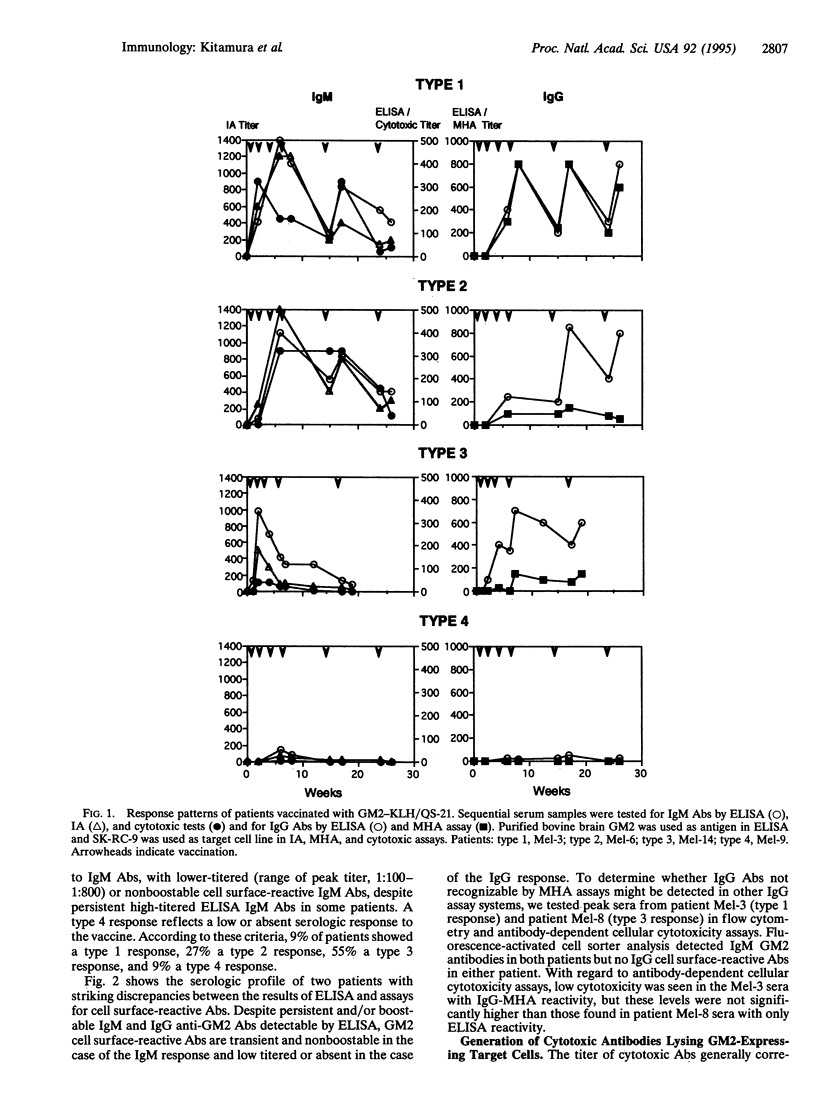
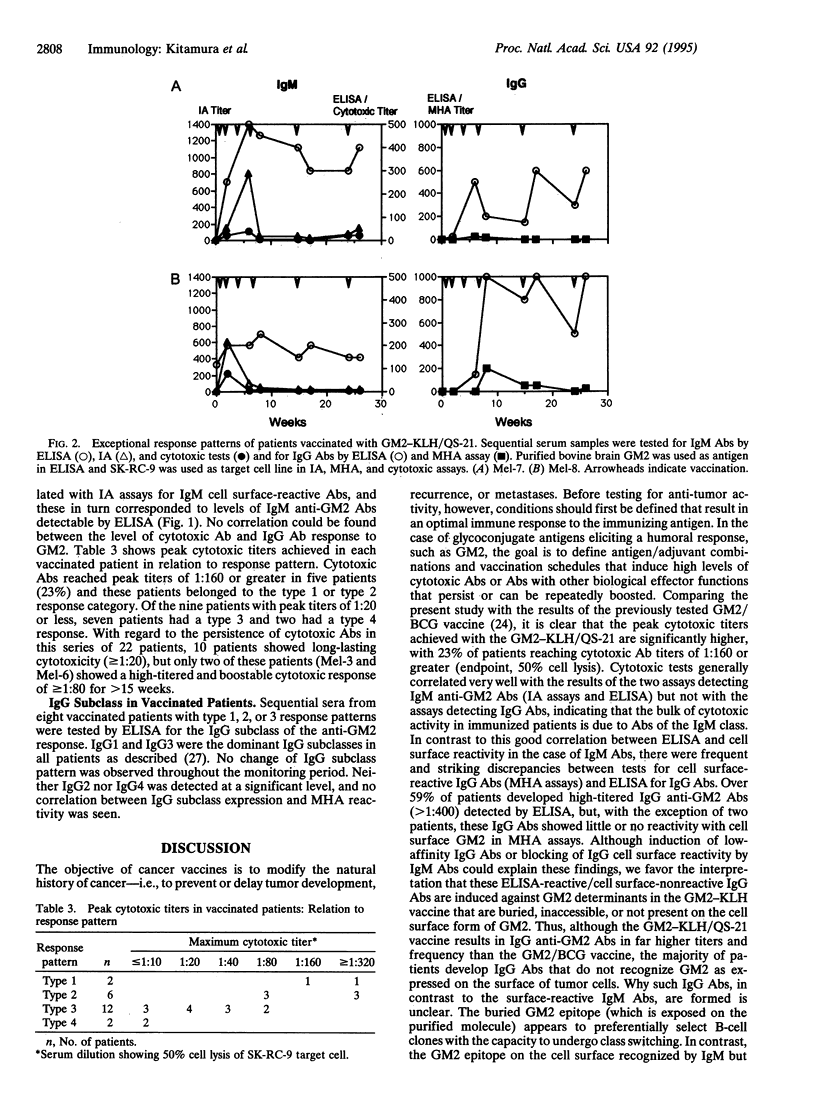
Abstract
Gangliosides, such as GM2, GD2, GD3, and 9-O-acetyl GD3, are receiving attention as targets for antibody-based and vaccine-based therapies of melanoma. GM2 appears to be a particularly immunogenic ganglioside in humans, as indicated by the presence of naturally occurring IgM anti-GM2 antibodies in approximately 5% of humans and the fact that immunization with irradiated GM2-expressing melanoma cells or purified GM2 adherent to bacillus Calmette-Guérin elicits GM2 antibodies of low to moderate titers in a high proportion of vaccinated patients. To develop vaccines that consistently induce high titers of IgM as well as IgG anti-GM2 antibodies, vaccines containing GM2 conjugated to keyhole limpet hemocyanin as the carrier protein and QS-21 as the adjuvant have been constructed. The serological response of vaccinated patients was monitored by ELISA using purified GM2 ganglioside for IgM and IgG anti-GM2 antibodies and for GM2 cell surface-reactive antibodies by immune adherence assays and cytotoxic tests (IgM antibodies) and mixed hemadsorption assays (IgG antibodies). The majority of vaccinated patients developed IgM and IgG antibodies detectable by ELISA. In most cases, the results of IgM ELISA correlated with assays for cell surface-reactive IgM antibodies. This was not true for IgG anti-GM2 antibodies, where strong discrepancies were seen between high titers in ELISA and little or no reactivity in mixed hemadsorption tests for cell surface-reactive antibodies. These IgG antibodies (and the less frequent IgM antibodies that show similar discrepancies) may be directed against GM2 determinants that are buried, hidden, or not present on GM2-expressing target cells. With regard to a major objective of ganglioside vaccines--i.e., generation of cytotoxic antibodies--the GM2-keyhole limpet hemocyanin/QS-21 vaccine is clearly superior to the previously tested GM2/bacillus Calmette-Guérin vaccine. However, variability in patient response and lack of persistence of high-titered IgM cytotoxic antibodies in many patients are problems that remain to be solved.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carey T. E., Takahashi T., Resnick L. A., Oettgen H. F., Old L. J. Cell surface antigens of human malignant melanoma: mixed hemadsorption assays for humoral immunity to cultured autologous melanoma cells. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3278–3282. doi: 10.1073/pnas.73.9.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheresh D. A., Harper J. R., Schulz G., Reisfeld R. A. Localization of the gangliosides GD2 and GD3 in adhesion plaques and on the surface of human melanoma cells. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5767–5771. doi: 10.1073/pnas.81.18.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheresh D. A., Honsik C. J., Staffileno L. K., Jung G., Reisfeld R. A. Disialoganglioside GD3 on human melanoma serves as a relevant target antigen for monoclonal antibody-mediated tumor cytolysis. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5155–5159. doi: 10.1073/pnas.82.15.5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung N. K., Lazarus H., Miraldi F. D., Abramowsky C. R., Kallick S., Saarinen U. M., Spitzer T., Strandjord S. E., Coccia P. F., Berger N. A. Ganglioside GD2 specific monoclonal antibody 3F8: a phase I study in patients with neuroblastoma and malignant melanoma. J Clin Oncol. 1987 Sep;5(9):1430–1440. doi: 10.1200/JCO.1987.5.9.1430. [DOI] [PubMed] [Google Scholar]
- Cheung N. K., Saarinen U. M., Neely J. E., Landmeier B., Donovan D., Coccia P. F. Monoclonal antibodies to a glycolipid antigen on human neuroblastoma cells. Cancer Res. 1985 Jun;45(6):2642–2649. [PubMed] [Google Scholar]
- Dippold W. G., Lloyd K. O., Li L. T., Ikeda H., Oettgen H. F., Old L. J. Cell surface antigens of human malignant melanoma: definition of six antigenic systems with mouse monoclonal antibodies. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6114–6118. doi: 10.1073/pnas.77.10.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garin-Chesa P., Beresford H. R., Carrato-Mena A., Oettgen H. F., Old L. J., Melamed M. R., Rettig W. J. Cell surface molecules of human melanoma. Immunohistochemical analysis of the gp57, GD3, and mel-CSPG antigenic systems. Am J Pathol. 1989 Feb;134(2):295–303. [PMC free article] [PubMed] [Google Scholar]
- Hamilton W. B., Helling F., Lloyd K. O., Livingston P. O. Ganglioside expression on human malignant melanoma assessed by quantitative immune thin-layer chromatography. Int J Cancer. 1993 Feb 20;53(4):566–573. doi: 10.1002/ijc.2910530407. [DOI] [PubMed] [Google Scholar]
- Helling F., Shang A., Calves M., Zhang S., Ren S., Yu R. K., Oettgen H. F., Livingston P. O. GD3 vaccines for melanoma: superior immunogenicity of keyhole limpet hemocyanin conjugate vaccines. Cancer Res. 1994 Jan 1;54(1):197–203. [PubMed] [Google Scholar]
- Hellström I., Brankovan V., Hellström K. E. Strong antitumor activities of IgG3 antibodies to a human melanoma-associated ganglioside. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1499–1502. doi: 10.1073/pnas.82.5.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton A. N., Mintzer D., Cordon-Cardo C., Welt S., Fliegel B., Vadhan S., Carswell E., Melamed M. R., Oettgen H. F., Old L. J. Mouse monoclonal IgG3 antibody detecting GD3 ganglioside: a phase I trial in patients with malignant melanoma. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1242–1246. doi: 10.1073/pnas.82.4.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensil C. R., Patel U., Lennick M., Marciani D. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex. J Immunol. 1991 Jan 15;146(2):431–437. [PubMed] [Google Scholar]
- Kitamura K., Stockert E., Garin-Chesa P., Welt S., Lloyd K. O., Armour K. L., Wallace T. P., Harris W. J., Carr F. J., Old L. J. Specificity analysis of blood group Lewis-y (Le(y)) antibodies generatedagainst synthetic and natural Le(y) determinants. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12957–12961. doi: 10.1073/pnas.91.26.12957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston P. O., Adluri S., Helling F., Yao T. J., Kensil C. R., Newman M. J., Marciani D. Phase 1 trial of immunological adjuvant QS-21 with a GM2 ganglioside-keyhole limpet haemocyanin conjugate vaccine in patients with malignant melanoma. Vaccine. 1994 Nov;12(14):1275–1280. doi: 10.1016/s0264-410x(94)80052-2. [DOI] [PubMed] [Google Scholar]
- Livingston P. O., Natoli E. J., Calves M. J., Stockert E., Oettgen H. F., Old L. J. Vaccines containing purified GM2 ganglioside elicit GM2 antibodies in melanoma patients. Proc Natl Acad Sci U S A. 1987 May;84(9):2911–2915. doi: 10.1073/pnas.84.9.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston P. O., Ritter G., Srivastava P., Padavan M., Calves M. J., Oettgen H. F., Old L. J. Characterization of IgG and IgM antibodies induced in melanoma patients by immunization with purified GM2 ganglioside. Cancer Res. 1989 Dec 15;49(24 Pt 1):7045–7050. [PubMed] [Google Scholar]
- Livingston P. O., Wong G. Y., Adluri S., Tao Y., Padavan M., Parente R., Hanlon C., Calves M. J., Helling F., Ritter G. Improved survival in stage III melanoma patients with GM2 antibodies: a randomized trial of adjuvant vaccination with GM2 ganglioside. J Clin Oncol. 1994 May;12(5):1036–1044. doi: 10.1200/JCO.1994.12.5.1036. [DOI] [PubMed] [Google Scholar]
- Lloyd K. O., Old L. J. Human monoclonal antibodies to glycolipids and other carbohydrate antigens: dissection of the humoral immune response in cancer patients. Cancer Res. 1989 Jul 1;49(13):3445–3451. [PubMed] [Google Scholar]
- Magnani J. L., Smith D. F., Ginsburg V. Detection of gangliosides that bind cholera toxin: direct binding of 125I-labeled toxin to thin-layer chromatograms. Anal Biochem. 1980 Dec;109(2):399–402. doi: 10.1016/0003-2697(80)90667-3. [DOI] [PubMed] [Google Scholar]
- Natoli E. J., Jr, Livingston P. O., Pukel C. S., Lloyd K. O., Wiegandt H., Szalay J., Oettgen H. F., Old L. J. A murine monoclonal antibody detecting N-acetyl- and N-glycolyl-GM2: characterization of cell surface reactivity. Cancer Res. 1986 Aug;46(8):4116–4120. [PubMed] [Google Scholar]
- Pukel C. S., Lloyd K. O., Travassos L. R., Dippold W. G., Oettgen H. F., Old L. J. GD3, a prominent ganglioside of human melanoma. Detection and characterisation by mouse monoclonal antibody. J Exp Med. 1982 Apr 1;155(4):1133–1147. doi: 10.1084/jem.155.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindranath M. H., Morton D. L., Irie R. F. An epitope common to gangliosides O-acetyl-GD3 and GD3 recognized by antibodies in melanoma patients after active specific immunotherapy. Cancer Res. 1989 Jul 15;49(14):3891–3897. [PubMed] [Google Scholar]
- Real F. X., Mattes M. J., Houghton A. N., Oettgen H. F., Lloyd K. O., Old L. J. Class 1 (unique) tumor antigens of human melanoma. Identification of a 90,000 dalton cell surface glycoprotein by autologous antibody. J Exp Med. 1984 Oct 1;160(4):1219–1233. doi: 10.1084/jem.160.4.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter G., Boosfeld E., Adluri R., Calves M., Oettgen H. F., Old L. J., Livingston P. Antibody response to immunization with ganglioside GD3 and GD3 congeners (lactones, amide and gangliosidol) in patients with malignant melanoma. Int J Cancer. 1991 May 30;48(3):379–385. doi: 10.1002/ijc.2910480312. [DOI] [PubMed] [Google Scholar]
- Ritter G., Krause W., Geyer R., Stirm S., Wiegandt H. Glycosphingolipid composition of human semen. Arch Biochem Biophys. 1987 Sep;257(2):370–378. doi: 10.1016/0003-9861(87)90579-0. [DOI] [PubMed] [Google Scholar]
- Ritter G., Livingston P. O. Ganglioside antigens expressed by human cancer cells. Semin Cancer Biol. 1991 Dec;2(6):401–409. [PubMed] [Google Scholar]
- SVENNERHOLM L. CHROMATOGRAPHIC SEPARATION OF HUMAN BRAIN GANGLIOSIDES. J Neurochem. 1963 Sep;10:613–623. doi: 10.1111/j.1471-4159.1963.tb08933.x. [DOI] [PubMed] [Google Scholar]
- Saarinen U. M., Coccia P. F., Gerson S. L., Pelley R., Cheung N. K. Eradication of neuroblastoma cells in vitro by monoclonal antibody and human complement: method for purging autologous bone marrow. Cancer Res. 1985 Nov;45(11 Pt 2):5969–5975. [PubMed] [Google Scholar]
- Saito T., Hakomori S. I. Quantitative isolation of total glycosphingolipids from animal cells. J Lipid Res. 1971 Mar;12(2):257–259. [PubMed] [Google Scholar]
- Saleh M. N., Khazaeli M. B., Wheeler R. H., Allen L., Tilden A. B., Grizzle W., Reisfeld R. A., Yu A. L., Gillies S. D., LoBuglio A. F. Phase I trial of the chimeric anti-GD2 monoclonal antibody ch14.18 in patients with malignant melanoma. Hum Antibodies Hybridomas. 1992 Jan;3(1):19–24. [PubMed] [Google Scholar]
- Shiku H., Takahashi T., Oettgen H. F. Cell surface antigens of human malignant melanoma. II. Serological typing with immune adherence assays and definition of two new surface antigens. J Exp Med. 1976 Oct 1;144(4):873–881. doi: 10.1084/jem.144.4.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai T., Cahan L. D., Tsuchida T., Saxton R. E., Irie R. F., Morton D. L. Immunogenicity of melanoma-associated gangliosides in cancer patients. Int J Cancer. 1985 May 15;35(5):607–612. doi: 10.1002/ijc.2910350507. [DOI] [PubMed] [Google Scholar]
- Tai T., Paulson J. C., Cahan L. D., Irie R. F. Ganglioside GM2 as a human tumor antigen (OFA-I-1). Proc Natl Acad Sci U S A. 1983 Sep;80(17):5392–5396. doi: 10.1073/pnas.80.17.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida T., Saxton R. E., Morton D. L., Irie R. F. Gangliosides of human melanoma. J Natl Cancer Inst. 1987 Jan;78(1):45–54. doi: 10.1093/jnci/78.1.45. [DOI] [PubMed] [Google Scholar]
- Welt S., Carswell E. A., Vogel C. W., Oettgen H. F., Old L. J. Immune and nonimmune effector functions of IgG3 mouse monoclonal antibody R24 detecting the disialoganglioside GD3 on the surface of melanoma cells. Clin Immunol Immunopathol. 1987 Nov;45(2):214–229. doi: 10.1016/0090-1229(87)90036-5. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Furukawa K., Fortunato S. R., Livingston P. O., Lloyd K. O., Oettgen H. F., Old L. J. Human monoclonal antibody with dual GM2/GD2 specificity derived from an immunized melanoma patient. Proc Natl Acad Sci U S A. 1990 May;87(9):3333–3337. doi: 10.1073/pnas.87.9.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]



