Abstract
Ventilation-induced lung injury (VILI) of preterm neonates probably contributes to the pathogenesis of bronchopulmonary dysplasia (BPD). Erythropoietin (EPO) has been suggested as a therapy for BPD. The aim of this study was to determine whether prophylactic administration of EPO reduces VILI in preterm newborn lambs. Lambs at 126 days of gestation (term is 147 days) were delivered and ventilated with a high tidal volume strategy for 15 min to cause lung injury, then received gentle ventilation until 2 h of age. Lambs were randomized to receive intravenous EPO (5000 IU kg−1: Vent+EPO; n = 6) or phosphate-buffered saline (Vent; n = 7) soon after birth: unventilated controls (UVC; n = 8) did not receive ventilation or any treatment. Physiological parameters were recorded throughout the experimental procedure. Samples of lung were collected for histological and molecular assessment of inflammation and injury. Samples of liver were collected to assess the systemic acute phase response. Vent+EPO lambs received higher 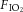 ,
, 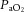 and oxygenation during the first 10 min than Vent lambs. There were no differences in physiological indices beyond this time. Total lung injury score, airway wall thickness, inflammation and haemorrhage were higher in Vent+EPO lambs than in Vent lambs. Lung inflammation and early markers of lung and systemic injury were elevated in ventilated lambs relative to unventilated lambs; EPO administration further increased lung inflammation and markers of lung and systemic injury. Prophylactic EPO exacerbates VILI, which may increase the incidence and severity of long-term respiratory disease. More studies are required before EPO can be used for lung protection in preterm infants.
and oxygenation during the first 10 min than Vent lambs. There were no differences in physiological indices beyond this time. Total lung injury score, airway wall thickness, inflammation and haemorrhage were higher in Vent+EPO lambs than in Vent lambs. Lung inflammation and early markers of lung and systemic injury were elevated in ventilated lambs relative to unventilated lambs; EPO administration further increased lung inflammation and markers of lung and systemic injury. Prophylactic EPO exacerbates VILI, which may increase the incidence and severity of long-term respiratory disease. More studies are required before EPO can be used for lung protection in preterm infants.
Introduction
The initiation of mechanical ventilation in preterm neonates causes lung inflammation and injury, particularly if high tidal volumes (VT) are delivered, potentially contributing to development of bronchopulmonary dysplasia (BPD) (Bjorklund et al. 1997; Wada et al. 1997; Polglase et al. 2008; Hillman et al. 2010). The initiation of ventilation in the delivery room is when preterm infants are most likely to inadvertently receive high VT ventilation because of the mechanical limitations of self-inflating bags and t-piece devices, and difficulties assessing adequate ventilation due to observation of rudimentary parameters, such as chest rise, without appropriate volume monitoring. Recent studies show that 85% of preterm infants inadvertently received high VT in the delivery room, with the range of delivered VT varying between 0 and >30 ml kg−1 (Schmolzer et al. 2010; Poulton et al. 2011). Ventilation-induced lung injury (VILI) can cause a systemic inflammatory response (Chiumello et al. 1999; Hillman et al. 2007; Polglase et al. 2009; Bohrer et al. 2010; Bose et al. 2013) and result in cerebral inflammation, leading to white matter injury (Khwaja & Volpe, 2008; Polglase et al. 2012a). Therefore, therapies targeted at reducing VILI could considerably reduce morbidity and mortality associated with preterm birth.
High-dose (500–5000 IU kg−1 per dose) human recombinant erythropoietin (EPO) has been investigated as a neuroprotective agent in numerous experimental and clinical studies (reviewed by McPherson & Juul, 2010; Juul, 2012), leading to its progression to clinical trials for term infants with hypoxic ischaemic encephalopathy (Elmahdy et al. 2010; Wang et al. 2011; Wu et al. 2012) and the treatment of preterm brain injury (Juul et al. 2008; McAdams et al. 2013). The ability of EPO to decrease inflammation, apoptosis and oxidative stress in organs such as the heart (Li et al. 2006) and to stimulate angiogenesis (Juul, 2012) have led to the suggestion that EPO may decrease VILI. A retrospective study demonstrated that low-dose (250–300 IU kg−1 per dose) EPO administration decreased the incidence of BPD in preterm infants, with the most profound effect observed in preterm infants who received EPO within the first weeks of life (Rayjada et al. 2012). In experimental studies, EPO improved alveolar structure, enhanced vascularity and decreased fibrosis during hyperoxia in neonatal rats (Ozer et al. 2005). However, the efficacy of EPO at reducing lung inflammation and injury in ventilated preterm neonates has not been investigated.
Given the current trials investigating early high-dose EPO administration for neuroprotection in preterm infants, we investigated the efficacy of early, high-dose EPO administration to reduce lung and systemic inflammation and injury resulting from injurious, high VT ventilation immediately after preterm birth. We hypothesised that early EPO administration would reduce pulmonary and systemic inflammation and injury.
Methods
All experimental procedures were approved by the relevant Monash University animal ethics committee, in accordance with the National Health and Medical Research Council (Australia) Australian code of practice for the care and use of animals for scientific purposes (7th Edition, 2004). The experiments conform to the principles of UK regulations, as described by Drummond (2009).
Delivery and ventilation
Ewes bearing twins at 126 ± 1 (mean ± SD) days of gestation (term is ∼148 days) were anaesthetized by i.v. injection of thiopentone sodium (20 mg kg−1), followed by tracheal intubation and maintenance with inhalational anaesthesia (isoflourane 1.5–2.5% in oxygen; Bomac Animal Health, NSW, Australia). A laparotomy was performed, the fetus was exposed and intubated with a cuffed endotracheal tube (4.0 mm), and lung liquid was drained passively. A transcutaneous oximeter (Masimo, Irvine, CA, USA) was attached around the right forelimb. The umbilical cord was then clamped and the lamb was delivered, dried and weighed and placed in an infant warmer (Fisher and Paykel Healthcare, East Tamaki, New Zealand) for initiation of ventilation. As ventilation was initiated, polyvinyl catheters were placed into an umbilical artery and vein to measure mean arterial pressure and administer drugs, respectively. All lambs received sedation (Alfaxane i.v. 5–15 mg kg−1 h−1; CenVet, Lynbrook, VIC, Australia) in 5% dextrose to minimize spontaneous breathing. Mean arterial pressure (DTX Plus Transducer; Becton Dickinson, Singapore), oximetry and ventilatory parameters were measured continuously (Powerlab; ADInstruments, Castle Hill, NSW, Australia). Injurious ventilation was initiated with a peak inspiratory pressure (PIP) of 40 cmH2O and a positive end-expiratory pressure (PEEP) of 0 cmH2O for the first 15 min using a neonatal positive pressure ventilator (Babylog 8000+; Dräger, Lübeck, Germany). PIP was adjusted (an upper limit of 50 cmH2O) to obtain a VT of 10–12 ml kg−1, as described previously (Polglase et al. 2012a). At the end of the 15 min injurious ventilation period, lambs were ventilated in volume-guarantee mode (VT 7 ml kg−1, PEEP 5 cmH2O, inspiratory time 0.5 s, expiratory time 0.5 s) for the remainder of the experiment (total 2 h). Lambs were ventilated with warmed, humidified air, with the fraction of inspired oxygen (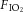 ) set initially at 0.4 but adjusted to maintain arterial oxygen saturation (
) set initially at 0.4 but adjusted to maintain arterial oxygen saturation (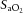 ) at 88–95%. Well-being of the newborn lambs was monitored by regular blood gas analysis (ABL30; Radiometer, Copenhagen, Denmark) and pre-ductal transcutaneous oxyhaemoglobin saturation. Lambs were humanely killed (sodium pentobarbitone: >100 mg kg−1
i.v.) at the end of the 2 h ventilation period. Unventilated control lambs (UVCs) were humanely killed immediately after delivery, without undergoing ventilation, for comparison of indices of injury and inflammation (n = 8). Ewes were humanely killed after delivery of the lambs.
) at 88–95%. Well-being of the newborn lambs was monitored by regular blood gas analysis (ABL30; Radiometer, Copenhagen, Denmark) and pre-ductal transcutaneous oxyhaemoglobin saturation. Lambs were humanely killed (sodium pentobarbitone: >100 mg kg−1
i.v.) at the end of the 2 h ventilation period. Unventilated control lambs (UVCs) were humanely killed immediately after delivery, without undergoing ventilation, for comparison of indices of injury and inflammation (n = 8). Ewes were humanely killed after delivery of the lambs.
Treatments
As soon as the umbilical line was inserted (∼2–3 min after ventilation was initiated), lambs were randomized to receive a bolus injection of 5000 IU kg−1 body weight of human recombinant EPO (Vent+EPO; n = 6; EPREX® Janssen, North Ryde, NSW, Australia) or an equivalent volume of vehicle (Vent: n = 7; pH neutral phosphate-buffered saline, pH 7.0) administered via the umbilical vein catheter. Plasma was collected immediately prior to and regularly during ventilation for later assessment of plasma EPO concentration using a human erythropoietin enzyme-linked immunosorbent assay kit (STEMCELL Technologies, Melbourne, Australia).
Measurements and calculations
Dynamic compliance (Cdyn), adjusted for body weight, was calculated as VT/kg/(PIP – PEEP). Ventilatory efficiency index (VEI) was calculated as 3800/(ΔP.f. 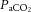 ) where 3800 is a carbon dioxide production constant (ml.mmHg kg−1 min−1), ΔP = PIP – PEEP and f is the respiratory frequency (Ikegami et al. 2004). The alveolar–arterial difference in oxygen (AaDO2) was calculated as AaDO2 = (
) where 3800 is a carbon dioxide production constant (ml.mmHg kg−1 min−1), ΔP = PIP – PEEP and f is the respiratory frequency (Ikegami et al. 2004). The alveolar–arterial difference in oxygen (AaDO2) was calculated as AaDO2 = (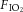 × (760 – 47 mmHg) –
× (760 – 47 mmHg) – 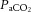 /RQ) –
/RQ) – 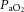 , where RQ is the respiratory quotient, which is ∼0.93 in newborn lambs.
, where RQ is the respiratory quotient, which is ∼0.93 in newborn lambs.
Tissue analyses
The right upper lung lobe was inflation fixed (at 20 cmH20) with 10% formalin and samples were embedded in paraffin. Three 5 μm sections randomly collected from different regions of the right upper lobe were stained with haematoxylin and eosin (H&E) and used to score lung injury. Fifteen random high power fields were scored on a 0–2 scale (0 being none, 2 being severe) for inflammation, haemorrhage, epithelial sloughing and airway wall thickness by an investigator blinded to the experimental group (G.R.P.) (Polglase et al. 2011). Sections of the right lower lobe were immediately frozen in liquid nitrogen for later assessment of mRNA levels of pro-inflammatory cytokines interleukin (IL)-1β, IL-6 and IL-8 and early markers of lung injury (connective tissue growth factor (CTGF), cysteine-rich 61 (CYR-61) and early growth response protein 1 (EGR-1)), using quantitative real-time PCR (qRT-PCR) as described previously (Wallace et al. 2009; Polglase et al. 2010). Samples of liver were immediately frozen in liquid nitrogen for later assessment of mRNA levels of serum amyloid A3 (SAA3), hepcidin and C-reactive protein using qRT-PCR.
Statistical analysis
Fetal umbilical cord blood gas variables and cytokine mRNA levels were compared between groups using one-way analysis of variance (SigmaPlot v12.0, Systat Software Inc., San Jose, CA, USA). Postnatal assessments were compared using two-way repeated-measures analysis of variance with time and group as factors. A Holm–Sidak multiple comparisons post hoc test was used to determine differences between groups. Statistical significance was accepted at P < 0.05. Data are presented as mean (SEM).
Results
Delivery
Umbilical cord blood gas status and birth weights were not different between groups (Table1). There was a higher proportion of females in the Vent+EPO group than in the UVC and Vent groups (Table1).
Table 1.
Birth characteristics and fetal umbilical arterial blood-gas variables at delivery
| UVCs | Vent | Vent + EPO | |
|---|---|---|---|
| Number | 8 | 7 | 6 |
| Male n (%) | 3 (38) | 3 (38) | 1 (17) |
| Birth weight (kg) | 3.5 ± 0.1 | 3.4 ± 0.4 | 3.3 ± 0.2 |
| pHa | 7.38 ± 0.02 | 7.33 ± 0.02 | 7.27 ± 0.03 |
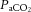 |
45.2 ± 3.0 | 54.0 ± 2.9 | 60.9 ± 4.6 |
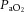 |
29.8 ± 6.8 | 26.4 ± 2.5 | 25.5 ± 1.4 |
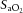 (%) (%) |
83.8 ± 6.1 | 73.2 ± 5.1 | 69.8 ± 3.7 |
Values are mean ± SD. pHa, arterial pH; 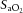 , arterial saturation;
, arterial saturation; 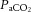 , partial pressure of arterial carbon dioxide;
, partial pressure of arterial carbon dioxide; 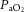 , partial pressure of arterial oxygen. *Significant difference (P < 0.001).
, partial pressure of arterial oxygen. *Significant difference (P < 0.001).
EPO administration to preterm lambs increased plasma concentration of EPO to ∼60,000 mU ml−1 by 10 min, and concentrations remained elevated throughout the ventilation period (Fig.1).
Figure 1. Plasma EPO concentrations.
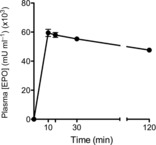
Plasma EPO concentration throughout the 2 h ventilation protocol after i.v. administration of 5000 IU kg−1 recombinant human EPO to ventilated preterm lambs.
Arterial blood gases and ventilation
Tidal volume was not different between groups during the initial 15 min injurious ventilation period or upon subsequent maintenance of ventilation (Fig.2A). Mean airway pressure (Paw; Fig.2B) and the change in pressure (ΔPressure Fig.2C) were not different between groups. Mean systolic and diastolic blood pressures (Fig.2D) were significantly higher in Vent lambs than in Vent+EPO lambs, between 45 and 105 min, but arterial pressures were not different at 2 h. Heart rate was not different between groups (data not shown).
Figure 2. Airway pressure, tidal volume and blood pressure during ventialtion.
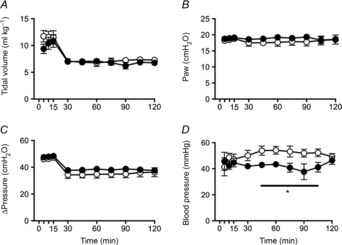
A, tidal volume; B, mean airway pressure (Paw); C, the difference between peak and end-expiratory pressure (ΔPressure); and D, mean arterial blood pressure in Vent + EPO (filled circles) and Vent (open circles) lambs. *Significantly different from the control group at the same time, P < 0.05.
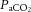 (Fig.3A) and
(Fig.3A) and 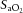 (data not shown) were maintained within the required limits and were not different between groups. To maintain
(data not shown) were maintained within the required limits and were not different between groups. To maintain 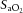 within target ranges, Vent+EPO lambs required significantly higher
within target ranges, Vent+EPO lambs required significantly higher 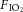 (Fig.3B) at 5 and 10 min compared with Vent only lambs, resulting in a significantly higher
(Fig.3B) at 5 and 10 min compared with Vent only lambs, resulting in a significantly higher 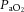 (Fig.3C) and AaDO2 (Fig.3D) during this time. There were no differences in these indices after this acute period.
(Fig.3C) and AaDO2 (Fig.3D) during this time. There were no differences in these indices after this acute period.
Figure 3. Blood-gas and oxygenation variables during ventilation.
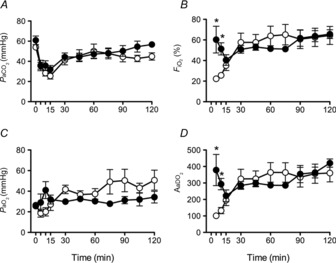
A, partial pressure of arterial oxygen (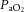 ); B, partial pressure of arterial carbon dioxide (
); B, partial pressure of arterial carbon dioxide (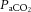 ); C, fraction of inspired oxygen (
); C, fraction of inspired oxygen ( ); and D, alveolar–arterial difference in oxygen (AaDO2) in Vent+EPO (filled circles) and Vent lambs (open circles). *Significantly different from values in the control group at the same time, P < 0.05.
); and D, alveolar–arterial difference in oxygen (AaDO2) in Vent+EPO (filled circles) and Vent lambs (open circles). *Significantly different from values in the control group at the same time, P < 0.05.
VEI (Fig.4A) and respiratory system compliance (Fig.4B) were not different between groups. Airway resistance was significantly higher in Vent+EPO lambs than in Vent lambs (Fig.4C; P < 0.001).
Figure 4. Respiratory indices during ventilation.
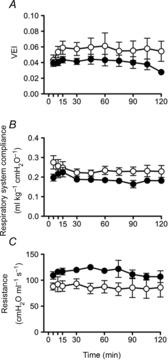
A, ventilator efficiency index (VEI); B, respiratory system compliance; and C, airway resistance in Vent+EPO lambs (filled circles) and Vent lambs (open circles). *Significantly different from the control group at the same time, P < 0.05.
Lung inflammation and injury
IL-1β, IL-6 and IL-8 mRNA levels were significantly higher in Vent lambs than in UVCs, but values in Vent + EPO lambs were significantly higher than both UVC and Vent+EPO groups (Fig.5A). Similarly, mRNA levels of early injury response genes EGR-1, CTGF and CYR-61 were significantly higher in Vent and EPO+Vent lambs than in UVCs, with CTGF mRNA levels significantly higher in EPO+Vent lambs than in both other groups (Fig.5B).
Figure 5. Lung inflammation and injury.
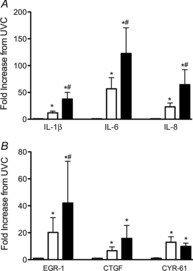
Messenger RNA levels of (A) the pro-inflammatory cytokines IL-1β, IL-6 and IL-8 and (B) early markers of lung injury: early growth response 1 (EGR-1), connective tissue growth factor (CTGF) and cysteine rich angiogenic factor 61 (CYR-61) in Vent (open bars) and Vent+EPO lambs (filled bars) relative to the levels in unventilated controls (UVC: grey bars). *P < 0.05 versus UVC, # P < 0.05 versus Vent.
Histological assessment of lung injury
Representative H&E-stained sections are shown in Fig.6. Vent and Vent+EPO lambs had significantly higher histological inflammation scores, airway wall thickness, epithelial sloughing and total injury compared to UVCs (Table2). Vent+EPO lambs had significantly greater airway wall thickness, inflammation and total injury score than Vent lambs. Vent + EPO lambs had significantly higher haemorrhage scores than UVCs; scores for Vent lambs were intermediate but not statistically different from either of the other groups.
Figure 6. Representative H&E-stained sections of the lung.
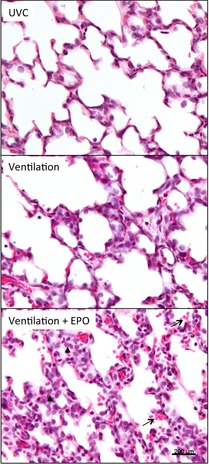
Sections of the right upper lung lobe of unventilated controls (UVC), ventilated lambs and lambs that were ventilated and received EPO (Ventilation + EPO). Note the increase in inflammation and alveolar wall thickness after ventilation, which is further exacerbated by EPO administration. Arrows indicate accumulation of red blood cells within the airways. Arrowhead indicates increased accumulation of inflammatory cells within the interstitium.
Table 2.
Histological lung injury scores
| UVCs | Vent | Vent + EPO | |
|---|---|---|---|
| Inflammation | 0.15 ± 0.06 | 0.66 ± 0.13* | 0.93 ± 0.38*# |
| Haemorrhage | 0.18 ± 0.06 | 0.66 ± 0.14 | 0.91 ± 0.37*† |
| Airway wall thickness | 0.12 ± 0.07 | 0.62 ± 0.13* | 0.76 ± 0.31*# |
| Epithelial sloughing | 0.01 ± 0.02 | 0.24 ± 0.08* | 0.25 ± 0.1* |
| Total injury score | 0.45 ± 0.13 | 2.15 ± 0.38* | 2.84 ± 1.16*# |
Values are mean ± SD. *Values in the Vent and Vent + EPO groups that are significantly different from those in the UVC group. #Values in the Vent + EPO group that are significantly different from those in the Vent group. †P = 0.07 from Vent.
Systemic inflammation
SAA-3 mRNA levels in the liver were significantly greater in Vent lambs than UVCs; values in Vent+EPO lambs were significantly higher than in UVC and Vent+EPO groups (Fig.7). Liver hepcidin (P = 0.76) and C-reactive protein (P = 0.36) mRNA levels were not different between groups (data not shown).
Figure 7. Early marker of systemic inflammation.
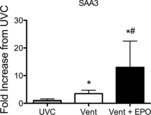
Serum amyloid A-3 (SAA-3) mRNA expression in Vent (white bars) and Vent + EPO lambs (filled bars) normalized to unventilated controls (grey bars). *P < 0.05 versus UVC, #P < 0.05 versus Vent.
Discussion
High-dose (>1000 IU kg−1 per dose) EPO administration has neuroprotective effects in various animal models of neonatal stroke (Gonzalez et al. 2009), perinatal hypoxia–ischemia (Liu et al. 2011) and inflammation-induced fetal brain injury (Rees et al. 2010). A retrospective study demonstrated that early low-dose (<300 IU kg−1 per dose) EPO administration decreased BPD in extremely preterm infants (Rayjada et al. 2012) and an experiment in mice showed that EPO can improve alveolarisation and vasculogenesis in a model of BPD (Ozer et al. 2005). Given that early high-dose EPO is being used for neuroprotection in preterm infants (McAdams et al. 2013) (and therefore this dose is most likely to be used in preterm babies) we assessed whether it could also provide lung protection in ventilated, preterm lambs. Contrary to our hypothesis, early high-dose EPO administration increased airway resistance, increased lung injury, and amplified pulmonary and systemic inflammation.
In term infants with neonatal encephalopathy, an EPO dose of 1000 IU kg−1 administered i.v. produced plasma concentrations consistent with neuroprotective levels (as indicated by preclinical animal studies) (Juul et al. 1999, 2004; Brines et al. 2000; Kellert et al. 2007; Statler et al. 2007; Rees et al. 2010). Only one study has investigated dosing of EPO in preterm infants, and demonstrated that 1000 and 2500 IU kg−1 EPO reached neuroprotective plasma levels (Juul et al. 2008). Clinical trials have used doses up to 3000 IU kg−1 for neuroprotection in preterm infants (Fauchere et al. 2008). In our experiment we administered a high dose of 5000 IU kg−1 EPO within the first minutes of ventilation to prevent the inflammatory cascade caused by high VT ventilation. We have previously demonstrated that 5000 IU kg−1 reduced the inflammatory response of preterm fetal lambs exposed to bolus doses of lipopolysaccharide (Rees et al. 2010). Our data show a single dose of 5000 IU kg−1 in preterm lambs resulted in plasma concentrations of EPO consistent with neuroprotective levels (Juul et al. 2004), by 10 min, but was twice that observed in preterm infants (Juul et al. 2008). Our data suggest that such levels do not confer lung protection. A study in preterm infants that used a low-dose strategy (250–300 IU kg−1 per dose, s.c., three times a week for 2 weeks) to decrease the requirement for early blood transfusions, rather than for lung or brain protection, found an unexpected reduction in BPD (Rayjada et al. 2012). Our findings and these clinical data suggest possible differential effects of EPO dose, but there are insufficient data upon which to base such a conclusion. The beneficial effect of low-dose EPO on the incidence of BPD may be due to its positive effects on vasculogenesis/angiogenesis (Foster et al. 2004; Ozer et al. 2005). Clearly, further research is required to determine optimal dosing of EPO in neonates.
Given that there is no current treatment or cure for BPD, prevention is the best approach to reduce its considerable adverse short- and long-term consequences. Indeed, the one study that demonstrated a reduction in BPD after EPO administration in humans showed benefit only if EPO was given early after birth (Fauchere et al. 2008). This was our rationale for administering EPO within the first 5 min of ventilation onset to try and reduce or prevent lung injury and the accompanying pulmonary and systemic inflammatory cascade. Clinical trials concluded that EPO is safe if administered at 24 h in preterm infants (Juul et al. 2008; McAdams et al. 2013) and only one clinical trial has evaluated the safety of high-dose EPO in preterm infants within the first hours after birth (Fauchere et al. 2008). A safety trial comparing administration of three injections of high-dose EPO (3000 IU kg−1 per dose) or placebo commencing 3 h after birth to preterm infants of ∼28 weeks’ gestation concluded there were no adverse side effects of EPO administration at this time (Fauchere et al. 2008). However, of the infants who received EPO, two died from severe respiratory failure and 48% had a patent ductus arteriosus requiring treatment (compared with 27% in the placebo group). Furthermore, white blood cell count at day 8 was 48% higher in the EPO group (15,615 ± 73 cells μl−1) than in the placebo group (10,535 ± 244 cells μl−1) (Fauchere et al. 2008). These observations show adverse effects of acute high-dose EPO on inflammation and cardiorespiratory outcomes consistent with our experimental observations.
Early EPO administration may be harmful because of its interaction with other processes occurring soon after birth. Bolus high-dose EPO (300 IU kg−1 per dose) increased tumour necrosis factor α (TNF-α), IL-6 and IL-1β, and aggravated endotoxic shock-induced markers of organ injury in conscious rats (Wu et al. 2010), suggesting that EPO may amplify existing inflammatory processes. Mechanical ventilation of preterm neonates results in an acute pulmonary inflammatory response that is maximal within the first few hours, but mRNA levels largely resolve within 6–24 h (Hillman et al. 2011). Therefore, EPO administration during this initial inflammatory response to mechanical ventilation after birth may amplify lung inflammation and injury. It would therefore appear that EPO therapy to prevent lung inflammation should be restricted to times later than the acute inflammatory cascade caused by ventilation. Regarding dose, the optimal timing of EPO administration to neonates is unknown.
To our knowledge, this is the first study to investigate an interaction between EPO administration and ventilation at birth in preterm neonates. Given the large number of extremely preterm infants who receive some form of respiratory support in the delivery room, we consider it critical to establish the safety and efficacy of EPO under these conditions. We used a high VT strategy that causes significant lung inflammation and injury in preterm lambs (Hillman et al. 2007; Polglase et al. 2008, 2010). While it is unlikely that infants would receive such injurious ventilation for this amount of time, it is evident that a significant proportion of preterm infants inadvertently receive high VT during positive pressure ventilation in the delivery room (Schmolzer et al. 2010; Poulton et al. 2011). Furthermore, studies have demonstrated that the initiation of ventilation resulting in pulmonary inflammation initiates a systemic inflammatory cascade (Chiumello et al. 1999; Hillman et al. 2007; Polglase et al. 2009; Quilez et al. 2011), resulting in multi-organ inflammation and injury (Quilez et al. 2011, 2012; Lopez-Aguilar et al. 2010), including in the preterm brain (Polglase et al. 2012a,b2012b). Term and late preterm infants have an acute systemic inflammatory response to ventilation, evident by increased plasma pro-inflammatory cytokines (Bohrer et al. 2010). We found that mRNA for the acute phase protein SAA-3 in the liver was significantly increased by ventilation and was further amplified with EPO administration. This systemic response is consistent with the increase in white blood cells after early EPO in preterm infants (Fauchere et al. 2008). Together, these findings suggest that early EPO administration can elicit a systemic inflammatory response. Given the demonstrated link between systemic inflammation and brain white matter injury in preterm infants (Volpe, 1998; Khwaja & Volpe, 2008), it is possible that early prophylactic high-dose EPO in ventilated preterm infants may in fact increase white matter injury rather than be neuroprotective.
The cause of the adverse response of the preterm lung to EPO in this study is not known. The fetal lung expresses the erythropoietin receptor (EPOR) from 6 weeks after conception (Juul et al. 1998), where it is localised to round interstitial cells, some of which were macrophages. A similar study in postnatal dogs found EPOR localised to discrete interstitial cells scattered throughout the lung parenchyma and also in the airway epithelium: in 10–20% EPOR was co-localized with a monocyte/macrophage marker (Foster et al. 2004). In activated macrophages, EPO impairs the formation of pro-inflammatory factors, including IL-6, by reducing activation of the NF-κB pathway (Nairz et al. 2011). Consistent with this, EPO administration reduced pulmonary inflammation by suppressing TNF and IL-1β during acute endotoxaemia in rats (Shang et al. 2009). However, EPOR activation on retinal vessels increased the NF-κB pathway in a mouse model of retinopathy (Chen et al. 2008), suggesting that EPO can be pro-inflammatory in some instances. Blood transfusion of packed red blood cells to extremely low birth weight infants may also increase pulmonary inflammation and increase BPD risk (Valieva et al. 2009). In our study, both groups received the same volume of either EPO or saline. It is unlikely that erythropoiesis would be stimulated to a significant extent within the 2 h time frame of this study.
In summary, we demonstrated that administration of high-dose EPO during an injurious ventilation strategy resulted in an amplification of lung inflammation and injury, with a subsequent amplification of systemic inflammation. These findings suggest that the early administration of EPO to ventilated preterm neonates may aggravate organ injury, particularly if given during an acute inflammatory response. We believe consideration should be given to the infants’ inflammatory status before high-dose EPO is given.
Acknowledgments
None Declared.
Glossary
- AaDO2
alveolar–arterial difference in oxygen
- BPD
bronchopulmonary dysplasia
- EPO
erythropoietin
- EPOR
erythropoietin receptor
- H&E
haematoxylin and eosin
- IL
interleukin
- PEEP
positive end-expiratory pressure
- PIP
peak inspiratory pressure
- qRT-PCR
quantitative real-time PCR
- SAA
serum amyloid A3
- UVC
unventilated control
- VEI
ventilatory efficiency index
- VILI
ventilation-induced lung injury
- VT
tidal volume
Key points
Erythropoietin (EPO) has been suggested as a potential treatment for bronchopulmonary dysplasia (BPD) in preterm infants.
Ventilation-induced lung injury (VILI) is a major cause of BPD in preterm neonates. We investigated whether early high-dose EPO (i.v. 5000 IU kg−1) administration can reduce lung inflammation and injury resultant from VILI in ventilated preterm lambs.
Early high-dose EPO administration increased mRNA expression of early markers of lung inflammation and injury and systemic injury controls.
Early high-dose EPO worsened histological assessment of inflammation, airway wall thickness, haemorrhage and total injury compared to controls.
Early high-dose EPO may increase the incidence and severity of respiratory disease in ventilated, preterm neonates.
Additional information
Conflicts of interest
None of the authors have any conflicts of interests to disclose.
Author contributions
All authors contributed to the conception and design or analysis and interpretation of data, drafting and revising the manuscript and gave final approval of the version to be published.
Funding
This research project was supported by an AVANT Innovative Research Grant awarded by the Research Foundation of Cerebral Palsy Alliance, NH&MRC Research Fellowships (G.R.P.: 1026890 and T.J.M.: 10043294) and Project grant (1021702), a Rebecca L. Cooper Medical Research Foundation Fellowship (G.R.P.) and the Victorian Government's Operational Infrastructure Support Program.
References
- Bjorklund LJ, Ingimarsson J, Curstedt T, John J, Robertson B, Werner O. Vilstrup CT. Manual ventilation with a few large breaths at birth compromises the therapeutic effect of subsequent surfactant replacement in immature lambs. Pediatr Res. 1997;42:348–355. doi: 10.1203/00006450-199709000-00016. [DOI] [PubMed] [Google Scholar]
- Bohrer B, Silveira RC, Neto EC. Procianoy RS. Mechanical ventilation of newborns infant changes in plasma pro- and anti-inflammatory cytokines. J Pediatr. 2010;156:16–19. doi: 10.1016/j.jpeds.2009.07.027. [DOI] [PubMed] [Google Scholar]
- Bose CL, Laughon MM, Allred EN, O'Shea TM, Van Marter LJ, Ehrenkranz RA, Fichorova RN. Leviton A. Systemic inflammation associated with mechanical ventilation among extremely preterm infants. Cytokine. 2013;61:315–322. doi: 10.1016/j.cyto.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, Itri LM. Cerami A. Erythropoietin crosses the blood–brain barrier to protect against experimental brain injury. Proc Natl Acad Sci U S A. 2000;97:10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Connor KM, Aderman CM. Smith LE. Erythropoietin deficiency decreases vascular stability in mice. J Clin Invest. 2008;118:526–533. doi: 10.1172/JCI33813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiumello D, Pristine G. Slutsky AS. Mechanical ventilation affects local and systemic cytokines in an animal model of acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160:109–116. doi: 10.1164/ajrccm.160.1.9803046. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in the Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmahdy H, El-Mashad AR, El-Bahrawy H, El-Gohary T, El-Barbary A. Aly H. Human recombinant erythropoietin in asphyxia neonatorum: pilot trial. Pediatrics. 2010;125:e1135–1142. doi: 10.1542/peds.2009-2268. [DOI] [PubMed] [Google Scholar]
- Fauchere JC, Dame C, Vonthein R, Koller B, Arri S, Wolf M. Bucher HU. An approach to using recombinant erythropoietin for neuroprotection in very preterm infants. Pediatrics. 2008;122:375–382. doi: 10.1542/peds.2007-2591. [DOI] [PubMed] [Google Scholar]
- Foster DJ, Moe OW. Hsia CC. Upregulation of erythropoietin receptor during postnatal and postpneumonectomy lung growth. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1107–1115. doi: 10.1152/ajplung.00119.2004. [DOI] [PubMed] [Google Scholar]
- Gonzalez FF, Abel R, Almli CR, Mu D, Wendland M. Ferriero DM. Erythropoietin sustains cognitive function and brain volume after neonatal stroke. Dev Neurosci. 2009;31:403–411. doi: 10.1159/000232558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman NH, Kallapur SG, Pillow JJ, Moss TJ, Polglase GR, Nitsos I. Jobe AH. Airway injury from initiating ventilation in preterm sheep. Pediatr Res. 2010;67:60–65. doi: 10.1203/PDR.0b013e3181c1b09e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman NH, Moss TJ, Kallapur SG, Bachurski C, Pillow JJ, Polglase GR, Nitsos I, Kramer BW. Jobe AH. Brief, large tidal volume ventilation initiates lung injury and a systemic response in fetal sheep. Am J Respir Crit Care Med. 2007;176:575–581. doi: 10.1164/rccm.200701-051OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman NH, Polglase GR, Jane Pillow J, Saito M, Kallapur SG. Jobe AH. Inflammation and lung maturation from stretch injury in preterm fetal sheep. Am J Physiol Lung Cell Mol Physiol. 2011;300:L232–241. doi: 10.1152/ajplung.00294.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami M, Kallapur SG. Jobe AH. Initial responses to ventilation of premature lambs exposed to intra-amniotic endotoxin 4 days before delivery. Am J Physiol Lung Cell Mol Physiol. 2004;286:L573–579. doi: 10.1152/ajplung.00211.2003. [DOI] [PubMed] [Google Scholar]
- Juul S. Neuroprotective role of erythropoietin in neonates. J Matern Fetal Neonatal Med. 2012;25(Suppl 4):105–107. doi: 10.3109/14767058.2012.715025. [DOI] [PubMed] [Google Scholar]
- Juul SE, McPherson RJ, Bauer LA, Ledbetter KJ, Gleason CA. Mayock DE. A phase I/II trial of high-dose erythropoietin in extremely low birth weight infants: pharmacokinetics and safety. Pediatrics. 2008;122:383–391. doi: 10.1542/peds.2007-2711. [DOI] [PubMed] [Google Scholar]
- Juul SE, McPherson RJ, Farrell FX, Jolliffe L, Ness DJ. Gleason CA. Erytropoietin concentrations in cerebrospinal fluid of nonhuman primates and fetal sheep following high-dose recombinant erythropoietin. Biol Neonate. 2004;85:138–144. doi: 10.1159/000074970. [DOI] [PubMed] [Google Scholar]
- Juul SE, Stallings SA. Christensen RD. Erythropoietin in the cerebrospinal fluid of neonates who sustained CNS injury. Pediatr Res. 1999;46:543–547. doi: 10.1203/00006450-199911000-00009. [DOI] [PubMed] [Google Scholar]
- Juul SE, Yachnis AT. Christensen RD. Tissue distribution of erythropoietin and erythropoietin receptor in the developing human fetus. Early Hum Dev. 1998;52:235–249. doi: 10.1016/s0378-3782(98)00030-9. [DOI] [PubMed] [Google Scholar]
- Kellert BA, McPherson RJ. Juul SE. A comparison of high-dose recombinant erythropoietin treatment regimens in brain-injured neonatal rats. Pediatr Res. 2007;61:451–455. doi: 10.1203/pdr.0b013e3180332cec. [DOI] [PubMed] [Google Scholar]
- Khwaja O. Volpe JJ. Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed. 2008;93:F153–161. doi: 10.1136/adc.2006.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Takemura G, Okada H, Miyata S, Maruyama R, Li L, Higuchi M, Minatoguchi S, Fujiwara T. Fujiwara H. Reduction of inflammatory cytokine expression and oxidative damage by erythropoietin in chronic heart failure. Cardiovasc Res. 2006;71:684–694. doi: 10.1016/j.cardiores.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Liu W, Shen Y, Plane JM, Pleasure DE. Deng W. Neuroprotective potential of erythropoietin and its derivative carbamylated erythropoietin in periventricular leukomalacia. Exp Neurol. 2011;230:227–239. doi: 10.1016/j.expneurol.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Aguilar J, Quilez ME, MartíSistac O, García-Martín C, Fuster G, Puig F, Flores C, Villar J, Artigas A. Blanch L. Early physiological and biological features in three animal models of induced acute lung injury. Intensive Care Med. 2010;36:347–355. doi: 10.1007/s00134-009-1695-x. doi: 10.1007/s00134-009-1695-x. [Epub 2009 Oct 17] [DOI] [PubMed] [Google Scholar]
- McAdams RM, McPherson RJ, Mayock DE. Juul SE. Outcomes of extremely low birth weight infants given early high-dose erythropoietin. J Perinatol. 2013;33:226–230. doi: 10.1038/jp.2012.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson RJ. Juul SE. Erythropoietin (Epo) for infants with hypoxic-ischemic encephalopathy (HIE) Curr Opin Pediatr. 2010;22:139–145. doi: 10.1097/MOP.0b013e328336eb57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairz M, Schroll A, Moschen AR, Sonnweber T, Theurl M, Theurl I, Taub N, Jamnig C, Neurauter D, Huber LA, Tilg H, Moser PL. Weiss G. Erythropoietin contrastingly affects bacterial infection and experimental colitis by inhibiting nuclear factor-κB-inducible immune pathways. Immunity. 2011;34:61–74. doi: 10.1016/j.immuni.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer EA, Kumral A, Ozer E, Yilmaz O, Duman N, Ozkal S, Koroglu T. Ozkan H. Effects of erythropoietin on hyperoxic lung injury in neonatal rats. Pediatr Res. 2005;58:38–41. doi: 10.1203/01.PDR.0000163391.75389.52. [DOI] [PubMed] [Google Scholar]
- Polglase GR, Hillman NH, Ball MK, Kramer BW, Kallapur SG, Jobe AH. Pillow JJ. Lung and systemic inflammation in preterm lambs on continuous positive airway pressure or conventional ventilation. Pediatr Res. 2009;65:67–71. doi: 10.1203/PDR.0b013e318189487e. [DOI] [PubMed] [Google Scholar]
- Polglase GR, Hillman NH, Pillow JJ, Cheah FC, Nitsos I, Moss TJ, Kramer BW, Ikegami M, Kallapur SG. Jobe AH. Positive end-expiratory pressure and tidal volume during initial ventilation of preterm lambs. Pediatr Res. 2008;64:517–522. doi: 10.1203/PDR.0b013e3181841363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polglase GR, Hillman NH, Pillow JJ, Nitsos I, Newnham JP, Knox CL, Kallapur SG. Jobe AH. Ventilation-mediated injury after preterm delivery of Ureaplasma parvum colonized fetal lambs. Pediatr Res. 2010;67:630–635. doi: 10.1203/PDR.0b013e3181dbbd18. [DOI] [PubMed] [Google Scholar]
- Polglase GR, Kluckow M, Gill AW, Allison BJ, Moss TJ, Dalton RG, Pillow JJ, Andersen CC, Nitsos I. Hooper SB. Cardiopulmonary haemodynamics in lambs during induced capillary leakage immediately after preterm birth. Clin Exp Pharmacol Physiol. 2011;38:222–228. doi: 10.1111/j.1440-1681.2011.05489.x. [DOI] [PubMed] [Google Scholar]
- Polglase GR, Miller SL, Barton SK, Baburamani AA, Wong FY, Aridas JD, Gill AW, Moss TJ, Tolcos M, Kluckow M. Hooper SB. Initiation of resuscitation with high tidal volumes causes cerebral hemodynamic disturbance, brain inflammation and injury in preterm lambs. PLoS One. 2012a;7:e39535. doi: 10.1371/journal.pone.0039535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polglase GR, Nitsos I, Baburamani AA, Crossley KJ, Slater MK, Gill AW, Allison BJ, Moss TJ, Pillow JJ, Hooper SB. Kluckow M. Inflammation in utero exacerbates ventilation-induced brain injury in preterm lambs. J Appl Physiol. 2012b;112:481–489. doi: 10.1152/japplphysiol.00995.2011. [DOI] [PubMed] [Google Scholar]
- Poulton DA, Schmolzer GM, Morley CJ. Davis PG. Assessment of chest rise during mask ventilation of preterm infants in the delivery room. Resuscitation. 2011;82:175–179. doi: 10.1016/j.resuscitation.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Quilez ME, Fuster G, Villar J, Flores C, Marti-Sistac O, Blanch L. Lopez-Aguilar J. Injurious mechanical ventilation affects neuronal activation in ventilated rats. Crit Care. 2011;15:R124. doi: 10.1186/cc10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quilez ME, Lopez-Aguilar J. Blanch L. Organ crosstalk during acute lung injury, acute respiratory distress syndrome, and mechanical ventilation. Curr Opin Crit Care. 2012;18:23–28. doi: 10.1097/MCC.0b013e32834ef3ea. [DOI] [PubMed] [Google Scholar]
- Rayjada N, Barton L, Chan LS, Plasencia S, Biniwale M. Bui KC. Decrease in incidence of bronchopulmonary dysplasia with erythropoietin administration in preterm infants: a retrospective study. Neonatology. 2012;102:287–292. doi: 10.1159/000341615. [DOI] [PubMed] [Google Scholar]
- Rees S, Hale N, De Matteo R, Cardamone L, Tolcos M, Loeliger M, Mackintosh A, Shields A, Probyn M, Greenwood D. Harding R. Erythropoietin is neuroprotective in a preterm ovine model of endotoxin-induced brain injury. J Neuropathol Exp Neurol. 2010;69:306–319. doi: 10.1097/NEN.0b013e3181d27138. [DOI] [PubMed] [Google Scholar]
- Schmolzer GM, Kamlin OC, O'Donnell CP, Dawson JA, Morley CJ. Davis PG. Assessment of tidal volume and gas leak during mask ventilation of preterm infants in the delivery room. Arch Dis Child Fetal Neonatal Ed. 2010;95:F393–397. doi: 10.1136/adc.2009.174003. [DOI] [PubMed] [Google Scholar]
- Shang Y, Li X, Prasad PV, Xu S, Yao S, Liu D, Yuan S. Feng D. Erythropoietin attenuates lung injury in lipopolysaccharide treated rats. J Surg Res. 2009;155:104–110. doi: 10.1016/j.jss.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Statler PA, McPherson RJ, Bauer LA, Kellert BA. Juul SE. Pharmacokinetics of high-dose recombinant erythropoietin in plasma and brain of neonatal rats. Pediatr Res. 2007;61:671–675. doi: 10.1203/pdr.0b013e31805341dc. [DOI] [PubMed] [Google Scholar]
- Valieva OA, Strandjord TP, Mayock DE. Juul SE. Effects of transfusions in extremely low birth weight infants: a retrospective study. J Pediatr. 2009;155:331–337. doi: 10.1016/j.jpeds.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe JJ. Brain injury in the premature infant: overview of clinical aspects, neuropathology, and pathogenesis. Semin Pediatr Neurol. 1998;5:135–151. doi: 10.1016/s1071-9091(98)80030-2. [DOI] [PubMed] [Google Scholar]
- Wada K, Jobe AH. Ikegami M. Tidal volume effects on surfactant treatment responses with the initiation of ventilation in preterm lambs. J Appl Physiol. 1997;83:1054–1061. doi: 10.1152/jappl.1997.83.4.1054. [DOI] [PubMed] [Google Scholar]
- Wallace MJ, Probyn ME, Zahra VA, Crossley K, Cole TJ, Davis PG, Morley CJ. Hooper SB. Early biomarkers and potential mediators of ventilation-induced lung injury in very preterm lambs. Respir Res. 2009;10:19. doi: 10.1186/1465-9921-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YJ, Pan KL, Zhao XL, Qiang H. Cheng SQ. [Therapeutic effects of erythropoietin on hypoxic-ischemic encephalopathy in neonates] Zhongguo Dang Dai Er Ke Za Zhi. 2011;13:855–858. (in Chinese) [PubMed] [Google Scholar]
- Wu WT, Hu TM, Lin NT, Subeq YM, Lee RP. Hsu BG. Low-dose erythropoietin aggravates endotoxin-induced organ damage in conscious rats. Cytokine. 2010;49:155–162. doi: 10.1016/j.cyto.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Wu YW, Bauer LA, Ballard RA, Ferriero DM, Glidden DV, Mayock DE, Chang T, Durand DJ, Song D, Bonifacio SL, Gonzalez FF, Glass HC. Juul SE. Erythropoietin for neuroprotection in neonatal encephalopathy: safety and pharmacokinetics. Pediatrics. 2012;130:683–691. doi: 10.1542/peds.2012-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]


