Abstract
Synapses may represent a key nidus for dementia including Alzheimer's disease (AD) pathogenesis. Here we review published studies and present new ideas related to the question of the specificity of synapse loss in AD. Currently, AD is defined by the regional presence of neuritic plaques and neurofibrillary tangles in the brain. The severity of involvement by those pathological hallmarks tends to correlate both with antemortem cognitive status, and also with synapse loss in multiple brain areas. Recent studies from large autopsy series have led to a new standard of excellence with regard to clinical–pathological correlation and to improved comprehension of the numerous brain diseases of the elderly. These studies have provided evidence that it is the rule rather than the exception for brains of aged individuals to demonstrate pathologies (often multiple) other than AD plaques and tangles. For many of these comorbid pathologies, the extent of synapse loss is imperfectly understood but could be substantial. These findings indicate that synapse loss is probably not a hallmark specific to AD but rather a change common to many diseases associated with dementia.
Keywords: TDP-43, Neuropathology, Cerebrovascular, Hippocampal sclerosis, HS-Aging
1. Introduction
Compelling data from many sources support the hypothesis that synaptic changes play a key role in Alzheimer's disease (AD), probably contributing directly to the profound cognitive impairment that is characteristic of the disease. However, there are also key unanswered questions in the field. Here we address the issue of the specificity of synapse loss to AD. We review some of the published data with a focus on human studies. We include figures and photomicrographs to help express how non-AD brain diseases can produce synapse loss that render the understanding of AD-specific changes very challenging. Before addressing the data related to synapse loss in AD and other dementias, it is worth considering some of the recent advancements in the field.
2. Evolution of clinical-pathologic correlations
The question of whether or not synapse changes in AD correlate with disease severity (i.e. cognitive status) is essentially one of clinical–pathological correlation (CPC). The past decade has seen important research advancements in the area of CPC. These advancements are directly relevant to the current review and bear consideration. In recent years, better quality data and more sophisticated analyses from many different research centers have enabled new insights and more valid research conclusions. These improvements constitute a truly new standard of research excellence. Some of the features that characterize the new standard for research in this area are:
higher-quality research cohorts incorporating a fuller spectrum of disease;
longitudinal assessment of patients, rather than only cross-sectional analyses;
improving clinical & neurocognitive evaluation;
more universally applied pathological evaluation (new pathologic biomarkers have found evidence for “new” diseases as described below);
increasingly quantitative assessment of pathological markers with less bias;
better statistical power due to larger cohorts;
more variables gathered and factored into quantitative correlation;
increased focus on the full spectrum of advanced age.
These technical and infrastructural advancements have enabled the field to move past the prior stumbling blocks linked to overdichotomization (dementia/nondemented, AD/“control”), ignoring comorbid diseases, and a general oversimplification of the concept of brain diseases among the elderly.
As a specific example of how far we have advanced in recent years, it is instructive to compare our level of insights relative to the seminal work in CPC performed by Tomlinson, Blessed, and Roth in the 1960s and early 1970s [1–3]. While these works provided breakthrough new insights at the time, there were also necessary limitations. For example, the research cohorts were relatively young (average age at death ~74 years); specific pathologic markers for AD (tau and Aβ markers) were not yet developed; and there was poor knowledge about frequently seen neurodegenerative disease comorbidities. Coupled with this was the fact that good pathologic markers for α-synuclein and TDP-43 were not available [4,5]. Knowledge of the molecular pathogenesis or markers for frontotemporal lobar degeneration (FLTD) was mostly non-existent. The patients in the classic CPC studies also had cross-sectional (not longitudinal) assessment of cognitive status, not necessarily proximal to death, and there were neither epidemiologic nor community-based cohorts to study to advanced age. The application of advanced statistical approaches with greater statistical power was still awaiting both the mathematical expertise and larger research cohorts so the more sophisticated approaches could pay off. Although the early CPC studies provided key insights, one should not remain tethered to those conclusions. New technology and approaches have enabled new insights with new basic assumptions that now can lead us forward. Among these new insights is the important realization that comorbid pathologies are the rule, rather than the exception, in the aged human brain.
3. Brain pathologies linked to aging: Prevalence and impact on cognitive status
The gold standard for describing the presence and severity of brain disease is neuropathologic evaluation. Pathology can provide insights about disease mechanisms when the molecular pathways are modeled in other experimental systems. However, pathological assessments themselves are cross-sectional (generally seen at autopsy) and relate to the visual manifestation of histochemical or immunohistochemical staining often observed at the end stage of the disease.
3.1. Multiple and comorbid pathologies in aging brain
Dementia is a significant problem worldwide especially in older individuals and a clinical condition that is expanding at a very rapid rate [6]. It consists of a variety of different clinical syndromes including Alzheimer's disease (AD), dementia with Lewy bodies (DLB) and Parkinson's disease dementia (PDD), frontotemporal lobar degeneration (FTLD), Huntington's disease (HD), and cerebrovascular disease (CVD). Relative to younger individuals, there are alterations that are observed consistently in the aged human brain, some of which are seen in increased abundance in individuals with dementia. However, there has been substantial focus on less disease-specific brain changes such as myelinopathy, neuroinflammation, glial activation, and the oxidation of proteins, lipids, and nucleic acids [7–10]. One of the major hypotheses of the current review is that synapse changes may belong in this category, i.e. changes seen in multiple different neurodegenerative diseases. Some subtypes of those changes may in the future be proven to be restricted to specific diseases, or the aging process itself, but more work needs to be performed in those areas. By contrast, there are AD-linked brain changes such as Hirano bodies, granulovacuolar degeneration, and cerebral amyloid angiopathy (CAA) which are often seen in AD brains [11] but their correlative impact on cognitive loss is a matter of debate.
The abovementioned advancements in CPC studies are directly relevant to a discussion of the past and future studies addressing the association between synaptic changes and cognitive status in AD. We now know that a large proportion of cognitive decline in the elderly is explained by diseases other than AD, a fact which was unknown at the time of some of the classic studies that addressed the same question.
3.2. Alzheimer's disease
The most common type of dementia appears to be AD, which is a progressive neurodegenerative dementing disorder that was first described by Alois Alzheimer in 1907. Clinically it is characterized very early by a decline in recent memory function often coupled with changes in verbal fluency and problems with visuospatial abilities. Subsequently there is depression, problems with activities of daily living (ADL) including sleep and increased anxiety. Eventually there is a significant decline in frontal and executive functions and severe psychosis leading to an inability to perform any ADL. These cognitive changes can be differentiated from those in other types of dementia [12] and appear to involve multiple regions of the cortex.
3.3. AD neuropathologic changes
Neurofibrillary pathology comprises aberrant, partly-insoluble, protease-resistant, hyperphosphorylated tau aggregates – some with paired helical filament appearance by electron microscopy [13] – inside various cellular compartments or extracellular after death of the parent cell. Neurofibrillary tangle (NFT) is the term that describes neurofibrillary pathology found in nerve cell bodies. NFTs are not specific for AD [14–16]; indeed, they are found in almost every class of brain disease and are observed universally (usually topographically and quantitatively restricted) in normal aging subjects [17].
NFTs are also found in the brains of individuals who suffered from FTLD with tauopathy (FTLD-MAPT), myotonic dystrophy, prion diseases, metabolic diseases, some brain tumors, chronic traumatic encephalopathy, viral encephalitis, and other brain diseases [18–21] (conditions collectively termed “tauopathies”). This suggests that NFTs are, at least under some conditions, a secondary response to injury. Tau gene (MAPT) mutations can produce clinical dementia, which establishes that under some conditions NFTs may be directly linked to the primary or at least proximal neurodegenerative changes [15,16,19]. There is no condition with widespread neocortical NFTs that lacks cognitive impairment [22,23].
In contrast to NFTs, Aβ amyloid plaques (AβPs) are extracellular [24,25], often roughly spherical structures containing Aβ peptide and other material. A particularly important subtype of AβPs is “neuritic plaques” (NPs), which have also been referred to as “senile plaques”, and which are more likely to be associated with cognitive impairment than diffuse AβPs [26–28]. NPs are AβPs that are surrounded by degenerating axons and dendrites that often contain hyperphosphorylated tau aggregates. In elderly individuals, widespread neocortical NFTs are virtually nonexistent without the presence of widespread AβPs, except in the minority of cases with well defined tauopathy (e.g. FTLD-MAPT). These observation and others (see below) have led researchers to hypothesize that AβP development is “upstream” of neocortical NFTs in AD pathogenesis [29]. Although the clinical–pathological data are complex, it should be stressed that the extant literature does indeed indicate a specific disease exists that is characterized by the presence of AβPs and NFTs [23]. Coupled with the presence of AβPs and NFTs is significant neuron loss in the hippocampal formation and various associative cortical regions. Primary sensory and motor regions of the neocortex appear to be largely spared until very late in the disease progression. In addition, there is widespread loss of synapses throughout the cortex and hippocampus.
An important step forward for neuropathologists is the most recent (2012) revision of recommendations for the neuropathologic approach to AD diagnoses [30,31]. These recent consensus guidelines have advanced the field in at least three ways: (1) Removed the necessity of documented antemortem cognitive impairment so that AD (like any other disease) can be diagnosed using the pathological “gold standard” alone; (2) Provided greater guidance for the diagnoses of non-AD comorbid pathologies such as HS-Aging, DLB, and CVD pathologies; (3) Provided guidance to pathologists for anatomical regions to sample and stains to employ in the assessment of neurodegenerative diseases. Accurate clinical–pathological correlation requires that both components – clinical workup and pathological analyses – are performed optimally. Both of these become potential obstacles in extremely old individuals for whom clinical assessments are challenging and autopsy rates are generally low.
According to many different studies, dementia prevalence in Western populations is approximately 2% at age 65 and then doubles every five years thereafter [32–35]. Dementia incidence appears to level off after age 90 [34,36–39] although clinical dementia prevalence probably does keep increasing [40,41]. In contrast to the increased prevalence of dementia with advanced age (a clinical observation), the appearance of neuritic AβPs and NFTs pathology seems to level off in older cognitively impaired individuals according to multiple autopsy series [42–45], with the caveat that not all studies agree (discussed in [46,47]). Further, as discussed below, there is a greater “background” of hippocampal and brainstem NFTs in chronologically old individuals' brains. These phenomena have been interpreted to indicate a “dissociation” between AD neuropathologic change and cognitive status in extreme old age. However, even in extreme old age, the presence of many neocortical NFTs (Braak neurofibrillary stage VI) correlates with antemortem cognitive decline [48–50]. Thus, no “dissociation” exists between the AD neuropathologic change and cognitive impairment. Unsurprisingly, other diseases can also induce cognitive impairment in the absence of AD pathology at autopsy. Many studies have now stated that in addition to the accumulation and distribution of NFT and NPs as hallmarks of AD, the loss of synapses is also a prevalent neuropathologic finding and should also be considered a hallmark of the disease. This is based on the fact that several studies have reported a strong association between a subject's cognitive ability and their synaptic numbers [51–58].
The first study to report possible synaptic decline in AD was an ultrastructural assessment by Gibson [59]. Although the study was not specifically designed to study AD, Gibson reported no decline in the superior frontal cortex for a few subjects with AD compared to neurologically normal controls. The first study specifically designed to assess possible AD-related synaptic change [60] reported a loss of the synaptic protein, synapsin I, only in the hippocampus and not in numerous other regions including the cingulate gyrus. Subsequent studies have used immunohistochemistry, ultrastructure, and a variety of other techniques to evaluate possible synaptic changes in multiple regions of the cortex (for extensive reviews see [10,61]). Multiple studies have reported AD-associated changes in specific synaptic proteins in the hippocampus [62–65], frontal [66–72], temporal [64,66,70,72–74], and parietal lobes [66,67,71]. These changes include both presynaptic (synaptophysin, synaptobrevin, SNAP 25, synaptotagmin, syntaxin, rab3a, synapsin I) and postsynaptic proteins (PSD-95, drebrin). The overall picture from these studies is that the loss of synapses and/or synaptic proteins is extremely widespread in AD and not limited to a specific region of the cortex. This may underlie the multiple aspects of the diverse neurocognitive changes observed with the progression of the disease.
Numerous neuropathologic studies have documented the presence of both NFT and AβPs in the cerebral cortex of non-demented elderly individuals. In some cases the densities of these “lesions” approach those observed in demented individuals [75] suggesting that some of these lesions may be a common feature of normal brain aging. Many elderly individuals with NFT and NPs have been labeled as “preclinical AD”, with the idea that they would eventually progress to AD if they had lived long enough. Goldman et al. [76] found that neuropathologic changes in preclinical AD did not produce measureable cognitive decline on standard tests. Nevertheless, there are elderly individuals who manifest some cognitive deficits that are atypical of those observed in normal aging [77,78] but are not clinically demented. These individuals are thought to be in the transitional zone between dementia and normal aging. Through a variety of different research endeavors, Petersen [79] coined the term “mild cognitive impairment” (MCI) to describe this cohort. Individuals with amnestic MCI (aMCI) have been shown to have a higher risk of progressing to clinically probable AD [80].
Possible synaptic change in aMCI has been evaluated with ultrastructure and changes in synaptic proteins. Scheff et al. [56,81], using ultrastructural techniques, reported a significant decline in total synapse numbers in the hippocampal CA1 region and the inferior temporal cortex in aMCI, but a failure to observe a significant decline in either the outer molecular layer of the hippocampal dentate gyrus or the precuneus cortex [82,83]. Relatively few synaptic protein studies have been reported for individuals with aMCI. A recent study [84] using a very small cohort reported a loss of PSD-95 in the hippocampus compared to an age-matched control group. Counts and colleagues [66,85] reported a significant loss of the post synaptic protein, drebrin, in the superior temporal cortex and the hippocampus in a relatively large cohort of aMCI subjects. It is interesting to note that in aMCI, the presynaptic protein, synaptophysin, was largely preserved in both of these regions.
3.4. Dementia with synucleinopathy—Dementia with Lewy bodies and Parkinson's disease dementia
Lewy bodies (LB) are made up of peptide polymers derived from alpha-synuclein (α-SN). In Parkinson's disease (PD), there is a preponderance of brainstem LBs (see Fig. 1), and often an evolution of clinical manifestations that evolve from “parkinsonism” to include dementia (PDD). Individuals with LB often present with dementia but LB can occur without dementia. Dementia with Lewy bodies (DLB) often includes parkinsonism during the course of the disease with more pathology in the cerebral cortex including α-SN immunoreactive LB and Lewy neurites (LN). Neuropathological data are especially important for cases with mixed pathology, such as AD with concomitant DLB, where precise clinical diagnostic criteria are lacking. Remarkably, some individuals with amyloid precursor protein mutations have early-onset cognitive symptoms with AD + DLB pathology [86,87]. It is also important to note that “pure” DLB (extensive neocortical LB pathology in individuals lacking AD pathology) is relatively rare, and is far more often seen in males than in females, but has a definite cognitive impact [49,88–91].
Fig. 1.
Human frontal cortex (Brodmann area 9) brain sections stained with hematoxylin and eosin allows comparison between normal cortex at 80 years of age ((A) and (B)) to a person with autopsy-confirmed dementia with Lewy bodies (DLB) at 71 years of age ((C) and (D)). Pia mater indicated ((A) and (B)) with a “p”. The homogeneous, mildly eosinophilic background in brain without neurodegenerative disease ((A) and (B)) indicates relatively intact neuropil, which is made up largely of synaptic ending, which differs from the DLB case which has some rarefaction and vacuolation, particularly near the pial surface (asterisk). ((A) and (C)) scale bar = 150 μm; ((B) and (D)) scale bar = 75 μm.
Parkinson's disease (PD) is a progressive disorder characterized by a resting tremor, bradykinesia, and postural instability. These motor symptoms are primarily the result of pathology in the striatal circuitry predominately due to a loss of neurons in the substantia nigra and a reduction in dopamine. There are numerous reports in PD of significant cell loss in the nucleus basalis of Meynert (nbM) [92] showing a pathological signature similar to that in AD. This is in contrast to progressive supranuclear palsy (PSP) where the nbM and cortical cholinergic projections are preserved [93]. In addition to extrapyramidal motor dysfunction, there are disturbances related to numerous cortical and subcortical regions that can give rise to dementia. Estimates of dementia associated with PD are as high as 75% for individuals with an exacerbated disease progression [94]. There are only two studies that have evaluated possible synaptic loss in PD in the absence of dementia. In an early study by Zhan et al. [95] three different regions of the brain were evaluated for possible changes in synaptophysin intensity staining. The PD group was divided into a cohort with dementia (PDD) or without dementia and compared to a group consisting of control individuals with no cognitive impairment (NCI) and a cohort of AD subjects. In the PDD cases, there was a significant decline in both the frontal and occipital cortical regions while the PD cases without dementia failed to show any cortical decline compared to controls. In the hippocampus, both PD subgroups displayed a significant loss in staining compared to the controls but values substantially higher than in the AD group. There did not appear to be a significant difference between the two PD subgroups. It is unclear whether or not the PDD subjects also had AD or if there was other contributing pathology such as LB. In the study by Baloyannis et al. [96] evaluation was limited to the locus coeruleus (LC) and used a Golgi technique to count dendritic spines as a measure of synaptic density. A NCI control cohort was compared to the same type of PD subgroups in the above study. There was a significant loss of spines reported for both subgroups with the PDD cohort manifesting greater spine loss. Unfortunately there is limited information about the subject in the different cohorts and it is unclear if the PDD subjects also had AD. Problems with sampling and statistical treatment of the data make the results difficult to interpret. It does appear that PD alone may be result in LC spine loss.
A variety of imaging studies have probed various aspects of different neurotransmitters in PD. While there does appear to be some differences in the motor cortex, frontal cortex is not significantly different from normal healthy individuals [97]. It is now clear that some of the executive/cognitive dysfunction observed in PD is most likely associated with significant alterations to both the dopaminergic and cholinergic innervations of the cortex [92]. There is currently good evidence that cortical LBs are not the “cause” of dementia in cases of PD [98]. While one common idea is that α-SN accumulation leads to neuronal death, several studies suggest the opposite might be the case [98–100].
Dementia with Lewy bodies (DLB) [101], is cognitive dysfunction associated with LB and was originally described by Okazaki [102]. In the original report, the brains of two progressively dementing individuals upon autopsy revealed neurons throughout the cerebral cortex containing numerous “intraganglionic inclusion bodies” that appeared to be Lewy bodies. There are a variety of different terms that have been associated with LB disease [103] and include diffuse Lewy body disease [104,105], Lewy body variant (LBV) of Alzheimer's disease [106], senile disease of the Lewy body type [107], Parkinson disease features in AD [108], and subsequently dementia with Lewy bodies [101]. Fig. 1 shows a case with LB pathology in the frontal cortex (Brodmann area 9) and rarefaction of synapse-rich neuropil as is often observed at autopsy. Masliah and colleagues [109], using a dot-blot technique, compared synaptophysin levels in both the midfrontal cortex and hippocampus from subjects with LBV and compared it to both age-matched controls and AD subjects. Individuals considered to be LBV have wide spread LB and sufficient numbers of cortical NFT and AβPs to meet the AD criteria. Both dementia groups were found to have a significant loss compared to controls but were not different from each other. It was unclear whether or not the synaptophysin loss resulted from the presence of LB or from other pathology attributed to AD. A subsequent study [110] from this same group used optical density measurements to determine if individuals with DLB and dementia (termed “DLBD” in this study) were different from individuals with LBV. The DLB subjects did not have sufficient numbers of NFT and AβPs to satisfy the criteria for AD but were demented. Numerous regions of the hippocampal formation were evaluated for possible changes in a variety of different synaptic proteins. Only the antibody for synaptophysin showed a loss in AD and the LBV cohort compared to the control group, while the DLB subjects showed essentially no change. This study is difficult to interpret because the number of subjects in the DLB group was extremely small and the control group was significantly younger than the other cohorts. Other studies have supported the idea that synaptophysin levels are significantly lower in a LBV cohort compared to NCI [111].
Further evidence supporting the lack of a possible relationship between LB and synapses can be found in a study by Samuel et al. [112] who evaluated a larger cohort of DLB subjects and compared the results to that of individuals with LBV, AD without LB, and age-matched NCI controls. There was an expected decline in synaptophysin levels in the MFC in both the AD and LBV groups, but no difference was observed between the DLB cohort and controls despite the fact that the DLB subjects were demented. They also reported a failure to observe any association between synaptophysin levels and LB counts or NFT and plaque counts. Whether or not synapse loss plays a role in DLB is unclear since levels of choline acetyltransferase were significantly lower in all dementia groups compared to controls. A rather elaborate study supporting the idea that LBs alone do not result in significant synapse loss was reported by Hansen et al. [113]. Evaluating the frontal cortex with a dot-blot immunoassay for synaptophysin, they reported a failure to find a significant loss with pure LB subjects regardless of whether or not they had dementia. They concluded that the dementia in DLB is not due to the loss of synaptophysin levels in the MFC. If the subject has LBV, then there is a significant loss. Lippa [114] also reported minimal loss of synaptophysin staining in the hippocampus when comparing DLB subjects without significant AD type pathology with age-matched controls, but did observe a significant loss in an AD cohort. Revuelta et al. [115] stained hippocampal sections for synaptophysin from individuals with DLB and attempted to correlate optical density staining with LNs. There was no distinction made between individuals that were LBV and DLB. Although they reported variable loss of immunostaining, there did not appear to be a significant association with LN. A more recent study [116] examined an extremely large number of cases in an attempt to compare possible differences between individuals with DLB and AD. This study reported a loss of synaptophysin puncta in the frontal cortex similar to that in AD. Unfortunately there was no attempt to differentiate individuals with LBV from those with DLB and overall the subject populations were very poorly described. The number of subjects representing various age groups was extremely disparate which further complicates interpretation of the data.
3.5. Tauopathies
Frontotemporal lobar degeneration (FTLD) refers to pathological manifestations associated with a clinical state termed frontotemporal dementia (FTD), a progressive degenerative disorder that is distinct from AD [117,118]. Both FTLD (pathology) and FTD (clinical aspects) refer to a broad category of neurodegenerative processes that primarily affect the frontal cortex and the anterior portion of the temporal lobe. From a pathology viewpoint, FTLD is quite distinct from AD in that the posterior cingulate and occipital cortex are not involved [119]. There is a modest atrophy of the frontal and anterior temporal cortex with a sparing of the superior temporal cortex. The primary neuron loss is in lamina II and III with an obvious sparing of lamina V. From a clinical aspect there are early signs of social disinhibition, mental rigidity/inflexibility, and expressive speech impairment. Patients with FTD are distinguishable from those with AD on neuropsychological tests in that memory is initially preserved in the early stages [120,121].
A very early study by Brun et al. [122] evaluated possible differences between FTLD and individuals with AD with a primary focus on the molecular layer (lamina I) of the frontal and parietal cortex. Optical density measurements were made of possible changes in synaptophysin staining. Compared to age-matched control subjects, both the AD and FTLD cohorts showed almost equivalent loss of staining in the frontal region but only the AD cohort demonstrated a loss in the parietal cortex. This was somewhat surprising since the amount of atrophy in the FTLD was not significantly different from the AD group. The AD subjects showed significant increases in astrocytes in both regions while this type of gliosis was only observed in the frontal region in FTLD. In two follow up studies from this same group [123,124], analysis included all cortical layers with both additional subjects and two additional cortical regions, the inferior temporal and posterior cingulate, areas also known to be heavily involved in AD. As expected, there was a significant loss of synaptophysin staining in all cortical regions throughout the cortical mantel in the AD cohort. The FTLD subjects only displayed a significant decline in the upper three lamina of the frontal cortex while the other three cortical regions showed staining equivalent to age-matched controls. These results support the idea that the underlying mechanism responsible for dementia may not be related primarily to widespread synaptic loss and clearly differentiates AD from FTD. Lipton and colleagues [57] reported somewhat different results than the Swedish group. Using a rather heterogeneous limited group of FTD subjects that included individuals with corticobasal ganglion degeneration, Pick's disease (PiD), and sporadic multiple system tauopathy with dementia, they reported a significant loss of synaptophysin in frontal, temporal and parietal cortex that was greater than that observed in an AD cohort. Since this group of FTD subjects was quite different from the previous studies reporting no change, it is difficult to compare the two. The greatest loss was observed in the single case with PiD.
Pick's disease (PiD) is a tauopathic subtype of FTLD [125] that is characterized by extreme cortical atrophy of the frontal and temporal regions and the presence of neurons with well circumscribed amphophilic spherical inclusions (balloons) that stain positive with tau antibodies (Pick bodies) [126]. The affected cortex has diminished pyramidal neurons and increased gliosis. The amygdala and hippocampus are among the most heavily affected regions of the limbic system. The parietal and occipital cortex is rarely involved. Clinically it is a slow progressive dementia with emotional blunting, disinhibition, and marked memory impairment which is distinct from AD [35]. A study by Sparks and Markesbery [127] evaluated serotonin, imipramine binding and choline acetyltransferase levels in the frontal pole, temporal pole, hypothalamus and nbM in five individuals diagnosed with PiD at autopsy. Serotonin binding was significantly down in all regions in the PiD compared to normal controls while imipramine binding was increased although not significantly. The previously mentioned study by Lippa [114] also evaluated a number of cases of PiD for possible changes in hippocampal synaptophysin. Individuals with PiD displayed a significant loss of staining compared to the age-matched controls with the staining being equivalent between the AD and PiD groups.
Progressive supranuclear palsy (PSP) [128], also known as Steele–Richardson–Olszewski syndrome, is primarily a basal ganglia, brainstem and cerebellar degenerative disorder and another pathological subtype of FTLD tauopathy. These individuals have Parkinson's type characteristics including bradykinesia and downward gaze impairments. As the disease progresses there are significant frontal lobe problems coupled with dysarthria and dysphasia. While cognitive dysfunction is evident, it doesn't result in severe dementia [129,130]. From a neuropathologic perspective, there are numerous NFT in both the basal ganglia and the brainstem and it is common to observe NFT in the motor and parietal areas. Neuronal loss is often observed in the frontal and parietal cortex accompanied by increased microglial activation [131–134]. Bigio et al. [135] hypothesized that frontal cortex would show synaptic loss in individuals with PSP and dementia while individuals with only PSP would not. They evaluated multiple different regions of the cortex and cerebellum. In all cortical regions there was a significant decline in synaptophysin levels in all PSP subjects that compared very closely with the AD group. The cerebellum was not different from NCI controls. Surprisingly the synaptophysin levels were higher in the PSP dementia group than the PSP non-demented cohort. It should be noted however that the number of PSP cases evaluated was extremely small. Nevertheless, this is evidence to support the idea that levels of synaptophysin do not necessarily closely associate with dementia even though there may be a loss throughout the cortex. A rather interesting imaging study [93] reported a loss of cholinergic innervation to the thalamus in PSP with a preservation of cholinergic cortical innervation.
3.6. Dementia with TDP-43 pathology—ALS + MND, FTLD-TDP, and HS-Aging
TDP-43 is an RNA-binding protein, normally localized to the cell nucleus, that is linked pathogenetically with amyotropic lateral sclerosis (ALS), frontotemporal lobar degeneration (FTLD-TDP), hippocampal sclerosis of aging (HS-Aging), and other brain diseases. Recent studies have supported the hypothesis that ALS and FTLD-TDP exist across a spectrum of disease that incorporates many different genetic etiologies. Specifically, FTLD-TDP represents a spectrum of disease that has been linked to mutations in a number of different genes [136]. The pathologic manifestations of FTLD-TDP are associated with disturbances of language, executive function, and memory. In addition to being linked to FTLD, which mostly affects people before advanced old age. TDP-43 pathology is also seen in hippocampal sclerosis (HS) [137,138]. Hippocampal sclerosis (HS) refers to neuronal cell loss and astrocytosis in subiculum and cornu ammonis subfields of the hippocampal formation unrelated to AD pathology (Fig. 2). In contrast to the disease that affects younger adults and is also referred to as “hippocampal sclerosis” [139], HS in older individuals is associated with significant antemortem cognitive dysfunction [49,140] but not necessarily with epilepsy [141]. There is no universally-applied specific nosology for these cases and the term “HS-Aging” is used to refer to the disease with HS pathology in aging individuals [138]. In the largest autopsy series of HS-Aging to date, we found that approximately 90% of HS patients had aberrant TDP-43 immunohistochemical staining, in comparison to ~10% in older controls irrespective of the presence of other pathologies [138]. Currently there are no published studies that have evaluated possible synaptic changes specifically associated with TDP-43, although the synapse-rich neuropil in HS-Aging brain appears disrupted (Fig. 2). Presumably, a disease with the high prevalence of HS-Aging would directly impact AD CPC, by altering cognition without plaques and tangles.
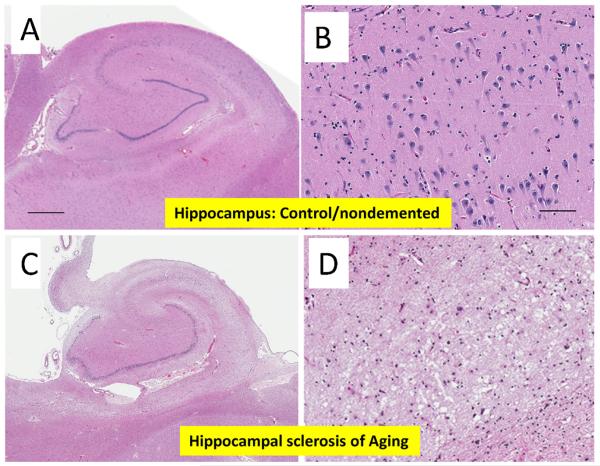
Fig. 2.
Comparison of sections, stained with hematoxylin and eosin, representing normal hippocampus ((A) and (C)) and a hippocampus with hippocampal sclerosis of aging (HS-Aging) ((B) and (D)). These two patients were both 91 years of age at death. Panels (C) and (D) highlight differences in the CA1 region at 10× magnification (scale bar 100 μm); note rarefaction of neuropil in panel (D).
3.7. Huntington's disease
Huntington's disease (HD) is an autosomal dominant disorder, caused by a trinucleotide (CAG) repeat expression in the huntingtin (htt) gene. This disease is primarily characterized by marked degeneration and atrophy in the striatum resulting in motor abnormalities. This disorder also presents with the loss of medium spiny neurons in the striatum and pyramidal cell loss in the cortex. Cortical atrophy is located primarily in the occipital lobe but occurs in other areas also. The cortical involvement including cortical atrophy, which is a hallmark of the disease, is believed to underlie the cognitive deficits and dementia [142–145]. Zahn and colleagues [95] were among the first to study possible synaptic changes in the cortex and hippocampus in HD. They evaluated five autopsy cases of HD with dementia with an age range of 22–73 years and compared these to NCI control. The HD subjects displayed a significant loss of staining in both the frontal and occipital regions with deficits primarily confined to the pyramidal layers of the cortex. The molecular layer of the dentate gyrus also displayed a decline in synaptophysin staining and the loss was comparable to age-matched AD subjects. Morton et al. [146] evaluated both the striatum and hippocampus of a relatively large number of HD subjects using immunoblotting and a variety of different synaptic proteins. The results were mixed depending upon the brain region and the synaptic protein being evaluated. The striatum in HD showed a significant decline in presynaptic soluble-N-ethylmaleimide-sensitive fusion attachment receptor (SNARE) proteins such as complexin II and Rab3A. In contrast, the hippocampus appeared to be spared and in some cases showed a slight increase. Synaptophysin levels did not appear to change in either the striatum or hippocampus. In a relatively recent study, Smith et al. [147] used the Western blotting technique to study possible synaptic protein changes in the frontal cortex in HD. They reported a loss of one of the SNARE proteins, SNAP-25 and a loss of rabphilin 3a primarily in HD individuals with the most progressive form of the disease. There was no apparent loss of synaptophysin, synataxin I or synaptobrevin II, or rab3a.
3.8. Cerebrovascular disease
Cerebrovascular disease (CVD) although not necessarily considered a “neurodegenerative disease”, comprises the most prevalent non-AD pathology in advanced aged brains and is directly relevant to any CPC study related to dementia among the elderly [22,50]. The reason CVD is directly relevant in the current review is that CVD will presumably cause synapse loss independent of AD or other neurodegenerative conditions (see for example Fig. 3). For CPC studies, the difficulties introduced by this prevalent, multifaceted, and clinically unpredictable disease category in aged individuals have been discussed previously [148–157]. In individuals beyond the age of 90 years, some degree of CVD pathology is universal [50,158–160]. We note the lack of an ideal rubric for CVD clinical–pathological correlation, although the recent consensus-based criteria attempted to systematize neuropathologic assessment of this complex form of brain injury [30]. Despite its high prevalence and profound impact, researchers tend to under-appreciate the importance of CVD pathology [161–163].
Fig. 3.
Low-power photomicrographs show portions of a brain section from a 95-year old woman with multiple, small gray matter subacute infarcts. Both photomicrographs show areas of the same slide, and both were taken at same magnification (scale bar = 500 μm for both photomicrographs; the cortex is thinner in panel (B)), of a section stained using immunohistochemistry for synaptophysin. The external portion of the brain (pia mater) is indicated in both photomicrographs with a “p”. The normal-appearing neocortex (A) is relatively thick (>2.5 mm) and there is diffuse, strong immunopositivity for synaptophysin. By contrast, the area with the subacute infarct (B) shows thinning of the gray matter (<2 mm thickness), with some rarefaction (green asterisk) of the synaptophysin immunoreactivity.
The pathologic findings attributed to CVD are very broad, ranging from lobar hemorrhage/infarction to mild alterations in small vessel morphology [164]. Large caliber vessel involvement typically consists of atherosclerosis of the cerebral vessels at various branchpoints around the Circle of Willis. While the morphologic changes resemble atherosclerotic lesions seen systemically, its severity is usually much less than that seen in the systemic circulation, often falling within the mild category [165]. Blockage of these vessels, by either local disease or emboli from elsewhere, lead to ischemia and, without restoration of blood flow, infarction. Systemic metabolic, toxic, or anoxic insults can also induce pathologies that resemble histomorphologic changes that are due to disrupted blood flow.
Arguably the most prevalent, and least quantifiable subtype of CVD pathologies, are the small vessel changes noted in the subcortical white matter, deep gray nuclei, and brainstem. Thickening of the smaller arterioles and capillaries, due to arteriolosclerosis or lipohyalinosis, is often observed. Disruption of the smaller perforating vessels in the subcortical gray matter and brainstem (a process exacerbated by hypertension), can induce lacunar type infarcts [166]. The Virchow–Robin spaces, normally a narrow compartment around the perimeter of the blood vessels, can vary dramatically in size. White matter is considered to be adversely affected by small vessel CVD and can take on a ragged or rarefied appearance (Fig. 3). These changes, seen both microscopically and to some extent via imaging modalities (where they are known as leukoariosis), are found more commonly with increasing age. At the present time, their relative abundance, size, or location cannot be correlated consistently with clinical symptomatology [167,168]. Currently there are no published studies that have specifically evaluated possible synaptic changes associated with CVD.
4. Note on animal models and timing of synaptic changes
Although outside the focus of the current review of human subject studies, animal models have been developed which recapitulate some of the features of specific biochemical pathways relevant to human diseases. The animal studies provide an experimental context to study mechanisms and timing of synaptic changes in the brain. These data may relate to the importance of synapse changes in AD, but not necessarily the specificity of synapse changes in AD, because animal brains do not manifest the comorbidities of the aged human brain. Some studies in animal models have indicated that synaptic changes can precede other manifestations of neurodegeneration. However, those observations do not modify our underlying hypothesis about the lack of proven specificity of synaptic changes in relationship to AD. In other words, while synapse changes appear to be an “early” component of AD, there is nothing to suggest synapse changes are not also an “early” component of FTLD or other neurodegenerative diseases as well. At this time, an animal model with synapse changes is not necessarily a model of AD any more than it is a model of any other neurodegenerative condition. In the future we may find specific subtypes of synaptic changes in AD (or other conditions) that can be better addressed in experimental models.
5. Summary and conclusions
Our review addresses whether or not synapse loss is a specific feature of AD and we conclude that the specificity of synaptic loss in AD has not yet been proven. Recent CPC studies help to greatly expand the available information about brain diseases of the elderly and provide a better basis for studying the biology of synapse changes in AD. Recent studies also help explain why there is an imperfect correlation between AD-type pathology per se (plaques and tangles) and cognitive status. It is important to scrutinize carefully the prior studies that have evaluated synaptic change in regards to different dementias. Synapse loss may be a nonspecific proxy for multiple diseases, a good indicator for neurodegeneration without specifically indicating any disease subtype. For a visual depiction of this phenomenologically, see the diagrams and explanation in Fig. 4. We conclude that there is much additional work to be performed studying the ways that synapse loss can occur in different brain diseases, but the prior published studies help explain why a non-specific proximal marker/cause of neurodegeneration (synapse loss, or neuronal loss) would paradoxically have a better correlation with cognitive status than the specific pathological markers related to any one disease entity. More work is required to highlight specific subtypes of synaptic changes that can be linked more specifically to particular diseases.
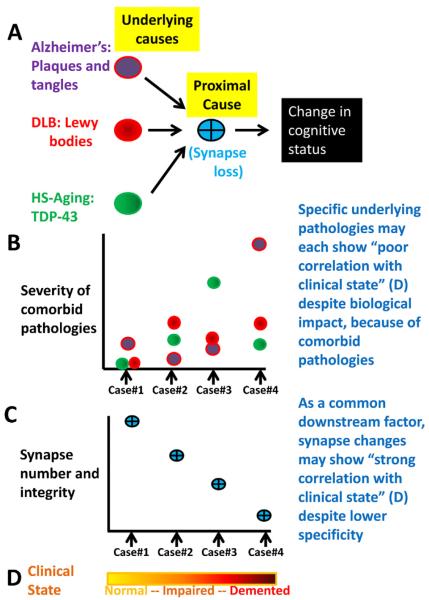
Fig. 4.
A summary schematic diagram helps depict how the severity of common comorbid underlying diseases, including Alzheimer's disease, dementia with Lewy bodies (DLB), and hippocampal sclerosis of aging (HS-Aging), can each have apparent poor correlation with cognitive status, unlike a more proximal change such as synapse loss (panels (A)–(C)). For panels (B) and (C), four cases are presented with pathologic findings that are frequently seen in practice (see Refs. [50,169–171]). Note that a common downstream “proximal cause”, such as synapse loss (C), could show stronger correlation with cognitive status ((D); an “impaired case” may qualify as mild cognitive impairment, MCI) despite representing a less specific component of the pathogenesis.
Acknowledgments
Funding/support This study was supported by grant 5-P30-AG028383, R01AG042475 and K08 NS050110 from the National Institutes of Health, Bethesda, MD.
Nomenclature
- AβPs
a-beta plaques
- AD
Alzheimer's disease
- ADL
activities of daily living
- CAA
cerebral amyloid angiopathy
- CPC
clinical-pathologic correlation
- CVD
cerebrovascular disease
- DLB
dementia with Lewy bodies (some studies use “DLBD” to refer to the dementia of DLB)
- FTD
frontotemporal dementia (a clinical diagnosis)
- FTLD
frontotemporal lobar degeneration (a pathological diagnosis)
- HD
Huntington's disease
- HS-Aging
hippocampal sclerosis of aging
- LB
Lewy body
- LBV
Lewy body variant (of Alzheimer's disease)
- LN
Lewy neurites
- MAPT
microtubule associated protein tau
- MCI
mild cognitive impairment (aMCI—amnestic MCI)
- nbM
nucleus basalis of Meynert
- NCI
no cognitive impairment
- NFT
neurofibrillary tangle
- NP
neuritic amyloid plaque
- PD
Parkinson's disease
- PDD
Parkinson's disease dementia
- PiD
Pick's disease
- PSP
progressive supranuclear palsy
- α-SN
alpha-synuclein
- TDP-43
TAR-DNA binding protein 43
References
- [1].Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- [2].Roth M, Tomlinson BE, Blessed G. Correlation between scores for dementia and counts of `senile plaques' in cerebral grey matter of elderly subjects. Nature. 1966;209:109–10. doi: 10.1038/209109a0. [DOI] [PubMed] [Google Scholar]
- [3].Tomlinson BE, Blessed G, Roth M. Observations on the brains of demented old people. J Neurol Sci. 1970;11:205–42. doi: 10.1016/0022-510x(70)90063-8. [DOI] [PubMed] [Google Scholar]
- [4].Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–3. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- [5].Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–7. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- [6].Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimer's Dementia. 2013;9(63–75):e62. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- [7].Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer's disease. Neurobiol Aging. 2004;25:5–18. doi: 10.1016/j.neurobiolaging.2003.03.001. author reply 49–62. [DOI] [PubMed] [Google Scholar]
- [8].Butterfield DA, Perluigi M, Sultana R. Oxidative stress in Alzheimer's disease brain: new insights from redox proteomics. Eur J Pharmacol. 2006;545:39–50. doi: 10.1016/j.ejphar.2006.06.026. [DOI] [PubMed] [Google Scholar]
- [9].Masliah E. Mechanisms of synaptic dysfunction in Alzheimer's disease. Histol Histopathol. 1995;10:509–19. [PubMed] [Google Scholar]
- [10].Scheff SW, Price DA. Synaptic pathology in Alzheimer's disease: a review of ultrastructural studies. Neurobiol Aging. 2003;24:1029–46. doi: 10.1016/j.neurobiolaging.2003.08.002. [DOI] [PubMed] [Google Scholar]
- [11].Duyckaerts C, Delatour B, Potier MC. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 2009;118:5–36. doi: 10.1007/s00401-009-0532-1. [DOI] [PubMed] [Google Scholar]
- [12].Weintraub S, Wicklund AH, Salmon DP. The neuropsychological profile of Alzheimer disease. Cold Spring Harbor Perspect Med. 2012;2:a006171. doi: 10.1101/cshperspect.a006171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Terry RD. The fine structure of neurofibrillary tangles in Alzheimer's disease. J Neuropathol Exp Neurol. 1963;22:629–42. doi: 10.1097/00005072-196310000-00005. [DOI] [PubMed] [Google Scholar]
- [14].Arai T, Ikeda K, Akiyama H, Shikamoto Y, Tsuchiya K, Yagishita S, et al. Distinct isoforms of tau aggregated in neurons and glial cells in brains of patients with Pick's disease, corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol. 2001;101:167–73. doi: 10.1007/s004010000283. [DOI] [PubMed] [Google Scholar]
- [15].Buee L, Bussiere T, Buee-Scherrer V, Delacourte A, Hof PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev. 2000;33:95–130. doi: 10.1016/s0165-0173(00)00019-9. [DOI] [PubMed] [Google Scholar]
- [16].Goedert M. Tau protein and neurodegeneration. Semin Cell Dev Biol. 2004;15:45–9. doi: 10.1016/j.semcdb.2003.12.015. [DOI] [PubMed] [Google Scholar]
- [17].Bouras C, Hof PR, Giannakopoulos P, Michel JP, Morrison JH. Regional distribution of neurofibrillary tangles and senile plaques in the cerebral cortex of elderly patients: a quantitative evaluation of a one-year autopsy population from a geriatric hospital. Cerebral Cortex. 1994;4:138–50. doi: 10.1093/cercor/4.2.138. [DOI] [PubMed] [Google Scholar]
- [18].Bancher C, Leitner H, Jellinger K, Eder H, Setinek U, Fischer P, et al. On the relationship between measles virus and Alzheimer neurofibrillary tangles in subacute sclerosing panencephalitis. Neurobiol Aging. 1996;17:527–33. doi: 10.1016/0197-4580(96)00069-3. [DOI] [PubMed] [Google Scholar]
- [19].Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114:5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tabaton M, Mandybur TI, Perry G, Onorato M, Autilio-Gambetti L, Gambetti P. The widespread alteration of neurites in Alzheimer's disease may be unrelated to amyloid deposition. Ann Neurol. 1989;26:771–8. doi: 10.1002/ana.410260614. [DOI] [PubMed] [Google Scholar]
- [21].Wang XF, Dong CF, Zhang J, Wan YZ, Li F, Huang YX, et al. Human tau protein forms complex with PrP and some GSS- and fCJD-related PrP mutants possess stronger binding activities with tau in vitro. Mol Cell Biochem. 2008;310:49–55. doi: 10.1007/s11010-007-9664-6. [DOI] [PubMed] [Google Scholar]
- [22].Abner EL, Kryscio RJ, Schmitt FA, Santacruz KS, Jicha GA, Lin Y, et al. “End-stage” neurofibrillary tangle pathology in preclinical Alzheimer's disease: fact or fiction? J Alzheimer's Dis. 2011;25:445–53. doi: 10.3233/JAD-2011-101980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71:362–81. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dickson DW. Neuropathological diagnosis of Alzheimer's disease: a perspective from longitudinal clinicopathological studies. Neurobiol Aging. 1997;18:S21–6. doi: 10.1016/s0197-4580(97)00065-1. [DOI] [PubMed] [Google Scholar]
- [25].Selkoe DJ. Biochemistry and molecular biology of amyloid beta-protein and the mechanism of Alzheimer's disease. Handb Clin Neurol. 2008;89:245–60. doi: 10.1016/S0072-9752(07)01223-7. [DOI] [PubMed] [Google Scholar]
- [26].Mirra SS. The CERAD neuropathology protocol and consensus recommendations for the postmortem diagnosis of Alzheimer's disease: a commentary. Neurobiol Aging. 1997;18:S91–4. doi: 10.1016/s0197-4580(97)00058-4. [DOI] [PubMed] [Google Scholar]
- [27].Terry RD, Masliah E, Hansen L. Structural basis of the cognitive alterations in Alzheimer disease. In: Terry RD, Katzman R, Bick KL, editors. Alzheimer disease. Raven Press; New York, NY: 1994. pp. 179–96. [Google Scholar]
- [28].Wisniewski HM, Vorbrodt AW, Moretz RC, Lossinsky AS, Grundke-Iqbal I. Pathogenesis of neuritic (senile) and amyloid plaque formation. Exp Brain Res. 1982;5(Suppl):3–9. doi: 10.1007/978-3-642-68507-1_1. [DOI] [PubMed] [Google Scholar]
- [29].Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- [30].Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimer's Dementia. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW. National Institute on Aging-Alzheimer's Association guidelines for the neuropatho-logic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gao S, Hendrie HC, Hall KS, Hui S. The relationships between age, sex, and the incidence of dementia and Alzheimer disease: a meta-analysis. Arch Gen Psychiatry. 1998;55:809–15. doi: 10.1001/archpsyc.55.9.809. [DOI] [PubMed] [Google Scholar]
- [33].Katzman R, Kawas CH. The epidemiology of dementia and Alzheimer disease. In: Terry RD, Katzman R, Bick KL, editors. Alzheimer disease. Raven Press; New York, NY: 1994. pp. 105–22. [Google Scholar]
- [34].Kukull WA, Higdon R, Bowen JD, McCormick WC, Teri L, Schellenberg GD, et al. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol. 2002;59:1737–46. doi: 10.1001/archneur.59.11.1737. [DOI] [PubMed] [Google Scholar]
- [35].Mendez MF, Selwood A, Mastri AR, Frey WH., 2nd Pick's disease versus Alzheimer's disease: a comparison of clinical characteristics. Neurology. 1993;43:289–92. doi: 10.1212/wnl.43.2.289. [DOI] [PubMed] [Google Scholar]
- [36].Hall CB, Verghese J, Sliwinski M, Chen Z, Katz M, Derby C, et al. Dementia incidence may increase more slowly after age 90: results from the Bronx Aging Study. Neurology. 2005;65:882–6. doi: 10.1212/01.wnl.0000176053.98907.3f. [DOI] [PubMed] [Google Scholar]
- [37].Launer LJ. Counting dementia: there is no one “best” way. Alzheimer's Dementia. 2011;7:10–4. doi: 10.1016/j.jalz.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rocca WA, Cha RH, Waring SC, Kokmen E. Incidence of dementia and Alzheimer's disease: a reanalysis of data from Rochester, Minnesota, 1975–1984. Am J Epidemiol. 1998;148:51–62. doi: 10.1093/oxfordjournals.aje.a009560. [DOI] [PubMed] [Google Scholar]
- [39].Rocca WA, Petersen RC, Knopman DS, Hebert LE, Evans DA, Hall KS, et al. Trends in the incidence and prevalence of Alzheimer's disease, dementia, and cognitive impairment in the United States. Alzheimer's Dementia. 2011;7:80–93. doi: 10.1016/j.jalz.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Corrada MM, Brookmeyer R, Paganini-Hill A, Berlau D, Kawas CH. Dementia incidence continues to increase with age in the oldest old: the 90+ study. Ann Neurol. 2010;67:114–21. doi: 10.1002/ana.21915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Seshadri S, Beiser A, Au R, Wolf PA, Evans DA, Wilson RS, et al. Operationalizing diagnostic criteria for Alzheimer's disease and other age-related cognitive impairment—Part 2. Alzheimer's Dementia. 2011;7:35–52. doi: 10.1016/j.jalz.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Crystal HA, Dickson D, Davies P, Masur D, Grober E, Lipton RB. The relative frequency of “dementia of unknown etiology” increases with age and is nearly 50% in nonagenarians. Arch Neurol. 2000;57:713–9. doi: 10.1001/archneur.57.5.713. [DOI] [PubMed] [Google Scholar]
- [43].Haroutunian V, Schnaider-Beeri M, Schmeidler J, Wysocki M, Purohit DP, Perl DP, et al. Role of the neuropathology of Alzheimer disease in dementia in the oldest-old. Arch Neurol. 2008;65:1211–7. doi: 10.1001/archneur.65.9.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C, et al. Age, neuropathology, and dementia. N Engl J Med. 2009;360:2302–9. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- [45].Neuropathology Group of Medical Research Council Cognitive Function and Aging Study Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) Lancet. 2001;357:169–75. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- [46].Imhof A, Kovari E, von Gunten A, Gold G, Rivara CB, Herrmann FR, et al. Morphological substrates of cognitive decline in nonagenarians and centenarians: a new paradigm? J Neurol Sci. 2007;257:72–9. doi: 10.1016/j.jns.2007.01.025. [DOI] [PubMed] [Google Scholar]
- [47].Jellinger KA, Attems J. Prevalence of dementia disorders in the oldest-old: an autopsy study. Acta Neuropathol. 2010;119:421–33. doi: 10.1007/s00401-010-0654-5. [DOI] [PubMed] [Google Scholar]
- [48].Dolan D, Troncoso J, Resnick SM, Crain BJ, Zonderman AB, O'Brien RJ. Age, Alzheimer's disease and dementia in the Baltimore Longitudinal Study of Ageing. Brain. 2010;133:2225–31. doi: 10.1093/brain/awq141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Nelson PT, Abner EL, Schmitt FA, Kryscio RJ, Jicha GA, Smith CD, et al. Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons. Brain Pathol. 2010;20:66–79. doi: 10.1111/j.1750-3639.2008.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Nelson PT, Jicha GA, Schmitt FA, Liu H, Davis DG, Mendiondo MS, et al. Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: neuritic plaques and neurofibrillary tangles “do count” when staging disease severity. J Neuropathol Exp Neurol. 2007;66:1136–46. doi: 10.1097/nen.0b013e31815c5efb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann Neurol. 1990;27:457–64. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- [52].Scheff SW, DeKosky ST, Price DA. Quantitative assessment of cortical synaptic density in Alzheimer's disease. Neurobiol Aging. 1990;11:29–37. doi: 10.1016/0197-4580(90)90059-9. [DOI] [PubMed] [Google Scholar]
- [53].Scheff SW, Price D. Synapse loss in the temporal lobe in Alzheimer's disease. Ann Neurol. 1993;33:190–9. doi: 10.1002/ana.410330209. [DOI] [PubMed] [Google Scholar]
- [54].Terry RD, Masliah E, Hansen L. Structural basis of the congnitive alterations in Alzheimer disease. In: Terry RD, Katzman R, Bick KL, editors. Alzheimer disease. Raven Press; New York, NY: 1994. pp. 179–96. [Google Scholar]
- [55].Masliah E, Mallory M, Hansen L, DeTeresa R, Alford M, Terry R. Synaptic and neuritic alterations during the progression of Alzheimer's disease. Neurosci Lett. 1994;174:67–72. doi: 10.1016/0304-3940(94)90121-x. [DOI] [PubMed] [Google Scholar]
- [56].Scheff SW, Price DA, Schmitt FA, Scheff MA, Mufson EJ. Synaptic loss in the inferior temporal gyrus in mild cognitive impairment and Alzheimer's disease. J Alzheimer's Dis. 2011;24:547–57. doi: 10.3233/JAD-2011-101782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lipton AM, Cullum CM, Satumtira S, Sontag E, Hynan LS, White CL, 3rd, et al. Contribution of asymmetric synapse loss to lateralizing clinical deficits in frontotemporal dementias. Arch Neurol. 2001;58:1233–9. doi: 10.1001/archneur.58.8.1233. [DOI] [PubMed] [Google Scholar]
- [58].Samuel W, Masliah E, Hill LR, Butters N, Terry R. Hippocampal connectivity and Alzheimer's dementia: effects of synapse loss and tangle frequency in a two-component model. Neurology. 1994;44:2081–8. doi: 10.1212/wnl.44.11.2081. [DOI] [PubMed] [Google Scholar]
- [59].Gibson PH. EM study of the numbers of cortical synapses in the brains of aging people and people with Alzheimer-type dementia. Acta Neuropathol (Berlin) 1983;62:127–33. doi: 10.1007/BF00684929. [DOI] [PubMed] [Google Scholar]
- [60].Perdahl E, Adolfsson R, Alafuzoff I, Albert KA, Nestler EJ, Greengard P, et al. Synapsin I (protein I) in different brain regions in senile dementia of Alzheimer type and in multiinfarct dementia. J Neural Transm. 1984;60:133–41. doi: 10.1007/BF01245030. [DOI] [PubMed] [Google Scholar]
- [61].Scheff SW, Price DA. Alzheimer's disease-related alterations in synaptic density: neocortex and hippocampus. J Alzheimer's Dis. 2006;9:101–15. doi: 10.3233/jad-2006-9s312. [DOI] [PubMed] [Google Scholar]
- [62].Harigaya Y, Shoji M, Shirao T, Hirai S. Disappearance of actin-binding protein, drebrin, from hippocampal synapses in Alzheimer's disease. J Neurosci Res. 1996;43:87–92. doi: 10.1002/jnr.490430111. [DOI] [PubMed] [Google Scholar]
- [63].Sze CI, Bi H, Kleinschmidt-DeMasters BK, Filley CM, Martin LJ. Selective regional loss of exocytotic presynaptic vesicle proteins in Alzheimer's disease brains. J Neurol Sci. 2000;175:81–90. doi: 10.1016/s0022-510x(00)00285-9. [DOI] [PubMed] [Google Scholar]
- [64].Tannenberg RK, Scott HL, Tannenberg AE, Dodd PR. Selective loss of synaptic proteins in Alzheimer's disease: evidence for an increased severity with APOE varepsilon4. Neurochem Int. 2006;49:631–9. doi: 10.1016/j.neuint.2006.05.004. [DOI] [PubMed] [Google Scholar]
- [65].Marksteiner J, Kaufmann WA, Gurka P, Humpel C. Synaptic proteins in Alzheimer's disease. J Mol Neurosci. 2002;18:53–63. doi: 10.1385/JMN:18:1-2:53. [DOI] [PubMed] [Google Scholar]
- [66].Counts SE, Nadeem M, Lad SP, Wuu J, Mufson EJ. Differential expression of synaptic proteins in the frontal and temporal cortex of elderly subjects with mild cognitive impairment. J Neuropathol Exp Neurol. 2006;65:592–601. doi: 10.1097/00005072-200606000-00007. [DOI] [PubMed] [Google Scholar]
- [67].Gylys KH, Fein JA, Yang F, Wiley DJ, Miller CA, Cole GM. Synaptic changes in Alzheimer's disease: increased amyloid-beta and gliosis in surviving terminals is accompanied by decreased PSD-95 fluorescence. Am J Pathol. 2004;165:1809–17. doi: 10.1016/s0002-9440(10)63436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Leuba G, Savioz A, Vernay A, Carnal B, Kraftsik R, Tardif E, et al. Differential changes in synaptic proteins in the Alzheimer frontal cortex with marked increase in PSD-95 postsynaptic protein. J Alzheimer's Dis. 2008;15:139–51. doi: 10.3233/jad-2008-15112. [DOI] [PubMed] [Google Scholar]
- [69].Minger SL, Honer WG, Esiri MM, McDonald B, Keene J, Nicoll JA, et al. Synaptic pathology in prefrontal cortex is present only with severe dementia in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60:929–36. doi: 10.1093/jnen/60.10.929. [DOI] [PubMed] [Google Scholar]
- [70].Mukaetova-Ladinska EB, Garcia-Siera F, Hurt J, Gertz HJ, Xuereb JH, Hills R, et al. Staging of cytoskeletal and beta-amyloid changes in human isocortex reveals biphasic synaptic protein response during progression of Alzheimer's disease. Am J Pathol. 2000;157:623–36. doi: 10.1016/s0002-9440(10)64573-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Reddy PH, Mani G, Park BS, Jacques J, Murdoch G, Whetsell W, Jr, et al. Differential loss of synaptic proteins in Alzheimer's disease: implications for synaptic dysfunction. J Alzheimer's Dis. 2005;7:103–17. doi: 10.3233/jad-2005-7203. discussion 173–180. [DOI] [PubMed] [Google Scholar]
- [72].Shim KS, Lubec G. Drebrin, a dendritic spine protein, is manifold decreased in brains of patients with Alzheimer's disease and Down syndrome. Neurosci Lett. 2002;324:209–12. doi: 10.1016/s0304-3940(02)00210-0. [DOI] [PubMed] [Google Scholar]
- [73].Hatanpaa K, Isaacs KR, Shirao T, Brady DR, Rapoport SI. Loss of proteins regulating synaptic plasticity in normal aging of the human brain and in Alzheimer disease. J Neuropathol Exp Neurol. 1999;58:637–43. doi: 10.1097/00005072-199906000-00008. [DOI] [PubMed] [Google Scholar]
- [74].Love S, Siew LK, Dawbarn D, Wilcock GK, Ben-Shlomo Y, Allen SJ. Premorbid effects of APOE on synaptic proteins in human temporal neocortex. Neurobiol Aging. 2006;27:797–803. doi: 10.1016/j.neurobiolaging.2005.04.008. [DOI] [PubMed] [Google Scholar]
- [75].Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harbor Perspect Med. 2011;1:a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Goldman WP, Price JL, Storandt M, Grant EA, McKeel DW, Jr, Rubin EH, et al. Absence of cognitive impairment or decline in preclinical Alzheimer's disease. Neurology. 2001;56:361–7. doi: 10.1212/wnl.56.3.361. [DOI] [PubMed] [Google Scholar]
- [77].Carlesimo GA, Sabbadini M, Fadda L, Caltagirone C. Word-list forgetting in young and elderly subjects: evidence for age-related decline in transferring information from transitory to permanent memory condition. Cortex. 1997;33:155–66. doi: 10.1016/s0010-9452(97)80011-1. [DOI] [PubMed] [Google Scholar]
- [78].Parkin AJ, Lawrence A. A dissociation in the relation between memory tasks and frontal lobe tests in the normal elderly. Neuropsychologia. 1994;32:1523–32. doi: 10.1016/0028-3932(94)90124-4. [DOI] [PubMed] [Google Scholar]
- [79].Petersen RC. Conceptual overview. In: Petersen RC, editor. Mild cognitive impairment. Oxford University Press; New York, NY: 2003. pp. 1–14. [Google Scholar]
- [80].Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–94. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- [81].Scheff SW, Price DA, Schmitt FA, DeKosky ST, Mufson EJ. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology. 2007;68:1501–8. doi: 10.1212/01.wnl.0000260698.46517.8f. [DOI] [PubMed] [Google Scholar]
- [82].Scheff SW, Price DA, Schmitt FA, Mufson EJ. Hippocampal synaptic loss in early Alzheimer's disease and mild cognitive impairment. Neurobiol Aging. 2006;27:1372–84. doi: 10.1016/j.neurobiolaging.2005.09.012. [DOI] [PubMed] [Google Scholar]
- [83].Scheff SW, Price DA, Schmitt FA, Roberts KN, Ikonomovic MD, Mufson EJ. Synapse stability in the precuneus early in the progression of Alzheimer's disease. J Alzheimer's Dis. 2013;35:599–609. doi: 10.3233/JAD-122353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Sultana R, Banks WA, Butterfield DA. Decreased levels of PSD95 and two associated proteins and increased levels of BCl2 and caspase 3 in hippocampus from subjects with amnestic mild cognitive impairment: insights into their potential roles for loss of synapses and memory, accumulation of Abeta, and neurodegeneration in a prodromal stage of Alzheimer's disease. J Neurosci Res. 2010;88:469–77. doi: 10.1002/jnr.22227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Counts SE, He B, Nadeem M, Wuu J, Scheff SW, Mufson EJ. Hippocampal drebrin loss in mild cognitive impairment. Neurodegener Dis. 2012;10:216–9. doi: 10.1159/000333122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Guyant-Marechal I, Berger E, Laquerriere A, Rovelet-Lecrux A, Viennet G, Frebourg T, et al. Intrafamilial diversity of phenotype associated with app duplication. Neurology. 2008;71:1925–6. doi: 10.1212/01.wnl.0000339400.64213.56. [DOI] [PubMed] [Google Scholar]
- [87].Rosenberg CK, Pericak-Vance MA, Saunders AM, Gilbert JR, Gaskell PC, Hulette CM. Lewy body and Alzheimer pathology in a family with the amyloid-beta precursor protein APP717 gene mutation. Acta Neuropathol. 2000;100:145–52. doi: 10.1007/s004019900155. [DOI] [PubMed] [Google Scholar]
- [88].Gnanalingham KK, Byrne EJ, Thornton A, Sambrook MA, Bannister P. Motor and cognitive function in Lewy body dementia: comparison with Alzheimer's and Parkinson's diseases. J Neurol Neurosurg Psychiatry. 1997;62:243–52. doi: 10.1136/jnnp.62.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Nelson PT, Jicha GA, Kryscio RJ, Abner EL, Schmitt FA, Cooper G, et al. Low sensitivity in clinical diagnoses of dementia with Lewy bodies. J Neurol. 2010;257:359–66. doi: 10.1007/s00415-009-5324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Nelson PT, Kryscio RJ, Jicha GA, Abner EL, Schmitt FA, Xu LO, et al. Relative preservation of MMSE scores in autopsy-proven dementia with Lewy bodies. Neurology. 2009;73:1127–33. doi: 10.1212/WNL.0b013e3181bacf9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Nelson PT, Schmitt FA, Jicha GA, Kryscio RJ, Abner EL, Smith CD, et al. Association between male gender and cortical Lewy body pathology in large autopsy series. J Neurol. 2010;257:1875–81. doi: 10.1007/s00415-010-5630-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Bohnen NI, Albin RL. The cholinergic system and Parkinson disease. Behav Brain Res. 2011;221:564–73. doi: 10.1016/j.bbr.2009.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Shinotoh H, Namba H, Yamaguchi M, Fukushi K, Nagatsuka S, Iyo M, et al. Positron emission tomographic measurement of acetylcholinesterase activity reveals differential loss of ascending cholinergic systems in Parkinson's disease and progressive supranuclear palsy. Ann Neurol. 1999;46:62–9. [PubMed] [Google Scholar]
- [94].Aarsland D, Kurz MW. The epidemiology of dementia associated with Parkinson disease. J Neurol Sci. 2010;289:18–22. doi: 10.1016/j.jns.2009.08.034. [DOI] [PubMed] [Google Scholar]
- [95].Zhan SS, Beyreuther K, Schmitt HP. Quantitative assessment of the synaptophysin immuno-reactivity of the cortical neuropil in various neurodegenerative disorders with dementia. Dementia. 1993;4:66–74. doi: 10.1159/000107299. [DOI] [PubMed] [Google Scholar]
- [96].Baloyannis SJ, Costa V, Baloyannis IS. Morphological alterations of the synapses in the locus coeruleus in Parkinson's disease. J Neurol Sci. 2006;248:35–41. doi: 10.1016/j.jns.2006.05.006. [DOI] [PubMed] [Google Scholar]
- [97].Nikolaus S, Antke C, Muller HW. In vivo imaging of synaptic function in the central nervous system: I. Movement disorders and dementia. Behav Brain Res. 2009;204:1–31. doi: 10.1016/j.bbr.2009.06.008. [DOI] [PubMed] [Google Scholar]
- [98].Parkkinen L, Pirttila T, Alafuzoff I. Applicability of current staging/categorization of alpha-synuclein pathology and their clinical relevance. Acta Neuropathol. 2008;115:399–407. doi: 10.1007/s00401-008-0346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Bodner RA, Outeiro TF, Altmann S, Maxwell MM, Cho SH, Hyman BT, et al. Pharmacological promotion of inclusion formation: a therapeutic approach for Huntington's and Parkinson's diseases. PNAS. 2006;103:4246–51. doi: 10.1073/pnas.0511256103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Garske AL, Smith BC, Denu JM. Linking SIRT2 to Parkinson's disease. ACS Chem Biol. 2007;2:529–32. doi: 10.1021/cb700160d. [DOI] [PubMed] [Google Scholar]
- [101].McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–24. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- [102].Okazaki H, Lipkin LE, Aronson SM. Diffuse intracytoplasmic ganglionic inclusions (Lewy type) associated with progressive dementia and quadriparesis in flexion. J Neuropathol Exp Neurol. 1961;20:237–44. doi: 10.1097/00005072-196104000-00007. [DOI] [PubMed] [Google Scholar]
- [103].Kosaka K, Yoshimura M, Ikeda K, Budka H. Diffuse type of Lewy body disease: progressive dementia with abundant cortical Lewy bodies and senile changes of varying degree—a new disease? Clin Neuropathol. 1984;3:185–92. [PubMed] [Google Scholar]
- [104].Gibb WR, Esiri MM, Lees AJ. Clinical and pathological features of diffuse cortical Lewy body disease (Lewy body dementia) Brain. 1987;110(Pt 5):1131–53. doi: 10.1093/brain/110.5.1131. [DOI] [PubMed] [Google Scholar]
- [105].Lennox G, Lowe J, Landon M, Byrne EJ, Mayer RJ, Godwin-Austen RB. Diffuse Lewy body disease: correlative neuropathology using anti-ubiquitin immunocytochemistry. J Neurol Neurosurg Psychiatry. 1989;52:1236–47. doi: 10.1136/jnnp.52.11.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Hansen L, Salmon D, Galasko D, Masliah E, Katzman R, DeTeresa R, et al. The Lewy body variant of Alzheimer's disease: a clinical and pathologic entity. Neurology. 1990;40:1–8. doi: 10.1212/wnl.40.1.1. [DOI] [PubMed] [Google Scholar]
- [107].Perry RH, Irving D, Blessed G, Fairbairn A, Perry EK. Senile dementia of Lewy body type. A clinically and neuropathologically distinct form of Lewy body dementia in the elderly. J Neurol Sci. 1990;95:119–39. doi: 10.1016/0022-510x(90)90236-g. [DOI] [PubMed] [Google Scholar]
- [108].Ditter SM, Mirra SS. Neuropathologic and clinical features of Parkinson's disease in Alzheimer's disease patients. Neurology. 1987;37:754–60. doi: 10.1212/wnl.37.5.754. [DOI] [PubMed] [Google Scholar]
- [109].Masliah E, Mallory M, DeTeresa R, Alford M, Hansen L. Differing patterns of aberrant neuronal sprouting in Alzheimer's disease with and without Lewy bodies. Brain Res. 1993;617:258–66. doi: 10.1016/0006-8993(93)91093-8. [DOI] [PubMed] [Google Scholar]
- [110].Wakabayashi K, Honer WG, Masliah E. Synapse alterations in the hippocampal-entorhinal formation in Alzheimer's disease with and without Lewy body disease. Brain Res. 1994;667:24–32. doi: 10.1016/0006-8993(94)91709-4. [DOI] [PubMed] [Google Scholar]
- [111].Brown DF, Risser RC, Bigio EH, Tripp P, Stiegler A, Welch E, et al. Neocortical synapse density and Braak stage in the Lewy body variant of Alzheimer disease: a comparison with classic Alzheimer disease and normal aging. J Neuropathol Exp Neurol. 1998;57:955–60. doi: 10.1097/00005072-199810000-00007. [DOI] [PubMed] [Google Scholar]
- [112].Samuel W, Alford M, Hofstetter CR, Hansen L. Dementia with Lewy bodies versus pure Alzheimer disease: differences in cognition, neuropathology, cholinergic dysfunction, and synapse density. J Neuropathol Exp Neurol. 1997;56:499–508. doi: 10.1097/00005072-199705000-00006. [DOI] [PubMed] [Google Scholar]
- [113].Hansen LA, Daniel SE, Wilcock GK, Love S. Frontal cortical synaptophysin in Lewy body diseases: relation to Alzheimer's disease and dementia. J Neurol Neurosurg Psychiatry. 1998;64:653–6. doi: 10.1136/jnnp.64.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Lippa CF. Synaptophysin immunoreactivity in Pick's disease: comparison with Alzheimer's disease and dementia with Lewy bodies. Am J Alzheimer's Dis Other Dementia. 2004;19:341–4. doi: 10.1177/153331750401900606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Revuelta GJ, Rosso A, Lippa CF. Neuritic pathology as a correlate of synaptic loss in dementia with Lewy bodies. Am J Alzheimer's Dis Other Dementia. 2008;23:97–102. doi: 10.1177/1533317507310565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Ubhi K, Peng K, Lessig S, Estrella J, Adame A, Galasko D, et al. Neuropathology of dementia with Lewy bodies in advanced age: a comparison with Alzheimer disease. Neurosci Lett. 2010;485:222–7. doi: 10.1016/j.neulet.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Gustafson L. Frontal lobe degeneration of non-Alzheimer type. II. Clinical picture and differential diagnosis. Arch Gerontol Geriat. 1987;6:209–23. doi: 10.1016/0167-4943(87)90022-7. [DOI] [PubMed] [Google Scholar]
- [118].Gustafson L, Brun A, Passant U. Frontal lobe degeneration of non-Alzheimer type. Baillieres Clin Neurol. 1992;1:559–82. [PubMed] [Google Scholar]
- [119].Clinical and neuropathological criteria for frontotemporal dementia. The Lund and Manchester Groups. J Neurol Neurosurg Psychiatry. 1994;57:416–8. doi: 10.1136/jnnp.57.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Elfgren C, Brun A, Gustafson L, Johanson A, Minthon L, Passant U, Risberg J. Neuropsychological tests as discriminators between dementia of Alzheimer type and frontotemporal dementia. Int J Geriatr Psychiatr. 1994;9:635–42. [Google Scholar]
- [121].Neary D. Dementia of frontal lobe type. J Am Geriat Soc. 1990;38:71–2. doi: 10.1111/j.1532-5415.1990.tb01601.x. [DOI] [PubMed] [Google Scholar]
- [122].Brun A, Liu X, Erikson C. Synapse loss and gliosis in the molecular layer of the cerebral cortex in Alzheimer's disease and in frontal lobe degeneration. Neurodegeneration. 1995;4:171–7. doi: 10.1006/neur.1995.0021. [DOI] [PubMed] [Google Scholar]
- [123].Liu X, Brun A. Regional and laminar synaptic pathology in frontal lobe degeneration of non-Alzheimer type. Int J Geriat Psychiatry. 1996;11:47–55. [Google Scholar]
- [124].Liu X, Erikson C, Brun A. Cortical synaptic changes and gliosis in normal aging, Alzheimer's disease and frontal lobe degeneration. Dementia. 1996;7:128–34. doi: 10.1159/000106867. [DOI] [PubMed] [Google Scholar]
- [125].Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–59. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- [126].Dickson DW. Neuropathology of Pick's disease. Neurology. 2001;56:S16–20. doi: 10.1212/wnl.56.suppl_4.s16. [DOI] [PubMed] [Google Scholar]
- [127].Sparks DL, Markesbery WR. Altered serotonergic and cholinergic synaptic markers in Pick's disease. Arch Neurol. 1991;48:796–9. doi: 10.1001/archneur.1991.00530200032014. [DOI] [PubMed] [Google Scholar]
- [128].Steele JC, Richardson JC, Olszewski J. Progressive supranuclear palsy. A heterogeneous degeneration involving the brain stem, basal ganglia and cerebellum with vertical gaze and pseudobulbar palsy, nuchal dystonia and dementia. Arch Neurol. 1964;10:333–59. doi: 10.1001/archneur.1964.00460160003001. [DOI] [PubMed] [Google Scholar]
- [129].Grafman J, Litvan I, Gomez C, Chase TN. Frontal lobe function in progressive supranuclear palsy. Arch Neurol. 1990;47:553–8. doi: 10.1001/archneur.1990.00530050077015. [DOI] [PubMed] [Google Scholar]
- [130].Maher ER, Smith EM, Lees AJ. Cognitive deficits in the Steele–Richardson–Olszewski syndrome (progressive supranuclear palsy) J Neurol Neurosurg Psychiatry. 1985;48:1234–9. doi: 10.1136/jnnp.48.12.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Dickson DW. Neuropathologic differentiation of progressive supranuclear palsy and corticobasal degeneration. J Neurol. 1999;246(Suppl 2):II6–15. doi: 10.1007/BF03161076. [DOI] [PubMed] [Google Scholar]
- [132].Hauw JJ, Daniel SE, Dickson D, Horoupian DS, Jellinger K, Lantos PL, et al. Preliminary NINDS neuropathologic criteria for Steele–Richardson–Olszewski syndrome (progressive supranuclear palsy) Neurology. 1994;44:2015–9. doi: 10.1212/wnl.44.11.2015. [DOI] [PubMed] [Google Scholar]
- [133].Ishizawa K, Dickson DW. Microglial activation parallels system degeneration in progressive supranuclear palsy and corticobasal degeneration. J Neuropathol Exp Neurol. 2001;60:647–57. doi: 10.1093/jnen/60.6.647. [DOI] [PubMed] [Google Scholar]
- [134].Gerhard A, Trender-Gerhard I, Turkheimer F, Quinn NP, Bhatia KP, Brooks DJ. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in progressive supranuclear palsy. Mov Disord. 2006;21:89–93. doi: 10.1002/mds.20668. [DOI] [PubMed] [Google Scholar]
- [135].Bigio EH, Vono MB, Satumtira S, Adamson J, Sontag E, Hynan LS, et al. Cortical synapse loss in progressive supranuclear palsy. J Neuropathol Exp Neurol. 2001;60:403–10. doi: 10.1093/jnen/60.5.403. [DOI] [PubMed] [Google Scholar]
- [136].Van Langenhove T, van der Zee J, Van Broeckhoven C. The molecular basis of the frontotemporal lobar degeneration-amyotrophic lateral sclerosis spectrum. Ann Med. 2012;44:817–28. doi: 10.3109/07853890.2012.665471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, et al. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer's disease. Ann Neurol. 2007;61:435–45. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Nelson PT, Schmitt FA, Lin Y, Abner EL, Jicha GA, Patel E, et al. Hippocampal sclerosis in advanced age: clinical and pathological features. Brain. 2011;134:1506–18. doi: 10.1093/brain/awr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Thom M. Hippocampal sclerosis: progress since Sommer. Brain Pathol. 2009;19:565–72. doi: 10.1111/j.1750-3639.2008.00201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Corey-Bloom J, Sabbagh MN, Bondi MW, Hansen L, Alford MF, Masliah E, et al. Hippocampal sclerosis contributes to dementia in the elderly. Neurology. 1997;48:154–60. doi: 10.1212/wnl.48.1.154. [DOI] [PubMed] [Google Scholar]
- [141].Nelson PT, Smith CD, Abner EL, Wilfred BJ, Wang WX, Neltner JH, et al. Hippocampal sclerosis of aging, a prevalent and high-morbidity brain disease. Acta Neuropathol. 2013;126:161–77. doi: 10.1007/s00401-013-1154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Shin H, Kim MH, Lee SJ, Lee KH, Kim MJ, Kim JS, et al. Decreased metabolism in the cerebral cortex in early-stage Huntington's disease: a possible biomarker of disease progression? J Clin Neurol. 2013;9:21–5. doi: 10.3988/jcn.2013.9.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Spampanato J, Gu X, Yang XW, Mody I. Progressive synaptic pathology of motor cortical neurons in a BAC transgenic mouse model of Huntington's disease. Neuroscience. 2008;157:606–20. doi: 10.1016/j.neuroscience.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Estrada-Sanchez AM, Rebec GV. Role of cerebral cortex in the neuropathology of Huntington's disease. Front Neural Circuits. 2013;7:19. doi: 10.3389/fncir.2013.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Robins Wahlin TB, Lundin A, Dear K. Early cognitive deficits in Swedish gene carriers of Huntington's disease. Neuropsychology. 2007;21:31–44. doi: 10.1037/0894-4105.21.1.31. [DOI] [PubMed] [Google Scholar]
- [146].Morton AJ, Faull RL, Edwardson JM. Abnormalities in the synaptic vesicle fusion machinery in Huntington's disease. Brain Res Bull. 2001;56:111–7. doi: 10.1016/s0361-9230(01)00611-6. [DOI] [PubMed] [Google Scholar]
- [147].Smith R, Klein P, Koc-Schmitz Y, Waldvogel HJ, Faull RL, Brundin P, et al. Loss of SNAP-25 and rabphilin 3a in sensory-motor cortex in Huntington's disease. J Neurochem. 2007;103:115–23. doi: 10.1111/j.1471-4159.2007.04703.x. [DOI] [PubMed] [Google Scholar]
- [148].Bowler JV, Munoz DG, Merskey H, Hachinski V. Fallacies in the pathological confirmation of the diagnosis of Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1998;64:18–24. doi: 10.1136/jnnp.64.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Chui HC, Zarow C, Mack WJ, Ellis WG, Zheng L, Jagust WJ, et al. Cognitive impact of subcortical vascular and Alzheimer's disease pathology. Ann Neurol. 2006;60:677–87. doi: 10.1002/ana.21009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Jellinger K, Danielczyk W, Fischer P, Gabriel E. Clinicopathological analysis of dementia disorders in the elderly. J Neurol Sci. 1990;95:239–58. doi: 10.1016/0022-510x(90)90072-u. [DOI] [PubMed] [Google Scholar]
- [151].Jellinger KA, Attems J. Neuropathological evaluation of mixed dementia. J Neurol Sci. 2007;257:80–7. doi: 10.1016/j.jns.2007.01.045. [DOI] [PubMed] [Google Scholar]
- [152].Jicha GA, Parisi JE, Dickson DW, Johnson K, Cha R, Ivnik RJ, et al. Neuropathologic outcome of mild cognitive impairment following progression to clinical dementia. Arch Neurol. 2006;63:674–81. doi: 10.1001/archneur.63.5.674. [DOI] [PubMed] [Google Scholar]
- [153].Kovari E, Gold G, Herrmann FR, Canuto A, Hof PR, Bouras C, et al. Cortical microinfarcts and demyelination affect cognition in cases at high risk for dementia. Neurology. 2007;68:927–31. doi: 10.1212/01.wnl.0000257094.10655.9a. [DOI] [PubMed] [Google Scholar]
- [154].Rockwood K. Mixed dementia: Alzheimer's and cerebrovascular disease. Int Psychogeriatr. 2003;15(Suppl 1):39–46. doi: 10.1017/S1041610203008949. [DOI] [PubMed] [Google Scholar]
- [155].Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- [156].Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology. 2004;62:1148–55. doi: 10.1212/01.wnl.0000118211.78503.f5. [DOI] [PubMed] [Google Scholar]
- [157].Todorov AB, Go RC, Constantinidis J, Elston RC. Specificity of the clinical diagnosis of dementia. J Neurol Sci. 1975;26:81–98. doi: 10.1016/0022-510x(75)90116-1. [DOI] [PubMed] [Google Scholar]
- [158].Polvikoski TM, van Straaten EC, Barkhof F, Sulkava R, Aronen HJ, Niinisto L, et al. Frontal lobe white matter hyperintensities and neurofibrillary pathology in the oldest old. Neurology. 2010;75:2071–8. doi: 10.1212/WNL.0b013e318200d6f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].White L, Petrovitch H, Hardman J, Nelson J, Davis DG, Ross GW, et al. Cerebrovascular pathology and dementia in autopsied Honolulu-Asia Aging Study participants. Ann NY Acad Sci. 2002;977:9–23. doi: 10.1111/j.1749-6632.2002.tb04794.x. [DOI] [PubMed] [Google Scholar]
- [160].White L, Small BJ, Petrovitch H, Ross GW, Masaki K, Abbott RD, et al. Recent clinical-pathologic research on the causes of dementia in late life: update from the Honolulu–Asia Aging Study. J Geriat Psychiatry Neurol. 2005;18:224–7. doi: 10.1177/0891988705281872. [DOI] [PubMed] [Google Scholar]
- [161].Cordonnier C, van der Flier WM. Brain microbleeds and Alzheimer's disease: innocent observation or key player? Brain. 2011;134:335–44. doi: 10.1093/brain/awq321. [DOI] [PubMed] [Google Scholar]
- [162].Korczyn AD. Mixed dementia—the most common cause of dementia. Ann NY Acad Sci. 2002;977:129–34. doi: 10.1111/j.1749-6632.2002.tb04807.x. [DOI] [PubMed] [Google Scholar]
- [163].Thal DR, Ghebremedhin E, Orantes M, Wiestler OD. Vascular pathology in Alzheimer disease: correlation of cerebral amyloid angiopathy and arteriosclerosis/lipohyalinosis with cognitive decline. J Neuropathol Exp Neurol. 2003;62:1287–301. doi: 10.1093/jnen/62.12.1287. [DOI] [PubMed] [Google Scholar]
- [164].Jellinger K. The neuropathologic substrates of vascular-ischemic dementia. In: Paul RH, Cohen R, Ott BR, Salloway S, editors. Vascular dementia. Humana Press; Totowa, NJ: 2005. pp. 23–56. [Google Scholar]
- [165].Jellinger KA, Attems J. Neuropathology and general autopsy findings in nondemented aged subjects. Clin Neuropathol. 2012;31:87–98. doi: 10.5414/np300418. [DOI] [PubMed] [Google Scholar]
- [166].Fisher CM. The arterial lesions underlying lacunes. Acta Neuropathol. 1968;12:1–15. doi: 10.1007/BF00685305. [DOI] [PubMed] [Google Scholar]
- [167].Esiri MM, Wilcock GK, Morris JH. Neuropathological assessment of the lesions of significance in vascular dementia. J Neurol Neurosurg Psychiatry. 1997;63:749–53. doi: 10.1136/jnnp.63.6.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Jicha GA, Abner EL, Schmitt FA, Kryscio RJ, Riley KP, Cooper GE, Stiles N, Mendiondo MS, Smith CD, Van Eldik LJ, Nelson PT. Preclinical AD Workgroup staging: pathological correlates and potential challenges. Neurobiol Aging. 2012;33(3):622.e1–622.e16. doi: 10.1016/j.neurobiolaging.2011.02.018. http://dx.doi.org/10.1016/j.neurobiolaging. 2011.02.018. Epub 2011 Apr 19, PMID: 21507528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Nelson PT, Braak H, Markesbery WR. Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship. J Neuropathol Exp Neurol. 2009;68:1–14. doi: 10.1097/NEN.0b013e3181919a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Petrovitch H, Ross GW, He Q, Uyehara-Lock J, Markesbery W, Davis D, et al. Characterization of Japanese–American men with a single neocortical AD lesion type. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]