Abstract
Eosinophilic esophagitis (EoE) is a recently recognized allergic disorder, characterized by eosophageal dysfunction, accumulation of ≥15 eosinophils/high-powered field, eosinophil microabssess, basal cell hyperplasia, extracellular eosinophilic granules in the esophageal epithelial mucosal biopsy and a lack of response to a 8-week proton pump inhibitor treatment. Despite the increased incidences and considerable progress made in understanding EoE pathogenesis, there are limited diagnostic and therapeutic options available for EoE. Currently, the only criterion for diagnosing EoE is repetitive esophageal endoscopic biopsies and histopathological evaluation. Antigen elimination or corticosteroid therapies are effective therapies for EoE but are expensive and have limitations, if continued in the long term. Hence, there is a great necessity for novel noninvasive diagnostic biomarkers that can easily diagnose EoE and assess effectiveness of therapy. Herein, we have provided an update on key molecules involved in the disease initiation, and progression and proposed novel noninvasive diagnostic molecules and strategies for EoE therapy.
Keywords: basophils, eosinophil, esophagitis, interleukin, invariant natural killer T cells, mast cell
Eosinophilic esophagitis (EoE, earlier also referred as EE) was first described in 1978 [1]. However, EoE was clinically recognized in 1994 [2], broadly accepted with defined characteristics and well-accepted nomenclature in 2007 [3], then further updated in 2011 [4]. EoE pathogenesis is still not well understood. In the past, patients with EoE were incorrectly diagnosed as gastroesophageal reflux disease (GERD). EoE is an allergen-induced chronic inflammatory esophageal disorder that is characterized by: symptoms of esophageal dysfunction; the infiltration of large numbers of eosinophils (≥15 eosinophils/high-powered field) in the epithelial mucosa of the esophagus, which is typically devoid of eosinophils at baseline in healthy state [5,6]; and absence of response to a 8-week proton-pump inhibitor (PPI) trial [4]. While EoE was previously thought to be a rare disease, now it is recognized as one of the most common causes of difficulty swallowing and food impaction in the pediatric population. This has become a global trend with increased cases of EoE being reported from almost each continent of the world from developed to developing countries [3,7–8]. Despite the increased incidence of EoE, it has limited, established diagnostic criteria based on esophageal biopsies and pathological characteristics, and at least 8 weeks of adequate dosages of PPIs to block gastric acid secretion to exclude GERD and PPI-responsive esophageal eosinophilia [3,9]. The major clinical symptoms of EoE in adults are difficulty in swallowing solid food (dysphagia) and food impaction including heartburn and chest pain as less common symptoms. In children, the most common symptoms are abdominal pain, nausea, vomiting, coughing and failure to thrive. The clinical symptoms of EoE mimics GERD, such as heartburn, swallowing problem, food refusal, dysphagia, and food impaction [3,10]. These similarities often make the diagnosis more difficult [3,10]. Lack of response to high-dose PPI therapy or normal pH monitoring in the GI tract differentiates EoE to PPI-responsive esophageal eosinophilia (REE) and GERD [4,9].
The histological analysis of the esophageal biopsies in EoE [11,12] demonstrate that a number of leukocytes infiltrate in the esophageal mucosa including eosinophils, mast cells [13–16], basophils [17] and T-cell subsets, such as CD4+, CD8+ and invariant natural killer T (iNKT) cells [18]. These inflammatory cells promote proliferation of the esophageal epithelial cells [3,19] and collagen deposition that causes tissue remodeling and fibrosis [20–24]. In addition, thickened muscularis mucosa, muscular hypertrophy, esophageal motility dysfunction and stricture are also reported in experimental and human EoE [10,23,25]. Stricture formation is a major complication of EoE. Recent studies of large EoE cohort suggest the progression of EoE with time from an inflammatory to a fibrostenotic disease [24]. These studies suggest that patients develop fibrotic endoscopic features late in the disease course, in addition to purely inflammatory endoscopic features of EoE that are present in early disease stages [23,24]. Furthermore, there is significant association of risk of development of esophageal stricture with the delay in diagnosis of EoE [23].
Eosinophils, mast cells and basophils are considered to be major effector cells of tissue fibrosis [26] in a variety of other inflammatory cell-associated allergic diseases and hypereosinophilic syndromes including asthma [27], eosinophil myalgia syndrome [28,29], eosinophilic endomyocardial fibrosis [30,31], idiopathic pulmonary fibrosis [32–36] and scleroderma [28,37–38]. Both human and animal model studies provide compelling evidence for eosinophils as an effector cell for tissue remodeling and fibrosis. Studies with respective cell-deficient mice provide direct evidence for the induction of remodeling and fibrosis by these inflammatory cells. A number of studies demonstrated the essential role of these cells in the development of airway remodeling, including mucus (goblet) cell metaplasia, smooth muscle cell hyperplasia and subepithelial fibrosis [39–41]. Additionally, multiple growth factors and cytokines expressed by these inflammatory cells [42,43] are implicated in tissue remodeling and fibrosis. Eosinophils and mast cells are a major source of a highly fibrogenic growth factor, TGF-β, both in the asthmatic lungs and in the esophagus of EoE patients. TGF-β-induced fibroblasts are implicated in the over-production of collagens, tissue inhibitors of metallo-proteinases, and glycosaminoglycans in fibrogenesis [44,45].
Of note, studies of eosinophil-mediated tissue remodeling and fibrosis in EoE are very much limited, due to the difficulties obtaining sufficient biopsies containing esophageal lamina propria below the stiffened hyperplastic epithelium. However, the evidence for progressive remodeling and fibrosis of the esophagus is well documented by endoscopic and radiologic features of the disease [46]. Even with the limited tissues obtained from biopsies, our previous studies, as well as others [20,25], have demonstrated that pediatric EoE esophageal biopsies show increased levels of subepithelial fibrosis, induced expression of profibrotic cytokine and increased expression of TGF-β1 and its signaling molecule phospho-SMAD2/3 compared with the normal control subjects [20]. Furthermore, the genome-wide expression profiling of EoE esophageal biopsies identified increased expression of eotaxin-3 (mediator of eosinophil recruitment) [47] and periostin (a gene that participates in tissue remodeling and fibrosis). Collagen-rich fibrous connective tissues that adapt to mechanical stress and are involved in wound healing, mainly express periostin. The role of periostin in the development of subepithelial fibrosis in bronchial asthma has also been reported as downstream to the IL-4 and −13 signaling [48]. Periostin is expressed in esophageal vascular papillae and is secreted from primary esophageal fibroblasts when cultured with IL-13 and TGF-β [49].
The field of EoE research has expanded greatly in recent years, but mostly restricted to its pathogenesis [10,47], including esophageal remodeling [20,23–25]. Estimated occurrences of EoE in pediatric and adults population are 43–57 per 100,000 people (similar to that of Crohn’s disease in pediatric population – a well recognized gastrointestinal disorder) [50–52]. Earlier, our and other investigators’ efforts have made significant progress in understanding EoE pathogenesis; however, novel therapy is still lacking. A diet consisting exclusively of an elemental (amino acid-based) formula frequently improves symptoms and normalizes esophageal pathology [9,53–59]; however, the approach is often intolerable and expensive for patients. Systemic steroids are used for acute exacerbations while topical glucocorticoids are used to provide long-term control [60,61]. The anti-IL-5 antibody therapy is also not promising as it was initially thought. This may be because IL-5 is only a survival factor for eosinophils but not an initiator of human EoE. As indicated, eotaxin-3 is a highly induced gene in human EoE [47]; however, eotaxin-3 is absent in the mouse genome and its critical role in the experimental model of EoE cannot be tested. Moreover, even if, eotaxin-3 can be blocked by the anti-eotaxin-3 neutralizing antibody in patients, there is a possibility that eotaxin-1 and eotaxin-2 may compensate for the activity of eotaxin-3. Furthermore, the anti-IL-13 antibody therapy might also not work effectively in human, as we have recently shown that IL-13 is not critical for allergen-induced experimental EoE [62]. These concerns highlight the importance of uncovering other potential target molecules for EoE therapy. In the present review, we provide a detailed overview of the current understanding of the pathogenesis, diagnosis and therapeutic strategy for EoE.
Significance of food & aeroallergens in EoE pathogenesis
Allergy is characterized by an over-reaction of the human immune system to a foreign protein substance (‘allergen’) that is eaten, inhaled into the lungs, injected or touched. An estimated 50 million Americans suffer from all types of allergies including indoor/outdoor (aeroallergens), food and drug, latex, insect, skin and eye allergies. Approximately 6% of allergy sufferers have food/drug allergies and approximately 40 million Americans have indoor/outdoor (aeroallergens) allergies as their primary allergy. Food allergy is more common among children than adults. Milk, soy, eggs, wheat, peanuts, tree nuts, fish and shellfish cause 90% of all food allergy reactions. The most common aeroallergens are: tree, grass, and weed pollen; mold spores; dust mite and cockroach allergen; and cat, dog and rodent dander.
EoE patients have the evidence of both food and aeroallergen hypersensitivity, and a relatively large fraction have food anaphylaxis [63]. Food allergies affect an estimated 6% of children and 3.7% of adults in the USA [8,64–65]. During the past decade, our understanding of food allergic diseases and manifestations has substantially increased. Recent literature on pediatric and adult patients with EoE confirms that nearly all patients respond to an elemental diet with resolution of symptoms and normalization of biopsies [53,66–67]. Presence of seasonal variation in esophageal eosinophils and in symptoms of EoE suggests the role of aeroallergens in the pathogenesis of EoE in addition to food allergens [66,68]. Furthermore, several studies with human subjects and animal models confirm the link between EoE with aeroallergens and allergies that suggests the involvement of the sensitization pathway and antigen-presenting cells in the pathogenesis of EoE [69–72]. Initial studies suggest a linkage of an average of three to six foods per patient for development of EoE. But current studies with a high response rate of elemental diet (96%) versus six-food elimination diet (SFED; 70%) treatment suggest that food allergens are causative agents for the development of EoE [73–76]. However, some human studies suggest the involvement of aeroallergens and the possibility of crossreactivity of aeroallergens with food antigens for development of EoE [65,68–69,71–72,77]. These observations provide evidence for the involvement of both food and aeroallergens in the pathogenesis of EoE. In addition, the data from murine model also complement the findings of human subjects and suggests that Aspergillus, peanut and dust mite intranasal exposure induces pathogenesis of EoE [5,78–79].
Role of T cells, B cells & IgE in EoE pathogenesis
The analysis of esophageal biopsies of EoE patients [80–83] or experimental allergen-induced EoE [84] of mice provides details of cellular infiltrates present in the disease condition versus healthy state. Apart from enhanced infiltration of eosinophils (characteristic feature of EoE) in the esophagus, the presence of a large number of other immune cells, including intraepithelial T-cell subsets such as CD3+, CD4+, and CD8+, are also reported in the esophageal biopsies of EoE patients [81]. Furthermore, there were increased percentages of peripheral blood CD4+ T cells expressing IL-5 in active EoE patients compared with nonatopic control children. In this study, the percentage of IL-5+ CD4+ T cells in EoE was correlated with the degree of tissue eosinophilia. Additionally, the percentages of peripheral blood eosinophils, eosinophil CCR3 expression, and percentage of CD4+ T cells are significantly lower in patients with improved EoE than in patients with active EoE [82]. These data provide strong evidence that CD4+ IL-5+ T cells contribute to EoE pathogenesis. B cells are also present in enhanced numbers in the esophageal mucosa of EoE [80,83] and GERD patients [81,83]. Interestingly, normal subjects have no intraepithelial B cells [80,83]. Surprisingly, the intraepithelial B cell number correlates well with mast cells, but not with eosinophils [80]. In a subset of EoE patients, B-cell class switch to IgE was also observed [80].
Furthermore, to understand the function(s) of these cells in the development of the pathogenesis of EoE, Mishra and colleagues have developed a murine model of allergen-induced EoE [84]. The murine model of EoE mimics most of the pathophysiological changes observed in human subjects with various forms of EoE features characterized by intraepithelial eosinophils, extracellular granule deposition and epithelial cell hyperplasia [84]. However, murine models of EoE have a few features that are not similar with human EoE (Table 1). Using this murine model, they demonstrated that esophageal eosinophils and eosinophil-specific Th2 cytokines (IL-5, IL-4 and IL-13) play an important role in disease pathogenesis [10,18]. The observation that following allergen exposure there is enhanced number of B cells, CD4+ and CD8+ in the esophagus implicates the role of adaptive T-cell immunity in initiating experimental EoE. Studies with the RAG1 gene-deficient mice, B-cell-deficient (IgH6−/−) mice and T-cell-deficient (forkhead box N1−/−; Foxn1−/−) confirmed that T cells but not B cells are responsible in the pathogenesis of EoE that was assessed by ablation of eosinophils and epithelial cell proliferation in the esophagus [18]. It is likely that B cells have some role in EoE pathogenesis, although the data suggest that they are not critically involved in the development of disease pathogenesis. Data from CD8α-deficient mice, and CD4-deficient mice provide additional evidence that there is a partial role of CD4+ T cells but no role of CD8+ T or B cells in the experimental model of EoE.
Table 1.
Similarities and dissimilarities between features of human and murine eosinophilic esophagitis.
| Serial number | Characteristic features of EoE | Human | Mouse |
|---|---|---|---|
| 1 | History of atopy | + | NF |
| 2 | Proton pump inhibitor response | − | NF |
| 3 | History of food impaction | + | NF |
| 4 | Endoscopic findings: linear furrowing, loss of vascularity, ring-like structures, and the presence of white exudate on the esophageal epithelium | + | NP |
| 5 | Intraepithelial eosinophils | ≥15 eosinophils/high-powered field | ~40 eosinophils/mm2 |
| 6 | Eosinophilic microabscesses, | + | + |
| 7 | Basal zone hyperplasia, papillary elongation | + | +/− |
| 8 | Esophageal strictures in chronic EoE | + | + |
| 9 | Extracellular granules | + | + |
+: Present; −: Absent; +/−: Present or absent; EoE: Eosinophilic esophagitis; NF: Not found; NP: Not possible.
Both clinical studies and murine models provide evidence for the strong association between EoE and atopy [4,85–88]. Most of the adult and pediatric EoE patients (50–60%) have prior history of atopy and an additional allergic disease [68,77,89–92]. However, IgE-mediated hypersensitivity is reported only in 15–43% of EoE patients. IgE plays an essential role in atopic disorders, [93,94]; however, its role in EoE is still unclear. Sometimes, EoE patients have another IgE-mediated disorder of the airway or GI tract [95]. Immunohistological analyses of esophageal biopsies have shown increased numbers of IgE-positive cells only in some EoE patients, [80,96] but not in normal subjects, [83] GERD patients [81] and EoE subjects on fluticasone therapy [81]. IgE-positive cells are identified as mast cells (c-kit+IgE+) and IgE-secreting B cells (IgE+ckit−) in these EoE patients [80]. However, this is an indirect evidence for the presence of IgE-secreting B cells in EoE [80]. Avoiding food antigens with an amino acid-based elemental diet is an effective treatment in some EoE patients with an established IgE-mediated food allergy. This observation suggests that IgE may have role in the pathophysiology of EoE. It is well known that elemental diets not only remove antigenic peptides but also have additional effects. However, an experimental murine model of EoE demonstrated that an antigen-specific antibody has no role in pathogenesis of EoE [18]. Future studies will be required to fully understand the contribution of IgE in EoE, and to identify potential food or environmental antigens that trigger the IgE-dependent inflammatory response in individual patients.
Role of eosinophil-active cytokines in EoE pathogenesis
Eosinophils are the major cellular infiltrate present in the esophagus in EoE and play a critical role in the pathogenesis of EoE. Eosinophils are multifunctional, immunomodulatory and proinflammatory leukocytes that produce a wide range of inflammatory cytokines (IL-1, IL-3, IL-4, IL-5, IL-13, TNF-α, chemokines (eotaxin), growth factors (GM-CSF, TGF-α, TGF-β) [97–99] and toxic granule components [100–103]. These eosinophilic granules contain a crystalloid core, composed of MBP-1 and MBP-2, ECP, eosinophilic-derived neurotoxin (EDN) and EPO with numerous proinflammatory and cytotoxic properties [5,97,100,103–104]. These eosinophil-secreted products contribute to the development of pathogenesis of EoE, including tissue remodeling. The most important step of EoE pathogenesis is the trafficking of eosinophils to the tissue and is selectively regulated by IL-5. Genome-wide microarray expression profile of esophageal biopsies from EoE subjects identified a striking EoE transcript signature, involving approximately 1% of the human genome. The most highly expressed cytokine gene detected in EoE patients is IL-15 and its receptor IL-15Rα [47]. Our studies with IL-15Rα-deficient mice demonstrated that IL-15 is required for development of allergen-induced esophageal eosinophilia [105]. While IL-5 is a cytokine that is required for eosinophil survival and development, IL-15 is required for the differentiation and survival of T-cell subsets, as well as activation to produce Th2 cytokines including IL-5 [105]. Notably, another inflammatory cytokine induced in EoE patients is IL-13, which causes airway eosinophilia when given intratracheally [21]. However, its role in EoE pathogenesis is not supported by the experimental model of EoE [62]. By contrast, an important role of both IL-5 and IL-15 is shown in the pathogenesis of EoE by the experimental EoE murine models [105]. Furthermore, incubation of primary humans and mice esophageal epithelial cells with IL-15 caused enhanced expression of the transcript and protein levels of eotaxin-1, −2 and −3 on these cells [105]. Eotaxins also selectively participate in esophageal eosinophil recruitment [47].
IL-5 in EoE pathogenesis
Th2 cells mainly produce IL-5 but eosinophils and mast cells also produce IL-5 in chronic allergic reactions. IL-5 is an essential factor for eosinophil differentiation, growth, activation and survival [106]. This cytokine may regulate eosinophil trafficking to the esophagus either by enhancing esophageal eotaxin, or by upregulating homing receptors for eosinophils in the esophagus [106]. Both IL-5 mRNA and protein levels are significantly overexpressed in esophageal epithelial biopsies, and its critical role in development of EoE has been established from various studies [25,83,106]. IL-5-deficient or anti-IL-5 antibody-treated mice demonstrated a marked reduction in esophageal eosinophilia but IL-5 overexpressing mice promoted experimental EoE even in the absence of eotaxin [106]. In addition, IL-5 overexpression-induced eosinophilia promotes esophageal tissue remodeling (a thickened basal layer and collagen accumulation in the lamina propria of esophageal tissue sections) in CD2-IL-5 transgenic mice but not in IL-5-deficient allergen-challenged mice [25]. These histological findings are also supported by several clinical reports [25].
IL-13 in EoE pathogenesis
Earlier reports utilizing murine models and in vitro studies demonstrated that IL-13 appears to be an important component of EoE pathogenesis [21,107]. These earlier studies indicated that intratracheal IL-13 delivery promotes EoE, and anti-human IL-13 antibodies block EoE induction in experimental EoE [21,108]. However, it has also been shown that IL-13-induced EoE is dependent on IL-5, as IL-13 failed to induce EoE in IL-5-deficient mice [21]. The IL-13 mRNA level was markedly increased (16-fold) in esophageal biopsies from EoE patients compared with control individuals. IL-13 also upregulates gene expression of the EoE transcriptome of esophageal biopsy tissues and increases eotaxin-3 expression on human esophageal epithelial cells [109]. In addition, the role of IL-13 in promoting fibrosis, angiogenesis and epithelial cell hyperplasia in IL-13-overexpressed mice is also reported [21]. However, a recent report indicated that IL-13 is not essential, as IL-13-, IL-4/IL-13- or STAT6-deficient mice do not show impaired EoE development following allergen challenge [62]. Therefore, it might be possible that IL-13 has a role in the pathogenesis of EoE but is not critical for the induction/initiation of EoE.
IL-15 in EoE pathogenesis
Most recently, the critical role of IL-15 has been shown in allergen-induced experimental EoE. Genome-wide microarray expression profiling showed increased IL-15 mRNA expression in the esophageal biopsies of EoE patients [47]. IL-15 is a pleiotropic cytokine and is similar in structure to IL-2. Both IL-15 and IL-2 share a number of biological activities, including the ability to stimulate the proliferation and differentiation of activated T cells [110,111]. In addition, IL-15 is required in the maintenance of natural killer (NK) cells and some T-cell subsets, including their activation in an antigen-independent manner [110,111]. This process is believed to contribute to intestinal inflammatory responses, including those found in celiac disease, a disease that shares features with EoE, such as being triggered by food antigens, the involvement of epithelial cells (although squamous epithelium in EoE), and the overexpression of NK cell activation antigens such as the MHC-like molecule MIC [47,112]. Notably, mice deficient in IL-15 or the IL-15 receptor (IL-15R−/−) have defective naive and memory CD8+ T cells, intestinal intraepithelial lymphocytes and NK cells [113]. The quantitative PCR analyses showed that levels of IL-15 and its receptor IL-15Rα were increased in tissues from patients with EoE, as well as in a murine model of EoE. Interestingly, IL-15 mRNA levels correlated with esophageal eosinophilia in human EoE and IL-15 levels reduced in EoE improved patients [105]. Additionally, evidence of the critical role of IL-15 comes from studies where IL-15Rα-deficient mice were protected from allergen-induced esophageal eosinophilia [105]. Furthermore, the IL-15 lung overexpressed mice showed increased esophageal eosinophilia [Mishra A et al., Unpublished Data], which confirms the role of IL-15 in the development of EoE.
Role of eosinophil-active chemokines (eotaxins) in EoE pathogenesis
Eotaxin is an eosinophil-selective CC chemokine, constitutively expressed in the GI tract, and is critical for the maintenance of eosinophils in this tissue [5,97]. Although eotaxin is also expressed in the esophagus but no eosinophils are present in esophagus in healthy state. Eosinophils have enhanced responsiveness to eotaxin when primed with IL-5 [5]. Humans express eotoaxin-1, −2 and −3 while mice only express eotaxin-1 and −2. Mice do not express analogous of human eotaxin-3. Eotaxin-3 (in contrast with eotaxin-1 or eotaxin-2) is specifically overexpressed in EoE in humans [47]. Furthermore, the levels of this eotaxin-3 expression in the esophagus strongly correlated with disease severity based on basal layer expansion and levels of eosinophils and mast cells [47]. In addition, the evidence of the critical role of eotaxin in the pathogenesis of EoE comes from studies with eotaxin-deficient IL-5 transgenic mice that show that esophageal eosinophilia is markedly impaired compared with eotaxin-intact IL-5 transgenic mice [114].
Role of mast cells & basophils in EoE pathogenesis
Several clinical and experimental murine studies have shown an accumulation and degranulation of mast cells in the esophagus of EoE patients [13,63,83,115]. Significantly increased numbers of tryptase-positive mast cells and TGF-β1+ cells in smooth muscles of EoE patients were detected compared with those seen in control subjects. Tryptase-positive mast cells expressed TGF-β1 and increased the contractility of cultured primary human esophageal smooth muscle cells in vitro [13]. Hence, mast cells may participate in disease pathogenesis by generating a number of proinflammatory mediators that activate eosinophils and promote tissue remodeling [13,103,115]. Similar to eosinophils, mast cells also express CCR3 [25]; therefore, induced eotaxin-3 expression may also be responsible for mast cell recruitment in the esophagus in EoE. A recent report indicates that both eosinophils and mast cells correlate with disease severity in human [47]. Studies with a murine model shows that mast cells are critical in promoting muscle cell hyperplasia and hypertrophy in experimental EoE [115]. Furthermore, most recently elevated basophil levels have been shown in human EoE [17] and basophil depletion in experimental setup ameliorated EoE [17]. Taken together, this suggests that mast cells and basophils contribute to the disease pathogenesis and may have a significant role in promoting esophageal functional abnormalities in EoE.
Role of iNKT cells in EoE pathogenesis
The iNKT cells are a nonconventional population of T cells that express a canonical invariant TCR-α chain (Vα14-Jα18 for mice and Vα24-Jα18 for humans) and a TCR-β chain using limited Vβ segments (Vβ8.2 and 7 for mice and Vβ11 for humans)[116–119]. The iNKT subsets are well characterized in health and disease and its activation, releases a number of inflammatory Th1, Th2 and Th17 cytokines including IFN-γ, IL-4, [120,121], IL-5 and IL-13 [118,120,122–123]. Previously, iNKT cells were implicated in the induction of number of allergic diseases including asthma [124,125] and recently, we demonstrated that iNKT cell-deficient mice (CD1d−/−) are protected from allergen (peanut and Aspergillus)-induced experimental EoE [78]. The increased number of iNKT cells, its receptors Vα24 and Vβ11, and associated chemokine CXCL16 are also found in esophageal biopsies of EoE patients. Additionally, we also showed that the activation of iNKT cells in vivo is sufficient to induce EoE in mice [78]. Of note, the significance of iNKT cells in EoE was further established by treating the mice with anti-CD1d or anti-Vα24/Jα18 neutralizing antibodies in allergen-induced experimental EoE [126].
Role of experimental models in dissecting the EoE pathogenesis & proposing a treatment strategy
Eosinophils reside in all segments of the GI tract, except the esophagus from the prenatal to adult stage in both mice and humans at healthy state [5,127] and their number increases in the GI tract at disease state [128–131]. That indicates a very different mechanism that is operational in the esophagus for eosinophil accumulation compared with the other segments of GI tract. Therefore, to understand the mechanism of eosinophil trafficking into the esophagus, we developed the first EoE experimental model in 2001 by intranasal challenging of the mice with aeroallergens. The Aspergillus-induced EoE mouse model showed almost all the characteristic features of human EoE [131], such as intraepithelial eosinophilia, extracellular eosinophilic granules, basal cell hyperplasia and eosinophilic microabscesses. These models also show esophageal remodeling and fibrosis that are commonly observed in chronic human EoE [20,25]. Esophageal remodeling leads to a number of esophageal disorders, such as esophageal stricture (narrowing), peristaltic dysfunction, esophageal rings, tissue scars, and epithelial and muscle cell hyperplasia/hypertrophy, which are commonly observed in human EoE. Apart from the Aspergillus-induced EoE mouse model, the oral intragastric ovalbumin (OVA) beads [132] or OVA-sensitized and -challenged mouse models were also developed [133]. However, in both these OVA mouse models, there is a low magnitude of esophageal eosinophilia and the absence of a number of human EoE characteristics, such as intraepithelial eosinophils, eosinophilic microabscess, basal cell hyperplasia, and extracellular eosinophilic granules in the esophageal epithelium are not shown or reported. Furthermore, a mouse model of a food allergen (i.e., peanut or corn)-sensitized and -challenged EoE mouse model was also reported that indicated a critical role of T-cell subset, iNKT cells in EoE pathogenesis [134]. Both peanut and corn allergens promoted a large number of intraepithelial esophageal eosinophilia, eosinophilic microabscess and extracellular eosinophilic granules in the esophageal epithelium. In addition, using the mouse models, indoor insect allergens, such as cockroaches and dust mites, are also implicated for the EoE pathogenesis [79]. All these models dissected the significant role of iNKT cells and eosinophil active cytokines, such as IL-5, IL-13, IL-15 and chemokines such as eotaxin in EoE pathogenesis (Figure 1) [79,135]. Hence, a number of specific cytokine and chemokine genes (involved in EoE) transgenic mice that were created by either constitutive transgene overexpression, tissue-specific overexpression or comprising gene-inducible and gene-deficient mice [25,136]. Interestingly, most of the developed mouse models mimic the disease characteristics observed in humans (Table 1). Collectively, these mouse models provide us with a useful tool not only for understanding the mechanism of pathogenesis but also for predicting the future therapeutic drug trials for EoE.
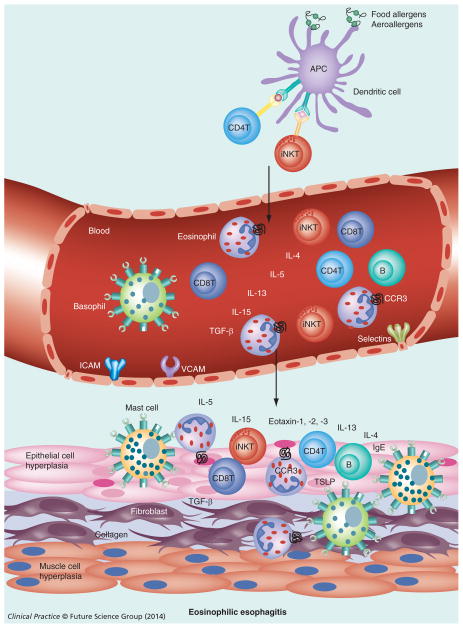
Figure 1. Allergen-induced esophageal eosinophilic esophagitis.
APCs process the allergens (food allergens/aeroallergens) and present antigens to T cells (iNKT cells in eosinophilic esophagitis, CD4 T cells in case of other allergic diseases). These T cells home to the esophagus via blood circulation and upon activation release Th2 cytokines (IL-4, IL-13 and IL-5) that induce IL-15 and eotaxin in the esophageal epithelium that attracts eosinophils into the epithelium (≥15 eosinophils/high-powered field).
Additionally, in response to allergens, iNKT cells, CD4+ T cells, CD8+ T cells, B cells, mast cells and basophils are also recruited to the esophagus. B cells synthesize IgE locally or systemically that may activate mast cells and basophils. Basophils and TSLP also contributes to EoE pathogenesis independently of IgE. Activated eosinophils and mast cells secrete TGF-β and other factors that induce esophageal remodeling, including fibrosis, collagen accumulation, muscle cell hyperplasia and epithelial cell hyperplasia that finally cause esophageal dysfunction and lead to eosinophilic esophagitis.
APC: Antigen-presenting cell; iNKT: Invariant natural killer T cell; TSLP: Thymic stromal lymphopoietin.
Current diagnosis & therapy
Current diagnosis for EoE is based on painful, expensive, risky and repetitive invasive endoscopy examinations followed by the histopathological evaluation of esophageal biopsies. The endoscopic evaluation includes the presence of longitudinal furrowing, white exudates, edema, longitudinal shearing, corrugated or ringed esophagus in combination with pathological biopsies examination that provide diagnostic features of EoE such as: at least 15 eosinophils/high-powered field, basal layer hyperplasia, eosinophilic microabscesses and extracellular eosinophilic granules [137]. However, most of the symptoms of EoE are similar to GERD and sometimes it is very difficult to differentiate between GERD and EoE. Therefore, the first criterion for diagnosis of EoE is nonresponsive PPI therapy and repetitive eosinophil counts [138–140]. Classically, PPIs have had a role in distinguishing GERD from EoE. Once the diagnosis of EoE is made, the main goal of treatment is to control the symptoms and is decided after knowing the allergic status of the patients. Moreover, it is still debatable whether the goal of treatment should be histologic remission or clinical improvement. Most of the patients are asymptomatic at diagnosis, and the treatment approach to these patients is still unknown [137]. Herein, we list the most common treatment strategies that are recommended for EoE.
Dietary manipulation
Food allergy is extremely common in EoE patients; therefore, a dietary manipulation is the first basis of treatment options. The dietary restriction has three main practices; an elemental diet completely devoid of allergens (amino acid-based formula administered either orally or by nasogastric tube), the six-food elimination diet (SFED), most likely removal of cow’s milk, soy, wheat, eggs, seafood and tree nuts, and the elimination of the food allergen based on allergy testing (either skin prick test [SPT] or allergen specific IgE). Although the elemental diet approach has been shown to be an effective treatment in up to 96% of patients [73–76], the major issue with this approach is the adherence to therapy and the cost of the elemental formula. The drawback of this strategy is that patients have to live all their lives on the restricted diet. Although these approaches have been used with success in pediatric populations, current data on adult patients suggest that the symptoms improved by 68% in targeted elimination diet and by 78% in SFED but endoscopic appearance improved only by 53% in targeted elimination diet and 56% in SFED. Only 32% in targeted elimination diet and 56% in SFED responded to ≤15 eosinophils/high powered field [53]. Similarly, a systematic review and meta-analysis of 33 studies of different dietary treatments that included 1317 patients with EoE (1128 children and 189 adults) verified that elemental diets were effective for 90.8% of cases, SFED for 72.1% and allergy test result-directed food elimination for 45.5% of cases. This study’s result shows that dietary therapy provided symptom relief to the majority of patients and resulted in a substantial (78–43 mean eosinophils/high-powered field) but not a complete decrease in eosinophil counts [56]. Because of the poor palatability of elemental formulas, elimination diets based on SPTs and atopy patch tests (APTs) or removal of the most common food allergens have been tried and resulted in a similar rate (75%) of improvement. The study of predictive values for SPT and APT for eosinophilic esophagitis by Spergel et al. [66,141] suggests that elimination diets based on positive foods found on APT and SPT and milk elimination regardless of the testing results can prevent the need for an elemental diet in a majority of children with EoE. Furthermore, the combination of SPT and APT can identify potential causative foods that might contribute to the pathogenesis of eosinophilic esophagitis.
Steroid therapy: topical & oral systemic therapy
The second treatment regimen is the topical/systemic steroids treatment for EoE. Steroids treatment significantly reduces both clinical and histological symptoms. Interestingly, steroid treatment also shows reversal of tissue fibrosis in children [142]. Esophageal remodeling is associated with the upregulated gene expression of profibrogenic cytokines in adults with EoE, and prolonged fluticasone propionate treatment leads to a nonsignificant reduction in subepithelial collagen deposition accompanied by the downregulation of profibrogenic cytokine gene expression, specifically CCL18 [143].
Swallowed steroid therapy was found to be more effective with the complete remission in a significant number of patients compared with dietary therapies [144]. However, there are limitations that exist with oral swallowed steroids therapy: dependency on treatment (reoccurrence of EoE symptoms within few months after discontinuation of therapy) and oral fungal infections [4,145–146]. However, no systemic side effects have been reported with this regimen.
Antileukotriene therapy
Human eosinophils express both heptahelical G protein- coupled receptors, cysLT1 receptor and cysLT2 receptor. Montelukast (a leukotriene D receptor, cysLT1 receptor, antagonist) has an effect on eosinophil function, but not in eosinophil recruitment. An adult trial of EoE has shown some clinical but no histological improvement [147–149]; therefore, the current guidelines do not recommended antileukotriene therapy for EoE [4].
Anti-IL-5 & anti-IL-13 therapy
A humanized anti-IL-5 antibody (mepolizumab [150–152] or resalizumab [153] treatment) clinical trial indicates that a substantial disease component is reversible in adults with severe manifestations of patients with longstanding EoE symptoms and severe tissue pathology. The anti-IL-5 antibody treatment reduces EoE symptoms including the eosinophil load in the blood and in the tissue, as well as eosinophil activation but could not reduce eosinophilic count as much as an elimination diet treatment (less than four eosinophils/high-powered field). In addition, these studies demonstrated a trend of improvement of clinical symptoms that did not reach statistical significance [153,154]. However, the drawback of anti-IL-5 antibody therapy is similar to the elimination diet treatment; the EoE symptoms come back as soon as therapy is withdrawn. Another potential pharmacological agent currently on trial is anti-IL-13 neutralizing antibody treatments for EoE; however, it is debatable whether an anti-IL-13 treatment strategy will be successful. Recently, it has been shown that IL-13-induced EoE is dependent on IL-5 and IL-13 gene deficiency does not impair antigen-induced EoE [62].
Esophageal dilation
The effective therapy to relieve dysphagia is dilation of esophageal strictures. This therapy, however, has no effect on the inflammation of esophagus. This therapy is often used for patients who failed other medical therapies and sometimes it is also used for patients who have high-grade strictures. Clinical outcome of dysphagia varies after dilation, with symptomatic recurrence occurring within 3–12 months to longer than a year. The main concern with dilation is perforation. However, a meta-analysis suggests that this risk is minimal [155]. Dilation is a safe procedure with a low rate of serious complications (<1%), and provides at least a short-term improvement of symptoms in the majority of patients. Interestingly, a recent study suggested that dilation was less economical than treatment with swallowed aerosolized steroids in EoE [156]. Patients should be informed that dilation itself will not be sufficient for treatment, and topical therapy in addition to dilation should be recommended.
Future diagnosis & therapy
We and other investigators have made significant progress in understanding EoE pathogenesis; however, there is still a lack of novel noninvasive biomarkers for precise diagnosis of EoE. Thus, there is a great need for innovative approaches to uncover new possibilities for diagnostic and therapeutic interventions. The identification of a reliable noninvasive EoE biomarker would advance EoE treatment, as it would allow for more accurate and timely detection of changes resulting from therapy alteration. The key molecules that participate in the pathophysiology can be used as noninvasive biomarkers to diagnose EoE. Earlier, a number of biomarkers are proposed but most of them are the byproducts of eosinophils, which are not specific to EoE and do not differentiate EoE from GERD [157]. We recently established that allergen-induced IL-15 and its responsive T-cell subset (iNKT cell) are induced in human EoE and have a role in the initiation and progression of EoE pathogenesis. Furthermore, the iNKT neutralization provides protection from the induction of EoE in an experimental model of EoE [78,126]. In addition, we also found that the mRNA levels of IgE receptors FcεRI, FcεRII and IL-15 responsive T-cell subset receptors (TCR) such as CXCR6, Vα24, γ and δ, are differentially expressed in the blood of normal individuals, EoE and GERD patients. These molecules may be novel noninvasive biomarkers for EoE [158] and are critical for future therapeutic intervention.
Conclusion
In summary, we have described in this review the progress in understanding the development of pathogenesis of EoE. In brief, we have discussed the critical role of T cells, specifically its subset iNKT cells, in the pathogenesis of EoE. Although B-cell levels are increased in EoE patients and in a murine model, B-cell-deficient mice data showed that they are not required for development of EoE. Interestingly, FcεRI and FcεRII receptors on blood cells are differentially expressed in EoE and GERD compared with normal subjects. We have also provided the evidence for the involvement of eosinophils, mast cells and basophils in tissue remodeling during the development of pathogenesis of EoE. Furthermore, we have also discussed the major role of Th2 cytokines, IL-5 and IL-13, chemokines and eotaxins in the development of symptoms of EoE. Apart from eotaxin, IL-5 and IL-13, we have described newly identified key targets, IL-15 and its responsive iNKT cells and their receptors (Vα24 and Vβ11) that play a critical role in initiation and progression of EoE. Data from overexpressing IL-15-transgenic mice, IL-15Rα-deficient mice, iNKT cell-deficient mice, and depletion of iNKT cells via anti-CD1d or anti-Vα24/Jα18 antibodies demonstrate the critical role of IL-15, iNKT cells and their receptors in the development of characteristic features of EoE in a murine model (Figure 1). These data provided us with novel therapeutic targets for EoE treatment and diagnosis. Studies with human subjects confirmed their association with disease pathogenesis. Hence, these target molecules can be utilized for validation of diagnosis and therapeutic treatment strategies.
Future perspective
Eosinophilic esophagitis (EoE) is a chronic immune-or allergen-mediated esophageal disease characterized by clinical symptoms (related to esophageal dysfunction such as dysphagia, heart burn and chest pain), endoscopic evaluation (esophageal rings, narrowing or strictures, linear furrows, white plaques or exudates, decreased vasculature, edema and mucosal fragility) or by pathologic assessment (esophageal eosinophilia; ≥15 intraepithelial eosinophils/high-powered field). Recent consensus guidelines, based on the expert opinions of pediatric and adult gastroenterologists, allergists and pathologists, provide standardized diagnostic criteria for EoE. However, still there is no single clinical, endoscopic, or histologic characteristic feature identified for the diagnosis of EoE. Current challenges for EoE investigators are: in clinical symptoms that distinguish EoE from GERD and PPI-REE; endoscopic evaluation indicating that in some patients (5–10%) where the esophageal mucosa appears normal and if a biopsy is not taken from them, there will be a chance of missed diagnosis of EoE patient; and endoscopic and histologic characteristic features described for EoE cannot distinguish GERD or PPI-REE from EoE. Yet, there is no validated tool to assess the effectiveness of EoE treatment other than the above-described diagnostic criteria by clinical and repetitive endoscopic and histologic evaluation for management of EoE. There is a great concern of the long-term effect of anesthesia used when obtaining repetitive endoscopic biopsies on the development of children. Currently, steroid and elemental/elimination diet therapies are effective for both adult and pediatric EoE but these therapies have a number of limitations. Therefore, there is a need for a better noninvasive diagnostic biomarker that can be utilized for assessing the effectiveness of therapy for the management of EoE. Utilizing human and murine models, few new molecules involved in EoE pathogenesis have been identified that include thymic stromal lymphopoietin, basophils, IL-15, iNKT cells and IL-15 responsive cell surface receptors. We hope that in next few years, a novel reliable noninvasive diagnostic or therapeutic molecule will be established for EoE management that will not only reduce the risk and pain associated with endoscopic biopsies but also the economic burden of patients and their families.
Practice points.
Eosinophilic esophagitis (EoE) is an allergen-induced T-cell mediated chronic allergic inflammatory disease.
Characteristic features of EoE pathogenesis are esophageal dysfunction, infiltration of esophagus with eosinophils (>15 eosinophils/high-powered field), extracellular eosinophilic granules, eosinophil microabssess and tissue remodeling, including epithelial cell hyperplasia, and tissue fibrosis and a lack of response to a 8-week proton-pump inhibitor treatment.
CD4+ T cells including invariant natural killer T cells, basophils and mast cells promote EoE pathogenesis.
IL-15, IL-5, IL-13, eotaxin-1 and eotaxin-3 play a critical role in EoE pathogenesis.
The current diagnosis criteria for EoE is invasive multiple biopsies of esophagus for histological determination of eosinophil numbers in the esophagus and lack of acid suppression (proton pump inhibitor) to differentiate EoE from gastroesophageal reflux disease.
There is a great necessity for novel noninvasive diagnosis biomarkers for EoE that differentiate EoE from gastroesophageal reflux disease.
The most common therapy for EoE is elemental diet and corticosteroid treatments that are effective but are expensive, uncomfortable and have side effects.
Anti-IL-5 therapy is effective but does not have promising results in clinics. The reason may be that it is a survival factor but not an initiator of disease.
Anti-IL-13 and anti-eotaxin-3 therapies are currently being investigated. Recently anti-IL-13 therapy has been terminated because of the side effects.
Esophageal dilation does improve dysphagia but has no effect on inflammation.
The evidence provided for the critical role of IL-15, its responsive invariant natural killer T cells and their receptors in the pathogenesis of EoE suggest that these molecules could be new targets for novel noninvasive diagnosis and therapy modality.
Acknowledgments
The authors would like to thank ME Rothenberg for his guidance and support, and LL Hamm and JA Lasky for providing the facility at Tulane University School of Medicine to continue their eosinophilic esophagitis research work.
Footnotes
Disclosure
A Mishra is the senior author and Endowed Schlieder Chair and Professor of Medicine at Tulane Eosinophilic Disorders Center and Section of Pulmonary Diseases.
Financial & competing interests disclosure
This work was supported in part by the grants NIH R01 DK067255 (A Mishra) and NIH R01 AI080581 (A Mishra). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest;
•• of considerable interest
- 1.Landres RT, Kuster GG, Strum WB. Eosinophilic esophagitis in a patient with vigorous achalasia. Gastroenterology. 1978;74(6):1298–1301. [PubMed] [Google Scholar]
- 2•.Attwood SE, Smyrk TC, Demeester TR, Jones JB. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Digest Dis Sci. 1993;38(1):109–116. doi: 10.1007/BF01296781. Primarily defines eosinophilic esophagitis (EoE) as a characteristic clinicopathological syndrome. [DOI] [PubMed] [Google Scholar]
- 3.Furuta GT, Liacouras CA, Collins MH, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133(4):1342–1363. doi: 10.1053/j.gastro.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 4••.Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128(1):3–20. e26. doi: 10.1016/j.jaci.2011.02.040. quiz 21–22. Provides the updated consensus recommendations for EoE of children and adults, and discussed further advances and controversies regarding diagnostic methods, surrogate disease markers, allergy testing and treatment approaches. [DOI] [PubMed] [Google Scholar]
- 5•.Mishra A, Hogan SP, Lee JJ, Foster PS, Rothenberg ME. Fundamental signals that regulate eosinophil homing to the gastrointestinal tract. J Clin Invest. 1999;103(12):1719–1727. doi: 10.1172/JCI6560. Describes eotaxin as the primary regulator of eosinophil gastrointestinal homing under homeostatic states. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keshishian J, Vrcel V, Boyce HW, Estores D, Serrano J, Richter JE. Eosinophilic esophagitis: a paradigm shift for pathology. J Clin Gastroenterol. 2013 doi: 10.1097/MCG.0b013e3182a9a9cc. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 7•.Soon IS, Butzner JD, Kaplan GG, Debruyn JC. Incidence and prevalence of eosinophilic esophagitis in children. J Pediat Gastroenterol Nutr. 2013;57(1):72–80. doi: 10.1097/MPG.0b013e318291fee2. Provides a systematic review with meta-analysis on the epidemiology of EoE in children and concluded that the incidence and prevalence of EoE in children have increased significantly; however, the population-based incidence and prevalence of EoE vary widely across geographic variations. [DOI] [PubMed] [Google Scholar]
- 8.Sorser SA, Barawi M, Hagglund K, Almojaned M, Lyons H. Eosinophilic esophagitis in children and adolescents: epidemiology, clinical presentation and seasonal variation. J Gastroenterol. 2013;48(1):81–85. doi: 10.1007/s00535-012-0608-x. [DOI] [PubMed] [Google Scholar]
- 9••.Dellon ES, Gonsalves N, Hirano I, et al. ACG clinical guideline: evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE) Am J Gastroenterol. 2013;108(5):679–692. doi: 10.1038/ajg.2013.71. quiz 693. Emphasizes the concepts of esophageal eosinophilia and proton-pump inhibitor-responsive esophageal eosinophilia (PPI-REE) as entities distinct from EoE. [DOI] [PubMed] [Google Scholar]
- 10•.Rothenberg ME, Mishra A, Collins MH, Putnam PE. Pathogenesis and clinical features of eosinophilic esophagitis. J Allergy Clin Immunol. 2001;108(6):891–894. doi: 10.1067/mai.2001.120095. Defines the clinical feature of EoE based on the number of eosinophils in the esophagus. [DOI] [PubMed] [Google Scholar]
- 11.Fox VL, Nurko S, Teitelbaum JE, Badizadegan K, Furuta GT. High-resolution EUS in children with eosinophilic ‘allergic’ esophagitis. Gastrointest Endosc. 2003;57(1):30–36. doi: 10.1067/mge.2003.33. [DOI] [PubMed] [Google Scholar]
- 12.Stevoff C, Rao S, Parsons W, Kahrilas PJ, Hirano I. EUS and histopathologic correlates in eosinophilic esophagitis. Gastrointest Endosc. 2001;54(3):373–377. doi: 10.1067/mge.2001.116569. [DOI] [PubMed] [Google Scholar]
- 13•.Aceves SS, Chen D, Newbury RO, Dohil R, Bastian JF, Broide DH. Mast cells infiltrate the esophageal smooth muscle in patients with eosinophilic esophagitis, express TGFbeta1, and increase esophageal smooth muscle contraction. J Allergy Clin Immunol. 2010;126(6):1198–1204. e1194. doi: 10.1016/j.jaci.2010.08.050. Describes the mast cell infiltration into the esophageal lamina propria and smooth muscle and the effects of their products on smooth muscle function. [DOI] [PubMed] [Google Scholar]
- 14.Dellon ES, Chen X, Miller CR, et al. Tryptase staining of mast cells may differentiate eosinophilic esophagitis from gastroesophageal reflux disease. Am J Gastroenterol. 2011;106(2):264–271. doi: 10.1038/ajg.2010.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lucendo AJ, Bellon T, Lucendo B. The role of mast cells in eosinophilic esophagitis. Pediatr Allergy Immunol. 2009;20(6):512–518. doi: 10.1111/j.1399-3038.2008.00798.x. [DOI] [PubMed] [Google Scholar]
- 16•.Mavi P, Rajavelu P, Rayapudi M, Paul RJ, Mishra A. Esophageal functional impairments in experimental eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol. 2012;302(11):G1347–1355. doi: 10.1152/ajpgi.00013.2012. Implicates chronic eosinophilic inflammation in the development of the esophageal structural impairments of experimental EoE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noti M, Wojno ED, Kim BS, et al. Thymic stromal lymphopoietin-elicited basophil responses promote eosinophilic esophagitis. Nat Med. 2013;19(8):1005–1013. doi: 10.1038/nm.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18••.Mishra A, Schlotman J, Wang M, Rothenberg ME. Critical role for adaptive T cell immunity in experimental eosinophilic esophagitis in mice. J Leukoc Biol. 2007;81(4):916–924. doi: 10.1189/jlb.1106653. Indicates a role for CD4+ and CD4− cell populations in EoE pathogenesis and demonstrates that experimental allergen-induced EoE is dependent on adaptive T-cell immunity. [DOI] [PubMed] [Google Scholar]
- 19•.Noel RJ, Putnam PE, Collins MH, et al. Clinical and immunopathologic effects of swallowed fluticasone for eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2004;2(7):568–575. doi: 10.1016/s1542-3565(04)00240-x. Suggests that patients treated with swallowed fluticasone have improved endoscopic, histologic and immunologic parameters associated with EoE. [DOI] [PubMed] [Google Scholar]
- 20•.Aceves SS, Newbury RO, Dohil R, Bastian JF, Broide DH. Esophageal remodeling in pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2007;119(1):206–212. doi: 10.1016/j.jaci.2006.10.016. This study of pediatric patients with EoE confirmed the presence of esophageal remodeling demonstrated by increased fibrosis, vascularity and vascular activation in the esophagus that may contribute to stricture formation. [DOI] [PubMed] [Google Scholar]
- 21.Mishra A, Rothenberg ME. Intratracheal IL-13 induces eosinophilic esophagitis by an IL-5, eotaxin-1, and STAT6-dependent mechanism. Gastroenterology. 2003;125(5):1419–1427. doi: 10.1016/j.gastro.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Straumann A, Kristl J, Conus S, et al. Cytokine expression in healthy and inflamed mucosa: probing the role of eosinophils in the digestive tract. Inflamm Bowel Dis. 2005;11(8):720–726. doi: 10.1097/01.mib.0000172557.39767.53. [DOI] [PubMed] [Google Scholar]
- 23.Schoepfer AM, Safroneeva E, Bussmann C, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology. 2013;145(6):1230–1236. e1231–1232. doi: 10.1053/j.gastro.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 24.Dellon ES, Kim HP, Sperry SL, Rybnicek DA, Woosley JT, Shaheen NJ. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest Endosc. 2013;79(4):577–585.e4. doi: 10.1016/j.gie.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25••.Mishra A, Wang M, Pemmaraju VR, et al. Esophageal remodeling develops as a consequence of tissue specific IL-5-induced eosinophilia. Gastroenterology. 2008;134(1):204–214. doi: 10.1053/j.gastro.2007.10.002. Provides evidence that local IL-5-mediated eosinophilia is essential in the induction of esophageal remodeling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gharaee-Kermani M, Phan SH. The role of eosinophils in pulmonary fibrosis (review) Int J Mol Med. 1998;1(1):43–53. [PubMed] [Google Scholar]
- 27.Kay AB. The role of eosinophils in the pathogenesis of asthma. Trends Mol Med. 2005;11(4):148–152. doi: 10.1016/j.molmed.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Varga J, Kahari VM. Eosinophilia-myalgia syndrome, eosinophilic fasciitis, and related fibrosing disorders. Curr Opin Rheumatol. 1997;9(6):562–570. doi: 10.1097/00002281-199711000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol. 2013;13(1):9–22. doi: 10.1038/nri3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spry CJ. The pathogenesis of endomyocardial fibrosis: the role of the eosinophil. Spger Sem Immunopathol. 1989;11(4):471–477. doi: 10.1007/BF00201883. [DOI] [PubMed] [Google Scholar]
- 31.Valent P. Pathogenesis, classification, and therapy of eosinophilia and eosinophil disorders. Blood Rev. 2009;23(4):157–165. doi: 10.1016/j.blre.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Gharaee-Kermani M, Phan SH. Molecular mechanisms of and possible treatment strategies for idiopathic pulmonary fibrosis. Curr Pharm Des. 2005;11(30):3943–3971. doi: 10.2174/138161205774580561. [DOI] [PubMed] [Google Scholar]
- 33.Cha SI, Chang CS, Kim EK, et al. Lung mast cell density defines a subpopulation of patients with idiopathic pulmonary fibrosis. Histopathology. 2012;61(1):98–106. doi: 10.1111/j.1365-2559.2012.04197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kosanovic D, Dahal BK, Wygrecka M, et al. Mast cell chymase: an indispensable instrument in the pathological symphony of idiopathic pulmonary fibrosis? Histol Histopathol. 2013;28(6):691–699. doi: 10.14670/HH-28.691. [DOI] [PubMed] [Google Scholar]
- 35.Wygrecka M, Dahal BK, Kosanovic D, et al. Mast cells and fibroblasts work in concert to aggravate pulmonary fibrosis: role of transmembrane SCF and the PAR-2/PKC-alpha/Raf-1/p44/42 signaling pathway. Am J Pathol. 2013;182(6):2094–2108. doi: 10.1016/j.ajpath.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Noguchi H, Kephart GM, Colby TV, Gleich GJ. Tissue eosinophilia and eosinophil degranulation in syndromes associated with fibrosis. Am J Pathol. 1992;140(2):521–528. [PMC free article] [PubMed] [Google Scholar]
- 37.Atamas SP, White B. Cytokine regulation of pulmonary fibrosis in scleroderma. Cytoke Growth Factor Rev. 2003;14(6):537–550. doi: 10.1016/s1359-6101(03)00060-1. [DOI] [PubMed] [Google Scholar]
- 38.Gruber BL. Mast cells in the pathogenesis of fibrosis. Curr Rheumatol Rep. 2003;5(2):147–153. doi: 10.1007/s11926-003-0043-3. [DOI] [PubMed] [Google Scholar]
- 39.Lee JJ, Dimina D, Macias MP, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305(5691):1773–1776. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- 40.Humbles AA, Lloyd CM, Mcmillan SJ, et al. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305(5691):1776–1779. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- 41.Cho JY, Miller M, Baek KJ, et al. Inhibition of airway remodeling in IL-5-deficient mice. J Clin Invest. 2004;113(4):551–560. doi: 10.1172/JCI19133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lacy P, Moqbel R. Eosinophil cytokines. Chem Immunol. 2000;76:134–155. doi: 10.1159/000058782. [DOI] [PubMed] [Google Scholar]
- 43.Lambrecht BN, Hammad H. Asthma: the importance of dysregulated barrier immunity. Eur J Immunol. 2013;43(12):3125–3137. doi: 10.1002/eji.201343730. [DOI] [PubMed] [Google Scholar]
- 44.Varga J, Jimenez SA. Modulation of collagen gene expression: its relation to fibrosis in systemic sclerosis and other disorders. Annals Inter Med. 1995;122(1):60–62. doi: 10.7326/0003-4819-122-1-199501010-00010. [DOI] [PubMed] [Google Scholar]
- 45.Eickelberg O, Pansky A, Mussmann R, et al. Transforming growth factor-beta1 induces interleukin-6 expression via activating protein-1 consisting of JunD homodimers in primary human lung fibroblasts. J Biol Chem. 1999;274(18):12933–12938. doi: 10.1074/jbc.274.18.12933. [DOI] [PubMed] [Google Scholar]
- 46••.Straumann A, Spichtin HP, Grize L, Bucher KA, Beglinger C, Simon HU. Natural history of primary eosinophilic esophagitis: a follow-up of 30 adult patients for up to 11.5 years. Gastroenterology. 2003;125(6):1660–1669. doi: 10.1053/j.gastro.2003.09.024. Demonstrates that eosinophilic esophagitis, a primary and chronic disease restricted to the esophagus, leads to persistent dysphagia and structural esophageal alterations but does not impact the nutritional state. [DOI] [PubMed] [Google Scholar]
- 47••.Blanchard C, Wang N, Stringer KF, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest. 2006;116(2):536–547. doi: 10.1172/JCI26679. Provides the genome-wide microarray expression analysis of esophageal tissue from EoE patients and defined the striking transcript signature of EoE patients involving 1% of the human genome. Demonstrates the enhanced expression of mRNA of eotaxin-3 and IL-15 in EoE patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takayama G, Arima K, Kanaji T, et al. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol. 2006;118(1):98–104. doi: 10.1016/j.jaci.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 49.Blanchard C, Mingler MK, Mcbride M, et al. Periostin facilitates eosinophil tissue infiltration in allergic lung and esophageal responses. Mucosal Immunol. 2008;1(4):289–296. doi: 10.1038/mi.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hruz P, Straumann A, Bussmann C, et al. Escalating incidence of eosinophilic esophagitis: a 20-year prospective, population-based study in Olten County, Switzerland. J Allergy Clin Immunol. 2011;128(6):1349–1350.e1345. doi: 10.1016/j.jaci.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 51.Arias A, Lucendo AJ. Prevalence of eosinophilic oesophagitis in adult patients in a central region of Spain. Eur J Gastroenterol Hepatol. 2013;25(2):208–212. doi: 10.1097/MEG.0b013e32835a4c95. [DOI] [PubMed] [Google Scholar]
- 52.Dellon ES, Jensen ET, Martin CF, Shaheen NJ, Kappelman MD. Prevalence of eosinophilic esophagitis in the United States. Clin Gastroenterol Hepatol. 2013;12(4):589–596.e1. doi: 10.1016/j.cgh.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wolf WA, Jerath MR, Sperry SL, Shaheen NJ, Dellon ES. Dietary elimination therapy is an effective option for adults with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2014 doi: 10.1016/j.cgh.2013.12.034. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lucendo AJ, Molina-Infante J. Emerging therapeutic strategies for eosinophilic esophagitis. Curr Treat Options Gastroenterol. 2014;12(1):1–17. doi: 10.1007/s11938-013-0001-8. [DOI] [PubMed] [Google Scholar]
- 55.Arias A, Lucendo AJ. Dietary therapies for eosinophilic esophagitis. Expert Rev Clin Immunol. 2014;10(1):133–142. doi: 10.1586/1744666X.2014.856263. [DOI] [PubMed] [Google Scholar]
- 56.Arias A, Gonzalez-Cervera J, Tenias JM, Lucendo AJ. Efficacy of dietary interventions in inducing histologic remission in patients with eosinophilic esophagitis: a systematic review and meta-analysis. Gastroenterology. 2014 doi: 10.1053/j.gastro.2014.02.006. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 57•.Spergel JM, Andrews T, Brown-Whitehorn TF, Beausoleil JL, Liacouras CA. Treatment of eosinophilic esophagitis with specific food elimination diet directed by a combination of skin prick and patch tests. Ann Allergy Asthma Immunol. 2005;95(4):336–343. doi: 10.1016/S1081-1206(10)61151-9. Shows that skin prick and atopy patch testing can help identify foods in most patients and dietary elimination of foods significantly improved both symptoms and esophageal inflammation. [DOI] [PubMed] [Google Scholar]
- 58.Kelly KJ, Lazenby AJ, Rowe PC, Yardley JH, Perman JA, Sampson HA. Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid-based formula. Gastroenterology. 1995;109(5):1503–1512. doi: 10.1016/0016-5085(95)90637-1. [DOI] [PubMed] [Google Scholar]
- 59.Kagalwalla AF, Sentongo TA, Ritz S, et al. Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2006;4(9):1097–1102. doi: 10.1016/j.cgh.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 60•.Faubion WA, Jr, Perrault J, Burgart LJ, Zein NN, Clawson M, Freese DK. Treatment of eosinophilic esophagitis with inhaled corticosteroids. J Pediat Gastroenterol Nutr. 1998;27(1):90–93. doi: 10.1097/00005176-199807000-00016. Provides a case report of four patients describing safety of inhaled corticosteroid as an effective alternative to oral therapy in patients with eosinophilic esophagitis that also attenuates the long-term side effects of conventional steroid therapy. [DOI] [PubMed] [Google Scholar]
- 61.Liacouras CA. Eosinophilic esophagitis: treatment in 2005. Curr Opin Gastroenterol. 2006;22(2):147–152. doi: 10.1097/01.mog.0000203863.40632.ba. [DOI] [PubMed] [Google Scholar]
- 62••.Niranjan R, Rayapudi M, Mishra A, Dutt P, Dynda S, Mishra A. Pathogenesis of allergen-induced eosinophilic esophagitis is independent of interleukin (IL)-13. Immunol Cell Biol. 2013;91(6):408–415. doi: 10.1038/icb.2013.21. Provides evidence that IL-13 is not involved in the pathogenesis of EoE by utilizing IL-13-deficient mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63•.Abonia JP, Blanchard C, Butz BB, et al. Involvement of mast cells in eosinophilic esophagitis. J Allergy Clin Immunol. 2010;126(1):140–149. doi: 10.1016/j.jaci.2010.04.009. Investigates the involvement of mast cells in disease pathology and identified an esophageal mast cell-associated transcriptome that is significantly divergent from the eosinophil-associated transcriptome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aceves SS. Food allergy testing in eosinophilic esophagitis: what the gastroenterologist needs to know. Clin Gastroenterol Hepatol. 2013 doi: 10.1016/j.cgh.2013.09.007. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Slack MA, Erwin EA, Cho CB, Raveendran R, Phillips G, Ogbogu PU. Food and aeroallergen sensitization in adult eosinophilic esophagitis. Ann Allergy Asthma Immunol. 2013;111(4):304–305. doi: 10.1016/j.anai.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 66•.Spergel JM. Eosinophilic esophagitis in adults and children: evidence for a food allergy component in many patients. Curr Opin Allergy Clin Immunol. 2007;7(3):274–278. doi: 10.1097/ACI.0b013e32813aee4a. Provides review on pediatric patients with eosinophilic esophagitis and response to elemental diet on symptoms. [DOI] [PubMed] [Google Scholar]
- 67.Peterson KA, Byrne KR, Vinson LA, et al. Elemental diet induces histologic response in adult eosinophilic esophagitis. Am J Gastroenterol. 2013;108(5):759–766. doi: 10.1038/ajg.2012.468. [DOI] [PubMed] [Google Scholar]
- 68.Almansa C, Krishna M, Buchner AM, et al. Seasonal distribution in newly diagnosed cases of eosinophilic esophagitis in adults. Am J Gastroenterol. 2009;104(4):828–833. doi: 10.1038/ajg.2008.169. [DOI] [PubMed] [Google Scholar]
- 69.Rezende ER, Barros CP, Ynoue LH, Santos AT, Pinto RM, Segundo GR. Clinical characteristics and sensitivity to food and inhalants among children with eosinophilic esophagitis. BMC Res Notes. 2014;7:47. doi: 10.1186/1756-0500-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wolf WA, Jerath MR, Dellon ES. De-novo onset of eosinophilic esophagitis after large volume allergen exposures. J Gastrointest Liver Dis. 2013;22(2):205–208. [PMC free article] [PubMed] [Google Scholar]
- 71.Van Rhijn BD, Van Ree R, Versteeg SA, et al. Birch pollen sensitization with cross-reactivity to food allergens predominates in adults with eosinophilic esophagitis. Allergy. 2013;68(11):1475–1481. doi: 10.1111/all.12257. [DOI] [PubMed] [Google Scholar]
- 72.Simon D, Straumann A, Dahinden C, Simon HU. Frequent sensitization to Candida albicans and profilins in adult eosinophilic esophagitis. Allergy. 2013;68(7):945–948. doi: 10.1111/all.12157. [DOI] [PubMed] [Google Scholar]
- 73.Henderson CJ, Abonia JP, King EC, et al. Comparative dietary therapy effectiveness in remission of pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2012;129(6):1570–1578. doi: 10.1016/j.jaci.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gonzalez-Cervera J, Angueira T, Rodriguez-Dominguez B, Arias A, Yague-Compadre JL, Lucendo AJ. Successful food elimination therapy in adult eosinophilic esophagitis: not all patients are the same. J Clin Gastroenterol. 2012;46(10):855–858. doi: 10.1097/MCG.0b013e3182432259. [DOI] [PubMed] [Google Scholar]
- 75.Gonsalves N, Yang GY, Doerfler B, Ritz S, Ditto AM, Hirano I. Elimination diet effectively treats eosinophilic esophagitis in adults; food reintroduction identifies causative factors. Gastroenterology. 2012;142(7):1451–1459. e1451. doi: 10.1053/j.gastro.2012.03.001. quiz e1414–e1455. [DOI] [PubMed] [Google Scholar]
- 76.Kagalwalla AF, Shah A, Li BU, et al. Identification of specific foods responsible for inflammation in children with eosinophilic esophagitis successfully treated with empiric elimination diet. J Pediat Gastroenterol Nutr. 2011;53(2):145–149. doi: 10.1097/MPG.0b013e31821cf503. [DOI] [PubMed] [Google Scholar]
- 77.Moawad FJ, Veerappan GR, Lake JM, et al. Correlation between eosinophilic oesophagitis and aeroallergens. Ailment Pharmacol Ther. 2010;31(4):509–515. doi: 10.1111/j.1365-2036.2009.04199.x. [DOI] [PubMed] [Google Scholar]
- 78•.Rajavelu P, Rayapudi M, Moffitt M, Mishra A, Mishra A. Significance of para-esophageal lymph nodes in food or aeroallergen-induced iNKT cell-mediated experimental eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol. 2012;302(7):G645–654. doi: 10.1152/ajpgi.00223.2011. Describes the involvement of paraesophageal lymph nodes, eotaxins and iNKT cells in pathogenesis of food- or aeroallergen-induced EoE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79••.Rayapudi M, Mavi P, Zhu X, et al. Indoor insect allergens are potent inducers of experimental eosinophilic esophagitis in mice. J Leukoc Biol. 2010;88(2):337–346. doi: 10.1189/jlb.0110025. Demonstrates that indoor insect allergens are potent inducers of IL-5 and eotaxin-mediated esophageal eosinophilia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vicario M, Blanchard C, Stringer KF, et al. Local B cells and IgE production in the oesophageal mucosa in eosinophilic oesophagitis. Gut. 2010;59(1):12–20. doi: 10.1136/gut.2009.178020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lucendo AJ, Navarro M, Comas C, et al. Immunophenotypic characterization and quantification of the epithelial inflammatory infiltrate in eosinophilic esophagitis through stereology: an analysis of the cellular mechanisms of the disease and the immunologic capacity of the esophagus. Am J Surg Pathol. 2007;31(4):598–606. doi: 10.1097/01.pas.0000213392.49698.8c. [DOI] [PubMed] [Google Scholar]
- 82.Bullock JZ, Villanueva JM, Blanchard C, et al. Interplay of adaptive Th2 immunity with eotaxin-3/c-C chemokine receptor 3 in eosinophilic esophagitis. J Pediat Gastroenterol Nutr. 2007;45(1):22–31. doi: 10.1097/MPG.0b013e318043c097. [DOI] [PubMed] [Google Scholar]
- 83.Straumann A, Bauer M, Fischer B, Blaser K, Simon HU. Idiopathic eosinophilic esophagitis is associated with a T(H)2-type allergic inflammatory response. J Allergy Clin Immunol. 2001;108(6):954–961. doi: 10.1067/mai.2001.119917. [DOI] [PubMed] [Google Scholar]
- 84••.Mishra A. Significance of mouse model in disecting the mechanism of human eosinophilic gastrointestinal diseases (EGID) J Gastroenterol Hepatol Res. 2013;2(11):845–853. doi: 10.6051/j.issn2224-3992.2013.02.343. Provides the overviews of the mouse models of gastrointestinal disorders that mimic the human eosinophilic gastrointestinal diseases and can be utilized as a tool for understanding the diseases pathogenesis and developing novel therapeutic targets. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sampson HA. Food allergy. Part 1: immunopathogenesis and clinical disorders. J Allergy Clin Immunol. 1999;103(5 Pt 1):717–728. doi: 10.1016/s0091-6749(99)70411-2. [DOI] [PubMed] [Google Scholar]
- 86.Spergel JM, Beausoleil JL, Mascarenhas M, Liacouras CA. The use of skin prick tests and patch tests to identify causative foods in eosinophilic esophagitis. J Allergy Clin Immunol. 2002;109(2):363–368. doi: 10.1067/mai.2002.121458. [DOI] [PubMed] [Google Scholar]
- 87.Assa’ad A. Eosinophilic esophagitis: association with allergic disorders. Gastrointest Endosc Clin North Am. 2008;18(1):119–132. x. doi: 10.1016/j.giec.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 88.Penfield JD, Lang DM, Goldblum JR, Lopez R, Falk GW. The role of allergy evaluation in adults with eosinophilic esophagitis. J Clin Gastroenterol. 2010;44(1):22–27. doi: 10.1097/MCG.0b013e3181a1bee5. [DOI] [PubMed] [Google Scholar]
- 89.Roy-Ghanta S, Larosa DF, Katzka DA. Atopic characteristics of adult patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2008;6(5):531–535. doi: 10.1016/j.cgh.2007.12.045. [DOI] [PubMed] [Google Scholar]
- 90.Prasad GA, Alexander JA, Schleck CD, et al. Epidemiology of eosinophilic esophagitis over three decades in Olmsted County, Minnesota. Clin Gastroenterol Hepatol. 2009;7(10):1055–1061. doi: 10.1016/j.cgh.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Erwin EA, James HR, Gutekunst HM, Russo JM, Kelleher KJ, Platts-Mills TA. Serum IgE measurement and detection of food allergy in pediatric patients with eosinophilic esophagitis. Ann Allergy Asthma Immunol. 2010;104(6):496–502. doi: 10.1016/j.anai.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Weinbrand-Goichberg J, Segal I, Ovadia A, Levine A, Dalal I. Eosinophilic esophagitis: an immune-mediated esophageal disease. Immunol Res. 2013;56(2–3):249–260. doi: 10.1007/s12026-013-8394-y. [DOI] [PubMed] [Google Scholar]
- 93.Macglashan DW., Jr IgE-dependent signaling as a therapeutic target for allergies. Trends Pharmacol Sci. 2012;33(9):502–509. doi: 10.1016/j.tips.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S73–80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95•.Simon D, Marti H, Heer P, Simon HU, Braathen LR, Straumann A. Eosinophilic esophagitis is frequently associated with IgE-mediated allergic airway diseases. J Allergy Clin Immunol. 2005;115(5):1090–1092. doi: 10.1016/j.jaci.2005.01.017. Discusses the findings that allergic airway diseases precede EoE suggest that the initial sensitization might take place in the airways and EoE should be considered an additional manifestation of atopy. [DOI] [PubMed] [Google Scholar]
- 96.Kirsch R, Bokhary R, Marcon MA, Cutz E. Activated mucosal mast cells differentiate eosinophilic (allergic) esophagitis from gastroesophageal reflux disease. J Pediat Gastroenterol Nutr. 2007;44(1):20–26. doi: 10.1097/MPG.0b013e31802c0d06. [DOI] [PubMed] [Google Scholar]
- 97•.Rothenberg ME, Mishra A, Brandt EB, Hogan SP. Gastrointestinal eosinophils. Immunol Rev. 2001;179:139–155. doi: 10.1034/j.1600-065x.2001.790114.x. Describes eosinophils as resident cells of the gastrointestinal immune system (innate, regulatory and inflammatory immune responses) and their levels are enhanced by antigen exposure under Th2 conditions and regulated by eotaxin and IL-5. [DOI] [PubMed] [Google Scholar]
- 98.Uhm TG, Kim BS, Chung IY. Eosinophil development, regulation of eosinophil-specific genes, and role of eosinophils in the pathogenesis of asthma. Allergy, Asthma Immunol Res. 2012;4(2):68–79. doi: 10.4168/aair.2012.4.2.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kita H. Eosinophils: multifunctional and distinctive properties. Int Arch Allergy Immunol. 2013;161(Suppl 2):3–9. doi: 10.1159/000350662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gleich GJ, Frigas E, Loegering DA, Wassom DL, Steinmuller D. Cytotoxic properties of the eosinophil major basic protein. J Immunol. 1979;123(6):2925–2927. [PubMed] [Google Scholar]
- 101.Abu-Ghazaleh RI, Dunnette SL, Loegering DA, et al. Eosinophil granule proteins in peripheral blood granulocytes. J Leukoc Biol. 1992;52(6):611–618. doi: 10.1002/jlb.52.6.611. [DOI] [PubMed] [Google Scholar]
- 102.Talley NJ, Kephart GM, Mcgovern TW, Carpenter HA, Gleich GJ. Deposition of eosinophil granule major basic protein in eosinophilic gastroenteritis and celiac disease. Gastroenterology. 1992;103(1):137–145. doi: 10.1016/0016-5085(92)91106-e. [DOI] [PubMed] [Google Scholar]
- 103•.Mueller S, Aigner T, Neureiter D, Stolte M. Eosinophil infiltration and degranulation in oesophageal mucosa from adult patients with eosinophilic oesophagitis: a retrospective and comparative study on pathological biopsy. J Clin Pathol. 2006;59(11):1175–1180. doi: 10.1136/jcp.2005.031922. Compares the detection of eosinophil numbers and degranulation via hematoxylin and eosin staining versus immunohistochemistry staining by monoclonal antibody for human eosinophilic major basic protein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rothenberg ME. Eosinophilic gastrointestinal disorders (EGID) J Allergy Clin Immunol. 2004;113(1):11–28. doi: 10.1016/j.jaci.2003.10.047. quiz 29. [DOI] [PubMed] [Google Scholar]
- 105••.Zhu X, Wang M, Mavi P, et al. Interleukin-15 expression is increased in human eosinophilic esophagitis and mediates pathogenesis in mice. Gastroenterology. 2010;139(1):182–193. e187. doi: 10.1053/j.gastro.2010.03.057. Reports the induction of IL-15 in human EoE biopsies and showed that IL-15 mediates pathogenesis of experimental EoE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mishra A, Hogan SP, Brandt EB, Rothenberg ME. IL-5 promotes eosinophil trafficking to the esophagus. J Immunol. 2002;168(5):2464–2469. doi: 10.4049/jimmunol.168.5.2464. [DOI] [PubMed] [Google Scholar]
- 107.Neilsen CV, Bryce PJ. Interleukin-13 directly promotes oesophagus production of CCL11 and CCL24 and the migration of eosinophils. Clin Exp Allergy. 2010;40(3):427–434. doi: 10.1111/j.1365-2222.2009.03419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Blanchard C, Mishra A, Saito-Akei H, Monk P, Anderson I, Rothenberg ME. Inhibition of human interleukin-13-induced respiratory and oesophageal inflammation by anti-humaninterleukin- 13 antibody (CAT-354) Clin Exp Allergy. 2005;35(8):1096–1103. doi: 10.1111/j.1365-2222.2005.02299.x. [DOI] [PubMed] [Google Scholar]
- 109.Blanchard C, Mingler MK, Vicario M, et al. IL-13 involvement in eosinophilic esophagitis: transcriptome analysis and reversibility with glucocorticoids. J Allergy Clin Immunol. 2007;120(6):1292–1300. doi: 10.1016/j.jaci.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 110.Burton JD, Bamford RN, Peters C, et al. A lymphokine, provisionally designated interleukin T and produced by a human adult T-cell leukemia line, stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc Natl Acad Sci USA. 1994;91(11):4935–4939. doi: 10.1073/pnas.91.11.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Grabstein KH, Eisenman J, Shanebeck K, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264(5161):965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 112.Meresse B, Chen Z, Ciszewski C, et al. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity. 2004;21(3):357–366. doi: 10.1016/j.immuni.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 113.Kennedy MK, Glaccum M, Brown SN, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191(5):771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hogan SP, Mishra A, Brandt EB, Foster PS, Rothenberg ME. A critical role for eotaxin in experimental oral antigen-induced eosinophilic gastrointestinal allergy. Proc Natl Acad Sci USA. 2000;97(12):6681–6686. doi: 10.1073/pnas.97.12.6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115•.Niranjan R, Mavi P, Rayapudi M, Dynda S, Mishra A. Pathogenic role of mast cells in experimental eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol. 2013;304(12):G1087–G1094. doi: 10.1152/ajpgi.00070.2013. Provides the direct evidence that mast cells promote muscle cell hyperplasia and hypertrophy, and may have a significant role in promoting esophageal functional abnormalities in EoE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bendelac A, Bonneville M, Kearney JF. Autoreactivity by design: innate B and T lymphocytes. Nat Rev Immunol. 2001;1(3):177–186. doi: 10.1038/35105052. [DOI] [PubMed] [Google Scholar]
- 117.Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annu Rev Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- 118.Wilson SB, Delovitch TL. Janus-like role of regulatory iNKT cells in autoimmune disease and tumour immunity. Nat Rev Immunol. 2003;3(3):211–222. doi: 10.1038/nri1028. [DOI] [PubMed] [Google Scholar]
- 119.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 120.Pellicci DG, Hammond KJ, Uldrich AP, Baxter AG, Smyth MJ, Godfrey DI. A natural killer T (NKT) cell developmental pathway involving a thymus-dependent NK1.1(−)CD4(+) CD1d-dependent precursor stage. J Exp Med. 2002;195(7):835–844. doi: 10.1084/jem.20011544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Watarai H, Sekine-Kondo E, Shigeura T, et al. Development and function of invariant natural killer T cells producing T(h)2- and T(h)17-cytokines. PLoS Biol. 2012;10(2):e1001255. doi: 10.1371/journal.pbio.1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sakuishi K, Oki S, Araki M, Porcelli SA, Miyake S, Yamamura T. Invariant NKT cells biased for IL-5 production act as crucial regulators of inflammation. J Immunol. 2007;179(6):3452–3462. doi: 10.4049/jimmunol.179.6.3452. [DOI] [PubMed] [Google Scholar]
- 123.Akbari O. The role of iNKT cells in development of bronchial asthma: a translational approach from animal models to human. Allergy. 2006;61(8):962–968. doi: 10.1111/j.1398-9995.2006.01124.x. [DOI] [PubMed] [Google Scholar]
- 124.Meyer EH, Dekruyff RH, Umetsu DT. iNKT cells in allergic disease. Curr Top Microbiol Immunol. 2007;314:269–291. doi: 10.1007/978-3-540-69511-0_11. [DOI] [PubMed] [Google Scholar]
- 125.Iwamura C, Nakayama T. Role of NKT cells in allergic asthma. Curr Opin Immunol. 2010;22(6):807–813. doi: 10.1016/j.coi.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 126••.Rayapudi M, Rajavelu P, Zhu X, et al. Invariant natural killer T-cell neutralization is a possible novel therapy for human eosinophilic esophagitis. Clin Translational Immunol. 2013 doi: 10.1038/cti.13. Shows for the first time that iNKT cells have a critical pathogenic role in human and experimental EoE, and anti-mCD1d- and anti-hVα24Jα18-neutralizing antibody treatment protects allergen-induced experimental EoE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Debrosse CW, Case JW, Putnam PE, Collins MH, Rothenberg ME. Quantity and distribution of eosinophils in the gastrointestinal tract of children. Pediatr Dev Pathol. 2006;9(3):210–218. doi: 10.2350/11-05-0130.1. [DOI] [PubMed] [Google Scholar]
- 128.Boyce JA. The pathobiology of eosinophilic inflammation. Allergy Asthma Proc. 1997;18(5):293–300. doi: 10.2500/108854197778590489. [DOI] [PubMed] [Google Scholar]
- 129.Desreumaux P, Capron M. Eosinophils in allergic reactions. Curr Opin Immunol. 1996;8(6):790–795. doi: 10.1016/s0952-7915(96)80006-9. [DOI] [PubMed] [Google Scholar]
- 130.Gleich GJ, Ottesen EA, Leiferman KM, Ackerman SJ. Eosinophils and human disease. Int Arch Allergy Appl Immunol. 1989;88(1–2):1–2. doi: 10.1159/000234749. [DOI] [PubMed] [Google Scholar]
- 131.Mishra A, Hogan SP, Brandt EB, Rothenberg ME. An etiological role for aeroallergens and eosinophils in experimental esophagitis. J Clin Invest. 2001;107(1):83–90. doi: 10.1172/JCI10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hogan SP, Mishra A, Brandt EB, et al. A pathological function for eotaxin and eosinophils in eosinophilic gastrointestinal inflammation. Nat Immunol. 2001;2(4):353–360. doi: 10.1038/86365. [DOI] [PubMed] [Google Scholar]
- 133.Rubinstein E, Cho JY, Rosenthal P, et al. Siglec-F inhibition reduces esophageal eosinophilia and angiogenesis in a mouse model of eosinophilic esophagitis. J Pediat Gastroenterol Nutr. 2011;53(4):409–416. doi: 10.1097/MPG.0b013e3182182ff8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rajavelu RRM, Zhu X, Narayanan S, et al. Induction and activation of invariant natural killer T cells is critical in the pathogenesis of eosinophilic esophagitis Mucosa Immunology. Am J Physiol Gastrointest Liver Physiol. 2012;302(7):645–654. [Google Scholar]
- 135.Blanchard C, Stucke EM, Rodriguez-Jimenez B, et al. A striking local esophageal cytokine expression profile in eosinophilic esophagitis. J Allergy Clin Immunol. 2011;127(1):208–217. 217 e201–e207. doi: 10.1016/j.jaci.2010.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mishra A, Hogan SP, Brandt EB, et al. Enterocyte expression of the eotaxin and interleukin-5 transgenes induces compartmentalized dysregulation of eosinophil trafficking. J Biol Chem. 2002;277(6):4406–4412. doi: 10.1074/jbc.M110424200. [DOI] [PubMed] [Google Scholar]
- 137.Straumann A. Eosinophilic esophagitis: a bulk of mysteries. Dig Dis. 2013;31(1):6–9. doi: 10.1159/000347095. [DOI] [PubMed] [Google Scholar]
- 138.Dellon ES. Eosinophilic esophagitis. Gastroenterol Clin North Am. 2013;42(1):133–153. doi: 10.1016/j.gtc.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Roman S, Savarino E, Savarino V, Mion F. Eosinophilic oesophagitis: from physiopathology to treatment. Dig Liver Dis. 2013;45(11):871–878. doi: 10.1016/j.dld.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 140.Schroeder S, Capocelli KE, Masterson JC, et al. Effect of proton pump inhibitor on esophageal eosinophilia. J Pediat Gastroenterol Nutr. 2013;56(2):166–172. doi: 10.1097/MPG.0b013e3182716b7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Spergel JM, Brown-Whitehorn T, Beausoleil JL, Shuker M, Liacouras CA. Predictive values for skin prick test and atopy patch test for eosinophilic esophagitis. J Allergy Clin Immunol. 2007;119(2):509–511. doi: 10.1016/j.jaci.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 142.Lieberman JA, Morotti RA, Konstantinou GN, Yershov O, Chehade M. Dietary therapy can reverse esophageal subepithelial fibrosis in patients with eosinophilic esophagitis: a historical cohort. Allergy. 2012;67(10):1299–1307. doi: 10.1111/j.1398-9995.2012.02881.x. [DOI] [PubMed] [Google Scholar]
- 143.Lucendo AJ, Arias A, De Rezende LC, et al. Subepithelial collagen deposition, profibrogenic cytokine gene expression, and changes after prolonged fluticasone propionate treatment in adult eosinophilic esophagitis: a prospective study. J Allergy Clin Immunol. 2011;128(5):1037–1046. doi: 10.1016/j.jaci.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 144.Dohil R, Newbury R, Fox L, Bastian J, Aceves S. Oral viscous budesonide is effective in children with eosinophilic esophagitis in a randomized, placebo-controlled trial. Gastroenterology. 2010;139(2):418–429. doi: 10.1053/j.gastro.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 145.Schaefer ET, Fitzgerald JF, Molleston JP, et al. Comparison of oral prednisone and topical fluticasone in the treatment of eosinophilic esophagitis: a randomized trial in children. Clin Gastroenterol Hepatol. 2008;6(2):165–173. doi: 10.1016/j.cgh.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 146•.Straumann A, Conus S, Degen L, et al. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology. 2010;139(5):1526–1537. 1537 e1521. doi: 10.1053/j.gastro.2010.07.048. Describes a 15-day course of treatment of adolescent and adult active EoE patients with budesonide that is well tolerated and highly effective in reducing a histologic and clinical symptoms. [DOI] [PubMed] [Google Scholar]
- 147.Lucendo AJ, De Rezende LC, Jimenez-Contreras S, et al. Montelukast was inefficient in maintaining steroid-induced remission in adult eosinophilic esophagitis. Digest Dis Sci. 2011;56(12):3551–3558. doi: 10.1007/s10620-011-1775-y. [DOI] [PubMed] [Google Scholar]
- 148.Attwood SE, Lewis CJ, Bronder CS, Morris CD, Armstrong GR, Whittam J. Eosinophilic oesophagitis: a novel treatment using Montelukast. Gut. 2003;52(2):181–185. doi: 10.1136/gut.52.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Filik L. Montelukast and maintenance of steroid-induced remission in eosinophilic esophagitis. Digest Dis Sci. 2012;57(1):258–259. doi: 10.1007/s10620-011-1923-4. [DOI] [PubMed] [Google Scholar]
- 150.Mepolizumab: 240563, anti-IL-5 monoclonal antibody – GlaxoSmithKline, anti-interleukin-5 monoclonal antibody – GlaxoSmithKline, SB 240563. Drugs RD. 2008;9(2):125–130. doi: 10.2165/00126839-200809020-00006. [DOI] [PubMed] [Google Scholar]
- 151.Stein ML, Villanueva JM, Buckmeier BK, et al. Anti-IL-5 (mepolizumab) therapy reduces eosinophil activation ex vivo and increases IL-5 and IL-5 receptor levels. J Allergy Clin Immunol. 2008;121(6):1473–1483. 1483 e1471–1474. doi: 10.1016/j.jaci.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Straumann A, Conus S, Grzonka P, et al. Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: a randomised, placebo-controlled, double-blind trial. Gut. 2010;59(1):21–30. doi: 10.1136/gut.2009.178558. [DOI] [PubMed] [Google Scholar]
- 153.Spergel JM, Rothenberg ME, Collins MH, et al. Reslizumab in children and adolescents with eosinophilic esophagitis: results of a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2012;129(2):456–463. 463 e451–453. doi: 10.1016/j.jaci.2011.11.044. [DOI] [PubMed] [Google Scholar]
- 154.Abonia JP, Rothenberg ME. Eosinophilic esophagitis: rapidly advancing insights. Annual Rev Med. 2012;63:421–434. doi: 10.1146/annurev-med-041610-134138. [DOI] [PubMed] [Google Scholar]
- 155.Moawad FJ, Cheatham JG, Dezee KJ. Meta-analysis: the safety and efficacy of dilation in eosinophilic oesophagitis. Ailment Pharmacol Ther. 2013;38(7):713–720. doi: 10.1111/apt.12438. [DOI] [PubMed] [Google Scholar]
- 156.Kavitt RT, Penson DF, Vaezi MF. Eosinophilic esophagitis: dilate or medicate? A cost analysis model of the choice of initial therapy. Dis Esophagus. 2012 doi: 10.1111/j.1442-2050.2012.01409.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Colombo JM, Neilan NA, Schurman JV, Friesen CA. Validation of methods to assess potential biomarkers in pediatric patients with esophageal eosinophilia. World J Gastrointest Pharmacol Ther. 2013;4(4):113–119. doi: 10.4292/wjgpt.v4.i4.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Children’s Hospital Medical Center. WO2013155010 A1. 2013