Abstract
Objective
Ovarian carcinosarcomas (OCS) are rare tumors composed of both malignant epithelial and mesenchymal elements. We compared the natural history and outcomes of OCS to serous carcinoma of the ovary.
Methods
Patients with OCS and serous carcinomas registered in the Surveillance, Epidemiology, and End Results (SEER) database between 1988-2007 were analyzed. Demographic and clinical characteristics were compared using chi square tests while survival was analyzed using Cox proportional hazards models and the Kaplan-Meier method.
Results
A total of 27,737 women, including 1763 (6.4%) with OCS and 25,974 (93.6%) with serous carcinomas, were identified. Patients with carcinosarcomas tended to be older and have unstaged tumors (P<0.0001). After adjusting for other prognostic factors, women with carcinosarcomas were 72% more likely to die from their tumors (HR=1.72; 95% CI, 1.52-1.96). Five-year survival for stage I carcinosarcomas was 65.2% (95% CI, 58.0-71.4%) vs. 80.6% (95% CI, 78.9-82.2%) for serous tumors. Similarly, five-year survival for stage IIIC patients was 18.2% (95% CI, 14.5-22.4%) for carcinosarcomas compared to 33.3% (95% 32.1-34.5%) for serous carcinomas.
Conclusions
Ovarian carcinosarcomas are aggressive tumors with a natural history that is distinct from serous cancers. The survival for both early and late stage carcinosarcoma is inferior to serous tumors.
Introduction
Ovarian cancer is the fifth leading cause of cancer-related death in women, with 22,280 cases and 15,500 deaths in 2012.(1) The majority of ovarian cancers are epithelial tumors, the most common of which are serous carcinomas. Ovarian carcinosarcomas are rare tumors composed of malignant epithelial and mesenchymal components.(2) It is estimated that carcinosarcomas account for 1-4% of malignant ovarian cancers.(2-7)
A number of observational studies have suggested that ovarian carcinosarcomas follow a distinct natural history compared to other more common epithelial carcinomas.(2, 5, 8) Although prospective data are lacking, the management of ovarian carcinosarcoma (OCS) is similar to other ovarian tumors and typically includes cytoreductive surgery followed by adjuvant chemotherapy for women with advanced stage disease.(2) Although platinum and taxane based chemotherapy are often used, the ideal chemotherapy regimen for OCS is not known.(2, 8-12) Based on the efficacy of ifosfamide for uterine carcinosarcoma, some studies have suggested that ifosfamide should be incorporated into the treatment of OCS.(10, 11)
Given that ovarian carcinosarcomas are rare, little is known about the disease course and outcome of women with these tumors. We performed a population-based analysis to examine the natural history and outcome of ovarian carcinosarcomas. Specifically, we compared women with serous carcinoma and carcinosarcoma of the ovary.
Methods
Data Source
The Surveillance, Epidemiology, and End Results (SEER) database of the National Cancer Institute was utilized for this analysis.(13-17) SEER is a population-based tumor registry that collects data on approximately 28% of the United States population.(18) SEER is composed of a number of geographically distinct registries and captures clinical and demographic data as well as tumor characteristics including stage, grade, histology, initial treatment, and follow-up. Exemption from the Columbia University Institutional Review Board was obtained.
Cohort Selection
Women with serous tumors or carcinosarcomas of the ovary diagnosed between 1988 and 2007 were included in the analysis. Clinical and pathological data, including age at diagnosis (< 50 years of age, 50-64 years of age, 65-74 years of age, and ≥75 years of age), race (white, black, other), and marital status (single, married, other), were collected. Year of diagnosis was classified as: 1988-1990, 1991-1993, 1994-1995, 1996-1997, 1998-1999, 2000-2001, 2002-2003, 2004-2005, 2006-2007. Subjects were categorized based on the geographic area of residence within the United States at the time of diagnosis: central (Detroit, Iowa, Kentucky, Louisiana, Utah), eastern (Connecticut, New Jersey, Atlanta, rural Georgia) and western (Alaska, California, Hawaii, Los Angeles, New Mexico, San Francisco, San Jose, Seattle).(19, 20) In addition to tumor histology, tumor grade was captured and grouped as well, moderately or poorly differentiated or unknown. Staging information was derived from the American Joint Cancer Committee staging information and recorded extent of disease codes.
Statistical Analysis
Frequency distributions between categorical variables were compared using χ2 tests. Survival was calculated as the number of months from cancer diagnosis to the date of death. Patients who were alive at last follow-up were censored. Cox proportional hazards models were developed to examine both cancer specific and overall survival after adjustment for clinical and demographic characteristics. The survival models included only women who underwent surgery as a part of their treatment. Five-year survival rates were calculated by stage and histology. Kaplan-Meier curves were developed to examine stage-specific survival and compared using the log-rank test. All hypothesis tests were two-sided. A P-value of <0.05 was considered statistically significant. All analyses were conducted using SAS version 9.2 (SAS Institute Inc, Cary, North Carolina).
Results
A total of 27,737 patients, including 1763 (6.4%) with carcinosarcomas (OCS) and 25,974 (93.6%) with serous carcinomas, were identified. The demographic and clinical characteristics of the cohort are displayed in Table 1. Patients with carcinosarcomas tended to be older than women with serous tumors; 58.6% with OCS were 65 and older compared to 45.3% with serous carcinomas (P<0.0001). At diagnosis, stage I tumors were noted in 11.0% of women with OCS and 10.3% of those with serous tumors, while stage IV neoplasms were documented in 22.6% of women with OCS and 28.8% of patients with serous tumors (P<0.0001). Stage was unknown in 15.8% of patients with OCS compared to 2.8% of women with serous tumors.
Table 1.
Clinical and demographic characteristics of the cohort.
| Carcinosarcoma | Serous | ||||
|---|---|---|---|---|---|
| N | (%) | N | (%) | P-value | |
| 1763 | 25,974 | ||||
| Age (years) | <0.0001 | ||||
| <50 | 186 | (10.6) | 4649 | (17.9) | |
| 50-64 | 544 | (30.9) | 9566 | (36.8) | |
| 65-74 | 522 | (29.6) | 6516 | (25.1) | |
| ≥75 | 511 | (29.0) | 5243 | (20.2) | |
| Race | 0.13 | ||||
| White | 1535 | (87.1) | 22,951 | (88.4) | |
| Black | 117 | (6.6) | 1431 | (5.5) | |
| Other/unknown | 111 | (6.3) | 1592 | (6.1) | |
|
Year of
diagnosis |
0.64 | ||||
| 1988-1990 | 131 | (7.4) | 1685 | (6.5) | |
| 1991-1993 | 162 | (9.2) | 2294 | (8.8) | |
| 1994-1995 | 132 | (7.5) | 1762 | (6.8) | |
| 1996-1997 | 125 | (7.1) | 1868 | (7.2) | |
| 1998-1999 | 127 | (7.2) | 2018 | (7.8) | |
| 2000-2001 | 262 | (14.9) | 4104 | (15.8) | |
| 2002-2003 | 271 | (15.4) | 4010 | (15.4) | |
| 2004-2005 | 278 | (15.8) | 3988 | (15.4) | |
| 2006-2007 | 275 | (15.6) | 4245 | (16.3) | |
| Marital status | 0.002 | ||||
| Married | 891 | (50.5) | 14,211 | (54.7) | |
| Unmarried | 809 | (45.9) | 11,028 | (42.5) | |
| Unknown | 63 | (3.6) | 735 | (2.8) | |
| SEER registry | 0.16 | ||||
| Eastern | 407 | (23.1) | 5495 | (21.2) | |
| Midwest | 432 | (24.5) | 6497 | (25.0) | |
| West | 924 | (52.4) | 13,982 | (53.8) | |
| Stage | <0.0001 | ||||
| IA | 117 | (6.6) | 1320 | (5.1) | |
| IB | 14 | (0.8) | 275 | (1.1) | |
| IC | 63 | (3.6) | 1075 | (4.1) | |
| INOS | 18 | (1.0) | 107 | (0.4) | |
| II | 187 | (10.6) | 1850 | (7.1) | |
| IIIA | 35 | (2.0) | 563 | (2.2) | |
| IIIB | 49 | (2.8) | 936 | (3.6) | |
| IIIC | 433 | (24.6) | 8778 | (33.8) | |
| IIINOS | 170 | (9.6) | 2864 | (11.0) | |
| IV | 398 | (22.6) | 7471 | (28.8) | |
| Unknown | 279 | (15.8) | 735 | (2.8) | |
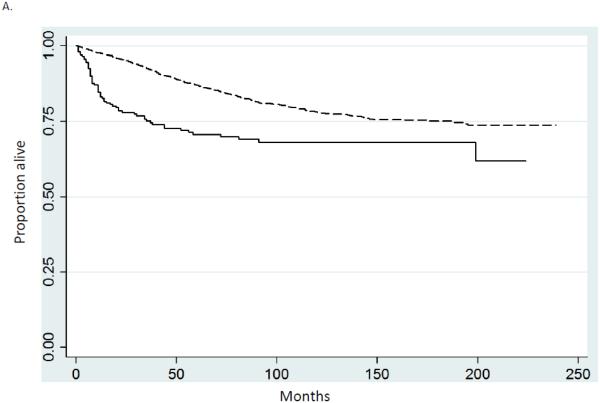
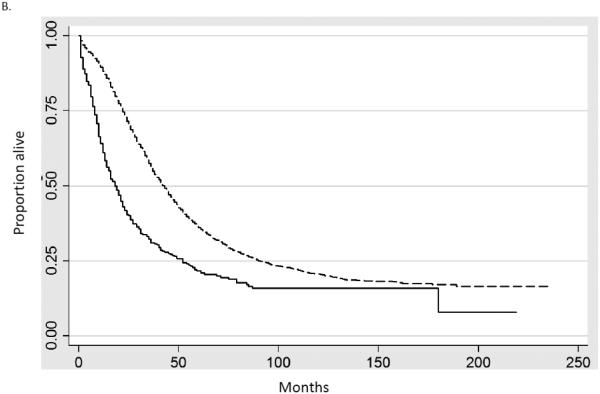
Multivariable Cox proportional hazards models including only women who underwent surgery were developed to examine survival while adjusting for other prognostic factors (Table 2). Cancer-directed surgery was performed in 93.1% of women with carcinosarcomas and 92.6% of patients with serous tumors. The hazard ratio for death for women with OCS compared to serous tumors was 1.76 (95% CI, 1.65-1.87) while the hazard ratio for death from cancer was 1.79 (95% CI, 1.68-1.91). Age, stage, area of residence, marital status and year of diagnosis were all statistically significantly associated with prognosis. Similar trends were seen in Kaplan-Meier analyses, survival was inferior for women with OCS for both early and late stage disease. Figure 1A compares survival for women with stage I tumors, while figure 1B displays survival for those with stage III neoplasms (P<0.0001 for both).
Table 2.
Cox proportional hazards models of cancer-specific and overall mortality.
| Cancer-specific survival |
Overall survival | |
|---|---|---|
| Histology | ||
| Serous | Referent | Referent |
| Carcinosarcoma | 1.79 (1.68-1.91)* | 1.76 (1.65-1.87)* |
| Age (years) | ||
| <50 | Referent | Referent |
| 50-64 | 1.36 (1.29-1.43)* | 1.41 (1.34-1.49)* |
| 65-74 | 1.73 (1.63-1.82)* | 1.88 (1.78-1.98)* |
| ≥75 | 2.39 (2.25-2.53)* | 2.77 (2.62-2.93)* |
| Race | ||
| White | Referent | Referent |
| Black | 1.11 (1.03-1.20)* | 1.16 (1.08-1.25)* |
| Other/unknown | 0.96 (0.88-1.03) | 0.99 (0.92-1.06) |
| Year of diagnosis | ||
| 1988-1990 | Referent | Referent |
| 1991-1993 | 0.97 (0.90-1.04) | 0.94 (0.88-1.01) |
| 1994-1995 | 0.90 (0.83-0.98)* | 0.87 (0.81-0.94)* |
| 1996-1997 | 0.89 (0.82-0.96)* | 0.85 (0.79-0.92)* |
| 1998-1999 | 0.90 (0.83-0.97)* | 0.85 (0.79-0.92)* |
| 2000-2001 | 0.89 (0.83-0.96)* | 0.85 (0.80-0.91)* |
| 2002-2003 | 0.82 (0.77-0.89)* | 0.79 (0.74-0.85)* |
| 2004-2005 | 0.81 (0.75-0.87)* | 0.78 (0.73-0.84)* |
| 2006-2007 | 0.76 (0.68-0.84)* | 0.73 (0.66-0.80)* |
| Marital status | ||
| Married | Referent | Referent |
| Unmarried | 1.13 (1.09-1.17)* | 1.16 (1.12-1.20)* |
| Unknown | 0.99 (0.88-1.11) | 1.02 (0.91-1.13) |
| SEER registry | ||
| Eastern | Referent | Referent |
| Midwest | 1.15 (1.10-1.21)* | 1.14 (1.08-1.19)* |
| West | 1.04 (1.00-1.09) | 1.04 (0.99-1.08) |
| Stage1 | ||
| I | Referent | Referent |
| II | 2.59 (2.30-2.92)* | 1.91 (1.74-2.11)* |
| IIIA | 4.02 (3.45-4.68)* | 2.65 (2.32-3.03)* |
| IIIB | 5.20 (4.57-5.91)* | 3.40 (3.04-3.79)* |
| IIIC | 6.56 (5.95-7.23)* | 4.26 (3.95-4.59)* |
| IIINOS | 7.06 (6.36-7.84)* | 4.59 (4.22-4.99)* |
| IV | 9.18 (8.33- 10.12)* |
5.88 (5.45-6.34)* |
| Unknown | 6.25 (5.41-7.22)* | 4.19 (3.70-4.75)* |
P<0.05
Figure 1.
Kaplan-Meier analysis of cancer-specific survival for women stratified by stage. A. Stage I (P<0.0001). B. Stage III (P<0.0001). Solid line carcinosarcoma, dashed line serous carcinoma.
When five-year survival was examined, similar findings of decreased survival for women with ovarian carcinosarcomas were identified (Table 3). Among women with stage I serous tumors five-year survival was 80.6% (95% CI, 78.9-82.2%) compared to 65.2% (95% CI, 58.0-71.4%) for OCS. Five-year survival for women with stage III neoplasams was 33.3% (95% CI, 32.1-34.5%) for serous carcinomas compared to 18.2% (95% CI, 14.5-22.4%) for ovarian carcinosarcomas. Similarly, among patients with stage IV neoplasms, five-year survival was 20.3% (95% CI, 19.2-21.3%) for serous tumors versus 11.2% (95% CI, 8.1-14.9%) for ovarian carcinosarcomas.
Table 3.
Five-year survival stratified by stage and histology.
| Carcinosarcoma | Serous | |
|---|---|---|
|
|
||
| Stage I | 65.2% (58.0-71.4%) | 80.6% (78.9-82.2%) |
| Stage II | 34.6% (27.2-42.0%) | 61.7% (59.1-64.2%) |
| Stage IIIC | 18.2% (14.5-22.4%) | 33.3% (32.1-34.5%) |
| Stage IV | 11.2% (8.1-14.9%) | 20.3% (19.2-21.3%) |
Discussion
Our findings suggest that ovarian carcinosarcomas are aggressive tumors with a natural history that is distinct from serous cancers. OCS tends to occur in older women and more often presents with disseminated disease. The survival for both early and late stage carcinosarcoma is inferior to serous tumors.
Prior studies have shown that ovarian carcinosarcomas are aggressive neoplasms with a predilection towards early dissemination.(2, 5, 7, 8, 21) Compared to serous and other epithelial tumors, OCS tends to occur in older women.(2, 5, 7, 8, 21) Likewise, at the time of presentation, women with OCS more often present with advanced stage disease.(2, 5, 7, 8, 21) Among women with advanced stage disease, optimal tumor cytoreduction appears to be an important determinant of survival.(4, 8, 9, 21-23) Rauh-Hain et al. found that patients with OCS who had only microscopic disease after cytoreduction had a median overall survival of 47 months compared to 18 months for those with optimal but macroscopic disease and 8 months for those with suboptimal disease after surgery.(8) We noted that OCS occurred in older women and also found that stage was unknown in a higher percentage of women with carcinosarcomas.
The optimal choice of chemotherapy for women with OCS remains to be determined. The Gynecologic Oncology Group (GOG) has undertaken several prospective studies of chemotherapy for women with OCS.(11, 12, 24) While doxorubicin appears to possess minimal activity, response rates of 20% were noted for both ifosfamide and cisplatin.(11, 12, 24) Given the activity of both cisplatin and ifosfamide, several institutional observational studies have compared outcomes of regimens containing the two agents.(2, 5, 7-9, 23, 25-28) Although limited by a small sample size, an analysis of 22 patients treated with either carboplatin and paclitaxel or cisplatin and ifosfamide found no difference in survival.(29) In contrast, Rutledge and co-workers noted improved survival in women with OCS who received ifosfamide as part of their treatment; among 31 patients with OCS the median progression free interval was 12 months in those who received carboplatin and paclitaxel but had not been reached in patients treated with a combination of cisplatin and ifosfamide.(10) Despite the potential efficacy of ifosfamide, the drug is often associated with substantial toxicity and further work is needed to examine the patterns of chemotherapy use in women with ovarian carcinosarcomas.
Regardless of treatment, outcomes appear to be poor for women with ovarian carcinosarcoma. A case-control study of 50 women with ovarian carcinosarcoma noted a median overall survival of 24 months, inferior to the 41 months noted for women with serous tumors.(8) A large study of women with OCS treated from 1988-1997 comparing survival for OCS and serous tumors noted inferior survival for OCS patients with advanced stage disease but found no statistically significant difference in survival between the two histologic subtypes for early stage tumors.(5) We noted that survival was inferior for all stages of women with OCS compared to serous tumors. For women with stage I tumors in our cohort, survival was only 65% for carcinosarcomas compared to 81% for those with serous tumors.
While our study includes a large number of patients, we acknowledge a number of important limitations. Perhaps most importantly, centralized pathology review was not available. This is particularly important in studies examining tumors that are uncommonly seen and, as such, we cannot exclude the possibility that a small number of neoplasms were misclassified. We lacked detailed data on residual disease, an important prognostic factor for ovarian cancer. Similarly, SEER lacks data on chemotherapy treatments and patterns and timing of recurrence. Lastly, as with any study of administrative data, we are unable to capture individual patient and physician preferences that certainly influenced the allocation of treatment.
In conclusion, our data suggests that ovarian carcinosarcomas have a distinct natural history compared to serous tumors. These neoplasms are associated with a poor outcome regardless of treatment. Given the differences in outcome for carcinosarcomas compared to more common histologic subtypes, future clinical trials focusing on this rare histology and alternate chemotherapy regimens should be considered. Further studies and the development of novel agents for the treatment of ovarian carcinosarcoma are warranted.(2)
Research Highlights.
-Ovarian carcinosarcomas are aggressive tumors with a natural history that is distinct from serous cancers.
-The survival for both early and late stage carcinosarcoma is inferior to serous tumors.
Acknowledgments
Dr. Wright (NCI R01CA169121-01A1) and Dr. Hershman (NCI R01CA134964) are recipients of grants from the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no conflicts of interest.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. Epub 2012/01/13. [DOI] [PubMed] [Google Scholar]
- 2.del Carmen MG, Birrer M, Schorge JO. Carcinosarcoma of the ovary: a review of the literature. Gynecol Oncol. 2012;125(1):271–7. doi: 10.1016/j.ygyno.2011.12.418. Epub 2011/12/14. [DOI] [PubMed] [Google Scholar]
- 3.Mano MS, Rosa DD, Azambuja E, Ismael G, Braga S, D'Hondt V, et al. Current management of ovarian carcinosarcoma. Int J Gynecol Cancer. 2007;17(2):316–24. doi: 10.1111/j.1525-1438.2006.00760.x. Epub 2007/03/17. [DOI] [PubMed] [Google Scholar]
- 4.Harris MA, Delap LM, Sengupta PS, Wilkinson PM, Welch RS, Swindell R, et al. Carcinosarcoma of the ovary. Br J Cancer. 2003;88(5):654–7. doi: 10.1038/sj.bjc.6600770. Epub 2003/03/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnholtz-Sloan JS, Morris R, Malone JM, Jr., Munkarah AR. Survival of women diagnosed with malignant, mixed mullerian tumors of the ovary (OMMMT) Gynecol Oncol. 2004;93(2):506–12. doi: 10.1016/j.ygyno.2004.02.016. Epub 2004/04/22. [DOI] [PubMed] [Google Scholar]
- 6.Chang J, Sharpe JC, A'Hern RP, Fisher C, Blake P, Shepherd J, et al. Carcinosarcoma of the ovary: incidence, prognosis, treatment and survival of patients. Ann Oncol. 1995;6(8):755–8. doi: 10.1093/oxfordjournals.annonc.a059312. Epub 1995/10/01. [DOI] [PubMed] [Google Scholar]
- 7.Brown E, Stewart M, Rye T, Al-Nafussi A, Williams AR, Bradburn M, et al. Carcinosarcoma of the ovary: 19 years of prospective data from a single center. Cancer. 2004;100(10):2148–53. doi: 10.1002/cncr.20256. Epub 2004/05/13. [DOI] [PubMed] [Google Scholar]
- 8.Rauh-Hain JA, Growdon WB, Rodriguez N, Goodman AK, Boruta DM, 2nd, Schorge JO, et al. Carcinosarcoma of the ovary: a case-control study. Gynecol Oncol. 2011;121(3):477–81. doi: 10.1016/j.ygyno.2011.02.023. Epub 2011/03/23. [DOI] [PubMed] [Google Scholar]
- 9.Duska LR, Garrett A, Eltabbakh GH, Oliva E, Penson R, Fuller AF. Paclitaxel and platinum chemotherapy for malignant mixed mullerian tumors of the ovary. Gynecol Oncol. 2002;85(3):459–63. doi: 10.1006/gyno.2002.6645. Epub 2002/06/08. [DOI] [PubMed] [Google Scholar]
- 10.Rutledge TL, Gold MA, McMeekin DS, Huh WK, Powell MA, Lewin SN, et al. Carcinosarcoma of the ovary-a case series. Gynecol Oncol. 2006;100(1):128–32. doi: 10.1016/j.ygyno.2005.07.119. Epub 2005/10/11. [DOI] [PubMed] [Google Scholar]
- 11.Sutton GP, Blessing JA, Homesley HD, Malfetano JH. A phase II trial of ifosfamide and mesna in patients with advanced or recurrent mixed mesodermal tumors of the ovary previously treated with platinum-based chemotherapy: a Gynecologic Oncology Group study. Gynecol Oncol. 1994;53(1):24–6. doi: 10.1006/gyno.1994.1081. Epub 1994/04/01. [DOI] [PubMed] [Google Scholar]
- 12.Tate Thigpen J, Blessing JA, DeGeest K, Look KY, Homesley HD. Cisplatin as initial chemotherapy in ovarian carcinosarcomas: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;93(2):336–9. doi: 10.1016/j.ygyno.2004.01.007. Epub 2004/04/22. [DOI] [PubMed] [Google Scholar]
- 13.Potosky AL, Riley GF, Lubitz JD, Mentnech RM, Kessler LG. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31(8):732–48. [PubMed] [Google Scholar]
- 14.Schiavone MB, Herzog TJ, Lewin SN, Deutsch I, Sun X, Burke WM, et al. Natural history and outcome of mucinous carcinoma of the ovary. Am J Obstet Gynecol. 2011;205(5):480 e1–8. doi: 10.1016/j.ajog.2011.06.049. Epub 2011/08/25. [DOI] [PubMed] [Google Scholar]
- 15.Wethington SL, Herzog TJ, Seshan VE, Bansal N, Schiff PB, Burke WM, et al. Improved survival for fallopian tube cancer: a comparison of clinical characteristics and outcome for primary fallopian tube and ovarian cancer. Cancer. 2008;113(12):3298–306. doi: 10.1002/cncr.23957. Epub 2008/11/14. [DOI] [PubMed] [Google Scholar]
- 16.Wright JD, Buck AM, Shah M, Burke WM, Schiff PB, Herzog TJ. Safety of ovarian preservation in premenopausal women with endometrial cancer. J Clin Oncol. 2009;27(8):1214–9. doi: 10.1200/JCO.2008.19.8150. Epub 2009/01/28. [DOI] [PubMed] [Google Scholar]
- 17.Wright JD, Shah M, Mathew L, Burke WM, Culhane J, Goldman N, et al. Fertility preservation in young women with epithelial ovarian cancer. Cancer. 2009;115(18):4118–26. doi: 10.1002/cncr.24461. Epub 2009/08/12. [DOI] [PubMed] [Google Scholar]
- 18.Surveillance EaERSPwscgRD-, National Cancer Institute, DCCPS Surveillance Research Program, Surveillance Systems Branch. released April 2012, based on the November 2011 submission. http://.seer.cancer.gov.
- 19.Spoozak L, Lewin SN, Burke WM, Deutsch I, Sun X, Herzog TJ, et al. Microinvasive adenocarcinoma of the cervix. Am J Obstet Gynecol. 2012;206(1):80 e1–6. doi: 10.1016/j.ajog.2011.07.029. Epub 2011/09/24. [DOI] [PubMed] [Google Scholar]
- 20.Wright JD, NathavithArana R, Lewin SN, Sun X, Deutsch I, Burke WM, et al. Fertility-conserving surgery for young women with stage IA1 cervical cancer: safety and access. Obstet Gynecol. 2010;115(3):585–90. doi: 10.1097/AOG.0b013e3181d06b68. Epub 2010/02/24. [DOI] [PubMed] [Google Scholar]
- 21.Sood AK, Sorosky JI, Gelder MS, Buller RE, Anderson B, Wilkinson EJ, et al. Primary ovarian sarcoma: analysis of prognostic variables and the role of surgical cytoreduction. Cancer. 1998;82(9):1731–7. Epub 1998/05/12. [PubMed] [Google Scholar]
- 22.Muntz HG, Jones MA, Goff BA, Fuller AF, Jr., Nikrui N, Rice LW, et al. Malignant mixed mullerian tumors of the ovary: experience with surgical cytoreduction and combination chemotherapy. Cancer. 1995;76(7):1209–13. doi: 10.1002/1097-0142(19951001)76:7<1209::aid-cncr2820760717>3.0.co;2-v. Epub 1995/10/01. [DOI] [PubMed] [Google Scholar]
- 23.Inthasorn P, Beale P, Dalrymple C, Carter J. Malignant mixed mullerian tumour of the ovary: prognostic factor and response of adjuvant platinum-based chemotherapy. Aust N Z J Obstet Gynaecol. 2003;43(1):61–4. doi: 10.1046/j.0004-8666.2003.00003.x. Epub 2003/05/21. [DOI] [PubMed] [Google Scholar]
- 24.Morrow CP, Bundy BN, Hoffman J, Sutton G, Homesley H. Adriamycin chemotherapy for malignant mixed mesodermal tumor of the ovary. A Gynecologic Oncology Group Study. Am J Clin Oncol. 1986;9(1):24–6. doi: 10.1097/00000421-198602000-00007. Epub 1986/02/01. [DOI] [PubMed] [Google Scholar]
- 25.Hellstrom AC, Tegerstedt G, Silfversward C, Pettersson F. Malignant mixed mullerian tumors of the ovary: histopathologic and clinical review of 36 cases. Int J Gynecol Cancer. 1999;9(4):312–6. doi: 10.1046/j.1525-1438.1999.99035.x. Epub 2001/03/10. [DOI] [PubMed] [Google Scholar]
- 26.Loizzi V, Cormio G, Camporeale A, Falagario M, De Mitri P, Scardigno D, et al. Carcinosarcoma of the ovary: analysis of 13 cases and review of the literature. Oncology. 2011;80(1-2):102–6. doi: 10.1159/000328794. Epub 2011/06/17. [DOI] [PubMed] [Google Scholar]
- 27.Cicin I, Saip P, Eralp Y, Selam M, Topuz S, Ozluk Y, et al. Ovarian carcinosarcomas: clinicopathological prognostic factors and evaluation of chemotherapy regimens containing platinum. Gynecol Oncol. 2008;108(1):136–40. doi: 10.1016/j.ygyno.2007.09.003. Epub 2007/10/16. [DOI] [PubMed] [Google Scholar]
- 28.Bilodeau L, Preese LM, Apple FS. Does low total creatine kinase rule out myocardial infarction? Ann Intern Med. 1992;116(6):523–4. doi: 10.7326/0003-4819-116-6-523_2. Epub 1992/03/15. [DOI] [PubMed] [Google Scholar]
- 29.Silasi DA, Illuzzi JL, Kelly MG, Rutherford TJ, Mor G, Azodi M, et al. Carcinosarcoma of the ovary. Int J Gynecol Cancer. 2008;18(1):22–9. doi: 10.1111/j.1525-1438.2007.00948.x. Epub 2007/04/25. [DOI] [PubMed] [Google Scholar]