Abstract
The incidence of melanoma is one of the fastest growing of all tumor types in the United States and the number of cases worldwide has doubled in the past 30 years. Melanoma, which arises from melanocytes, is an extremely aggressive tumor that invades the vascular and lymphatic systems to establish tumors elsewhere in the body. Melanoma is a particularly resilient cancer and systemic therapy approaches have achieved minimal success against metastatic melanoma resulting in only a few FDA-approved treatments with limited benefit. Leading treatments offer minimal efficacy with response rates generally under 15% in the long term with no clear effect on melanoma-related mortality. Even the recent success of the specific BRAF mutant inhibitor vemurafenib has been tempered somewhat since acquired resistance is rapidly observed. Thus, understanding the mechanism(s) of melanoma carcinogenesis is paramount to combating this deadly disease. Not only for the treatment of melanoma but, ultimately, for prevention. In this report, we will summarize our work to date regarding the characterization of ultraviolet radiation (UVR)-mediated melanomagenesis and highlight several promising avenues of ongoing research.
Keywords: Melanoma; ultraviolet radiation, photocarinogenesis
Introduction
Melanocytes are essential to protecting the skin from the harmful effects of UV radiation. Paradoxically, melanocytes are the precursors of the most deadly form of skin cancer, melanoma (1). Melanoma is the eighth most common U.S. malignancy, and the incidence is rising. In 1935, the lifetime risk of melanoma was 1 in 1500. Americans now have a greater than 1 in 50 chance of developing malignant melanoma. Data from the Surveillance, Epidemiology, and End Results (SEER) Program indicate that the incidence of melanoma is one of the fastest growing tumor types in the United States and the number of cases has doubled in the past 30 years (1, 2). SEER also suggests that melanoma incidence increases with age with altered patterns in men and women. Melanoma is an extremely aggressive tumor and highly resistant to current therapies (3). If melanoma is detected early, before the tumor becomes invasive, it can be cured through surgical resection. Unfortunately, melanoma lesions can remain unidentifiable or asymptomatic for long periods of time (3). Melanoma is a particularly resilient and aggressive cancer, accounting for only 4% of all skin cancers but responsible for 80% of skin cancer deaths (4). Further, only 14% of patients with metastatic melanoma survive for 5 years (4). Therefore, understanding the etiology of this disease is paramount.
Several epidemiological studies have investigated melanoma risk factors. These factors include family history of melanoma, number of dysplastic nevi, age, skin type and, of course, UVR exposure (2, 5). Evidence for the role of UVR in melanoma etiology is abundant. Fair-skinned people, particularly with blond or red hair that burn easily, have a higher risk of melanoma (6). Further, the incidence of melanoma among the white population correlates with location. The prime example is Australia, which has the world's highest melanoma incidence rate due to its subtropical climate with a largely Celtic population (6). Counterintuitively, sporadic UV-B exposure and not cumulative UVR exposure is a significant risk factor for melanoma. In particular, intense, intermittent exposure and blistering sunburns early in childhood and adolescence are associated with increased risk (2). However, the underlying mechanism(s) for this apparent dichotomy have not been elucidated.
Mechanisms underlying UV-mediated skin cancer have been the focus of intense research over the last 45 years or so ever since the seminal observation by Jim Cleaver and colleagues that people with the disease xeroderma pigmentosum develop fatal UV-mediated skin cancers (both non-melanoma and melanoma) due to defective DNA repair (7). Our lab has been among those investigating UV-mediated carcinogenesis over the last few decades, with a particular interest in melanoma. Here we review a swatch of our published research, present novel findings and discuss the ongoing elucidation of mechanisms underlying UV-mediated melanomagenesis.
RelA, p50 and inhibitor of Kappa B alpha are elevated in melanoma and respond aberrantly to UV-B
Our journey into the field of UV-B carcinogenesis began with our interest in nuclear factor kappa B (NFκB), which is known to play a vital role in the control of apoptosis (8). NFκB activation can be both pro- and anti-apoptotic in various cell types (8, 9). There are five mammalian NFκB/Rel family members, p50, p52, RelA, RelB and cRel that all share a highly conserved domain responsible for dimerization, nuclear localization and DNA binding (8-11). These proteins can form both homo- and heterodimers which yields differential induction of genes at NFκB binding sites in the promoter regions of a wide variety of genes (12). Several studies have shown NFκB transcription factors are associated with the genesis of several cancers including colon, breast and ovarian (13, 14).
Since all cancers must find a way to inhibit apoptosis our lab focused on NFκB regulation in normal melanocytes and melanoma. In 1999 we reported that NFκB expression and binding is altered in melanoma compared to normal melanocytes (15). Since UV-B is one environmental stress that can activate NFκB signaling, we next investigated the effect of UV-B irradiation on the regulation of the NFκB signaling pathway in human melanocytes and metastatic melanoma cell lines. In 2001, we reported that melanoma cell lines had higher nuclear levels of the NFκB subunits p50 and RelA as compared to normal human melanocytes (16). The increase in protein expression reported was 7-fold for p50 and 5-10-fold for RelA (16). This report also demonstrated that melanoma cells had higher cytoplasmic expression of RelA, p50 and of the inhibitor of kappa B alpha (IκBα) than melanocytes (16). Furthermore, we demonstrated that the response of p50 and IκBα protein levels to UV-B was dysregulated in melanoma compared to melanocytes. In melanocytes, UV-B exposure results in increased expression levels while, surprisingly, the levels of p50 and RelA decrease in response to UV-B in melanoma. Subsequently, we showed that inhibition of RelA via antisense RelA phosphorothioate oligonucleotides reduced melanoma viability (16). Thus, we concluded that constitutive activation of NFκB in melanoma cell cultures may be a therapeutically attractive target. Importantly, our recognition that UV-mediated signaling is dysfunctional led us to examine potential mechanisms for why/when UV-mediated responses begin to alter and to re-focus our investigations to early events in melanomagenesis.
Melanin as a Pro-oxidant
Melanin, the pigment produced in melanocytes, infuses color into all our skin and is responsible for protecting us from solar radiation. In normal melanocytes, melanin particles are generated in specialized organelles, termed melanosomes, by tyrosinase through successive oxidation of tyrosine (17). These melanosomes can also be transported to adjacent keratinocytes and accumulate in the perinuclear space of keratinocytes and melanocytes as UV-protective “caps” shielding cellular DNA (18). The effect of melanin here is two-pronged, acting as an absorbent filter of UV rays and a physical barrier that scatters UV rays (18). Melanin has also been shown to function as a free radical scavenger and superoxide dismutase in the reduction of reactive oxygen species (ROS) (18). However, if the melanosome is synthesized abnormally o r its structure is disrupted (which can occur for a multitude of reasons) free melanin can be released from its “solid state” into the cytoplasm and function as a pro-oxidant. This topic is very complex (see (18-20)). Paradoxically, generation of melanin is well accepted as a source of ROS and oxidative stress in melanoma (21, 22). In fact, melanosomes in melanoma are poorly formed, with abnormal membranes and granulized melanin (23-25). These irregularities allow release of ROS from the melanosomes into the cytosol (26, 27). Our lab and others have demonstrated that transformed melanocytes contain elevated levels of free radicals and ROS (27-30). Further, our lab extensively characterized the role of redox-responsive signaling pathways in melanoma; including NFκB (outlined above) and APE/Ref1 which are both markedly elevated in melanoma (15, 16, 31, 32). About that time a very interesting paper demonstrated abnormal melanin synthesis in dysplastic nevi; a precursor to melanoma in some cases (33). At the same time, our lab and others had postulated that this abnormal regulation of melanin results in a pro-oxidant activity for melanin (31, 34). In 2008, our lab provided a plausible mechanism for the basis of melanin ‘switching’ from its natural anti-oxidant state to the dysfunctional pro-oxidant form; the culprits…UV-B and metal ions (34). In this report, we demonstrated that UV-B exposure causes morphological changes and bleaching of the melanosome. Both of these effects were dramatically increased with co-treatment of the metal ions Cu (II) or Cd(II). Furthermore, we directly showed that UV-B + Cu (II) treatment caused bleaching of melanocytes through increased generation of hydroxyl radical (34). Thus, the course of our lab turned toward prevention of melanoma as we sought to understand better the causal relationship between UV-B, metal ions and initiation of melanoma.
The melanoma metal ion hypothesis; not just a ‘hip’ new theory
Perhaps the most striking epidemiological studies of disease where melanoma popped up unexpectedly, was in long-term follow up studies of patients with hip replacements (35-37). No increase of cancers (including melanoma) was observed in patients who had a metal-on-plastic hip replacement. In contrast, patients who received metal-on-metal hip replacements showed a significant increase in risk for 3 cancers, most notably melanoma (35). Melanoma risk was increased 23% while both prostate and kidney cancer risk increased by 13% (35). A large meta-analysis examining articles over a 38-year period ending in 2004 confirmed an increase in melanoma risk for metal-on-metal hip replacement patients (36). Furthermore, a subsequent study of the large Nordic inpatient registry also confirmed these findings (37). Studies focused on potential causes for this association and reveled that serum from these patients contained 5-10 times the normal levels of hexavalent chromium (Cr6+) and divalent Cobalt (Co2+) in the first 2 years after hip replacement and that levels of these metal ions remain 2-3-fold elevated indefinitely. These increases in circulating metal ions were not observed in patients who had received metal-on-plastic hip replacements (38).
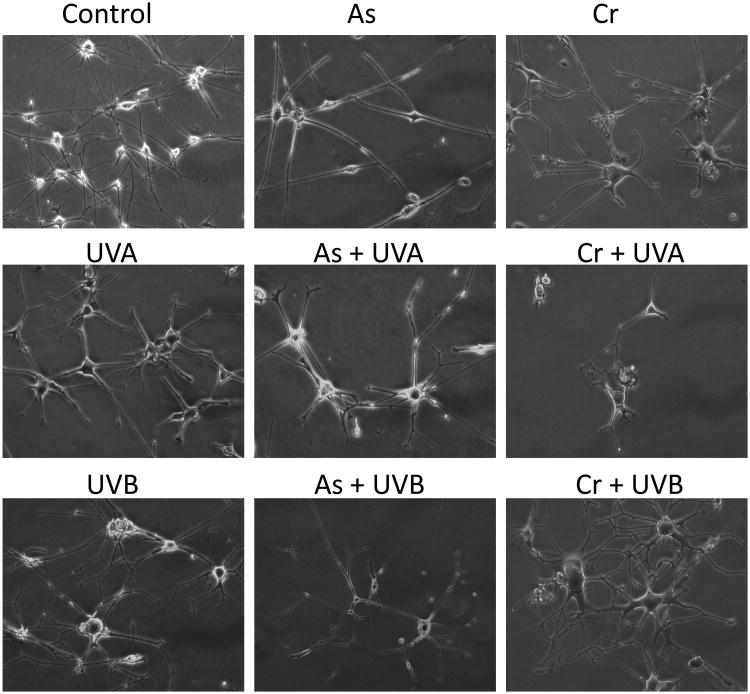
If hexavalent chromium sounds familiar that is most likely because you are a Julia Roberts fan and have seen ‘Erin Brockovich’. That movie was, of course, based on the infamous case of a town in California having an incredibly high cancer incidence rate which was attributed to their drinking water containing unsafe levels of Cr6+. Or it could be because Cr6+ is recognized as a carcinogen by the International Agency for Research on Cancer (IARC). Therefore, it is plausible that Cr6+ is the driving force behind the observed melanomas in metal-on-metal hip replacement patients. We have observed in the laboratory that the Cr6+-treated (1.0 μM) normal human melanocytes exhibited morphological changes after 6 weeks (Figure 1, unpublished data) and led to foci formation after 10 weeks (39). Co-treatment with UV-A (1 J/cm2, twice weekly) or UV-B (25 mJ/cm2, twice weekly) exacerbated the morphological deformity of these cells, namely, the cell body became larger while the number of dendrites increased (Figure 1). In contrast, cells treated with arsenic trioxide (2 μM) alone did not exhibit obvious changes, although co-treatment with UV-A and UV-B did (Figure 1). Treating with UV-A or UV-B alone for 6 weeks resulted different changes: UV-A increased the size of cell body which mimics senescence morphology while UV-B induced cell death in some cells and a more exacerbated morphological changes in the surviving cells (Figure 1).
Figure 1. Morphological changes of normal human melanocytes after treatment with arsenic trioxide or Cr6+ with or without UVA or UV-B radiation.
Cells were cultured in MCDB153 media and As was added into media at 2 μM final concentration or Cr6+ at 1 μM concentration. Cells were irradiated with UV-A (1 J/cm2) or UV-B (25 mJ/cm2) twice every week during the 6 week treatment period. Photos were taken at the end of the 6 week with initial 200× magnitude. UV-A and UV-B treatment alone caused different morphological changes in these cells, and co-treatment with As or Cr6+ modified the changes. Cr6+/UV-B treatment caused the most severe morphological changes.
Based on these findings, we proposed the overall theory that redox-active metals, which are widely dispersed in our environment, provide a basis for the “second” hit (UV being the first hit) and are the co-carcinogens in melanomagenesis (40). These reactive metals can lead to the generation of reactive oxygen species (ROS) in collaboration with melanin-bound iron present due to a blistering sunburn (maybe in childhood) that can give rise to DNA mutations and eventually melanoma.
Further evidence for this “hip” theory lies in a report indicating that metallothionein expression in primary melanomas is a strong prognostic factor for survival (41). This study examined 1270 patients prospectively and observed a dramatic decrease in survival for patients with high metallothioein expression (41). Since metallothioein regulates heavy metal uptake, this result strongly supports our melanoma metal ion hypothesis. The missing link is…where is the metal ion coming from? The easy answer is environmental exposure, but it could also be as simple as copper already present in the body. Large amounts of Cu2+ become available in the melanosome during melanin synthesis (which is governed by the copper dependent enzyme tyrosinase). If the melanosome becomes damaged release of Cu2+ occurs.
When a single bullet theory isn't logical, look for a second bullet
Based on our studies described above, the lack of “classical” UV-B induced DNA damage present in melanoma, and solid epidemiological studies indicating a lack of a direct causal relationship between UV-B exposure and melanoma incidence (40), we have postulated that a second co-carcinogen is required for melanomagenesis in many cases. In this section we will describe the interesting directions our lab has taken in pursuit of co-carcinogenic mechanisms of melanoma initiation and progression.
Unique gender difference in early onset melanoma
Another approach our laboratory took in our quest to elucidate the mechanisms of UV-mediated melanomagenesis was a purely epidemiological one. We mined the US SEER17 Registry database for age-specific melanoma incidence rates and compared males to females. We found that the relative risk (RR) for females was significantly higher for people 44 years old and younger as compared to males (42). The largest difference was observed for females 20-24 years old (RR=2.01, 95% CI= 1.21-3.33). Conversely, males exhibited higher melanoma incidence rates after age 44 (42). These results were confirmed using a second data set, the Nordic Cancer Registry. Importantly, the same bimodal gender effect was not observed for non-melanoma skin cancer incidence (NMSC), which is known to be strongly associated with cumulative solar UV exposure. Thus, we concluded that exposure to solar UV radiation is the major causative factor for melanoma at older age (>44 years), other factors may be playing a key role in early onset melanomas, especially in females (42). We hypothesize that these factors include estrogen and estrogen receptors, as well as insulin and insulin-like growth factor I (IGF1), a complex regulation of these hormones and growth factors during development or pregnancy may account for the dramatic changes of cell proliferation. Increased cell proliferation, if it goes awry, will lead to melanomagenesis.
UV-induced, NOX-mediated oxidative stress in melanomagenesis
While UV-B induced DNA signature mutations are not as common in melanoma as in NMSC, UV-A induced oxidative DNA damage has been assumed as a causative factor for melanomagenesis. Ninety percent of the solar UV radiation that reaches the earth's surface is UV-A. These longer wavelengths are able to penetrate skin deeper to reach melanocytes which lay between the epidermal and dermal junction. UV-A is known to induce reactive oxygen species, but how these ROS are generated was not clear. Our recent data showed that melanocytes express NADPH oxidase 1, a superoxide-generating enzyme (43), which is induced by UV-A and UV-B (our unpublished data). Nox1 was shown to be a major ROS source after UV radiation in human keratinocytes (44), and we speculate that this may also be true in melanocytes. If this is true, then a novel melanoma etiology pathway may be identifiable and can be engaged in future prevention studies.
UV-mediated regulation of the UDP-Glucuronosyltransferases (UGTs)
Recently, we identified three UGT family members (UGT2B7, UGT2B10 and UGT2B15) as being normally expressed in human melanocytes (45). The same three UGT family members were also expressed in the primary melanoma cell line WM115. No UGT expression was detected in another primary melanoma cell line, WM3211, or in any metastatic melanoma cell line examined. These results suggest that UGT expression is lost during melanoma progression (46).
The UGT family of enzymes catalyzes the glucuronidation of a wide range of xenobiotic and endogenous compounds. UGTs conjugate a glucuronic acid moiety to their substrates, altering the biological properties of the substrate and enhancing its excretion in urine or bile (47, 48). In general, glucuronidation converts substrates into less bioactive, more water soluble products facilitating their removal from the body. In this manner, glucuronidation is a major conjugation pathway that serves as a detoxification mechanism for numerous dietary and environmental chemicals including carcinogens (47, 49, 50). Genetic polymorphisms have been identified in several human UGT family members that alter their expression and/or activity (47, 49). Overwhelming epidemiological data has established the link between these polymorphisms and cancer risk. Case-control studies have demonstrated UGT polymorphisms that result in reduced glucuronidation activity have been linked to increased risk for several cancers including breast (51), colon (52), liver (53), oralaryngeal (54), pancreatic (55), and lung (56). Thus, it is clear that altered UGT function is a risk factor for cancer, most likely by increasing the cells exposure to carcinogens due to reduced clearance. Therefore, we hypothesized that the observed loss of UGTs during melanoma progression could be an early event in some melanomas and investigated whether UGT expression was regulated by UV-B radiation. This observation needs to be followed-up with a detailed moleculat epidemiologic study of UGT polymorphisms and melanoma risk.
Downregulation of UGT expression in human melanocytes following acute UV-B exposure
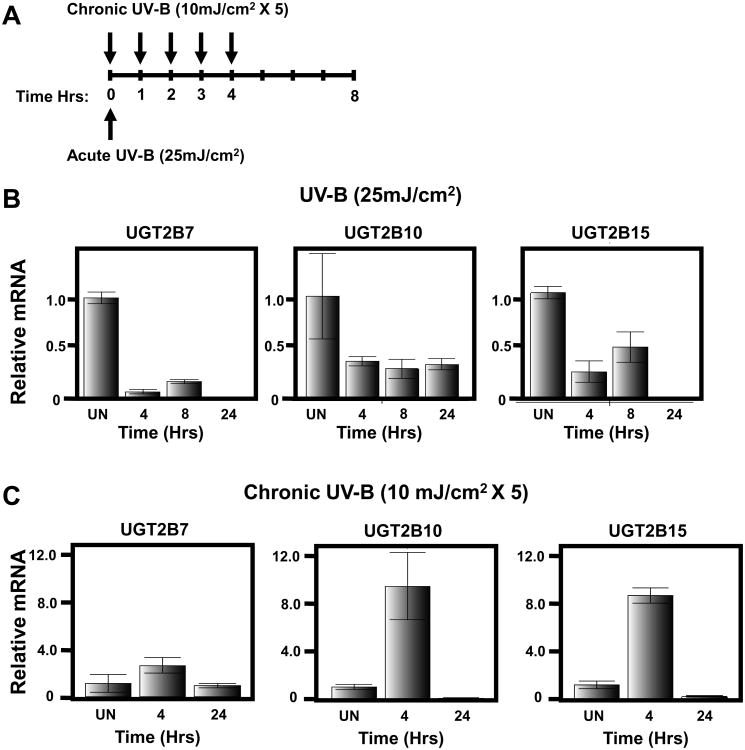
To determine if UV-B could regulate UGT expression in melanocytes, human melanocytes were isolated from de-identified neonatal foreskins and cultured as described previously (45). These cells were then exposed to a single dose of UV-B at 25 mJ/cm2, which approximates a sunburn dose (See Schematic Figure 2A). Cells were subsequently collected at 4, 8 and 24 hrs post irradiation and assayed for UGT expression using real-time PCR normalizing to GAPDH expression as described in materials and methods. Untreated melanocytes were also collected and assayed for UGT expression as a control. As shown in Figure 2B, expression levels of UGT2B7, UGT2B10 and UGT2B15 are all decreased in response to an acute UV-B dose. Specifically, UGT2B7 expression is significantly decreased 4 and 8 hrs post irradiation and is undetectable by 24 hours post exposure. UGT2B10 expression is decreased by 4 hrs and remains low 24 hours post treatment while UGT2B15 expression levels are reduced at 4 and 8 hrs, but undetectable by 24 hours post irradiation. This is the first demonstration that UGT expression can be regulated by UV-B exposure and is consistent with an increased risk of melanoma associated with UVR exposure.
Figure 2. Differential regulation of UGT expression by acute sunburn vs chronic suberythemal UV-B exposure.
(A) Schematic of exposure schedule for acute vs chronic UV-exposure. (B) The indicated predesigned Taqman gene expression assay was used to visualize individual UGT expression by real-time PCR following treatment of primary human melanocytes isolated from a Caucasian individual at 4, 8 and 24 hrs post acute, sunburn, UV-B irradiation (25 mJ/cm2). (C) UGT Taqman assays of Caucasian melanocytes at 4 and 24 hrs post chronic UV-B exposure (10 mJ/cm2 × 5 treatments). All assays are normalized to GAPDH and performed in triplicate. UN=Untreated.
Upregulation of UGT expression in human melanocytes following chronic UV-B exposure
To determine if chronic exposure to UV-B could also regulate UGT expression in human melanocytes, cultured melanocytes from the same subject as above (notably this infant was Caucasian) were used and exposed to a sub-erythemal UV-B dose of 10 mJ/cm2 every hour for a total of five treatments (See Schematic Figure 2A). Thus, the cumulative exposure to these cells is 50 mJ/cm2, twice that of the acute exposure. Cells were collected at 4 and 24 hrs post irradiation and untreated control cells were also collected and UGT expression was examined. In contrast to the acute expose results above, the expression of UGT2B7, UGT2B10 and UGT2B15 were upregulated in response to chronic UV-B exposure (Figure 2C). Specifically, UGT2B7, UGT2B10 and UGT2B15 levels were elevated by 4 hrs, but had returned to normal or reduced levels by 24 hrs.
Since UGTs detoxify carcinogens, we hypothesize that this observed reduction in UGT expression after acute UV-B exposure may account for the increased melanoma risk known to be associated with acute (but not chronic) exposures.
Moving forward, we will attempt to identify potential UGT substrates that could be acting as co-carcinogens in melanoma initiation. The UGTs detoxify environmental carcinogens as well as endogenously produced toxins and carcinogens. One excellent example of the latter would be the carcinogenic metabolites of estrogen. These are intriguing candidates as it could unify several of our lines of investigation. Catechol estrogens are major estrogen metabolites in mammals and they have been shown to be carcinogenic (57). Catechol estrogens are produced by cytochrome P450 oxidation of estrogen. Cytochrome P450 is a heme-dependent enzyme and thus would be dependent on oxygen and metal ions (58). Further oxidation of these catechols to estrogen-o-quinones is mediated through oxidative enzymes, metal ions and molecular oxygen. Estrogen-o-quinones have also been implicated in estrogen-linked carcinogenesis (58). Interestingly, UGT2B7 has been shown to have the highest activity of any UGT in the detoxification of catechol estrogens (59) and has high activity against other estrogen metabolites upstream of the catechols (60). Therefore, UGT2B7 expression in melanocytes may be vital to the normal regulation of estrogen in these cells, especially in young women, which in turn would prevent melanoma initiation. Our lab is currently following up on this hypothesis.
Summary
Our lab is actively pursuing several provocative avenues of investigation centered on characterizing the role of UV radiation in melanoma etiology. We are resolved to continue to shine a bright light on the field of UV-mediated carcinogenesis in (hopefully) a similar manner to how Jim Cleaver so eloquently has done throughout his career.
Methods
Reagents and Cell Culture
Normal human melanocytes were isolated from de-identified newborn foreskin from circumcision surgery in accordance with a protocol approved by UC Irvine's Internal Review Board. Melanocytes were isolated as previously described (61, 62) and cultured in MCDB153 media supplemented with 2% fetal bovine serum, 10 ng/ml of 12-O-tetradecanoylphobol-13-acetate and 0.15% bovine pituitary extract. Pterostilbene was obtained from ChromaDex, Inc. (Irvine, CA).
UV radiation of melanocytes
For UV-A treatment, culture media was removed and cells were kept in 1×PBS. UV-A lamps (peak at 340 nm) were turned on for a specified time so that the cells received the designated dose. Cells were then changed to culture media with or without metals and return to incubators. UV-A and UV-B treatment occurred every Tuesday and Friday during the treatment period. UV-B radiation was performed as previously described (63). Briefly, Cells were grown to about 70% confluence and media was removed completely for UV-B radiation. UV-B radiation was performed in a Stratagen crosslinker with peak wavelength at 312 nm. The UV intensity was measured by a radiometer with proper probes. The culture media was replaced immediately after radiation and cells were returned to a 37°C incubator to recover. For chronic exposures, this process was repeated every hour for a total of five exposures. Times indicated always reflect time following first exposure.
Total RNA Isolation, Reverse Transcription and Real-Time PCR
Total RNA was isolated from cells using the Arum total RNA mini Kit (BioRad) according to companies provided protocol. RNA was quantitated using a NanoDrop 1000 (Thermo/Fisher) cDNA was then made from 1.0 μg of RNA using the iScript Reverse Transcriptase Kit (BioRad) according to standard protocols. To analyze UGT mRNA expression levels in melanocytes real-time PCR was performed as previously described (50, 64). Briefly, pre-designed TaqMan Gene Expression Assays [Applied Biosystems (ID's Hs00426592_m1 for UGT2B7; Hs02556282_s1 for UGT2B10; Hs03008769_g1 for UGT2B15; Hs0016857_m1 for NQO1 and Hs99999905_m1 for GAPDH)] were used according to manufacturer's protocol. Real-time PCR was performed using a total volume of 20 μl containing 50 ng of cDNA using GAPDH as the normalizing ‘housekeeping’ gene. Real-time PCR was performed on a CFX96 Real-Time PCR machine (BioRad). Reported mRNA expression values are the average of at least 3 independent experiments with standard deviation.
Highlights.
Short review on ultraviolet radiation (UVR)-mediated melanomagenesis
Several promising avenues of ongoing research are highlighted
Role of metal ions as co-carcinogens for melanoma is discussed
Potential role of UGTs in the initiation of melanoma is discussed
Potential role of estrogen in early onset melanoma is discussed
Acknowledgments
This work was funded by the generous support of the Waltmar Foundation; grants from the National Institutes of Health: National Cancer Institute [P30CA62330] to FLM and [K07CA160756] to FLS as well as National Institute of Environmental Health Sciences [R03ES019668] to RWD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445(7130):851–857. doi: 10.1038/nature05661. [DOI] [PubMed] [Google Scholar]
- 2.Rigel DS. Epidemiology of melanoma. Semin Cutan Med Surg. 2010;29(4):204–209. doi: 10.1016/j.sder.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Soengas MS, Lowe SW. Apoptosis and melanoma chemoresistance. Oncogene. 2003;22(20):3138–3151. doi: 10.1038/sj.onc.1206454. [DOI] [PubMed] [Google Scholar]
- 4.Miller AJ, Mihm MC., Jr Melanoma. N Engl J Med. 2006;355(1):51–65. doi: 10.1056/NEJMra052166. [DOI] [PubMed] [Google Scholar]
- 5.Kricker A, et al. Ambient UV, personal sun exposure and risk of multiple primary melanomas. Cancer Causes Control. 2007;18(3):295–304. doi: 10.1007/s10552-006-0091-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilchrest BA, Eller MS, Geller AC, Yaar M. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 1999;340(17):1341–1348. doi: 10.1056/NEJM199904293401707. [DOI] [PubMed] [Google Scholar]
- 7.Cleaver JE. Defective repair replication of DNA in xeroderma pigmentosum. Nature. 1968;218(5142):652–656. doi: 10.1038/218652a0. [DOI] [PubMed] [Google Scholar]
- 8.Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87(1):13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 9.Baichwal VR, Baeuerle PA. Activate NF-kappa B or die? Current biology : CB. 1997;7(2):R94–96. doi: 10.1016/s0960-9822(06)00046-7. [DOI] [PubMed] [Google Scholar]
- 10.Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annual review of immunology. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 11.Verma IM, Stevenson JK, Schwarz EM, Van Antwerp D, Miyamoto S. Rel/NF-kappa B/I kappa B family: intimate tales of association and dissociation. Genes & development. 1995;9(22):2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 12.Lin R, Gewert D, Hiscott J. Differential transcriptional activation in vitro by NF-kappa B/Rel proteins. The Journal of biological chemistry. 1995;270(7):3123–3131. doi: 10.1074/jbc.270.7.3123. [DOI] [PubMed] [Google Scholar]
- 13.Dejardin E, et al. Highly-expressed p100/p52 (NFKB2) sequesters other NF-kappa B-related proteins in the cytoplasm of human breast cancer cells. Oncogene. 1995;11(9):1835–1841. [PubMed] [Google Scholar]
- 14.Bours V, Dejardin E, Goujon-Letawe F, Merville MP, Castronovo V. The NF-kappa B transcription factor and cancer: high expression of NF-kappa B- and I kappa B-related proteins in tumor cell lines. Biochemical pharmacology. 1994;47(1):145–149. doi: 10.1016/0006-2952(94)90448-0. [DOI] [PubMed] [Google Scholar]
- 15.Meyskens FL, Jr, Buckmeier JA, McNulty SE, Tohidian NB. Activation of nuclear factor-kappa B in human metastatic melanomacells and the effect of oxidative stress. Clinical cancer research : an official journal of the American Association for Cancer Research. 1999;5(5):1197–1202. [PubMed] [Google Scholar]
- 16.McNulty SE, Tohidian NB, Meyskens FL., Jr RelA, p50 and inhibitor of kappa B alpha are elevated in human metastatic melanoma cells and respond aberrantly to ultraviolet light B. Pigment cell research / sponsored by the European Society for Pigment Cell Research and the International Pigment Cell Society. 2001;14(6):456–465. doi: 10.1034/j.1600-0749.2001.140606.x. [DOI] [PubMed] [Google Scholar]
- 17.Crippa R VHG, Prota P, Sworonos P, Wolfram L. Chemistry of melanins. Academic Press; New York: 1989. [Google Scholar]
- 18.Brenner M, Hearing VJ. The protective role of melanin against UV damage in human skin. Photochemistry and photobiology. 2008;84(3):539–549. doi: 10.1111/j.1751-1097.2007.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meredith P, Sarna T. The physical and chemical properties of eumelanin. Pigment cell research / sponsored by the European Society for Pigment Cell Research and the International Pigment Cell Society. 2006;19(6):572–594. doi: 10.1111/j.1600-0749.2006.00345.x. [DOI] [PubMed] [Google Scholar]
- 20.Plonka PM, et al. What are melanocytes really doing all day long…? Experimental dermatology. 2009;18(9):799–819. doi: 10.1111/j.1600-0625.2009.00912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobson ES, Jenkins ND, Todd JM. Relationship between superoxide dismutase and melanin in a pathogenic fungus. Infection and immunity. 1994;62(9):4085–4086. doi: 10.1128/iai.62.9.4085-4086.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sotomatsu A, Tanaka M, Hirai S. Synthetic melanin and ferric ions promote superoxide anion-mediated lipid peroxidation. FEBS letters. 1994;342(2):105–108. doi: 10.1016/0014-5793(94)80481-8. [DOI] [PubMed] [Google Scholar]
- 23.Curran RC, McCann BG. The ultrastructure of benign pigmented naevi and melanocarcinomas in man. The Journal of pathology. 1976;119(3):135–146. doi: 10.1002/path.1711190303. [DOI] [PubMed] [Google Scholar]
- 24.Rhodes AR, Seki Y, Fitzpatrick TB, Stern RS. Melanosomal alterations in dysplastic melanocytic nevi. A quantitative, ultrastructural investigation. Cancer. 1988;61(2):358–369. doi: 10.1002/1097-0142(19880115)61:2<358::aid-cncr2820610227>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 25.Borovansky J, Mirejovsky P, Riley PA. Possible relationship between abnormal melanosome structure and cytotoxic phenomena in malignant melanoma. Neoplasma. 1991;38(4):393–400. [PubMed] [Google Scholar]
- 26.Jimbow K, et al. Exploitation of pigment biosynthesis pathway as a selective chemotherapeutic approach for malignant melanoma. The Journal of investigative dermatology. 1993;100(2 Suppl):231S–238S. [PubMed] [Google Scholar]
- 27.Riley PA, et al. Melanogenesis-targeted anti-melanoma pro-drug development: effect of side-chain variations on the cytotoxicity of tyrosinase-generated ortho-quinones in a model screening system. European journal of cancer. 1997;33(1):135–143. doi: 10.1016/s0959-8049(96)00340-1. [DOI] [PubMed] [Google Scholar]
- 28.Ito S, Palumbo A, Prota G. Tyrosinase-catalyzed conjugation of dopa with glutathione. Experientia. 1985;41(7):960–961. doi: 10.1007/BF01970033. [DOI] [PubMed] [Google Scholar]
- 29.Zareba M, Bober A, Korytowski W, Zecca L, Sarna T. The effect of a synthetic neuromelanin on yield of free hydroxyl radicals generated in model systems. Biochimica et biophysica acta. 1995;1271(2-3):343–348. doi: 10.1016/0925-4439(95)00058-c. [DOI] [PubMed] [Google Scholar]
- 30.Meyskens FL, Jr, Chau HV, Tohidian N, Buckmeier J. Luminol-enhanced chemiluminescent response of human melanocytes and melanoma cells to hydrogen peroxide stress. Pigment cell research / sponsored by the European Society for Pigment Cell Research and the International Pigment Cell Society. 1997;10(3):184–189. doi: 10.1111/j.1600-0749.1997.tb00482.x. [DOI] [PubMed] [Google Scholar]
- 31.McNulty SE, del Rosario R, Cen D, Meyskens FL, Jr, Yang S. Comparative expression of NFkappaB proteins in melanocytes of normal skin vs. benign intradermal naevus and human metastatic melanoma biopsies. Pigment cell research / sponsored by the European Society for Pigment Cell Research and the International Pigment Cell Society. 2004;17(2):173–180. doi: 10.1111/j.1600-0749.2004.00128.x. [DOI] [PubMed] [Google Scholar]
- 32.Yang S, McNulty S, Meyskens FL., Jr During human melanoma progression AP-1 binding pairs are altered with loss of c-Jun in vitro. Pigment cell research / sponsored by the European Society for Pigment Cell Research and the International Pigment Cell Society. 2004;17(1):74–83. doi: 10.1046/j.1600-0749.2003.00114.x. [DOI] [PubMed] [Google Scholar]
- 33.Pavel S, et al. Disturbed melanin synthesis and chronic oxidative stress in dysplastic naevi. European journal of cancer. 2004;40(9):1423–1430. doi: 10.1016/j.ejca.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 34.Gidanian S, Mentelle M, Meyskens FL, Jr, Farmer PJ. Melanosomal damage in normal human melanocytes induced by UVB and metal uptake--a basis for the pro-oxidant state of melanoma. Photochemistry and photobiology. 2008;84(3):556–564. doi: 10.1111/j.1751-1097.2008.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nyren O, et al. Cancer risk after hip replacement with metal implants: a population-based cohort study in Sweden. Journal of the National Cancer Institute. 1995;87(1):28–33. doi: 10.1093/jnci/87.1.28. [DOI] [PubMed] [Google Scholar]
- 36.Onega T, Baron J, MacKenzie T. Cancer after total joint arthroplasty: a meta-analysis. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2006;15(8):1532–1537. doi: 10.1158/1055-9965.EPI-06-0127. [DOI] [PubMed] [Google Scholar]
- 37.Visuri TI, Pukkala E, Pulkkinen P, Paavolainen P. Cancer incidence and causes of death among total hip replacement patients: a review based on Nordic cohorts with a special emphasis on metal-on-metal bearings. Proceedings of the Institution of Mechanical Engineers Part H, Journal of engineering in medicine. 2006;220(2):399–407. doi: 10.1243/095441105X63282. [DOI] [PubMed] [Google Scholar]
- 38.Silva M, Heisel C, Schmalzried TP. Metal-on-metal total hip replacement. Clinical orthopaedics and related research. 2005;(430):53–61. doi: 10.1097/01.blo.0000149995.84350.d7. [DOI] [PubMed] [Google Scholar]
- 39.Meyskens FL, Yang S. Thinking about the role (largely ignored) of heavy metals in cancer prevention: hexavalent chromium and melanoma as a case in point. Recent Results Cancer Res. 2011;188:65–74. doi: 10.1007/978-3-642-10858-7_5. [DOI] [PubMed] [Google Scholar]
- 40.Meyskens FL, Jr, Berwick M. UV or not UV: metals are the answer. Cancer Epidemiol Biomarkers Prev. 2008;17(2):268–270. doi: 10.1158/1055-9965.EPI-07-0653. [DOI] [PubMed] [Google Scholar]
- 41.Weinlich G, et al. Metallothionein - overexpression as a highly significant prognostic factor in melanoma: a prospective study on 1270 patients. Br J Cancer. 2006;94(6):835–841. doi: 10.1038/sj.bjc.6603028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu F, et al. A unique gender difference in early onset melanoma implies that in addition to ultraviolet light exposure other causative factors are important. Pigment Cell Melanoma Res. 2013;26(1):128–135. doi: 10.1111/pcmr.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu F, Gomez Garcia AM, Meyskens FL., Jr NADPH oxidase 1 overexpression enhances invasion via matrix metalloproteinase-2 and epithelial-mesenchymal transition in melanoma cells. The Journal of investigative dermatology. 2012;132(8):2033–2041. doi: 10.1038/jid.2012.119. [DOI] [PubMed] [Google Scholar]
- 44.Valencia A, Kochevar IE. Nox1-based NADPH oxidase is the major source of UVA-induced reactive oxygen species in human keratinocytes. The Journal of investigative dermatology. 2008;128(1):214–222. doi: 10.1038/sj.jid.5700960. [DOI] [PubMed] [Google Scholar]
- 45.Dellinger RW, Matundan HH, Ahmed AS, Duong PH, Meyskens FL. Anti-Cancer Drugs Elicit Re-expression of UDP-glucuronosyltransferases in Melanoma Cells. Submitted. 2012 doi: 10.1371/journal.pone.0047696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dellinger RW, Matundan HH, Ahmed AS, Duong PH, Meyskens FL., Jr Anti-cancer drugs elicit re-expression of UDP-glucuronosyltransferases in melanoma cells. PLoS One. 2012;7(10):e47696. doi: 10.1371/journal.pone.0047696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagar S, Remmel RP. Uridine diphosphoglucuronosyltransferase pharmacogenetics and cancer. Oncogene. 2006;25(11):1659–1672. doi: 10.1038/sj.onc.1209375. [DOI] [PubMed] [Google Scholar]
- 48.Tukey RH, Strassburg CP. Human UDP-glucuronosyltransferases: metabolism, expression, and disease. Annu Rev Pharmacol Toxicol. 2000;40:581–616. doi: 10.1146/annurev.pharmtox.40.1.581. [DOI] [PubMed] [Google Scholar]
- 49.Desai AA, Innocenti F, Ratain MJ. UGT pharmacogenomics: implications for cancer risk and cancer therapeutics. Pharmacogenetics. 2003;13(8):517–523. doi: 10.1097/01.fpc.0000054116.14659.e5. [DOI] [PubMed] [Google Scholar]
- 50.Dellinger RW, et al. Glucuronidation of PhIP and N-OH-PhIP by UDP-glucuronosyltransferase 1A10. Carcinogenesis. 2007;28(11):2412–2418. doi: 10.1093/carcin/bgm164. [DOI] [PubMed] [Google Scholar]
- 51.Guillemette C, Millikan RC, Newman B, Housman DE. Genetic polymorphisms in uridine diphospho-glucuronosyltransferase 1A1 and association with breast cancer among African Americans. Cancer Res. 2000;60(4):950–956. [PubMed] [Google Scholar]
- 52.Butler LM, et al. Joint effects between UDP-glucuronosyltransferase 1A7 genotype and dietary carcinogen exposure on risk of colon cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2005;14(7):1626–1632. doi: 10.1158/1055-9965.EPI-04-0682. [DOI] [PubMed] [Google Scholar]
- 53.Vogel A, et al. Genetic link of hepatocellular carcinoma with polymorphisms of the UDP-glucuronosyltransferase UGT1A7 gene. Gastroenterology. 2001;121(5):1136–1144. doi: 10.1053/gast.2001.28655. [DOI] [PubMed] [Google Scholar]
- 54.Zheng Z, Park JY, Guillemette C, Schantz SP, Lazarus P. Tobacco carcinogen-detoxifying enzyme UGT1A7 and its association with orolaryngeal cancer risk. Journal of the National Cancer Institute. 2001;93(18):1411–1418. doi: 10.1093/jnci/93.18.1411. [DOI] [PubMed] [Google Scholar]
- 55.Ockenga J, et al. UDP glucuronosyltransferase (UGT1A7) gene polymorphisms increase the risk of chronic pancreatitis and pancreatic cancer. Gastroenterology. 2003;124(7):1802–1808. doi: 10.1016/s0016-5085(03)00294-4. [DOI] [PubMed] [Google Scholar]
- 56.Araki J, et al. Polymorphism of UDP-glucuronosyltransferase 1A7 gene: a possible new risk factor for lung cancer. European journal of cancer. 2005;41(15):2360–2365. doi: 10.1016/j.ejca.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 57.Cavalieri EL, et al. Molecular origin of cancer: catechol estrogen-3, 4-quinones as endogenous tumor initiators. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(20):10937–10942. doi: 10.1073/pnas.94.20.10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bolton JL, Thatcher GR. Potential mechanisms of estrogen quinone carcinogenesis. Chemical research in toxicology. 2008;21(1):93–101. doi: 10.1021/tx700191p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheng Z, et al. Glucuronidation of catechol estrogens by expressed human UDP-glucuronosyltransferases (UGTs) 1A1, 1A3, and 2B7. Toxicological sciences : an official journal of the Society of Toxicology. 1998;45(1):52–57. doi: 10.1006/toxs.1998.2494. [DOI] [PubMed] [Google Scholar]
- 60.Guillemette C, Belanger A, Lepine J. Metabolic inactivation of estrogens in breast tissue by UDP-glucuronosyltransferase enzymes: an overview. Breast cancer research : BCR. 2004;6(6):246–254. doi: 10.1186/bcr936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eisinger M, Marko O. Selective proliferation of normal human melanocytes in vitro in the presence of phorbol ester and cholera toxin. Proc Natl Acad Sci U S A. 1982;79(6):2018–2022. doi: 10.1073/pnas.79.6.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu F, Fu Y, Meyskens FL., Jr MiTF regulates cellular response to reactive oxygen species through transcriptional regulation of APE-1/Ref-1. The Journal of investigative dermatology. 2009;129(2):422–431. doi: 10.1038/jid.2008.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu F, et al. MiTF links Erk1/2 kinase and p21 CIP1/WAF1 activation after UVC radiation in normal human melanocytes and melanoma cells. Mol Cancer. 2010;9:214. doi: 10.1186/1476-4598-9-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen G, Dellinger RW, Gallagher CJ, Sun D, Lazarus P. Identification of a prevalent functional missense polymorphism in the UGT2B10 gene and its association with UGT2B10 inactivation against tobacco-specific nitrosamines. Pharmacogenet Genomics. 2008;18(3):181–191. doi: 10.1097/FPC.0b013e3282f4dbdd. [DOI] [PubMed] [Google Scholar]