings to form the hair cast. This phenomenon could be considered a Nikolsky's sign in the scalp hair follicles12 and may be explained by the distribution of desmoglein 3 within outer root sheath. Desmoglein 3 is expressed throughout all layers of outer root sheath, in areas of tricholemmal keratinization, and in the basal layer of the outer root sheath in areas of epidermal keratinization.35 The hypothesis of Pirmez may be confirmed by the findings of Delmonte et al 12, who described three cases of pemphigus vulgaris in whom the hairs plucked from both lesional and perilesional skin were anagen hairs with intact root sheaths. In our study hair casts were observed in only 11.1% of patients with pemphigus vulgaris and in 20% of patients with pemphigus foliaceus. In all cases the presence of hair casts was associated with long-lasting diffuse scaling, but average PDAI for scalp was 4.3, what reflects intermediate activity of scalp involvement in the course pemphigus. Our data may indicate that hair casts in pemphigus are rather a sign of severe diffuse scaling (similar to Pityriasis amiantacea) than a direct reflection of acantholysis.36
In our study two types of hair casts could be distinguished: 1) poorly-defined, whitish, cotton-wool-like hair casts in pemphigus vulgaris and 2) well-defined, yellowish or whitish hair casts in pemphigus foliaceus.
The most consistent trichoscopy findings in patients with pemphigus were extravasations, appearing as red hemorrhagic polygonal structures. This feature was observed in all patients with pemphigus vulgaris and pemphigus foliaceus. This finding is not specific for pemphigus. Extravasations may be occasionally observed in other inflammatory scalp diseases, such as psoriasis, seborrheic dermatitis, pemphigoid, pediculosis and skin cancers.16,37,38 However, the results of this study and our experience show that an abundance of extravasations in all fields of view of a dermoscope may be indicative for an autoimmune bullous disease.
Yellow hemorrhagic crusts were observed frequently in the examined scalp lesions. The presence of yellow hemorrhagic crusts is not specific for pemphigus. It is a common trichoscopy finding in diseases associated with pus secretion, such as folliculitis decalvans or dissecting cellulitis.21,23 It may be considered whether such a trichoscopy finding in patients with pemphigus should be an indication for antibacterial treatment.
A common feature of scalp lesions in pemphigus was the presence of white diffuse scaling, occurring in 74% of patients (44% in pemphigus vulgaris and 100% in pemphigus foliaceus). Diffuse white scaling is a non-specific finding. It may occur in psoriasis, discoid lupus erythematosus, allergic dermatitis and dry skin.39,40 Yellow diffuse scaling was slightly more common in patients with pemphigus foliaceus, when compared to pemphigus vulgaris (40% and 22.2%, respectively). Yellow diffuse scaling also occurs in seborrheic dermatitis, discoid lupus erythematosus and ichthyosis.22,41,42 In 60% of patients with pemphigus foliaceus, the scaling had an appearance of white polygonal structures. Polygonal white structures were not observed in pemphigus vulgaris and were not previously described in other scalp disorders in the literature. This is a novel finding in our study.
In patients with pemphigus vulgaris, trichoscopy with immersion fluid revealed white cotton- wool-like areas, which correlated positively with the extension of the scalp involvement (PDAI 10). These type of structures have been shown to be a trichoscopy manifestation of scales observed with immersion fluid.16
Another common trichoscopy feature of scalp pemphigus was the presence of yellow dots. Yellow dots are a trichoscopy manifestation of empty hair follicles filled with keratinous material and sebum.27,43 In our study yellow dots were observed more frequently in pemphigus vulgaris (77.8%), when compared to pemphigus foliaceus (40%).
A novel trichoscopy finding in this study was the detection of yellow dots with a whitish halo. It may be hypothesized that these are empty hair follicles surrounded by an acanthotic outer root sheath. This trichoscopy finding has not been previouly described in the literature. We suggest that this finding is referred to as "fried egg sign". It is specific for pemphigus. A reversed version of this sign (white dots with yellow halo) were observed only in 20% of patients and only in pemphigus foliaceus.
Vascular abnormalities were more frequently observed in patients with pemphigus vulgaris, when compared to pemphigus foliaceus. Linear serpentine vessels occurred in pemphigus vulgaris and pemphigus foliaceus in 77.8% and 40% of patients, respectively. Moreover, the presence of vascular abnormalities (especially linear helical vessels and glomerular vessels) correlated with high Pemphigus Disease Area Index for the scalp and severe course of the disease. There are no literature data on dermoscopic or trichoscopic features of blood vessels in pemphigus. However, Kurzeja et al described vasodilatation in reflectance confocal microscopy of the upper dermis in 61%-86% of cutaneous lesions in patients with pemphigus.44
CONCLUSION
In conclusion, trichoscopy is a useful tool in the differential diagnosis of pemphigus, especially in cases of desquamative and exudative lesions limited to the scalp. Immunological examinations are the gold standard for diagnosing pemphigus, but trichoscopy may serve as a non-time consuming technique that may facilitate the initial diagnosis and help choosing an optimal site to obtain a biopsy specimen.45 Extravasations and yellow hemorrhagic crusts were the most frequent findings in scalp lesions of patients with pemphigus. However, the "fried egg sign" was identified as a new, trichoscopy- specific feature of pemphigus.
ACKNOWLEDGEMENTS
The study has been supported by the research grant No NN402371538 from the National Science Centre in Poland.
The authors thank Dr. Maria Victória Quaresma for her help in preparing the abstract in Portuguese
Footnotes
Conflict of interest: None
Financial funding: None
How to cite this article: Sar-Pomian M, Kurzeja M, Rudnicka L, Olszewska M. The value of trichoscopy in the differential diagnosis of scalp lesions in pemphigus vulgaris and pemphigus foliaceus. An Bras Dermatol. 2014;89(6):1007-12.
Study conducted at the Department of Dermatology, Medical University of Warsaw - Warszawa, Poland.
References
- 1.Cunha PR, Barraviera SR. Autoimmune bullous dermatoses. An Bras Dermatol. 2009;84:111–124. doi: 10.1590/s0365-05962009000200003. [DOI] [PubMed] [Google Scholar]
- 2.Porro AM, Caetano Lde V, Maehara Lde S, Enokihara MM. Non-classical forms of pemphigus: pemphigus herpetiformis, IgA pemphigus, paraneoplastic pemphigus and IgG/IgA pemphigus. An Bras Dermatol. 2014;89:96–106. doi: 10.1590/abd1806-4841.20142459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruocco V, Ruocco E, Lo Schiavo A, Brunetti G, Guerrera LP, Wolf R. Pemphigus: etiology, pathogenesis, and inducing or triggering factors: facts and controversies. Clin Dermatol. 2013;31:374–381. doi: 10.1016/j.clindermatol.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Sar-Pomian M, Kolacinska-Strasz Z, Labecka H, Krainska-Wojcik T, Olszewska M. Scalp lesions in pemphigus. Przegl Dermatol. 2010;97:14–20. [Google Scholar]
- 5.Oretti G, Giordano D, Di Lella F, Gradoni P, Zendri E, Ferri T. Unilesional pemphigus vulgaris of the scalp after cochlear implantation. Am J Otolaryngol. 2011;32:80–81. doi: 10.1016/j.amjoto.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Hadayer N, Ramot Y, Maly A, Zlotogorski A. Pemphigus vulgaris with loss of hair on the scalp. Int J Trichology. 2013;5:157–158. doi: 10.4103/0974-7753.125618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lehrhoff S, Miller K, Fischer M, Kamino H, Meehan S. Localized pemphigus with vegetative features. Dermatol Online J. 2012:18, 11. [PubMed] [Google Scholar]
- 8.Yamamoto S, Kanekura T, Gushi A, Sekiyama M, Shimada T, Shimada K, et al. A case of localized pemphigus foliaceus. J Dermatol. 1996;23:893–895. doi: 10.1111/j.1346-8138.1996.tb02721.x. [DOI] [PubMed] [Google Scholar]
- 9.Lapière K, Caers S, Lambert J. A case of long-lasting localized pemphigus vulgaris of the scalp. Dermatology. 2004;209:162–163. doi: 10.1159/000079607. [DOI] [PubMed] [Google Scholar]
- 10.Ko DK, Chae IS, Chung KH, Park JS, Chung H. Persistent pemphigus vulgaris showing features of tufted hair folliculitis. Ann Dermatol. 2011;23:523–525. doi: 10.5021/ad.2011.23.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olszewska M, Kolacinska-Strasz Z, Sulej J, Labecka H, Cwikla J, Natorska U, et al. Efficacy and safety of cyclophosphamide, azathioprine, and cyclosporine (ciclosporin) as adjuvant drugs in pemphigus vulgaris. Am J Clin Dermatol. 2007;8:85–92. doi: 10.2165/00128071-200708020-00004. [DOI] [PubMed] [Google Scholar]
- 12.Delmonte S, Semino MT, Parodi A, Rebora A. Normal anagen effluvium: a sign of pemphigus vulgaris. Br J Dermatol. 2000;142:1244–1245. doi: 10.1046/j.1365-2133.2000.03563.x. [DOI] [PubMed] [Google Scholar]
- 13.Petronić-Rosić V, Krunić A, Mijusković M, Vesić S. Tufted hair folliculitis: a pattern of scarring alopecia? J Am Acad Dermatol. 1999 Jul;41:112–114. doi: 10.1016/s0190-9622(99)70417-2. [DOI] [PubMed] [Google Scholar]
- 14.Saijyo S, Tagami H. Tufted hair folliculitis developing in a recalcitrant lesion of pemphigus vulgaris. J Am Acad Dermatol. 1998;38:857–859. doi: 10.1016/s0190-9622(98)70475-x. [DOI] [PubMed] [Google Scholar]
- 15.Rudnicka L, Olszewska M, Rakowska A, Kowalska-Oledzka E, Slowinska M. Trichoscopy: a new method for diagnosing hair loss. J Drugs Dermatol. 2008;7:651–654. [PubMed] [Google Scholar]
- 16.Rudnicka L, Olszewska M, Rakowska A. Atlas of trichoscopy: dermoscopy in hair and scalp disease. London: Springer; 2012. [Google Scholar]
- 17.Slowinska M, Rudnicka L, Schwartz RA, Kowalska-Oledzka E, Rakowska A, Sicinska J, et al. Comma hairs: a dermatoscopic marker for tinea capitis: a rapid diagnostic method. J Am Acad Dermatol. 2008;59:S77–S79. doi: 10.1016/j.jaad.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Pinheiro AM, Lobato LA, Varella TC. Dermoscopy findings in tinea capitis: case report and literature review. An Bras Dermatol. 2012;87:313–314. doi: 10.1590/s0365-05962012000200022. [DOI] [PubMed] [Google Scholar]
- 19.Abraham LS, Torres FN, Azulay-Abulafia L. Dermoscopic clues to distinguish trichotillomania from patchy alopecia areata. An Bras Dermatol. 2010;85:723–726. doi: 10.1590/s0365-05962010000500022. [DOI] [PubMed] [Google Scholar]
- 20.Rakowska A, Slowinska M, Kowalska-Oledzka E, Olszewska M, Rudnicka L. Dermoscopy in female androgenic alopecia: method standardization and diagnostic criteria. Int J Trichology. 2009;1:123–130. doi: 10.4103/0974-7753.58555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rakowska A, Slowinska M, Kowalska-Oledzka E, Warszawik O, Czuwara J, Olszewska M, et al. Trichoscopy of cicatricial alopecia. Drugs Dermatol. 2012;11:753–758. [PubMed] [Google Scholar]
- 22.Duque-Estrada B, Tamler C, Sodré CT, Barcaui CB, Pereira FB. Dermoscopy patterns of cicatricial alopecia resulting from discoid lupus erythematosus and lichen planopilaris. An Bras Dermatol. 2010;85:179–183. doi: 10.1590/s0365-05962010000200008. [DOI] [PubMed] [Google Scholar]
- 23.Fabris MR, Melo CP, Melo DF. Folliculitis decalvans: the use of dermatoscopy as an auxiliary tool in clinical diagnosis. An Bras Dermatol. 2013;88:814–816. doi: 10.1590/abd1806-4841.20132129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rudnicka L, Olszewska M, Rakowska A, Slowinska M. Trichoscopy update 2011. J Dermatol Case Rep. 2011;5:82–88. doi: 10.3315/jdcr.2011.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oliveira LC, Miranda AR, Pinto SA, Ianhez M. Case for diagnosis. An Bras Dermatol. 2014;89:353–355. doi: 10.1590/abd1806-4841.20142740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pirmez R, Piñeiro-Maceira J, Almeida BC, Sodré CT. Follicular red dots: a normal trichoscopy feature in patients with pigmentary disorders? An Bras Dermatol. 2013;88:459–461. doi: 10.1590/abd1806-4841.20132555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torres F, Tosti A. Trichoscopy: an update. G Ital Dermatol Venereol. 2014;149:83–91. [PubMed] [Google Scholar]
- 28.Pirmez R. Acantholytic hair casts: a dermoscopic sign of pemphigus vulgaris of the scalp. Int J Trichology. 2012;4:172–173. doi: 10.4103/0974-7753.100087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silva JA, Mesquita Kde C, Igreja AC, Lucas IC, Freitas AF, Oliveira SM, et al. Paraneoplastic cutaneous manifestations: concepts and updates. An Bras Dermatol. 2013;88:9–22. doi: 10.1590/S0365-05962013000100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rahbar Z, Daneshpazhooh M, Mirshams-Shahshahani M, Esmaili N, Heidari K, Aghazadeh N, et al. Pemphigus disease activity measurements: pemphigus disease area index, autoimmune bullous skin disorder intensity score, and pemphigus vulgaris activity score. JAMA Dermatol. 2014;150:266–272. doi: 10.1001/jamadermatol.2013.8175. [DOI] [PubMed] [Google Scholar]
- 31.Danopoulou I, Stavropoulos P, Stratigos A, Chatziolou E, Chiou A, Georgala S, et al. Pemphigus vegetans confined to the scalp. Int J Dermatol. 2006;45:1008–1009. doi: 10.1111/j.1365-4632.2006.02824.x. [DOI] [PubMed] [Google Scholar]
- 32.Veraitch O, Ohyama M, Yamagami J, Amagai M. Alopecia as a rare but distinct manifestation of pemphigus vulgaris. J Eur Acad Dermatol Venereol. 2013;27:86–91. doi: 10.1111/j.1468-3083.2011.04363.x. [DOI] [PubMed] [Google Scholar]
- 33.Gaitanis G, Patmanidis K, Skandalis K, Alexis I, Zioga A, Bassukas ID. Scaring alopecia in pemphigus vulgaris: a rare or underdiagnosed presentation? Eur J Dermatol. 2013;23:253–255. doi: 10.1684/ejd.2013.1954. [DOI] [PubMed] [Google Scholar]
- 34.França K, Villa RT, Silva IR, de Carvalho CA, Bedin V. Hair casts or pseudonits. Int J Trichology. 2011;3:121–122. doi: 10.4103/0974-7753.90834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu H, Stanley JR, Cotsarelis G. Desmoglein isotype expression in the hair follicle and its cysts correlates with type of keratinization and degree of differentiation. J Invest Dermatol. 2003;120:1052–1057. doi: 10.1046/j.1523-1747.2003.12234.x. [DOI] [PubMed] [Google Scholar]
- 36.Verardino GC, Azulay-Abulafia L, Macedo PM, Jeunon T. Pityriasis amiantacea: clinical-dermatoscopic features and microscopy of hair tufts. An Bras Dermatol. 2012;87:142–145. doi: 10.1590/s0365-05962012000100021. [DOI] [PubMed] [Google Scholar]
- 37.Martins LG, Bernardes F, Filho, Quaresma MV, Bellott TR, Botelho LN, Prata AC. Dermoscopy applied to pediculosis corporis diagnosis. An Bras Dermatol. 2014;89:513–514. doi: 10.1590/abd1806-4841.20142654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardynal A, Olszewska M. Modern non-invasive diagnostic techniques in the detection of early cutaneous melanoma. J Dermatol Case Rep. 2014;8:1–8. doi: 10.3315/jdcr.2014.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim KS, Shin MK, Ahn JJ, Haw CR, Park HK. Investigation of hair shaft in seborrheic dermatitis using atomic force microscopy. Skin Res Technol. 2011;17:288–294. doi: 10.1111/j.1600-0846.2010.00495.x. [DOI] [PubMed] [Google Scholar]
- 40.Miteva M, Tosti A. Dermatoscopy of hair shaft disorders. J Am Acad Dermatol. 2013;68:473–481. doi: 10.1016/j.jaad.2012.06.041. [DOI] [PubMed] [Google Scholar]
- 41.Haliasos EC, Kerner M, Jaimes-Lopez N, Rudnicka L, Zalaudek I, Malvehy J, et al. Dermoscopy for the pediatric dermatologist part I: dermoscopy of pediatric infectious and inflammatory skin lesions and hair disorders. Pediatr Dermatol. 2013;30:163–171. doi: 10.1111/pde.12097. [DOI] [PubMed] [Google Scholar]
- 42.Haliasos EC, Kerner M, Jaimes N, Zalaudek I, Malvehy J, Lanschuetzer CM, et al. Dermoscopy for the pediatric dermatologist, part ii: dermoscopy of genetic syndromes with cutaneous manifestations and pediatric vascular lesions. Pediatr Dermatol. 2013;30:172–181. doi: 10.1111/j.1525-1470.2012.01874.x. [DOI] [PubMed] [Google Scholar]
- 43.Ross EK, Vincenzi C, Tosti A. Videodermoscopy in the evaluation of hair and scalp disorders. J Am Acad Dermatol. 2006;55:799–806. doi: 10.1016/j.jaad.2006.04.058. [DOI] [PubMed] [Google Scholar]
- 44.Kurzeja M, Rakowska A, Rudnicka L, Olszewska M. Criteria for diagnosing pemphigus vulgaris and pemphigus foliaceus by reflectance confocal microscopy. Skin Res Technol. 2012;18:339–346. doi: 10.1111/j.1600-0846.2011.00574.x. [DOI] [PubMed] [Google Scholar]
- 45.Murrell DF, Dick S, Ahmed AR, Amagai M, Barnadas MA, Borradori L, et al. Consensus statement on definitions of disease, end points, and therapeutic response for pemphigus. J Am Acad Dermatol. 2008;58:1043–1046. doi: 10.1016/j.jaad.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]