Abstract
Objectives
To conduct outcome and process evaluations of school-located HPV vaccination clinics in partnership with a local health department.
Methods
Temporary clinics provided the HPV vaccine to middle school girls in Guilford County, North Carolina, in 2009–2010.
Results
HPV vaccine initiation was higher among girls attending host schools than satellite schools (6% vs. 1%, OR = 6.56, CI = 3.99–10.78). Of the girls who initiated HPV vaccine, 80% received all 3 doses. Private insurance or federal programs paid for most vaccine doses.
Conclusions
Lessons learned for creating more effective school-health department partnerships include focusing on host schools and delivering several vaccines to adolescents, not just HPV vaccine alone.
Keywords: HPV vaccine, vaccine clinic, schools
Human papillomavirus (HPV) vaccine prevents infection with HPV types that account for about 70% of cervical cancers in the US.1 National guidelines recommend HPV vaccine for routine use among 11-to-12-year-old adolescents, with catch-up vaccination for females up to age 26.2 However, only 35% of girls 13 to 17 years of age have received the full 3-dose series of HPV vaccine. 3 The low rate of coverage is surprising given that most adolescents in the US can receive free or low-cost HPV vaccine through private insurance or the federally-funded Vaccines for Children program that covers uninsured and Medicaid-eligible children.4
A promising approach for increasing adolescent vaccine uptake, particularly for HPV vaccine, is voluntary provision in schools. In some areas in the United Kingdom and Australia, HPV vaccine completion among 11-to-12-year-old girls exceeds 80%, largely due to voluntary school-located programs that provide HPV vaccine.5–9 In the US, voluntary, school-located programs, which often involve a partnership between a local school system and health department, have successfully increased uptake for several vaccines;10–12 however, few evaluations of school-located interventions to increase HPV vaccine provision exist,13 despite the finding from a national survey that 67% of mothers with daughters 11 to 14 years of age were supportive of school-located HPV vaccination.14
This project sought to create a partnership between schools and a health department to provide HPV vaccine in extramural school-located programs. To this end, the project focused on the local school system and health department in Guilford County, North Carolina. The 2000–2009 age-adjusted cervical cancer mortality rate in Guilford County is higher than the rate in the 4 adjacent counties of Alamance, Rockingham, Randolph, and Forsyth (6.1 vs. 3.5, 3.7, 4.1, and 5.3 cases per 100,000 women annually) and is the third highest in the state,15,16 which highlights the benefits of HPV vaccination for girls and women living in this area. Prior to the launch of the intervention, the Guilford County Health Department conducted a countywide education campaign called “Don’t Wait… Educate!” that reduced misconceptions about HPV and HPV vaccine among parents, healthcare providers, and middle school staff and increased school staffs’ support of school-located vaccination programs.17 Almost all parents surveyed (97%) supported offering HPV vaccination clinics at schools.17
Bolstered by these findings, the department launched an extramural school-located HPV vaccination campaign called “Don’t Wait… Vaccinate!” The present study sought to provide outcome and process evaluations of this intervention. The intervention goals for outcome measures were to administer HPV vaccine to at least 30% of middle school girls at a school-located clinic and have at least 90% of girls who initiate the vaccine complete the 3-dose series. Because this intervention began before the Centers for Disease Control and Prevention (CDC) recommended routine administration of HPV vaccine to boys,18 we targeted only girls. We hope that our report will assist others in developing and expanding school-located vaccine programs.
METHODS
The intervention provided HPV vaccine at temporary school-located clinics to Guilford County middle school girls who sought immunization accompanied by one of their parents. School officials identified 6 regions in the county, and for each they selected a middle school to host 4 one-day clinics that provided HPV vaccine (ie, “host schools”). The county’s 16 other middle schools served as “satellite schools” whose students could receive HPV vaccine at the host school clinics in their region. Vaccine doses were free for the girls and their parents.
Planning
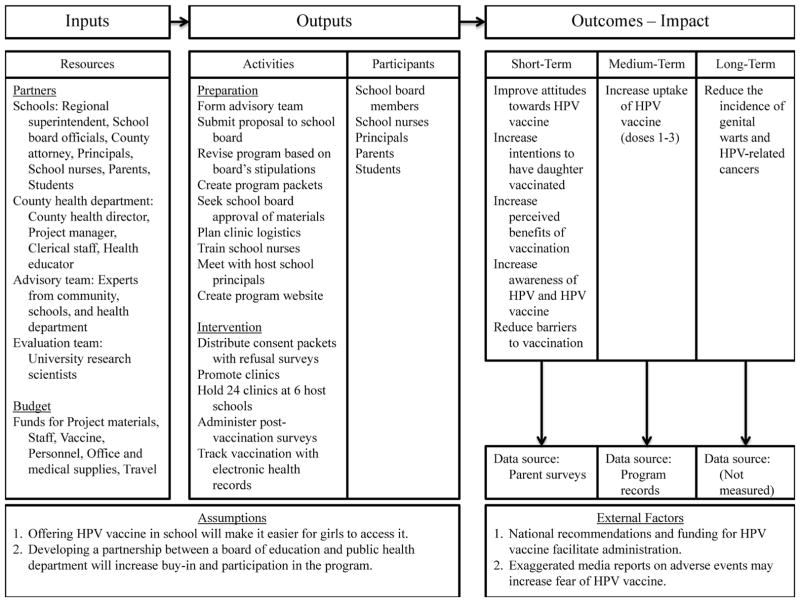
After securing grant funding for the intervention, the Guilford County Health Department made substantial initial efforts to create partnerships with key stakeholders to obtain access to the schools. Figure 1 shows the logic model guiding the development and implementation of this intervention, and below we provide more details about this process.
Figure 1.
Intervention Logic Model
Local school system
The health department developed a collaborative partnership with the Guilford County School System. Seven months prior to the desired start of the school-located vaccination clinics, they submitted a written proposal to the board of education describing HPV prevalence, risks, consequences of infection, and vaccination clinic execution plans. Once approved, the board members wrote guidelines for conducting the school-located vaccination clinics, described below. With assistance from the regional superintendent, they contacted the principals of the host schools to determine the dates of the clinics. The regional superintendent contacted the principals of the satellite schools to notify them that all of their female students would be eligible to attend the HPV vaccination clinics at the host schools.
Health department
The core intervention team consisted of a project manager, clerical support staff, and a health educator, all of whom were employees of the Guilford County Department of Public Health. The core team assigned school health nurses to staff the clinics and held in-service training sessions for the nurses. The core team also entered information on vaccines delivered in the clinics into the North Carolina Immunization Registry, a statewide electronic database that tracks the immunization status of children visiting public and private healthcare facilities.
The core team designed surveys assessing parents’ reasons for vaccinating their daughters, previous barriers to vaccination, and their overall assessment of the school-located vaccination clinics. Parents completed this survey during the post-vaccination observation period of the daughters’ third dose. For daughters who missed third doses, the team mailed their parents the surveys with prestamped return envelopes. By administering this survey at the end of the program, the study team was able to gather information on factors related to program completion, including reasons for participating and satisfaction with the program activities. The consent packet contained a separate survey on a postcard without postage for parents who chose not to vaccinate their daughters at the school-located clinics to determine their reasons for not using the clinics.
In addition to handling vaccination clinic logistics, the core team devised the information and consent packet for girls to take home to their parents. The packets contained a cover letter approved and signed by the Guilford County health director and Guilford County Schools’ superintendent, the HPV and HPV vaccine fact sheet, the HPV vaccination consent form, the HPV vaccination waiver, clinic schedules, a statement that the vaccination clinics would incur no out-of-pocket expense, and an unstamped postcard survey (as described above) for parents declining vaccination. The packets were written at the eighth-grade reading level, and Spanish translations appeared on the back of all materials. The intervention plan was to send the packets home with every girl 2 weeks prior to the first vaccination clinics to allow enough time for the parents to read the packets and make a decision. Parents deciding to vaccinate their daughters at a school-located clinic brought signed consent forms from the packet to the first clinic; vaccinations provided during subsequent second and third dose clinics did not require additional consent.
The core team also provided education and outreach. As described above, education was promoted largely by the earlier campaign, “Don’t Wait… Educate!”17 In addition, during the vaccination intervention, a web campaign appeared on the Guilford County Department of Public Health website that provided continuing education and outreach to parents, and the health educator provided educational HPV presentations upon request. Parents also were reminded about the vaccination clinics through ConnectEd, an automated calling service, and the media relations manager promoted the school-located clinics through local media outlets.
Advisory team
To assist the core team, the project manager formed an advisory team of 15 experts in various areas from the community, schools, and health department. All advisory team members worked part-time on the intervention and generally contributed one to 4 in-kind hours per week.
Implementation
The health department presented our proposal for school-located vaccination clinics to the county’s board of education in April 2009. The board approved our proposal by a contentious 6–5 vote with 5 major stipulations. First, the clinics had to take place during non-instructional hours. Second, parents had to be present for all of their daughters’ vaccinations, even if the parents signed a consent form. Third, the clinics could not put any additional burden or workload on school faculty and staff. Fourth, senior school staff had to approve materials sent home with students or mailed to parents. Fifth, senior school staff would determine how many and which schools would host the vaccination clinics.
Principals of the 6 designated host schools each agreed to offer a first dose clinic in October 2009, a second dose clinic in December 2009, and a third dose clinic in April 2010, in line with dosing recommendations. 2 Most principals of the host schools (6/6, or 100%) and satellite schools (15/16, or 94%) agreed to distribute HPV vaccine information and consent packets directly to the students. The information and consent packets took 2 months to create and receive approval from the local school system senior staff, local health director, and county attorney. Just prior to packet distribution, national media carried a negative story about the safety and potential side effects of the HPV vaccine. The officials who approved our packets asked our team to revise them to include a second, separate consent form that parents had to sign acknowledging the possibility of HPV vaccine side effects, including death. Due to concerns that the delayed packet distribution may have contributed to a low number of girls attending the first round of clinics offered in October, the team invited girls to receive their first dose of HPV vaccine at any of the clinics already planned in December 2009, and they added 3 second-dose clinics in February 2010 and 3 third-dose clinics in June 2010.
Evaluation
The Institutional Review Board at the University of North Carolina determined that analysis of data from this study did not require their approval, because it was an evaluation of a quality improvement program. We gathered data on HPV vaccine doses delivered and schools’ demographic information. For the outcome evaluation, the primary outcome of interest was the number of middle school girls receiving at least one dose of HPV vaccine at a vaccination clinic at a host school, based on program records. Other outcomes were the number of middle school girls receiving all 3 doses and the total number of doses delivered, also from program records. We examined whether HPV vaccine initiation differed between designated host and satellite schools using mixed logistic regression models, treating school as a random effect because girls were clustered within schools. We examined differences in school size using the median score test. The analysis was 2-tailed, with a critical alpha of .05, and conducted using SAS, version 9.2 (Cary, NC).
For the process evaluation, we characterized parents’ reasons for vaccination, barriers to prior vaccination, and evaluation of the school-located vaccination clinics among parents choosing to vaccinate their daughters. Postcard surveys of parents declining vaccination elicited their reasons for refusal. We documented the budgeted and actual expenses accrued, as well as in-kind expenses.
RESULTS
In total, the 6 host schools had 24 vaccination clinics between October 2009 and June 2010. Six middle schools hosted vaccination clinics, and 15 schools participated as satellite sites. Only one school that was eligible to be a satellite school declined to participate because the administration was concerned that parents would object. On average, satellite schools were 10 miles away from their associated host school (range, 2–17 miles). Students from 7 additional “ad-hoc” schools (6 high schools, one elementary school) attended school-located vaccination clinics at the host schools, and some students with unknown affiliations also attended the clinics. Staff provided these “ad-hoc” vaccinations as a courtesy to the parents.
The majority of host (67%), satellite (67%), and ad-hoc (57%) schools were city/urban schools (Table 1); the remaining schools were rural, except one that was suburban. The percentage of host and satellite schools with ≤ 40% economically disadvantaged students was similar (33% vs. 27%), as was median seventh grade class size (both 24). In addition, host and satellite schools had similar percentages of students testing at or above their grade-level on the Association of Boards of Certification (ABC) end-of-year tests for reading (67% and 69%) and math (84% and 82%). The median number of students attending the host (720), satellite (913), and ad-hoc schools (1160) did not differ (p = .14).
Table 1.
Characteristics of Participating Schools, Guilford County, 2009–2010
| Host Schools | Satellite Schools | Ad-hoc Schools | |
|---|---|---|---|
| Schools, N | |||
| Overall | 6 | 15 | 7 |
| Region | |||
| City | 4 | 10 | 4 |
| Suburbs | 0 | 1 | 0 |
| Rural | 2 | 4 | 3 |
| ≤40% students disadvantaged | 2 | 4 | 3 |
| Met state standards for adequate yearly progress a | 1 | 4 | 2 |
| Performance designation b | |||
| Honor or excellent schools | 2 | 4 | 1 |
| Progress schools | 2 | 9 | 4 |
| Non-recognition or priority schools | 2 | 2 | 2 |
| Students, Median | |||
| All grades (range) | 720 (233–829) | 913 (543–1104) | 1160 (118–1386) |
| Seventh grade (range) | 24 (17–26) | 24 (16–26) | NA |
| Tested at or above grade levelb | |||
| Reading | 67% | 69% | NA |
| Math | 84% | 82% | NA |
| Acts of violence (per 100 students) | 0 | 1 | 1 |
| Teachers, Median | |||
| ≥ 10 years of experience | 38% | 39% | 46% |
| Annual turnover | 13% | 11% | 12% |
Note.
Based on 29 goals that schools must meet under the No Child Left Behind Act.
Based on Association of Boards of Certification (ABCs) end-of-grade tests that are designed to measure student performance on the goals, objectives, and grade-level competencies specified in the North Carolina Standard Course of Study.
Outcome Evaluation
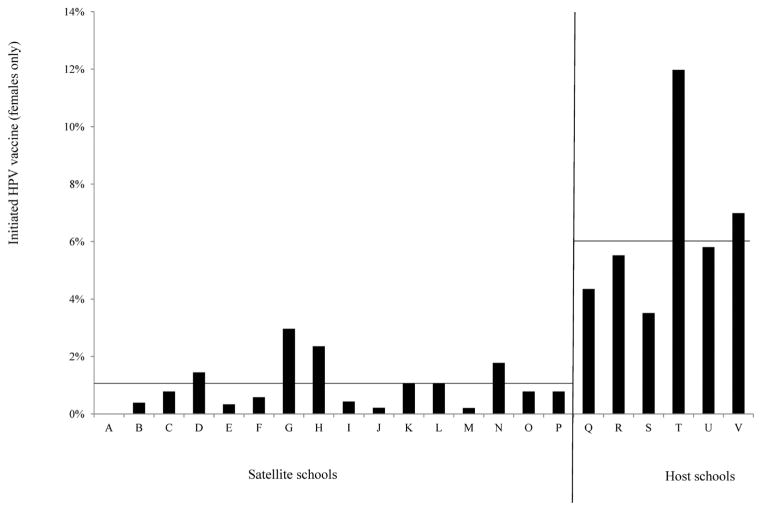
The median age of students served by the clinics was 12 years (range, 10–17 years), and the median grade in school was 7 (range, 4–12). The clinics provided HPV vaccine for 189 girls: 171 attended a host or satellite school; 11 attended an ad-hoc school; and 7 did not indicate the schools they attended. A larger percentage of girls attending a host school received at least one dose of HPV vaccine compared to those that attended satellite middle schools (6% [110/1781] vs. 1% [61/6135], Figure 2). A logistic regression analysis confirmed this difference was statistically significant (OR = 6.56, 95% CI = 3.99–10.78).
Figure 2.
HPV Vaccine Initiation at School-located Clinics
The 171 girls attending host or satellite schools who received the HPV vaccine represent about 2% (171/7916) of the total population of their schools. Of girls who initiated the HPV vaccine series in the clinics, 80% (137/181) received all 3 doses of the series (Table 2).
Table 2.
Doses of HPV Vaccine Provided to Middle School Girls at School-located Clinics (N=7916)
| N (%) | |
|---|---|
| No doses | 7745 (97.9) |
| One dose | 12 (0.2) |
| Two doses | 22 (0.3) |
| Three doses | 137 (1.7) |
Note.
HPV vaccine series completion requires 3 doses
Process Evaluation
Implementation
The intervention faced several implementation challenges. Two satellite schools did not distribute HPV vaccine information and consent packets to students as requested; instead, they sent a message informing parents to pick-up the packets from the school’s front office if they were interested in using the vaccination clinics. We estimate that parents of about 1000 (12%) girls in our target population of 7916 did not receive the material as a result. Difference in vaccine initiation between satellite and host schools remained statistically significant even after dropping these schools (p < .001).
The educational campaign included a Web presentation with voice narration that was removed from the Guilford County Department of Public Health website before the school-located vaccination clinics ended because the space was needed for other information. An additional unanticipated communication barrier was not being allowed to use the school system television stations to promote the clinics. The intervention team conducted 27 outreach presentations regarding HPV vaccine at the 21 host and satellite schools as part of this project (M = 1.3 presentations per school). They also conducted 18 outreach presentations to community groups, on request, including community organizations focusing on public health, medicine, and youth issues.
Parent surveys
Of 189 girls vaccinated, 63% (N = 119) of their parents completed post-vaccination surveys. The most common reasons parents gave for getting their daughters HPV vaccine were that it was the best way to prevent HPV infection (86%) and because the potential health consequences of HPV infection could be serious (52%) (Table 3). The most common reasons parents gave for not vaccinating previously were high HPV vaccine cost and lack of knowledge of HPV (both 33%).
Table 3.
Parent Responses about Participating in School-located HPV Vaccines Clinics
| % | |
|---|---|
| Why did you decide to get your daughter HPV vaccine? (N = 119) | |
| It is the best way to prevent HPV infection. | 86% |
| The potential health consequences of HPV infection could be serious. | 52% |
| My daughter’s healthcare provider recommended it. | 29% |
| My daughter is, or will be, at risk of acquiring HPV. | 24% |
| Why did you decide not to get your daughter HPV vaccine previously? (N = 119) | |
| HPV vaccine is too expensive. | 33% |
| I didn’t know enough about HPV. | 33% |
| It was inconvenient to make three appointments to receive all the shots. | 29% |
| I didn’t know enough about HPV vaccine. | 28% |
| HPV vaccine is not mandatory for school entry. | 24% |
| Feedback about school-located HPV vaccine clinic. (N = 119) | |
| I was pleased with the overall experience. | 100% |
| I want to see more partnering between the Guilford County School System and the Guilford County Department of Public Health in the future. | 99% |
| I would recommend school-located vaccination clinics to friends and family. | 100% |
| I felt that the information and consent packet was sufficient to make an informed decision. | 97% |
| The other information I received from this project helped me to make an informed decision. | 99% |
| Why did you choose not to have your daughter participate in our program? (N = 75) | |
| My daughter already received HPV vaccine. | 77% |
| I am not sure HPV vaccine is safe and effective. | 17% |
| I am worried about potential side effects of HPV vaccine. | 13% |
| I do not believe my daughter needs HPV vaccine. | 9% |
| I need more information before making a decision about HPV vaccine. | 7% |
All or almost all parents were pleased with their overall school-located clinic experiences (100%), wanted to see more partnering between the Guilford County School System and Guilford County Department of Public Health in the future (99%), and felt that the information provided by the intervention helped them to make informed decisions (97%–99%) (Table 3).
Of parents who chose not to have their daughters receive HPV vaccine through our program, 75 (~1%) returned a postcard survey. Most (77%) of these parents declined HPV vaccine at the school-located clinic because their daughters had already received HPV vaccine (Table 3). Other reasons parents gave were that they questioned the safety and effectiveness of the vaccine (17%) or worried about potential side effects (13%).
Costs
The school-located clinics used only 36% of the $376,104 budget. The largest percentage of the expended budget went to personnel costs (67%); the Guilford County Department of Public Health contributed an additional 48 in-kind clinic staff hours and 534 other in-kind staff hours. Whereas the second largest budget item was HPV vaccine itself (23%), vaccine expenses were lower than expected, which accounted for most of the budget surplus. The 2 main reasons for this difference between expected and actual costs were that the program delivered fewer vaccine doses than expected, and most doses were covered by either private insurance (46%) or the federal Vaccines for Children program (41%). Thus, the grant paid for doses of HPV vaccine for only 25 (13%) of the 189 participating girls.
DISCUSSION
We sought to create a model for partnership between a local school system and health department to provide administration of HPV vaccine. Although school-located HPV vaccine provision has been successful in other countries,5–9 the US has not examined it adequately as a way to increase vaccine coverage. Our extramural school-located HPV vaccination clinics provided HPV vaccine to 2% of girls attending middle school in Guilford County, North Carolina. Even accounting for the number of girls who had likely already initiated HPV vaccine prior to the campaign (around 50%, the rate of initiation of HPV vaccine among girls in 2009 in North Carolina19), which would reduce the number of eligible girls, vaccine initiation as part of the program was well short of our goal of 30%. The HPV vaccine initiation rate for the host schools of 6% was closer to the median 10% increase reported for other school-located HPV vaccine interventions in schools without health centers.13
More encouraging, completion of the 3-dose series was 80%, which was close to our goal of 90%. The high completion rate suggests that school-located programs could be quite successful in the US if vaccine initiation were higher.
Lessons Learned
We offer several insights that may have improved HPV vaccination rates and could help future school-located vaccination clinics achieve their targets. Given that Guilford County schools did not have health centers, developing a stronger partnership between the local school system and the health department was a promising approach to increasing HPV vaccination. However, it would have been beneficial to include leaders in the school system much earlier in the planning process, perhaps as early as the grant-writing phase of planning. Some school system leaders were resistant to efforts to provide HPV vaccine to middle school girls, which prevented the intervention team from communicating freely with satellite schools and from using certain communication resources, such as the school system television station. Involving school leaders in project development may have helped gain trust, understanding, and the support necessary to better promote HPV vaccine uptake at school-located vaccination clinics.
Focusing more generally on adolescent vaccines may elicit less resistance to HPV vaccine during planning of future interventions.13,20 The US Advisory Committee on Immunization Practices (ACIP) currently recommends administering tetanus booster and meningococcal vaccines during the same healthcare visit if both are indicated and available.21 HPV vaccine also can be given to adolescents at the same visit as other vaccines are provided, as doing so likely will increase the number of adolescents receiving vaccines on schedule.1 Because tetanus, diphtheria, and a cellular pertussis (Tdap) vaccine is required for entry into sixth grade in North Carolina and many other states,22 offering it in conjunction with other recommended vaccines during the transition from fifth to sixth grade would likely increase participation at clinics and vaccination rates for other recommended vaccines. Parents have expressed concerns about concomitant administration of vaccines,23–25 but most have allowed their children to receive multiple recommended vaccines during the same visit.26 In addition, programs in other countries that offer multiple adolescent vaccines concurrently have been successful in achieving high rates of uptake.7,8,27
Given that parents of younger students may participate more frequently in school events than parents of older students, it may be helpful to offer HPV education to parents of fifth-grade students. As HPV vaccine is recommended for ages 11 or 12, and approved for ages 9 through 26,1 most fifth graders (usually ages 10 to 11 years) would be eligible to receive HPV vaccinations at clinics held in their school. Finally, school-located clinics that occur during school hours (eg, lunchtime) may increase the ability to vaccinate children who might otherwise miss recommended but non-mandatory vaccines like HPV. For this intervention, the local school system required the presence of parents during all vaccinations, so the intervention team chose to offer them after school. The requirement that parents accompany their daughters may have contributed to low participation in our intervention.
Based on our results that revealed that the largest percentage of girls receiving vaccines at school-located clinics attended schools that hosted them, we recommend offering vaccination clinics in every school that contains the target population. As leaders from the local school system chose the number and schools that would host the vaccination clinics, we were unable to consider offering them at all 22 middle schools in the county. Given that the characteristics of host and satellite schools were similar, the lower participation rates for students at satellite schools may be due to the extra distance needed to travel to host schools, unfamiliarity with the host schools, and lack of visibility of the vaccination clinics.
Publicizing school-located vaccination clinics is critically important. Although we believe the health department’s previous HPV education campaign generated support for the HPV vaccination clinics,17 marketing about specific clinic dates and times still had to go through the school system. School nurses and directors of health services were important mediators among parents, school system leaders, and the core team. We recommend that schools deliver communication messages using a variety of methods, such as in-person presentations, letters, health department and school system websites, television broadcasts, newspaper articles, radio messages, and email, as schools did during our intervention.
Obtaining parental consent for daughters to receive HPV vaccine was a significant challenge. Because of requirements from the school board, parents had to sign 2 consent forms and accompany daughters to all clinic visits. Opt-in consent processes are associated with reduced uptake of preventive services,28–30 and requiring parents to attend afterschool clinics only increased barriers to participation. Persons planning future interventions should consider opt-out consent processes or eliminating requirements for parents to accompany children, or taking steps to eliminate the need for parental consent.20 In Ontario,31 school-located vaccination programs would waive the requirement for parental consent if program staff judged the student capable of providing consent, and in some US states, school health centers can provide preventive health services without notifying parents due to privacy laws.32 Other methods to increase the number of parents returning consent forms for school-located vaccination programs include offering incentives and creating consent forms that are colorful and attractive.10
Finally, project budgets should include funds to mail vaccine information and consent packets to parents, put postage on surveys that parents need to return by mail, and pay school nurses for additional hours worked. We learned that some of the vaccine information and consent packets distributed to the principals to give to girls in their school never made it home to parents. We believe that had the intervention team prioritized funding for sending the packets directly to the parents, they would have been more likely to receive them, and more girls may have attended the clinics. In addition, few parents who did not get HPV vaccine for their daughters in the extramural clinics returned the postcard survey, but we believe that the response rate could have been much higher if the surveys had postage attached. Unfortunately, the intervention team was not able to include postage for the postcard surveys because the original budget did not account for this cost. However, they mailed reminders to the home of each girl that initiated vaccination at a school-located clinic that reminded parents when she was due for a subsequent dose, and we believe these personal reminders helped the intervention increase series completion.
Limitations and Strengths
Several of the lessons learned described above reflect limitations to this intervention. Although this team did attempt to generate buy-in from stakeholders early in the intervention development process, school principals were still hesitant to participate fully. The intervention team worked closely with school officials at the district level, but expanding this outreach to principals will be important for future school-located vaccine programs.
In addition, the budget projections were different from the actual expenditures. The biggest discrepancy between projected and actual costs pertained to the cost of HPV vaccine doses provided by the program. Formative research to anticipate which portion of students would be able to receive HPV vaccine funded by federal programs or private insurance, instead of provided by the intervention, may have allowed us to allocate funds to increased advertising of the school-located clinics or postage for the surveys for non-participating parents. These efforts could have boosted participation in the program and the surveys, respectively, allowing us to have greater impact on HPV vaccination rates in this population as well as to draw stronger conclusions about why parents chose not to participate.
Another limitation was the low participation: 2% overall, with only 6% of girls at host schools receiving at least one dose of HPV vaccine. The reasons for this low rate of participation are unclear, but some possibilities include the barriers to participation (ie, 2 consent forms, requiring parents to accompany daughters to after school clinics), poor communication with and advertising to parents, resistance to HPV vaccination, and the novelty of delivering a healthcare service in a school setting. We discuss some of these possibilities in more detail below.
With only one percent of non-participating parents returning their surveys, we cannot draw conclusions about why so many parents chose not to participate. We intended for these surveys to communicate information about why parents chose not to participate in HPV vaccine clinics; however, we cannot generalize from the parents who returned the postcards to the entire sample of parents in the host and satellite schools.
The study design also has some limitations. Though the study was not a randomized trial with assignment to condition, satellite schools served as a comparison for host schools; girls and parents attending satellite schools faced similar challenges to receiving the HPV vaccine as girls attending healthcare clinics, such as locating and travelling to the venue. The comparison of the 2 rests on the question of how the school superintendent assigned schools to condition, a factor that was beyond our control and may reflect other differences between the schools.
This intervention may have limited generalizability to other contexts. For instance, the majority of schools participating in this program were urban, so how the intervention would work in rural schools is less certain. In addition, this program took place during the 2009–2010 school year, and in the time since then, the CDC has recommended that boys receive HPV vaccine as a routine part of preventive care.18 In addition, uptake of HPV vaccine has become more common, though coverage is still low.3 The intervention team did not use an “off-the-shelf” health behavior theory to guide the development of this program, though the intervention’s goals of increasing awareness and reducing barriers are consistent with a stage model of action. 33 Finally, this intervention involved a local health department partnering with a school, and partnerships with other types of organizations may have varying degrees of success. For instance, parents have low levels of trust towards drug companies, 34 so partnerships with such organizations may be less successful than partnerships with governmental or non-profit agencies.
The study has several strengths. We evaluated the program under real-life conditions similar to those that many public health departments around the country face: limited time, uncertain budget, and challenges in working with stakeholders and the public. Although this study did not have the experimental control a randomized efficacy trial would, it represents a more replicable model of partnership between public health departments and schools to provide a time-limited intervention to boost vaccination. In addition, the program had high buy-in from schools, with 21 out of 22 eligible schools taking part in the program.
This program offered several innovations over previous vaccine interventions. Few school-located HPV vaccine interventions have been conducted in the US.13 Compared to previous studies, this intervention was innovative in that it was the first to focus solely on HPV vaccine; it served a large number of students; and it built upon an educational campaign about HPV vaccine in the same county. This last innovation is especially important, given that parents commonly say that not knowing enough about HPV vaccine is a reason for not vaccinating their children.35–37 The program also focused on adolescents, whereas most previous school-located vaccination studies targeted younger children.10,38–41
The present study represents the first effort that we are aware of in the US to target a system of schools instead of intervening on single schools. Interventions in the United Kingdom and Australia, 5,7,8,42 which have exceptional rates of participation, operate under the auspices of national programs to boost HPV vaccination, which could suggest that political will and widespread, top-down dissemination could improve participation.
Conclusions
Whereas implementing school-located vaccination clinics delivered a modest number of HPV vaccine doses, we believe that such programs may improve HPV vaccination rates if intervention planning takes into account the aforementioned considerations. In particular, we believe that developing a partnership between local health departments and school systems when schools do not have pre-existing health centers is crucial to the success of school-located vaccination clinics, and that this partnership should be well-established before clinics begin. Although other countries have demonstrated the effectiveness of school-located vaccination clinics,5–9 our experiences provide important lessons for improving the effectiveness of future school-located vaccination clinics in the US. We hope that our report will assist others in developing and expanding school-located vaccination programs.
Footnotes
Human Subjects Statement
The Institutional Review Board at the University of North Carolina determined that this study was exempt from review because it was an evaluation of a quality improvement program.
Conflict of Interest Statement
C A Panozzo currently works for Global PharmacoVigilance, Sanofi Pasteur, but was not associated with this organization during the project or while she worked on the data analysis and manuscript writing. P L Reiter has received a past research grant from Merck Sharp & Dohme Corp. but has not received honoraria or consulting fees from this company. N T Brewer has received grants or served on paid advisory boards for GlaxoSmith- Kline and Merck Sharp & Dohme Corp and is a Consultant/Advisory Board member of Merck. No potential conflicts of interest were disclosed by the other authors.
Contributor Information
Brenda W. Stubbs, Community Health Educator, Guilford County Department of Public Health, Greensboro, NC.
Catherine A. Panozzo, Doctoral Candidate, Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, NC.
Jennifer L. Moss, Doctoral Student, Department of Health Behavior, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, NC.
Paul L. Reiter, Assistant Professor, Division of Cancer Prevention and Control, College of Medicine, and Comprehensive Cancer Center, The Ohio State University, Columbus, OH.
Dianne H. Whitesell, Nurse Practitioner and Project Director, Guilford HPV Campaign, Guilford County Department of Public Health, Greensboro, NC.
Noel T. Brewer, Associate Professor, Department of Health Behavior, and Lineberger Comprehensive Cancer Center, University of North Carolina, Chapel Hill, NC.
References
- 1.Markowitz LE, Dunne EF, Saraiya M, et al. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007;56(RR-2):1–24. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2010;59(20):626–629. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC). . National and state vaccination coverage among adolescents aged 13–17 years - United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:671–677. [PubMed] [Google Scholar]
- 4.Khan K, Curtis CR, Ekwueme DU, et al. Preventing cervical cancer: overviews of the National Breast and Cervical Cancer Early Detection Program and 2 US immunization programs. Cancer. 2008;113(10 Suppl):3004–3012. doi: 10.1002/cncr.23765. [DOI] [PubMed] [Google Scholar]
- 5.Brabin L, Roberts SA, Stretch R, et al. Uptake of first two doses of human papillomavirus vaccine by adolescent schoolgirls in Manchester: prospective cohort study. BMJ. 2008;336(7652):1056–1058. doi: 10.1136/bmj.39541.534109.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stretch R. Implementing a school-based HPV vaccination programme. Nurs Times. 2008;104(48):30–33. [PubMed] [Google Scholar]
- 7.Reeve C, De La Rue S, Pashen D, et al. School-based vaccinations delivered by general practice in rural north Queensland: an evaluation of a new human papilloma virus vaccination program. Commun Dis Intell. 2008;32(1):94–98. doi: 10.33321/cdi.2008.32.14. [DOI] [PubMed] [Google Scholar]
- 8.Watson M, Shaw D, Molchanoff L, McInnes C. Challenges, lessons learned and results following the implementation of a human papilloma virus school vaccination program in south Australia. Aust N Z J Public Health. 2009;33(4):365–370. doi: 10.1111/j.1753-6405.2009.00409.x. [DOI] [PubMed] [Google Scholar]
- 9.Brotherton JM, Deeks SL, Campbell-Lloyd S, et al. Interim estimates of human papillomavirus vaccination coverage in the school-based program in Australia. Commun Dis Intell. 2008;32(4):457–461. doi: 10.33321/cdi.2008.32.45. [DOI] [PubMed] [Google Scholar]
- 10.Cawley J, Hull HF, Rousculp MD. Strategies for implementing school-located influenza vaccination of children: a systematic literature review. J Sch Health. 2010;80(4):167–175. doi: 10.1111/j.1746-1561.2009.00482.x. [DOI] [PubMed] [Google Scholar]
- 11.Humiston SG, Rosenthal SL. Challenges to vaccinating adolescents: vaccine implementation issues. Pediatr Infect Dis J. 2005;24(6 Suppl):S134–S140. doi: 10.1097/01.inf.0000166161.12087.94. [DOI] [PubMed] [Google Scholar]
- 12.Lindley MC, Boyer-Chu L, Fishbein DB, et al. The role of schools in strengthening delivery of new adolescent vaccinations. Pediatrics. 2008;121(Suppl 1):S46–S54. doi: 10.1542/peds.2007-1115F. [DOI] [PubMed] [Google Scholar]
- 13.Hayes KA, Entzel P, Berger W, et al. Early lessons learned from extramural school programs that offer HPV vaccine. J Sch Health. 2013;83(2):119–126. doi: 10.1111/josh.12007. [DOI] [PubMed] [Google Scholar]
- 14.Kadis JA, McRee AL, Gottlieb SL, et al. Mothers’ support for voluntary provision of HPV vaccine in schools. Vaccine. 2011;29(14):2542–2547. doi: 10.1016/j.vaccine.2011.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.North Carolina State Center for Health Statistics. [Accessed January 5, 2011];Cancer incidence rates, North Carolina (on-line) Available at: http://www.epi.state.nc.us/SCHS/data/cancer.cfm.
- 16.US Census Bureau. [Accessed January 5, 2011];State and county QuickFacts: Guilford County, North Carolina (on-line) Available at: http://quickfacts.census.gov/qfd/states/37/37081.html.
- 17.Reiter PL, Stubbs B, Panozzo CA, et al. HPV and HPV vaccine education intervention: effects on parents, healthcare staff, and school staff. Cancer Epidemiol Biomarkers Prev. 2011;20(11):2354–2361. doi: 10.1158/1055-9965.EPI-11-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Recommendations on the use of quadrivalent human papillomavirus vaccine in males--Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60(50):1705–1708. [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. National, state, and local area vaccination coverage among adolescents aged 13–17 years --- United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59(32):1018–1023. [PubMed] [Google Scholar]
- 20.Reiter PL, McRee AL, Pepper JK, Brewer NT. Default policies and parents’ consent for school-located HPV vaccination. J Behav Med. 2012;35(6):651–657. doi: 10.1007/s10865-012-9397-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Broder KR, Cortese MM, Iskander JK, et al. Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55(RR-3):1–34. [PubMed] [Google Scholar]
- 22.Immunization Action Coalition. [Accessed April 1, 2011];State mandates on immunization and vaccine-preventable diseases: Tdap (on-line) Available at: http://www.immunize.org/laws/tdap.asp.
- 23.Madlon-Kay DJ, Harper PG. Too many shots? Parent, nurse, and physician attitudes toward multiple simultaneous childhood vaccinations. Arch Fam Med. 1994;3(7):610–613. doi: 10.1001/archfami.3.7.610. [DOI] [PubMed] [Google Scholar]
- 24.Woodin KA, Rodewald LE, Humiston SG, et al. Physician and parent opinions: are children becoming pincushions from immunizations? Arch Pediatr Adolesc Med. 1995;149(8):845–849. doi: 10.1001/archpedi.1995.02170210019003. [DOI] [PubMed] [Google Scholar]
- 25.Reiter PL, McRee AL, Pepper JK, et al. Improving human papillomavirus vaccine delivery: a national study of parents and their adolescent sons. J Adolesc Health. 2012;51(1):32–37. doi: 10.1016/j.jadohealth.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melman ST, Nguyen TT, Ehrlich E, et al. Parental compliance with multiple immunization injections. Arch Pediatr Adolesc Med. 1999;153(12):1289–1291. doi: 10.1001/archpedi.153.12.1289. [DOI] [PubMed] [Google Scholar]
- 27.Kessels SJ, Marshall HS, Watson M, et al. Factors associated with HPV vaccine uptake in teenage girls: a systematic review. Vaccine. 2012;30(24):3546–3556. doi: 10.1016/j.vaccine.2012.03.063. [DOI] [PubMed] [Google Scholar]
- 28.Johnson EJ, Goldstein D. Do defaults save lives? Science. 2003;302(5649):1338–1339. doi: 10.1126/science.1091721. [DOI] [PubMed] [Google Scholar]
- 29.Junghans C, Feder G, Hemingway H, et al. Recruiting patients to medical research: double blind randomised trial of “opt-in” versus “opt-out” strategies. BMJ. 2005;331(7522):940. doi: 10.1136/bmj.38583.625613.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nijkamp MD, Hollestelle ML, Zeegers MP, et al. To be(come) or not to be(come) an organ donor, that’s the question: a meta-analysis of determinant and intervention studies. Health Psychology Review. 2008;2(1):37–41. [Google Scholar]
- 31.Wilson SE, Karas E, Crowcroft NS, et al. Ontario’s school-based HPV immunization program: school board assent and parental consent. Can J Public Health. 2012;103(1):34–39. doi: 10.1007/BF03404066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.North Carolina General Assembly. [Accessed April 26, 2013];Practice of medicine: treatment of minors: 90–21.5: minor’s consent sufficient for certain medical health services. 2012 Available at: http://www.ncleg.net/gascripts/statutes/statutestoc.pl?chapter=0090.
- 33.Weinstein ND. The precaution adoption process. Health Psychol. 1988;7(4):355–386. doi: 10.1037//0278-6133.7.4.355. [DOI] [PubMed] [Google Scholar]
- 34.Pepper JK, Reiter PL, McRee AL, Brewer NT. Advertisements promoting human papillomavirus vaccine for adolescent boys: does source matter? Sex Transm Infect. 2012;88(4):264–265. doi: 10.1136/sextrans-2011-050197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bartlett JA, Peterson JA. The uptake of human papillomavirus (HPV) vaccine among adolescent females in the United States: a review of the literature. J Sch Nurs. 2011;27(6):434–446. doi: 10.1177/1059840511415861. [DOI] [PubMed] [Google Scholar]
- 36.Gilkey MB, Moss JL, McRee AL, Brewer NT. Do correlates of HPV vaccine initiation differ between adolescent boys and girls? Vaccine. 2012;30(41):5928–5934. doi: 10.1016/j.vaccine.2012.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brewer NT, Gottlieb SL, Reiter PL, et al. Longitudinal predictors of human papillomavirus vaccine initiation among adolescent girls in a high-risk geographic area. Sex Transm Dis. 2011;38(3):197–204. doi: 10.1097/OLQ.0b013e3181f12dbf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luthy KE, Thorpe A, Dymock LC, Connely S. Evaluation of an intervention program to increase immunization compliance among school children. J Sch Nurs. 2011;27(4):252–257. doi: 10.1177/1059840510393963. [DOI] [PubMed] [Google Scholar]
- 39.Glezen WP, Gaglani MJ, Kozinetz CA, Piedra PA. Direct and indirect effectiveness of influenza vaccination delivered to children at school preceding an epidemic caused by 3 new influenza virus variants. J Infect Dis. 2010;202(11):1626–1633. doi: 10.1086/657089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis MM, King JC, Jr, Moag L, et al. Countywide schoolbased influenza immunization: direct and indirect impact on student absenteeism. Pediatrics. 2008;122(1):e260–e265. doi: 10.1542/peds.2007-2963. [DOI] [PubMed] [Google Scholar]
- 41.King JC, Jr, Stoddard JJ, Gaglani MJ, et al. Effectiveness of school-based influenza vaccination. N Engl J Med. 2006;355(24):2523–2532. doi: 10.1056/NEJMoa055414. [DOI] [PubMed] [Google Scholar]
- 42.Immunise Australia Program. Human papillomavirus (HPV) - the national HPV vaccination program register. Department of Health & Ageing; 2011. [Google Scholar]