Abstract
Using the Surveillance, Epidemiology and End Results database we identified 43,882 (97.0%) women with endometrioid adenocarcinomas and 1374 (3.0%) with mucinous adenocarcinomas. Women with mucinous tumors were older (P<0.0001), more often white (P=0.04), and more often to present at advanced stage (P=0.001). Survival was similar for both histologies; the hazard ratio for cancer-specific survival for mucinous compared to endometrioid tumors was 0.90 (95% CI, 0.74-1.09) while the hazard ratio for overall survival was 0.95 (95% CI, 0.85-1.07). Five-year survival for stage I mucinous tumors was 89.9% (95% CI, 87.6-91.9%) compared to 89.0% (95% CI, 88.6-89.4%) for endometrioid tumors.
Introduction
Endometrial cancer is the most common gynecologic malignancy with over 47,000 cases diagnosed in 2012.1 Endometrial tumors may be broadly classified as endometriod or non-endometrioid. Endometrioid tumors are most prominent histologic subtype and are associated with a favorable prognosis when confined to the uterus. Mucinous tumors of the endometrium are a rare histologic variant.2,3 While case reports endometrial neoplasms with mucin production can be identified in the literature as early as 1950s, it was not until the three cases reported by Tiltman et al. that mucinous carcinoma of the endometrium was identified as a distinct clinical entity.4
The present diagnostic criteria for mucinous carcinoma of the endometrium was derived from the work of Ross et al. who published a series of 21 cases.3 The tumor architecture is usually glandular or villoglandular and consists of at least 50% columnar or pseudostratified epithelial cells containing intracytoplasmic mucin. These tumors closely resemble mucinous tumors of the ovary or endocervix. The cells are positive for carcinoembryonic antigen, mucicarmine, and periodic acid-Schiff stain and are diastase resistant. An endocervical sampling is necessary to distinguish mucinous endometrial tumor from similar appearing mucinous endocervical adenocarcinomas3. These tumors are generally well differentiated.2-4
On account of its rarity, much of what is known about the natural history and management of mucinous endometrial carcinomas has been derived from case series, most of which were published prior to the development of current standard treatment protocols. Musa et al. recently published a case control study consisting of 41 patients at a single institution treated according to current protocols. In this series, mucinous histology was independently associated with an increased risk of lymph node metastasis. Survival, however, was similar for the mucinous and endometrioid tumors.2 Given the paucity of data on the prognostic significance of mucinous endometrial carcinoma, we performed a population-based analysis to examine the natural history and outcome of mucinous carcinoma of the endometrium.
Materials and Methods
Data from the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) database was utilized. SEER is a population-based registry encompassing 17 geographically distinct tumor registries that include approximately 26% of the United States population.5 SEER collects all cases of incident cancer within the defined registries. SEER has been utilized in a number of studies examining quality of care and treatment outcomes.6-8
Women diagnosed with stage I-IV endometrial cancer 1988 to 2006 were analyzed. Patients were classified based on their tumor histology into the following groups: mucinous or endometrioid carcinoma. Demographic data collected included age at diagnosis (< 60, >60 years), race (white, black or other or unknown), and marital status (married, single, unknown). The year of diagnosis was classified as 1988–1994, 1995–2000, or 2001–2006. The geographic residence at the time of diagnosis was categorized into one of the following United States regions: Eastern (Connecticut, New Jersey, Atlanta, rural Georgia) Central (Detroit, Iowa, Kentucky, Louisiana, Utah), and Western (Alaska, California, Hawaii, Los Angeles, New Mexico, San Francisco, San Jose, Seattle). Tumor grade (1, 2, 3, or unknown) was recorded for each patient. Stage was assigned based on the reported SEER extent of disease codes and American Joint Cancer Committee (AJCC) criteria. Whether lymphadenectomy and adjuvant radiotherapy therapy were performed were also recorded.
Frequency distributions between categorical variables were compared using χ2 tests. The vital status of each patient was recorded. Survival was calculated as the number of months from cancer diagnosis to date of death. Patients who were alive at last follow-up were censored. Both overall and cancer-specific survivals were calculated. Cox proportional hazards models were developed to examine the influence of tumor histology on survival while correcting for other clinical and demographic variables. Additionally, survival was examined using the Kaplan-Meier method and compared using the log-rank tets. Separate Kaplan-Meier analyses were developed for stage I and III patients. All analyses were performed with SAS version 9.2 (SAS Institute Inc., Cary, North Carolina). A P-value of <0.05 was considered statistically significant.
Results
A total of 45,229 patients were identified including 1374 (3.0%) with mucinous endometrial tumors and 43,882 (97%) women with endometrioid adenocarcinoma. The demographic characteristics of this cohort are displayed in table 1. At the time of diagnosis, women with mucinous tumors were older (P<0.0001) and more often white (P=0.04) than those with endometrioid carcinomas. The frequency of endometrioid tumors increased over time. Patients in the mucinous group were more likely to have well to moderately differentiated tumors (P<0.0001). At diagnosis, 12.9% of women with mucinous tumors had stage III/IV tumors compared to 10.7% of those with endometrioid tumors (P=0.001). Those with mucinous tumors were less likely to have undergone lymphadenectomy (45.7% vs. 53.3% underwent lymphadenectomy) (P<0.0001).
Table 1.
Clinical and demographic characteristics of the cohort stratified by histology.
| Mucinous | Endometrioid | ||||
|---|---|---|---|---|---|
|
|
|||||
| N | (%) | N | (%) | P-value | |
| 1374 | (3.0) | 43,882 | (97.0) | ||
| Age | <0.0001 | ||||
| <60 years | 514 | (37.4) | 19,329 | (44.1) | |
| >60 years | 860 | (62.6) | 24,553 | (56.0) | |
| Race | 0.04 | ||||
| White | 1223 | (89.0) | 38,433 | (87.6) | |
| Black | 47 | (3.4) | 2220 | (5.1) | |
| Other | 100 | (7.3) | 3040 | (6.9) | |
| Unknown | 4 | (0.3) | 189 | (0.4) | |
| Year of diagnosis | <0.0001 | ||||
| 1988-1994 | 530 | (38.6) | 2232 | (5.1) | |
| 1995-2000 | 530 | (38.6) | 11,582 | (26.4) | |
| 2001-2006 | 552 | (40.2) | 30,068 | (68.5) | |
| Marital status | 0.17 | ||||
| Married | 770 | (56.0) | 23,471 | (53.5) | |
| Single | 558 | (40.6) | 18,793 | (42.8) | |
| Unknown | 46 | (3.4) | 1618 | (3.7) | |
| SEER registry | <0.0001 | ||||
| Western | 767 | (55.8) | 21,860 | (49.8) | |
| Central | 315 | (22.9) | 10,746 | (24.5) | |
| Eastern | 292 | (21.3) | 11,276 | (25.7) | |
| Grade | <0.0001 | ||||
| 1 | 716 | (52.1) | 19,819 | (45.2) | |
| 2 | 441 | (32.1) | 14,729 | (33.6) | |
| 3 | 111 | (8.1) | 6668 | (15.2) | |
| Unknown | 106 | (7.7) | 2666 | (6.1) | |
| Stage | 0.001 | ||||
| IA | 832 | (60.6) | 27,380 | (62.4) | |
| IB | 139 | (10.1) | 5114 | (11.7) | |
| I-II NOS | 164 | (11.9) | 4434 | (10.1) | |
| II | 24 | (1.8) | 1269 | (2.9) | |
| III | 132 | (9.6) | 3431 | (7.8) | |
| IV | 45 | (3.3) | 1271 | (2.9) | |
| Unknown | 38 | (2.8) | 983 | (2.2) | |
| Lymphadenectomy | <0.0001 | ||||
| No (0) | 746 | (54.3) | 20,506 | (46.7) | |
| Yes (1) | 628 | (45.7) | 23,376 | (53.3) | |
| Radiation | 0.01 | ||||
| External beam or external beam and brachytherapy |
276 | (20.1) | 8409 | (19.2) | |
| Brachytherapy | 47 | (3.4) | 2386 | (5.4) | |
| Other | 9 | (0.7) | 300 | (0.7) | |
| None/unknown | 1042 | (75.8) | 32,787 | (74.7) | |
After adjustment from differences in clinical and pathologic characteristics, there was no difference in survival between endometrioid and mucinous tumors. The hazard ratio for cancer-specific survival for mucinous compared to endometrioid tumors was 0.90 (95% CI, 0.74-1.09) while the hazard ratio for overall survival was 0.95 (95% CI, 0.85-1.07). Among women with mucinous tumors, stage and grade were the most important prognostic factors. Compared to women with stage IA mucinous tumors, the hazard ratio for cancer specific survival for women with stage III tumors was 8.28 (95% CI, 7.43-9.24). Similarly, women with grade 3 mucinous tumors were over four times more likely to die from their cancers than women with grade 1 lesions (HR=4.66; 95% CI, 4.16-5.22). Like endometrioid tumors, race was an important prognostic factor; compared to white women, black patients were 39% more likely to die from their neoplasms (HR=1.39; 95% CI, 1.22-1.59).
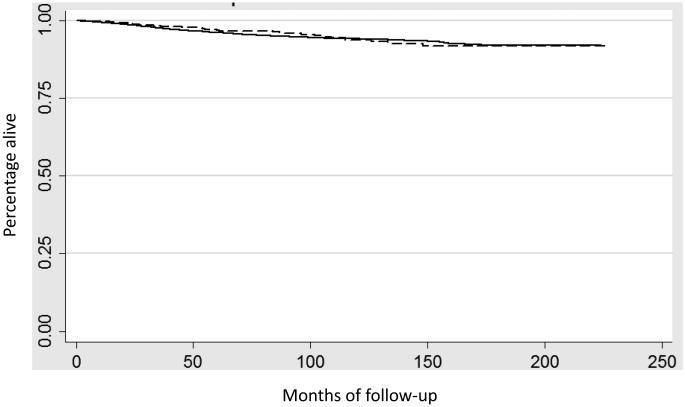
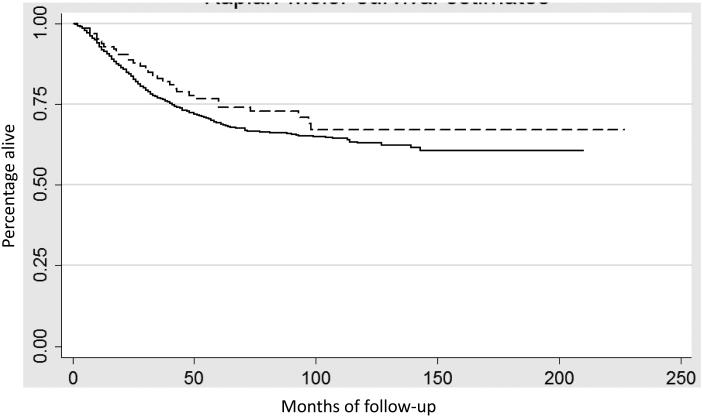
In a Kaplan-Meier analysis the results were similar. Figure 1 displays survival for uterine confined tumors (stage IA and IB) (P=0.54) while Figure 2 shows survival for women with stage III neoplasms (P=0.18). Five-year survival for stage I mucinous tumors was 89.9% (95% CI, 87.6-91.9%) compared to 89.0% (95% CI, 88.6-89.4%) for endometrioid tumors (Table 3). Likewise, five-year survival was 67.6% (95% CI, 58.2-75.4%) for stage III mucinous tumors versus 58.6% (95% CI, 56.5-61.0%) for similarly staged endometrioid tumors.
Figure 1.
Kaplan-Meier analysis of cancer-specific survival for stage I (IA and IB) stratified by histology (P=0.54). Solid line endometrioid tumors, dashed line mucinous tumors.
Figure 2.
Kaplan-Meier analysis of cancer-specific survival for stage III stratified by histology (P=0.18). Solid line endometrioid tumors, dashed line mucinous tumors.
Table 3.
Five-year survival stratified by stage and histology.
| Mucinous | Endometrioid | |||
|---|---|---|---|---|
|
|
||||
| N | 5-year survival | N | 5-year survival | |
| Stage I | 971 | 89.9% (87.6-91.9) | 32,494 | 89.0% (88.6-89.4) |
| Stage II | 24 | 68.6% (39.1-85.9) | 1269 | 76.4% (73.0-79.5) |
| Stage III | 132 | 67.6% (58.2-75.4) | 3431 | 58.6% (56.5-61.0) |
| Stage IV | 45 | 41.6% (26.7-55.9) | 1271 | 30.4% (27.4-33.5) |
(95% confidence interval)
Discussion
Our findings suggest that the outcomes of mucinous and endometrioid endometrial cancer are similar. While women with mucinous tumors more often present with advanced stage neoplasms, after correction for differences in clinical and demographic disparities survival is comparable to endometrioid tumors. Like endometrioid tumors, stage and grade are the most important prognostic factors and black women are more likely to die from their neoplasms than white women.
Mucinous endometrial carcinoma is rare, accounting for a small minority of endometrial adenocarcinomas. Since none of the major cooperative group surgicopathology trials considered mucinous carcinomas as a distinct entity, all data regarding its natural history and prognosis have been obtained from small case series, most derived from single institutions. A recent series of 41 patients with mucinous tumors, one of the largest studies published to date, suggested that women with mucinous tumors were more likely to present with nodal metastasis but found that survival for endometrioid and mucinous tumors was similar.2 Our population-based analysis of over 1300 cases noted similar findings, survival for stage matched women with endometrioid and mucinous tumors of the endometrium was similar.
Race is an important prognostic factor for women with mucinous endometrial tumors; compared to white women, black patients were nearly 40% more likely to die from their cancers. While race is an important prognostic factor for a number of tumors, uterine cancer is the tumor type with the strongest relationship between race and outcome 9-13 A prior study from the National Cancer Data Base noted that 5-year survival for early-stage endometrial tumors was 70% for black women compared to 95% for white patients.12 Despite the fact that race strongly influences outcomes for endometrial cancer, the underlying cause of these disparities has been more difficult to ascertain. Prior work has shown that outcomes are inferior for black women even after correction for differences in clinical factors and treatment.13 The current analysis suggests that racial differences also strongly influence outcomes for mucinous endometrial tumors.
Little is known about the natural history of mucinous endometrial tumors. Prior studies have shown that endometrial hyperplasia is a precursor lesion for endometrioid tumors and endometrial intraepithelial neoplasia precedes serous tumors of the endometrium.14-18 Yoo and coworkers postulated that papillary mucinous metaplasia iss a precursor of mucinous endometrial tumors.14 These authors demonstrated the association between mucinous metaplasia and mucinous adenocarcinomas in a molecular and immunohistochemical analysis.14 Further work to define the natural history and risk factors for mucinous tumors is clearly warranted.
Prior studies have shown that the various histological subtypes of endometrial cancer have distinct gene expression patterns. A high frequency of TP53 mutations is seen in uterine serous carcinomas.19,20 In the case of mucinous tumors of the ovary, KRAS mutations are frequent21 and KRAS mutations have also been demonstrated in mucinous endometrial carcinoma.14 KRAS mutations, which are found in 10-20% of endometrial cancers, have been associated with a longer disease free survival in early stage endometrioid endometrial cancers.22 Differential expression of genes such as KRAS may in part explain the clinical behavior of mucinous endometrial carcinoma. Even within mucinous carcinomas of the endometrium, variation exists. For example, an extremely rare type of mucinous endometrial carcinoma that mimics adenoma malignum of the uterine cervix, exhibits aggressive clinical behavior despite being low grade.23 The small numbers of these cases, however, precludes the study of these tumors as separate entities in large clinical trials and further studies are needed to characterize mucinous carcinomas of the endometrium on a molecular level.
While our analysis benefits from the inclusion of a large cohort of women, we recognize a number of important limitations. Central pathology review is not performed for patients registered in SEER. This is particularly important for uncommon histologic variants. While it is generally accepted that to be classified as mucinous, a tumor must have at least 50% of this component, we cannot exclude the possibility that a small number of patients would not have met these diagnostic criteria. SEER lacks some important pathologic data including lymphvascular space invasion as well as data on adjuvant cytotoxic and hormonal therapy. Finally, SEER lacks data on the timing and distribution and treatment of recurrences, and as such, our analysis is limited to survival.
The most important question raised by analyses of rare tumors such as mucinous endometrial carcinomas is whether these neoplasms should be treated differently than more common histologic subtypes (Arend, Cx Sarcoma). For ovarian cancer, mucinous tumors appear to follow a distinct course and some studies have suggested that alternative treatment strategies may be warranted.24 Adjuvant treatment for endometrial cancer remains controversial, and, as would be expected, prospective therapeutic trials for women with endometrial cancer have typically included few women with mucinous neoplasms.25 While our study suggests that the outcomes of mucinous and endometrioid endometrial cancer are similar, further work to examine the treatment of mucinous endometrial cancer are clearly needed.
Table 2.
Multivariable Cox proportional hazards models of death.
| Cancer specific survival | Overall survival | |
|---|---|---|
| Histology | ||
| Endometrioid | Referent | Referent |
| Mucinous | 0.90 (0.74-1.09) | 0.95 (0.85-1.07) |
| Age | ||
| <60 years | Referent | Referent |
| >60 years | 1.80 (1.66-1.96)* | 2.85 (2.69-3.03)* |
| Race | ||
| White | Referent | Referent |
| Black | 1.39 (1.22-1.59)* | 1.29 (1.18-1.42)* |
| Other | 1.04 (0.89-1.22) | 0.89 (0.80-1.01) |
| Unknown | 0.28 (0.07-1.11) | 0.60 (0.32-1.11) |
| Year of diagnosis | ||
| 1988-1994 | Referent | Referent |
| 1995-2000 | 0.99 (0.87-1.12) | 1.01 (0.93-1.09) |
| 2001-2006 | 0.98 (0.86-1.11) | 0.98 (0.90-1.06) |
| Marital status | ||
| Married | Referent | Referent |
| Single | 1.24 (1.15-1.34)* | 1.48 (1.41-1.55)* |
| Unknown | 0.99 (0.80-1.23) | 1.19 (1.05-1.36)* |
| SEER registry | ||
| Eastern | Referent | Referent |
| Central | 1.09 (0.99-1.20) | 1.09 (1.02-1.16)* |
| Western | 0.98 (0.90-1.07) | 0.95 (0.90-1.01) |
| Grade | ||
| 1 | Referent | Referent |
| 2 | 2.08 (1.86-2.32)* | 1.38 (1.29-1.46)* |
| 3 | 4.66 (4.16-5.22)* | 2.56 (2.39-2.73)* |
| Unknown | 2.52 (2.14-2.98)* | 1.58 (1.42-1.75)* |
| Stage | ||
| IA | Referent | Referent |
| IB | 2.14 (1.88-2.44)* | 1.72 (1.60-1.86)* |
| I-II NOS | 2.45 (2.14-2.79)* | 1.61 (1.49-1.74)* |
| II | 3.14 (2.53-3.90)* | 2.15 (1.86-2.49)* |
| III | 8.28 (7.43-9.24)* | 3.95 (3.67-4.26)* |
| IV | 20.77 (18.54-23.27)* | 8.34 (7.67-9.06)* |
| Unknown | 5.34 (4.45-6.41)* | 2.44 (2.12-2.81)* |
| Lymphadenectomy | ||
| No (0) | Referent | Referent |
| Yes (1) | 0.71 (0.66-0.77)* | 0.72 (0.68-0.76)* |
P<0.05
Acknowledgments
Dr. Wright (NCI R01CA169121-01A1) and Dr. Hershman (NCI R01CA134964) are recipients of grants from the National Cancer Institute.
Footnotes
Declaration of Interest
The authors have no conflicts of interest or disclosures.
References
- 1.Cancer Facts & Figures. American Cancer Society; Atlanta: 2012. 2012. [Google Scholar]
- 2.Musa F, Huang M, Adams B, Pirog E, Holcomb K. Mucinous histology is a risk factor for nodal metastases in endometrial cancer. Gynecol Oncol. 2012;125:541–5. doi: 10.1016/j.ygyno.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Ross JC, Eifel PJ, Cox RS, Kempson RL, Hendrickson MR. Primary mucinous adenocarcinoma of the endometrium. A clinicopathologic and histochemical study. Am J Surg Pathol. 1983;7:715–29. [PubMed] [Google Scholar]
- 4.Tiltman AJ. Mucinous carcinoma of the endometrium. Obstet Gynecol. 1980;55:244–7. [PubMed] [Google Scholar]
- 5.Hankey BF, Ries LA, Edwards BK. The surveillance, epidemiology, and end results program: a national resource. Cancer Epidemiol Biomarkers Prev. 1999;8:1117–21. [PubMed] [Google Scholar]
- 6.Wright JD, Buck AM, Shah M, Burke WM, Schiff PB, Herzog TJ. Safety of ovarian preservation in premenopausal women with endometrial cancer. J Clin Oncol. 2009;27:1214–9. doi: 10.1200/JCO.2008.19.8150. [DOI] [PubMed] [Google Scholar]
- 7.Wright JD, Shah M, Mathew L, et al. Fertility preservation in young women with epithelial ovarian cancer. Cancer. 2009;115:4118–26. doi: 10.1002/cncr.24461. [DOI] [PubMed] [Google Scholar]
- 8.Wright JD, NathavithArana R, Lewin SN, et al. Fertility-conserving surgery for young women with stage IA1 cervical cancer: safety and access. Obstet Gynecol. 2010;115:585–90. doi: 10.1097/AOG.0b013e3181d06b68. [DOI] [PubMed] [Google Scholar]
- 9.Bach PB, Schrag D, Brawley OW, Galaznik A, Yakren S, Begg CB. Survival of blacks and whites after a cancer diagnosis. Jama. 2002;287:2106–13. doi: 10.1001/jama.287.16.2106. [DOI] [PubMed] [Google Scholar]
- 10.Howard J, Hankey BF, Greenberg RS, et al. A collaborative study of differences in the survival rates of black patients and white patients with cancer. Cancer. 1992;69:2349–60. doi: 10.1002/1097-0142(19920501)69:9<2349::aid-cncr2820690925>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 11.Hill HA, Eley JW, Harlan LC, Greenberg RS, Barrett RJ, 2nd, Chen VW. Racial differences in endometrial cancer survival: the black/white cancer survival study. Obstet Gynecol. 1996;88:919–26. doi: 10.1016/s0029-7844(96)00341-9. [DOI] [PubMed] [Google Scholar]
- 12.Hicks ML, Phillips JL, Parham G, et al. The National Cancer Data Base report on endometrial carcinoma in African-American women. Cancer. 1998;83:2629–37. doi: 10.1002/(SICI)1097-0142(19981215)83:12<2629::AID-CNCR30>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 13.Wright JD, Fiorelli J, Schiff PB, et al. Racial disparities for uterine corpus tumors: changes in clinical characteristics and treatment over time. Cancer. 2009;115:1276–85. doi: 10.1002/cncr.24160. [DOI] [PubMed] [Google Scholar]
- 14.Yoo SH, Park BH, Choi J, et al. Papillary mucinous metaplasia of the endometrium as a possible precursor of endometrial mucinous adenocarcinoma. Mod Pathol. 2012;25:1496–507. doi: 10.1038/modpathol.2012.113. [DOI] [PubMed] [Google Scholar]
- 15.Trimble CL, Kauderer J, Zaino R, et al. Concurrent endometrial carcinoma in women with a biopsy diagnosis of atypical endometrial hyperplasia: a Gynecologic Oncology Group study. Cancer. 2006;106:812–9. doi: 10.1002/cncr.21650. [DOI] [PubMed] [Google Scholar]
- 16.Ambros RA, Sherman ME, Zahn CM, Bitterman P, Kurman RJ. Endometrial intraepithelial carcinoma: a distinctive lesion specifically associated with tumors displaying serous differentiation. Hum Pathol. 1995;26:1260–7. doi: 10.1016/0046-8177(95)90203-1. [DOI] [PubMed] [Google Scholar]
- 17.Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia. A long-term study of "untreated" hyperplasia in 170 patients. Cancer. 1985;56:403–12. doi: 10.1002/1097-0142(19850715)56:2<403::aid-cncr2820560233>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 18.Kuhn E, Wu RC, Guan B, et al. Identification of molecular pathway aberrations in uterine serous carcinoma by genome-wide analyses. J Natl Cancer Inst. 2012;104:1503–13. doi: 10.1093/jnci/djs345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhn E, Wu R-C, Guan B, et al. Identification of Molecular Pathway Aberrations in Uterine Serous Carcinoma by Genome-wide Analyses. Journal of the National Cancer Institute. 2012;104:1503–13. doi: 10.1093/jnci/djs345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayes MP, Wang H, Espinal-Witter R, et al. PIK3CA and. PTEN Mutations in Uterine Endometrioid Carcinoma and Complex Atypical Hyperplasia. Clinical Cancer Research. 2006;12:5932–5. doi: 10.1158/1078-0432.CCR-06-1375. [DOI] [PubMed] [Google Scholar]
- 21.Cuatrecasas M, Villanueva A, Matias-Guiu X, Prat J. K-ras mutations in mucinous ovarian tumors: a clinicopathologic and molecular study of 95 cases. Cancer. 1997;79:1581–6. doi: 10.1002/(sici)1097-0142(19970415)79:8<1581::aid-cncr21>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 22.Byron SA, Gartside M, Powell MA, et al. FGFR2 point mutations in 466 endometrioid endometrial tumors: relationship with MSI, KRAS, PIK3CA, CTNNB1 mutations and clinicopathological features. PLoS One. 2012;7:e30801. doi: 10.1371/journal.pone.0030801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujiwara M, Longacre TA. Low-grade mucinous adenocarcinoma of the uterine corpus: a rare and deceptively bland form of endometrial carcinoma. Am J Surg Pathol. 2011;35:537–44. doi: 10.1097/PAS.0b013e31820f1cc2. [DOI] [PubMed] [Google Scholar]
- 24.Schiavone MB, Herzog TJ, Lewin SN, et al. Natural history and outcome of mucinous carcinoma of the ovary. Am J Obstet Gynecol. 2011;205:480. doi: 10.1016/j.ajog.2011.06.049. e1-8. [DOI] [PubMed] [Google Scholar]
- 25.Fleming GF, Brunetto VL, Cella D, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2004;22:2159–66. doi: 10.1200/JCO.2004.07.184. [DOI] [PubMed] [Google Scholar]