Summary
The prognosis of patients with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL) is poor. Chemotherapy is rarely curative and tyrosine kinase inhibitors (TKIs) induce only transient responses. Heat shock protein 90 (Hsp90) is a chaperone protein that is important in signal transduction, cell cycle control, and transcription regulation in both normal and leukemia cells. In the current study, we tested the growth inhibitory and apoptotic effects of a novel Hsp90 inhibitor, EC141 on the Ph+ ALL lines Z-119, Z-181, and Z-33, as well as primary bone marrow-derived blasts from patients with newly diagnosed Ph+ ALL. We found that EC141 inhibited the growth of Ph+ ALL cells in a concentration-dependent manner with IC50 ranged from 1 to 10 nM. EC141 also inhibited the proliferation of primary bone marrow-derived blasts using the ALL blast colony assay. EC141 down-regulated Hsp90 and up-regulated Hsp70 protein levels, inhibited CrkL phosphorylation, and induced degradation of Bcr-Abl p190 protein through ubiquitin-dependent proteasomal pathway. Furthermore, exposure of Ph+ ALL cells to EC141 resulted in activation of caspase-3, cleavage of poly (ADP-ribose) polymerase (PARP), and induction of apoptosis. In conclusion, our data suggest that EC141 is a potent Hsp90 inhibitor with activity against Ph+ ALL. Further studies to investigate the anticancer effect of EC141 either as a single agent, or in combination in Ph+ ALL and other hematological malignancies are warranted.
Keywords: Leukemia, Hsp90 inhibitor, EC141, Apoptosis, Ph+ ALL
Introduction
The Philadelphia chromosome is the result of a reciprocal translocation that fuses the abl proto-oncogene from chromosome 9 with the bcr gene from chromosome 22 [1]. The Ph abnormality constitutes the most common cytogenetic abnormality in adult patients with ALL. It occurs in 20% to 30% of these patients overall, with an incidence of more than 50% in patients 50 years and older [2–4]. The location of the breakpoint within the bcr gene results in either the Bcr-Abl p190 fusion protein exclusively observed in Ph+ ALL or the Bcr-Abl p210 fusion protein that is seen in only 20% to 40% of patients with Ph+ ALL but in nearly all patients with Ph+ chronic myelogenous leukemia (CML) [5]. Before the advent of targeted therapy with the Abl tyrosine kinase inhibitors (TKIs), the prognosis for adult patients with this leukemia treated with conventional chemotherapy was poor. The long-term survival rate in patients with Ph+ ALL in the pre-imatinib era was less than 10%, and median survival durations ranged from 8 to 16 months largely because of relapse-related mortality [6, 7]. Recent data have shown that patients with newly diagnosed Ph+ ALL benefit from imatinib or newer generations of TKIs-based therapy [8, 9]. Nevertheless, the prognosis of these patients is still poor. New challenges have emerged with respect to the development of imatinib resistance through Abl kinase domain mutations and other mechanisms [10, 11]. The development of novel TKIs with increased potency against Abl kinase, or other novel therapeutic targets, and their incorporation into front-line therapy for Ph+ ALL may further improve clinical outcomes of these patients.
Hsp90 is a ubiquitous molecular chaperone of signal transduction proteins, cell cycle regulators, and transcription factors, and it accounts for 1% to 2% of all proteins in the cell [12, 13]. Hsp90 protects cells by interacting with and stabilizing its client proteins that are required for cell survival. Therefore, Hsp90 has been thought to be a target of active cancer therapy. Hsp90 client proteins include Bcr-Abl, c-Kit, epidermal growth factor receptor, ERB-B2, ZAP-70, Flt3, vascular endothelial growth factor receptor, androgen and estrogen receptors, hypoxia-inducible factor-1α, telomerase and many others [14, 15].
The importance of Hsp90 in the development and progression of malignant transformation led to the development of small-molecule Hsp90 inhibitors as potential anticancer therapeutic agents. The geldanamycin derivative 17-(allylamino)-17-demethoxygeldanamycin (17-AAG) is the first Hsp90 inhibitor to be extensively studied. A preclinical study using a prostate cancer xenograft model showed that intratumoral administration of 17-AAG inhibited tumor’s growth, induced pertinent client protein responses and apoptosis with minimal toxicity [16].
EC141 is a small-molecule, non-anasamycin Hsp90 inhibitor that blocks the chaperone activity of Hsp90 and induces degradation of its client proteins. The activity of this agent in human leukemia has not yet been studied. As part of the preclinical development of this agent, we studied the in vitro and ex vivo activity of EC141 in human Ph+ ALL cell lines as well as primary bone marrow-derived blasts obtained from patients with Ph+ ALL. Specifically, we investigated its effect on cell growth and apoptosis, modulation of Hsp90 and Hsp70 expression, and degradation of Bcr-Abl protein.
Materials and methods
Reagents
EC141 was developed and provided by Biogen Idec. (Cambridge, MA). The following commercially available products were used in this study: RPMI-1640 medium and penicillin-streptomycin from Gibco BRL (Grand Island, NJ), fetal calf serum (FCS) from Gemini Bio-Products (Woodland, CA), the anti-PARP antibody from Pharmingen (San Jose, CA), Ficoll-Hypaque 1077 and the anti-β-actin antibody from Sigma (St. Louis, MO), the anti-c-Abl (Ab-2) antibody from Calbiochem (San Jose, CA), the anti-phosphorylated CrkL antibody from Becton Dickinson (San Jose, CA), the anti-procaspase-3 and anti-caspase-3 antibodies from New England BioLabs (Ipswich, MA), the anti-Hsp70 and anti-Hsp90 antibodies from BD Biosciences (San Jose, CA), and the anti-ubiquitin antibody from Upstate Biotechnology (Billerica, MA).
Leukemia cell lines and primary bone marrow blasts
The following human Ph+ B-cell ALL cell lines were used in this study: Z-119, Z-181 and Z-33. These cell lines express a B-cell phenotype, carry the t(9:22) by standard cytogenetic analysis and expressed p190 Bcr-Abl protein as previously published [17, 18]. All of the cell lines were cultured in RPMI-1640 medium supplemented with 10% FCS. Mononuclear cells from fresh bone marrow samples were obtained from two patients with Ph+ ALL, the cells were fractionated using Ficoll-Hypaque 1077 according to a standard procedure under sterile conditions. All of the patients provided informed consent according to our institutional guidelines at the time of sample acquisition.
Analysis of cell proliferation using MTT assay
Exponentially growing leukemia cells or primary bone marrow blast cells obtained from patients with Ph+ ALL were incubated at 1×105 cells/well in six-well plates in RPMI-1640 medium supplemented with 10% FCS. EC141 was dissolved in dimethyl sulfoxide (DMSO) and was added at the initiation of the culture at concentrations indicated. Cell viability was assessed at various time points using an MTT-based cell proliferation/cytotoxicity assay from Promega (Madison, WI) according to the manufacturer’s instructions. Briefly, 20µL of CellTiter 96 One solution was added to each well after incubation of the cells with EC141. The plates were then incubated for an additional 60 min at 37°C in a humidified 5% CO2 atmosphere. Immediately after incubation, the absorbance was read using a 96-well plate reader at a wavelength of 490 nm. Each data point was determined six times before analysis. All experiments were performed in triplicate.
ALL blast colony assay
An ALL blast colony assay was performed as described previously [19, 20]. Briefly, 1×105 non-adherent T-cell-depleted bone marrow cells were plated in 0.8% methylcellulose in α-medium supplemented with 30% FCS, and irradiated (70 Gy) normal-donor low-density peripheral blood cells were used as feeder cells. EC141 was dissolved in DMSO and added at the initiation of cultures at concentrations ranging from 0 to 200 nM. The cells were then incubated in 35 mm Petri dishes in triplicate for 7 days at 37°C in a humidified atmosphere of 5% CO2 in air. ALL blast colonies were microscopically evaluated on day 7 of culture. A blast colony was defined as a cluster of 20 or more cells. Individual colonies were microaspirated, smeared on glass slides, and stained to confirm their leukemia cell composition. This ALL blast colony assay identified blasts rather than normal progenitors as demonstrated previously using cytogenetic and polymerase chain reaction analyses of colonies obtained using this assay [21].
Detection of apoptosis
Leukemia cells were stained with Annexin Vand propidium iodide using the Annexin V apoptosis detection kit from Pharmingen (San Jose, CA) according to the manufacturer’s protocol. Annexin V-positive cells were detected using flow cytometry with a FACSCalibur system and the CellQuest Pro software program from Becton Dickinson (San Jose, CA). All experiments were performed in triplicate.
Western blot and immunoprecipitation
The western blot and immunoprecipitation experiments were performed according to the standard protocol previously published by our group [19, 20].
Statistical analysis
We performed all statistical analyses using GraphPad Prism 4 from GraphPad Software (San Diego, CA). We used t test to evaluate the significance of differences between the groups. All computations were carried out on DELL PC using the Windows NT operating system. All reported p values were 2-sided, and p<0.05 was considered statistically significant.
Results
EC141 inhibits the growth of Ph+ ALL cells and primary bone marrow-derived ALL blast progenitors
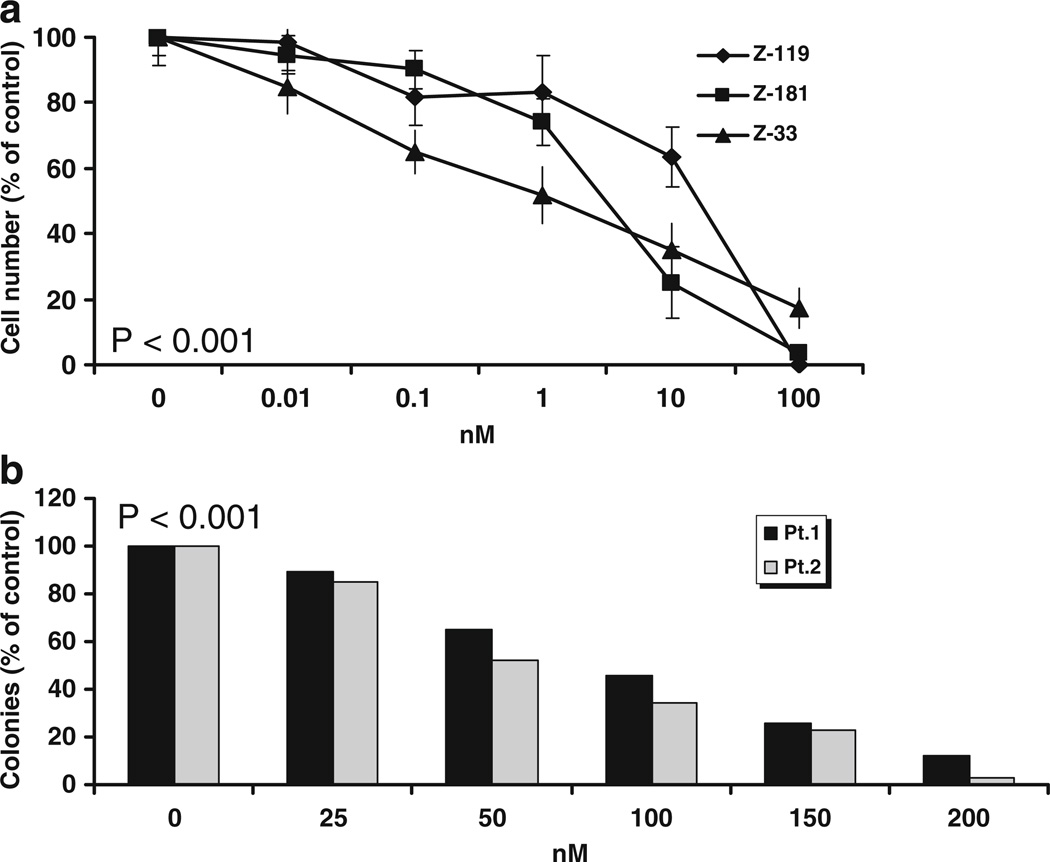
We first studied the effect of EC141 on the growth and survival of Z-33, Z-119 and Z-181 cells using MTT assay. The concentrations of EC141 we used for this assay ranged from 0.01 to 100 nM. We found a concentration-dependent inhibition of the growth of all three leukemia cell lines after 24-h of treatment with EC141. The IC50s ranged from 1 to 10 nM in these cells (Fig. 1a). Exposure to EC141 at 100 nM resulted in greater than 80% growth inhibition of these cells at 24-h. We then studied the ex vivo activity of EC141 in primary bone marrow-derived leukemia blast progenitors obtained from two patients with Ph+ ALL using the ALL blast colony assay. In order to explore concentration of EC141 above the IC50, we evaluated doses ranging from 25 to 200 nM in this assay. We observed a concentration-dependent inhibition of the growth of ALL blast colony forming cells obtained from both patients. The IC50 of EC141 was around 100 nM for patient 1 and 50 nM for patient 2, about 10-fold higher than the IC50 observed for the cell lines (Fig. 1b).
Fig. 1.
(a) growth-inhibitory effect of EC141 on the Ph+ ALL cell lines Z-33, Z-119, and Z-181. Cell viability was assessed after 24-h of treatment using an MTT assay. The results shown are the mean ± standard deviation for three different experiments. The P value is less than 0.001 in all three cell lines. (b) EC141 inhibits the growth of primary bone marrow-derived ALL blast progenitors. The P value is less than 0.001 in both patients
EC141 induces apoptosis in Ph+ ALL cells
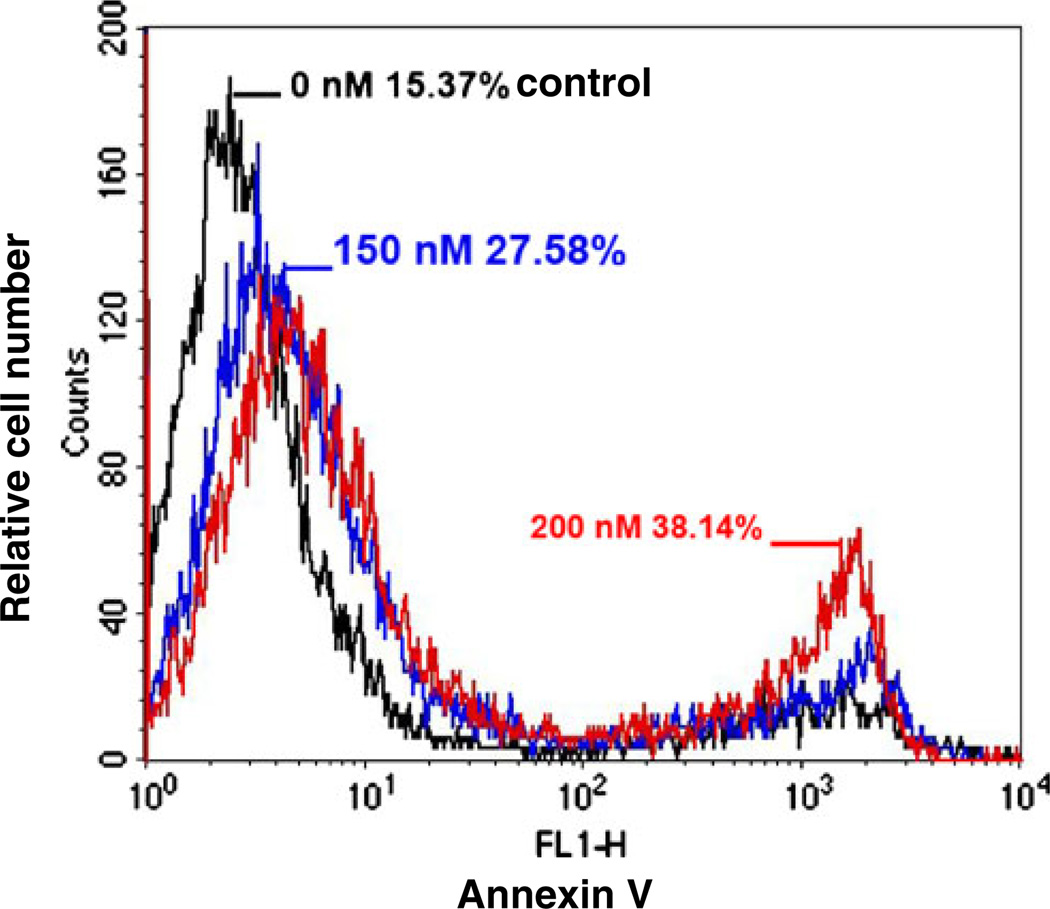
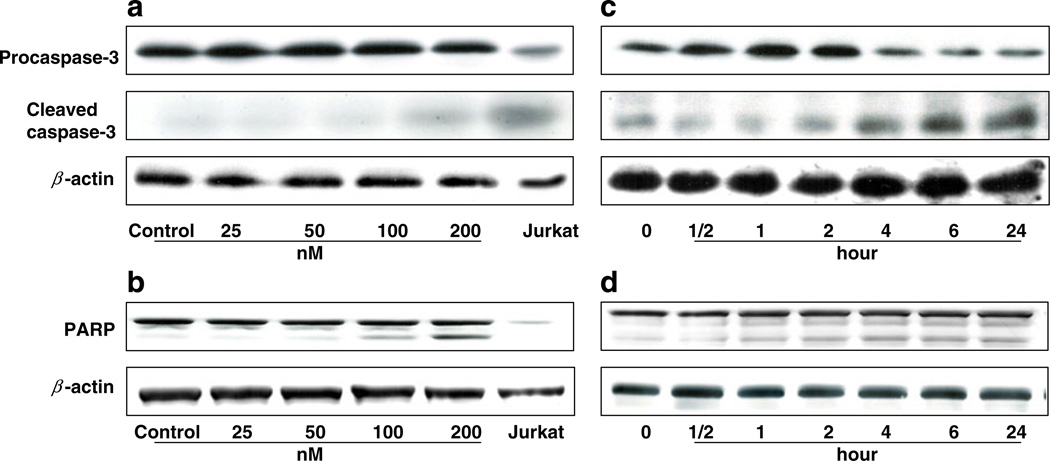
We studied the apoptotic effect of EC141 leukemia cells using an Annexin V assay. We observed a significant concentration-dependent increase in apoptosis after treatment with EC141 for 24-h in Z-181 cells (Fig. 2). The percentage of apoptotic cells among those treated with EC141 at 150 and 200 nM was 27.6% and 38.1%, respectively, whereas it was 15.4% in untreated cells. We then studied the changes in the expression levels of apoptosis-associated proteins in these leukemia cells. Following exposure to EC141 for 24-h at concentrations ranging from 50 to 200 nM, we observed in Z-181 cells a significant activation of caspase-3, and cleavage of its target molecule, PARP (Fig. 3a and b). Similarly, we observed a time-dependent effect on caspase-3 activation and PARP cleavage in Z-181 cells after treatment with EC141 at 100 nM for up to 24-h (Fig. 3c and d). Similar results were observed in Z-33 and Z-119 cells (data not shown).
Fig. 2.
EC141 induces apoptosis in Z-181 cells. Z-181 cells were treated with DMSO (vehicle control) or EC141 at concentrations of 150 nM and 200 nM. Annexin V positive cells were detected at the end of 24-h treatment by flow cytometry
Fig. 3.
(a, b) EC141 induces a concentration-dependent activation of caspase-3 and cleavage of PARP in Z-181 cells by western blot. Jurkat cells were used as a positive control. (c, d) EC141 induces a time-dependent activation of caspase-3 and cleavage of PARP in Z-181 cells by western blot. Expression of β-actin was used as a loading control. The results were representative of three separate experiments
EC141 down-regulates Hsp90 and up-regulates Hsp70 expression
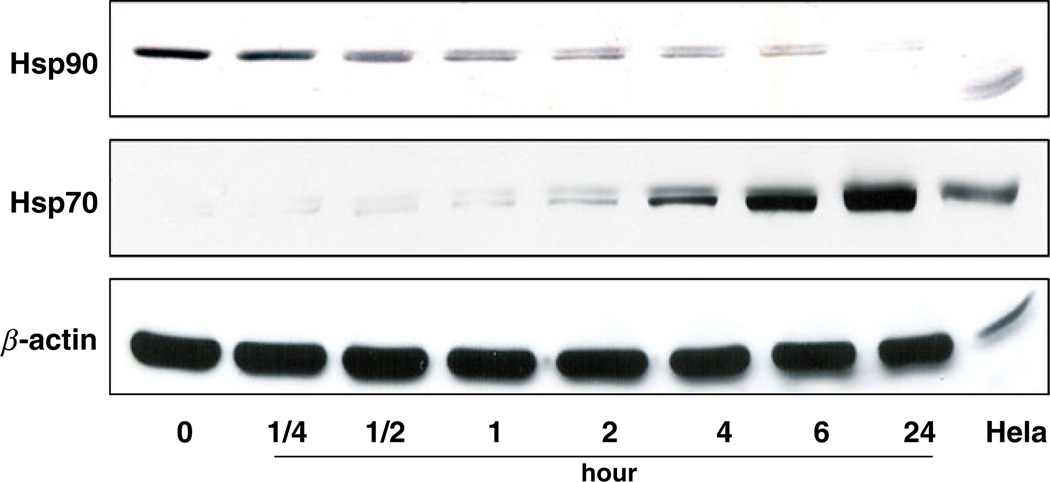
When we exposed Z-181 cells to EC141, we observed a time-dependent down-regulation in the expression level of Hsp90 protein. The expression level of Hsp90 protein was almost completely depleted after 24-h of treatment with EC141 at concentration of 100 nM (Fig. 4). In contrast, we observed a significant induction in the expression level of Hsp70 protein along with down-regulation of Hsp90 protein. Both changes were more pronounced after 24-h treatment with EC141. This was consistent with the results of previous studies showing that Hsp90 inhibition alters the chaperone association between Hsp90 and Bcr-Abl protein, up-regulates the expression of Hsp70 protein and promotes binding of Bcr-Abl to Hsp70, thus targeting Bcr-Abl for degradation [22, 23]. Similar results were observed in Z-33 and Z-119 cells (data not shown).
Fig. 4.
EC141 down-regulates the protein level of Hsp90 and up-regulates the protein level of Hsp70 in Z-181 cells by western blot. Hela cells were used as a positive control and β-actin was used as a loading control. The results were representative of three separate experiments
EC141 inhibits phosphorylation of CrkL, and degrades p190 Bcr-Abl protein through the ubiquitin-dependent proteasomal pathway
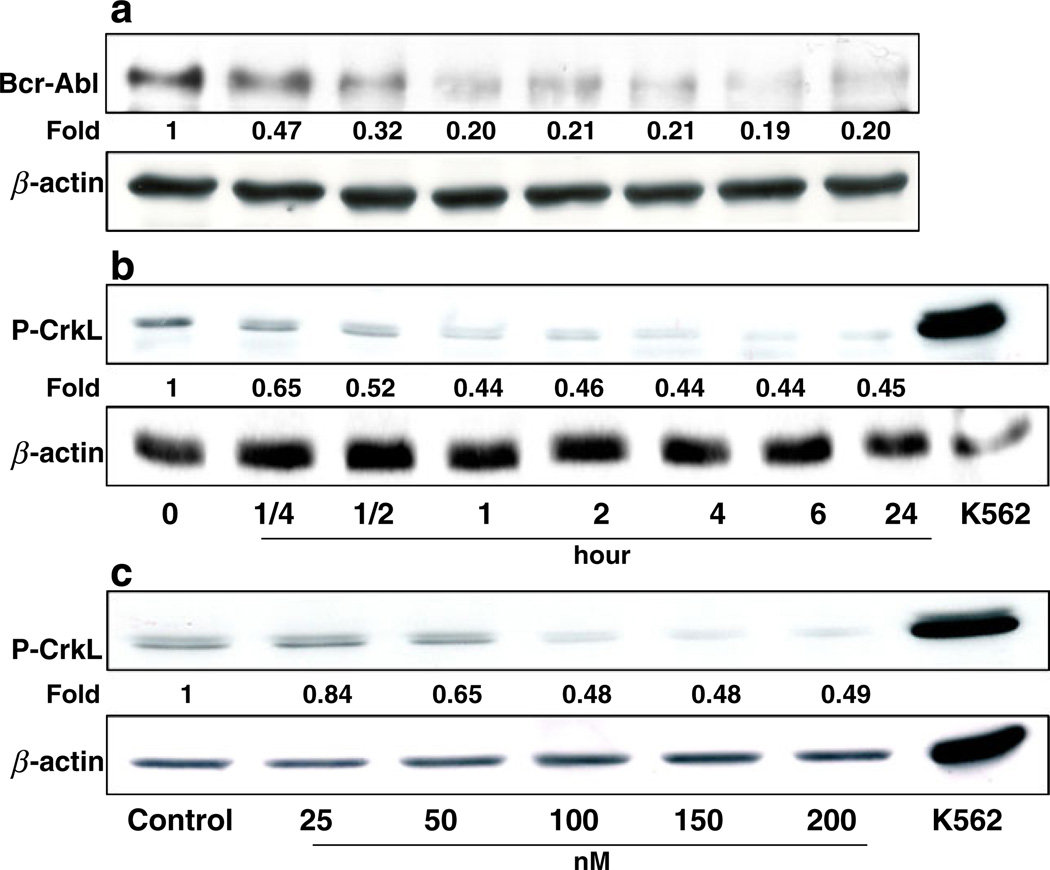
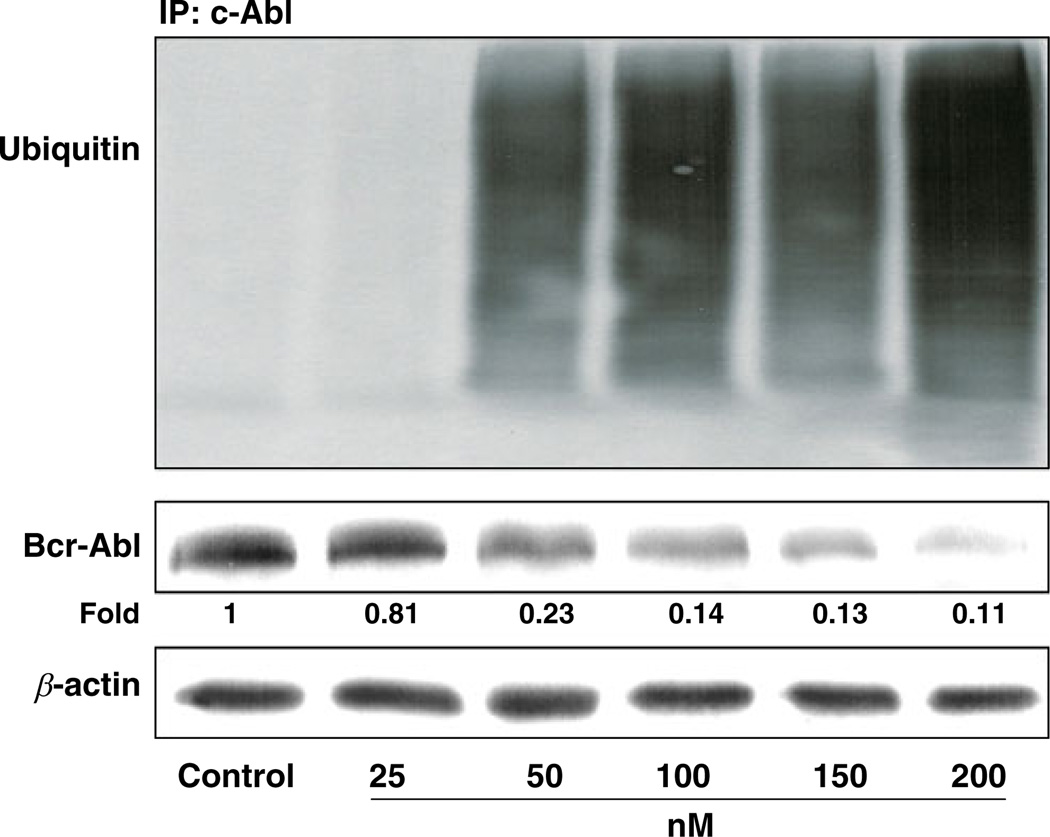
It has been shown that chaperone activity of Hsp90 is required for the stability and function of Bcr-Abl protein in CML cells, and rapid degradation of Bcr-Abl protein could be observed when these cells were treated with Hsp90 inhibitors [24]. In deed, we observed a time-dependent depletion of Bcr-Abl protein when the Z-181 cells were treated with EC141 at 100 nM (Figs. 5a). The depletion of p190 Bcr-Abl protein was accompanied by a concomitant decrease in phosphorylation of CrkL, a downstream target molecule in the Bcr-Abl tyrosine kinase pathway (Fig. 5b and c). To further assess whether the depletion of Bcr-Abl protein after EC141 treatment was linked to its ubiquitination, protein lysates from EC141-treated cells were immunoprecipitated with anti-c-Abl antibody and then followed by immunoblotting with anti-ubiquitin antibody. We found that EC141 treatment of Z-181 cells led to a marked concentration-dependent accumulation of polyubiquitinated c-Abl, in parallel with the depletion of the p190 Bcr-Abl protein level (Fig. 6). These data suggested that EC141-related degradation of p190 Bcr-Abl protein resulted from the activation of the ubiquitin-dependent proteasomal pathway. Similar results were observed in Z-33 and Z-119 cells (data not shown).
Fig. 5.
(a, b) EC141 depletes the protein level of Bcr-Abl and inhibits the phyosphorylation of CrkL (P-CrkL) in a time-dependent manner. (c) EC141 inhibits P-CrkL in a concentration-dependent manner. K562 cells were used as a positive control for P-CrkL and β-actin was used as a loading control. The numerical number below each band represents the fold change in the band density between each sample and the corresponding β-actin as measured by densitometry. The results were representative of three separate experiments
Fig. 6.
EC141 induces degradation of Bcr-Abl protein in a concentration-dependent manner through ubiquitination. The protein lysates were immunoprecipitated with c-Abl antibody and then immunoblotted with ubiquitin, c-Abl and β-actin antibodies. The numerical number below each band represents the fold change in the band density between each sample and the corresponding β-actin as measured by densitometry. IP, immunoprecipitation
Discussion
In the current study, we provided preclinical evidence of the potent anti-leukemia activity of EC141 in Ph+ ALL cells. The IC50s of EC141 ranged from 1 to 10 nM in cell lines and was about 10-fold higher in primary bone marrow-derived blast progenitors from patients with Ph+ ALL. This growth-inhibitory effect was associated with the down-regulation of Hsp90, up-regulation of Hsp70, and degradation of the p190 Bcr-Abl protein through activation of ubiquitin-dependent proteasomal pathway. This is the first report showing the potent anti-leukemia activity of this novel, small-molecule Hsp90 inhibitor. These data provided preclinical rationale to support further clinical investigation of EC141, either as a single agent or in combination, in patients with Ph+ ALL or other hematological malignancies.
Hsp90 is constitutively expressed at levels 2- to 10-fold higher in tumor cells than in normal cells, suggesting its relevant role in the survival and growth of these tumor cells [25]. Because of the wide array of Hsp90 client proteins, translational development of Hsp90 inhibitors has gone in many directions. One of client proteins for Hsp90 is the Bcr-Abl protein which is the most commonly observed abnormality in patients with CML and Ph+ ALL. The molecular effect of Hsp90 inhibitors on the Bcr-Abl protein is different from the direct inhibition of the kinase activity by tyrosine kinase inhibitors, such as imatinib. Down-regulation of Hsp90 protein level was accompanied by the up-regulation of Hsp70 after treatment with EC141 in Ph+ ALL cells. In this study, treatment of Ph+ ALL cells with EC141 at 200 nM for 24-h resulted in almost 90% reduction of the Bcr-Abl protein level and 50% reduction in the phosphorylation of CrkL. This was a very early event, since we observed a significant reduction in Bcr-Abl protein level and phosphorylation of CrkL as early as 30-min after the treatment with EC141. This change was then followed by the activation of caspase-3, cleavage of PARP, and finally induction of apoptosis in these cells. The leukemia cell lines and primary leukemia blasts used in the study expressed the p190 transcript of the Bcl-Abl protein. It is possible that the inhibitory effect of EC141 on the p210 transcript may be different. Thus we provided a rationale in this study for use of Hsp90 inhibitor, such as EC141 in combination with imatinib, or newer generations of TKIs in the treatment of patients who develop resistance to imatinib, since Hsp90 inhibitors may target those resistant clones and restore imatinib sensitivity of the cells.
Several Hsp90 inhibitors are currently in various stages of clinical development, and 17-AAG is one of the most extensively studied. Despite its potent in vitro anti-tumor activity, only mild clinical activity was observed in several phase I studies of this agent in patients with advanced solid tumors. Stable disease had been the best response reported, although it was well tolerated by all treated patients and the clinical toxicities were manageable [16, 26, 27]. Development of more potent inhibitors of the Hsp90 protein and appropriate pharmacokinetics-derived dosing schedule may further improve the clinical activity of these agents. Previous study had shown that the IC50 of 17-AAG in CML cells was around 1–2 µM. In the current study, EC141 has an IC50 ranging from 1 to 10 nM in Ph+ ALL cell lines, this is 1000-fold more potent than what was reported for 17-AAG. This makes EC141 an attractive agent for further pharmacokinetic and clinical development. Because the wide array of its client proteins, many of which are known oncogenes involved in survival of cancer cells, another strategy to improve clinical activity of Hsp90 inhibitors is to combine these agents with other chemotherapeutic agents with different mechanisms of action. This has been tested in several preclinical models with promising results. For example, combination of the histone deacetylase inhibitor, LBH589 and 17-AAG was highly active against human CML cells in blast phase and also AML cells carrying activating mutation of Flt-3 [28]. By disrupting the S-phase cell cycle checkpoint, 17-AAG can also enhance the antiproliferative effect of cytarabine in multiple human leukemia cell lines and cells from patients with AML [29].
In conclusion, our results indicated that EC141 had potent in vitro growth-inhibitory and apoptosis-inducing activity against Ph+ ALL cells. This effect was mediated through the down-regulation of Hsp90 and degradation of p190 Bcr-Abl protein. Further studies of this agent in patients with Ph+ ALL and other hematological malignancies are warranted.
Acknowledgments
We thank Biogen Idec. for providing EC141 for these studies, and Donald R. Norwood from the Department of Scientific Publication at the M.D. Anderson Cancer Center for editing the manuscript.
Footnotes
Disclosure of Potential Conflicts of Interest There is no conflict of interest to disclose.
References
- 1.Lugo TG, Pendergast AM, Muller AJ, Witte ON. Tyrosine kinase activity and transformation potency of bcr-abl oncogene products. Science. 1990;247:1079–1082. doi: 10.1126/science.2408149. [DOI] [PubMed] [Google Scholar]
- 2.Faderl S, Kantarjian HM, Talpaz M, Estrov Z. Clinical significance of cytogenetic abnormalities in adult acute lymphoblastic leukemia. Blood. 1998;91:3995–4019. [PubMed] [Google Scholar]
- 3.Larson RA. Management of acute lymphoblastic leukemia in older patients. Semin Hematol. 2006;43:126–133. doi: 10.1053/j.seminhematol.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Thomas DA. Philadelphia chromosome positive acute lymphocytic leukemia: a new era of challenges. Hematology Am Soc Hematol Educ Program. 2007;2007:435–443. doi: 10.1182/asheducation-2007.1.435. [DOI] [PubMed] [Google Scholar]
- 5.Voncken JW, Kaartinen V, Pattengale PK, Germeraad WT, Groffen J, Heisterkamp N. BCR/ABL P210 and P190 cause distinct leukemia in transgenic mice. Blood. 1995;86:4603–4611. [PubMed] [Google Scholar]
- 6.Faderl S, Kantarjian HM, Thomas DA. Outcome of Philadelphia chromosome-positive adult acute lymphoblastic leukemia. Leuk Lymphoma. 2000;36:263–273. doi: 10.3109/10428190009148847. [DOI] [PubMed] [Google Scholar]
- 7.Dombret H, Gabert J, Boiron JM. Outcome of treatment in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia-results of the prospective multicenter LALA-94 trial. Blood. 2002;100:2357–2366. doi: 10.1182/blood-2002-03-0704. [DOI] [PubMed] [Google Scholar]
- 8.Druker BJ, Sawyers CL, Kantarjian H. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001;344:1038–1042. doi: 10.1056/NEJM200104053441402. [DOI] [PubMed] [Google Scholar]
- 9.Thomas DA, Faderl S, Cortes J, Kantarjian HM. Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood. 2004;103:4396–4407. doi: 10.1182/blood-2003-08-2958. [DOI] [PubMed] [Google Scholar]
- 10.Gorre ME, Mohammed M, Ellwood K. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–880. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- 11.Shah NP, Nicoll JM, Nagar B. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002;2:117–125. doi: 10.1016/s1535-6108(02)00096-x. [DOI] [PubMed] [Google Scholar]
- 12.Welch WJ, Feramisco JR. Purification of the major mammalian heat shock proteins. J Biol Chem. 1982;257:14949–14959. [PubMed] [Google Scholar]
- 13.Workman P, Burrows F, Neckers L, Rosen N. Drugging the cancer chaperone HSP90: combinatorial therapeutic exploitation of oncogene addiction and tumor stress. Ann NY Acad Sci. 2007;1113:202–216. doi: 10.1196/annals.1391.012. [DOI] [PubMed] [Google Scholar]
- 14.Banerji U. Heat shock protein 90 as a drug target: some like it hot. Clin Cancer Res. 2009;15:9–14. doi: 10.1158/1078-0432.CCR-08-0132. [DOI] [PubMed] [Google Scholar]
- 15.Isaacs JS, Xu W, Neckers L. Heat shock protein 90 as a molecular target for cancer therapeutics. Cancer Cell. 2003;3:213–217. doi: 10.1016/s1535-6108(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 16.Williams CR, Tabios R, Linehan WM, Neckers L. Intra-tumor injection of the Hsp90 inhibitor 17AAG decreases tumor growth and induces apoptosis in a prostate cancer xenograft model. J Urol. 2007;178:1528–1532. doi: 10.1016/j.juro.2007.05.120. [DOI] [PubMed] [Google Scholar]
- 17.Estrov Z, Talpaz M, Zipf TF. Role of granulocyte-macrophage colony-stimulating factor in Philadelphia (Ph1)-positive acute lymphoblastic leukemia: studies on two newly established Ph1-positive acute lymphoblastic leukemia cell lines (Z-119 and Z-181) J Cell Physiol. 1996;166:618–630. doi: 10.1002/(SICI)1097-4652(199603)166:3<618::AID-JCP17>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 18.Estrov Z, Talpaz M, Ku S. Molecular and biologic characterization of a newly established Philadelphia-positive acute lymphoblastic leukemia cell line (Z-33) with an autocrine response to GM-CSF. Leukemia. 1996;10:1534–1543. [PubMed] [Google Scholar]
- 19.Estrov Z, Manna SK, Harris D. Phenylarsine oxide blocks interleukin-1beta-induced activation of the nuclear transcription factor NF-kappaB, inhibits proliferation, and induces apoptosis of acute myelogenous leukemia cells. Blood. 1999;94:2844–2853. [PubMed] [Google Scholar]
- 20.Faderl S, Lotan R, Kantarjian HM, Harris D, Van Q, Estrov Z. N-(4-Hydroxylphenyl)retinamide (fenretinide, 4-HPR), a retinoid compound with antileukemic and proapoptotic activity in acute lymphoblastic leukemia (ALL) Leuk Res. 2003;27:259–266. doi: 10.1016/s0145-2126(02)00162-5. [DOI] [PubMed] [Google Scholar]
- 21.Roberts WM, Estrov Z, Ouspenskaia MV, Johnston DA, McClain KL, Zipf TF. Measurement of residual leukemia during remission in childhood acute lymphoblastic leukemia. N Engl J Med. 1997;336:317–323. doi: 10.1056/NEJM199701303360501. [DOI] [PubMed] [Google Scholar]
- 22.Johnson AJ, Wagner AJ, Cheney CM. Rituximab and 17-allylamino-17-demethoxygeldanamycin induce synergistic apoptosis in B-cell chronic lymphocytic leukaemia. Br J Haematol. 2007;139:837–844. doi: 10.1111/j.1365-2141.2007.06878.x. [DOI] [PubMed] [Google Scholar]
- 23.Radujkovic A, Schad M, Topaly J. Synergistic activity of imatinib and 17-AAG in imatinib-resistant CML cells over-expressing BCR-ABL-Inhibition of P-glycoprotein function by 17-AAG. Leukemia. 2005;19:1198–1206. doi: 10.1038/sj.leu.2403764. [DOI] [PubMed] [Google Scholar]
- 24.Wu LX, Xu JH, Zhang KZ. Disruption of the Bcr-Abl/Hsp90 protein complex: a possible mechanism to inhibit Bcr-Abl-positive human leukemic blasts by novobiocin. Leukemia. 2008;22:1402–1409. doi: 10.1038/leu.2008.89. [DOI] [PubMed] [Google Scholar]
- 25.Neckers L. Heat shock protein 90: the cancer chaperone. J Biosci. 2007;32:517–530. doi: 10.1007/s12038-007-0051-y. [DOI] [PubMed] [Google Scholar]
- 26.Solit DB, Osman I, Polsky D. Phase II trial of 17-allylamino-17-demethoxygeldanamycin in patients with metastatic melanoma. Clin Cancer Res. 2008;14:8302–8307. doi: 10.1158/1078-0432.CCR-08-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neckers L. Heat shock protein 90 is a rational molecular target in breast cancer. Breast Dis. 2002;15:53–60. doi: 10.3233/bd-2002-15106. [DOI] [PubMed] [Google Scholar]
- 28.George P, Bali P, Annvarapu S. Combination of the histone deacetylase inhibitor LBH589 and the hsp90 inhibitor 17-AAG is highly active against human CML-BC cells and AML cells with activating mutation of the FLT-3. Blood. 2005;105:1768–1776. doi: 10.1182/blood-2004-09-3413. [DOI] [PubMed] [Google Scholar]
- 29.Mesa RA, Loegering D, Powell HL. Heat shock protein 90 inhibition sensitizes acute myelogenous leukemia cells to cytarabine. Blood. 2005;106:318–327. doi: 10.1182/blood-2004-09-3523. [DOI] [PMC free article] [PubMed] [Google Scholar]