Summary
We postulated that fludarabine (Flu) instead of cyclophosphamide (Cy) combined with IV busulfan (Bu) as preconditioning for allogeneic hematopoietic stem cell transplantation (HSCT) would improve safety and retain antileukemic efficacy. 67 patients received BuCy2 and subsequently 148 patients received Bu-Flu. We used a Bayesian method to compare outcomes between these non-randomized patients. The groups had comparable pretreatment characteristics, except that Bu-Flu patients were older (46 vs. 39 years, p< 0.01), more often had unrelated donors (47.3% vs. 20.9%, p< 0.0003), and had shorter median follow-up (39.7 vs. 74.6 months). To account for improved supportive care and other unidentified factors that may affect outcome (“period” effects), 78 AML patients receiving Melphalan-Flu (“MF”), treated in parallel during this time (1997 to 2004) were used to estimate the period effect; The MF patients’ outcomes worsened during this period. Therefore, the period effect is unlikely to explain the greatly improved outcome with Bu-Flu. Patients transplanted with Bu-Flu in CR1 had a 3-year overall survival and event-free-survival (EFS) of 78% and 74%, respectively, while CR1 patients younger than age 41 had a 3-year EFS of 89%. These results support replacing BuCy±ATG with Bu-Flu±rabbit-ATG, and warrant a prospective comparison between allogeneic HSCT and conventional induction/consolidation chemotherapy for AML in CR1.
Keywords: IV Busulfan, Fludarabine, Cyclophosphamide, AML, MDS, Allogeneic Stem Cell Transplantation
Introduction
Introduction of IV Busulfan (IV Bu) as an alternative to oral Bu [1] rekindled interest in optimizing the conditioning regimen to improve treatment outcome after allogeneic hematopoietic stem cell transplantation (HSCT) for myeloid leukemia [2–5]. Recent studies with IV Bu and cyclophosphamide (Cy) suggested a lower incidence of serious hepatic veno-occlusive disease (VOD) and other treatment related serious adverse events compared to what would be expected after oral BuCy2 [6, 7]. These risks are of particular concern since typically Bu is combined with other agent(s), e.g. Cy, known to cause VOD [8, 9]. However, not only regimen-related toxicity, but also engraftment and (acute) graft vs host disease (GVHD) may be influenced by variable systemic exposure [6, 7, 10, 11] and the relative timing of the individual cytotoxic agent(s) in a high-dose chemotherapy combination [12–14]. This is especially true when alkylating agents with partly overlapping dose-limiting toxicities are combined in myeloablative pretransplant conditioning therapy. We thus decided to combine IV Bu with an immunosuppressive agent having very limited hepatotoxic potential, fludarabine (Flu). A Bu-Flu combination has several appealing features; Flu is likely as immunosuppressive as Cy [15] and, when timed appropriately, it synergistically promotes Bu-induced cytotoxicity through interference with repair of XRT- and alkylator-induced DNA-damage [16]. Further, Flu does not cause VOD, and its long plasma half-life encourages once daily administration. We recently reported safety and outcome data after HSCT for AML/MDS with a myeloablative, once daily IV Bu-Flu regimen [17], and similarly encouraging data have been obtained in patients undergoing allogeneic HSCT for a variety of hematologic malignancies [18, 19]. These early reports demonstrate IV Bu-Flu combinations to be safe and efficacious, resulting in low treatment-related mortality (TRM) due to, at least in part, highly reproducible intra- and inter-patient systemic Bu exposure [17, 18, 20, 21]. Although the available safety and efficacy data appear promising, there was apprehension about possibly suboptimal antileukemic efficacy of Flu compared with Cy, particularly in patients with active leukemia at the time of transplant [4]. Ideally, this question would be addressed through a comparison of BuCy2 and Bu-Flu in a randomized phase III study, stratifying patients for clinical disease stage and other prognostic factors. However, aside from the large number of patients and long time needed to complete such a study, it would also be fraught with uncertainty as to whether fixed dose Bu delivery is optimal; an ongoing study at the MD Anderson Cancer Center is comparing fixed dose Bu with drug delivery based on patient-specific pharmacokinetic (PK) information. Further comparisons of clinical outcome and systemic Bu exposure suggest the presence of an optimal therapeutic interval for IV Bu in combination with either Cy or Flu [11, 22]. Because this issue is unresolved, and PK-guided dosing currently is being refined, it is premature to begin a long-term study of fixed dose Bu-Flu vs. BuCy2.
In the present analysis, we compared the outcomes of 67 patients receiving BuCy2 with 148 consecutive patients treated subsequently with the fixed-dose Bu-Flu combination. We observed a remarkable difference in TRM rates between the two groups, both within the first 100 days and at one year post transplant. This low early TRM after Bu-Flu was strikingly different from previous experience by both our group and that of others with alternative conditioning regimens [23–28].
However, comparison of Bu-Flu and BuCy2 based on these new data was complicated by several issues. First, there were significant differences in age and other characteristics of the two patient populations. Second, more importantly, patients were not randomized between the two conditioning regimens; rather, the programs were executed sequentially during an eight-year period, 1997 – 2001 for the Bu Cy2 trial and 2001 – 2005 for the Bu-Flu trial. Consequently, the difference between BuCy2 and Bu-Flu – the “treatment effect”-is confounded by possible differences between the patient groups or therapeutic environments in these two time periods that are unrelated to the preparative regimens, including changing practice patterns such as addition of new antibacterial-, antifungal-, and antiviral antibiotics, introduction of rabbit antithymocyte-globulin (ATG), changing referral patterns, differences in patient characteristics, or the effects of other, unknown variables. We will refer to the composite influence of all such confounding factors as the “period” effect. The statistical problem thus is to compare treatment effects between two treatment groups while accounting for the confounding between-trial effects. We will do this, using a Bayesian model and method [2, 29, 30], that deals with treatment-trial confounding, by estimating the period effect using a separate data set of 78 patients who received pretransplant conditioning therapy with Melphalan and Fludarabine (MF) at M.D. Anderson during the period 1997 to 2004 [26], and assuming that the period effect for the BuCy2 and Bu Flu patients was the same as for the MF patients. While this Bayesian approach is not a substitute for a randomized phase III trial, it can be used to obtain a reasonable estimate of treatment effect(s) under the assumption that period effect accounts for between-trial effects. The results may be used to decide whether to compare these regimens in a prospective randomized trial. Additionally, while ongoing studies of individualized, PK-guided IV Bu (combined with Flu±ATG) are completed, our analyses also can be used to support therapeutic decision-making in patients with AML/MDS who are considered for allogeneic HSCT using IV Bu-based conditioning therapy. Presently, our analyses strongly support a) Bu-Flu±ATG as a preferred regimen over IV BuCy2±ATG, and b) a prospective comparison of allogeneic HSCT with conventional chemotherapy in first complete remission (CR1) for patients with AML.
Patients and Methods
Patient Eligibility
AML patients should have failed initial induction chemotherapy, or have high-risk disease in CR1, characterized by cytogenetics other than translocation (t) (8;21), inversion (inv) 16, or t(15;17), or by the need for more than one cycle of chemotherapy to achieve CR [31]. Patients beyond CR1 were also eligible. Subjects with MDS were eligible if they had a high International Prognostic Score System (IPSS) score (≥ 2) [32], or if they progressed after chemotherapy.
The eligibility criteria included acceptable renal (creatinine ≤ 1.5 mg%) and hepatic function with normal bilirubin, SGPT ≤ 3 times the upper normal limit, a ZUBROD performance status ≤ 2, negative serology for hepatitis B, and C, and HIV, LVEF ≥ 45%, FEV1, FVC and DLCO ≥ 50% of predicted, absence of uncontrolled infection, and no chemotherapy within 30 days prior to entry. A Human Leukocyte Antigen (HLA) compatible related (fully matched or one antigen mismatched) or matched unrelated donor (MUD) was required. All patients signed informed consent according to institutional guidelines. One Bu-Flu patient was treated off protocol with Institutional Review Board approval, under a “compassionate plea” mechanism due to chronic renal failure developed after a previous non-myeloablative transplant. No patients treated after August 2005 were included in this study to allow for comparison of patient populations with a median follow-up greater than 2 years.
Conditioning regimens
IV Busulfan-Fludarabine (Bu-Flu)
The treatment has been previously described [17], and consisted of fludarabine (Fludara®, Berlex Laboratories, Inc., New Jersey, NJ) 40 mg/m2 given over 60 minutes daily for four days, each dose immediately followed by IV Busulfan (IV Busulfex® (busulfan) Injection, ESP Pharma, Inc., Edison, NJ), 130 mg/m2 over 3 hours daily (days −6 to −3).
IV Busulfan-Cyclophosphamide (IV BuCy2)
This regimen was also previously described [33]. Briefly, IV Bu was administered at 0.8 mg/kg (~32 mg/m2) over 2 hr every 6 hours for 16 doses (days −7 to −4) and Cy was then given at 60 mg/kg IV over one hr daily for 2 doses (days −3 and −2).
Melphalan-fludarabine (MF)
In the MF group the patients were treated with fludarabine 25 mg/m2 IV daily for 5 days and melphalan 90 mg/m2 or 70 mg/m2 daily for 2 days as described by Giralt et al [26]. Melphalan was given after fludarabine on the last two days of chemotherapy. Day zero was the day of transplantation in all protocols. In late 2004 this program was revised to incorporate gemtuzumab ozogamicin [34]. Patients treated on this revised MF protocol were not included in the current comparison. Patients were eligible for the MF program if they were older than 55 years and/or having at least one comorbid condition that made them ineligible for the front-line program (BuCy2 and Bu-Flu, respectively).
Supportive Care
All supportive care measures were utilized according to extant institutional protocols. All patients received Filgrastim (Neupogen®, Amgen, Inc., Thousand Oaks, CA) 5 μg/kg subcutaneously daily from day +7 until achieving an absolute neutrophil count (ANC) ≥1.5 × 109/L for three days. Phenytoin was used during and one day after IV Bu-based therapy.
The cytotoxic drugs were infused via a controlled-rate infusion pump through a central venous catheter. Fludarabine dosing was according to actual body weight. The alkylating agents were dosed per patients’ actual weight up to 120% of ideal body weight, above which the doses were based on adjusted ideal body weight (ideal weight plus 50% of the difference between ideal and actual weight). All groups received graft vs. host disease (GVHD) prophylaxis with tacrolimus (Prograf®, Fujisawa Healthcare, Inc., Deerfield, IL) and mini-dose methotrexate 5 mg/m2 on days 1, 3, 6 and 11 following transplant [35]. Tacrolimus was to be continued for 6–8 months. Patients with a one-antigen mismatched related or an unrelated donor received equine ATG (ATGAM®, Pharmacia & Upjohn Company, Kalamazoo, MI) 20 mg/kg daily (days −3 to −1, (in the BuCy2 group), or rabbit-ATG (Thymoglobulin®, Genzyme Inc., Cambridge, MA), 0.5 mg/kg on day −3, 1.5 mg/kg on day −2, and 2.0 mg/kg on day −1) (Bu-Flu group). In addition, Pentostatin (Nipent®, Supergen, Dublin, CA) was added in ten cases receiving unrelated (n=8) or one-antigen mismatched related donor grafts (n=2), under an investigational protocol (IV Bu-Flu arm only). The dose of pentostatin was 0.5 mg/m2 (n=2), 1 mg/m2 (n=4), and 1.5 mg/m2 (n=4) given on days 8, 15, 22 and 30 following HSCT.
Hematopoietic Stem cell Grafts
Procurement of donor peripheral blood progenitor cells (PBPC) has been described [36]. Donors were treated with Filgrastim 10–12 μg/kg every 12 hours over three days and in the morning of day four prior to PBPC collection. The donor’s total blood volume was processed three times per apheresis procedure. In case a second apheresis procedure was performed, Filgrastim treatment was continued through prior to the second procedure. The PBPC dose was targeted to approximately 5 × 106 CD34+ cells/Kg patient body weight, in keeping with the observation of a correlation between higher cell doses and incidence of GVHD [37]. Bone marrow or PBPC from unrelated donors were obtained through the National Marrow Donor Program.
Human Leukocyte Antigen Typing
HLA typing for class I antigens was performed using standard serological techniques. Class II alleles (HLA-DRB1) were resolved with low resolution molecular typing using sequence specific oligonucleotide primers for hybridization of amplified DNA, followed by high resolution typing in all patients and donors. Donor–recipient pairs were considered fully matched by compatibility for HLA-A, -B and -DRB1.
Analysis of Chimerism
Peripheral blood or bone marrow donor-recipient chimerism was evaluated by analysis of DNA microsatellite polymorphisms by polymerase chain reaction (PCR) with D6S264, D3S1282, D18S62 and D3S1300 fluorescence-labeled primers, and analyzed using GeneScan software (Applied Biosystems, Foster City, CA). In addition, we used conventional cytogenetic analysis with G-banding or fluorescent in-situ hybridization studies for the Y-chromosome in sex-mismatched cases. Mixed chimerism was defined as the presence of any detectable (≥1%) recipient DNA or -cells in addition to donor-derived DNA or -cells.
Clinical Outcome Variables
Time of engraftment was defined as the first of three consecutive days with ANC ≥0.5 × 109/L. Failure to engraft in the absence of malignancy by day +30 was considered primary engraftment failure. Secondary graft failure was initial engraftment with documented donor-derived hematopoiesis followed by loss of graft function without recurrent malignancy. Time of platelet engraftment was defined as the first of seven consecutive days with a platelet count ≥20 × 109/L without transfusion support. Criteria for CR prior to transplant included absence of circulating blasts, less than 5% marrow blasts, lack of chromosomal abnormalities, and platelet count ≥100 × 109/L. CR post transplant was defined using the same criteria except for platelet count, with documented donor cell engraftment.
Cytogenetics were considered prognostically favorable for patients with t(15,17), inv 16 or t(8,21); poor risk (“bad”) for patients with deletions of chromosome 5 and/or 7, multiple chromosomal abnormalities or trisomy of chromosome 8; and intermediate risk in all others [31]. Standard morphologic criteria, conventional cytogenetics, or both were used to diagnose recurrent disease. Cytogenetic relapse was documented by the presence of a clonal chromosomal abnormality in more than two consecutive tests, taken at least 4 weeks apart. Time to relapse/progressive disease was calculated from transplant to the day of documented event. Patients who did not achieve a CR after transplant were scored as failures at the date of documented persistent disease. Toxicity was scored using the modified NCI criteria (CTC 2.0).
Overall survival (OS) was calculated from the day of transplant, with patients alive at the time of last follow-up administratively censored. Treatment-related mortality (TRM) was defined as death due to any cause other than relapse, while non-relapse related survival (NRRS) was defined as the time from HSCT to death for reasons other than relapse, with relapse being a censoring event. Event-free survival (EFS) time was counted from day zero to relapse or death. Relapse-free survival (RFS) was defined as time from HSCT to relapse with death or time of last follow-up in complete remission (CR) counting as censoring events.
Adverse events and hematologic parameters were monitored daily, clinical chemistry parameters at least twice weekly during the initial hospitalization and then at least once weekly up to HSCT day+100. Subsequently, patients were followed at least quarterly during the first year, then at gradually increasing intervals.
Statistical Methods
General Methods
Patient characteristics were summarized using the median (range) for numerical variables or frequencies (percentages) for categorical variables. Differences in the distributions of patient characteristics between groups were assessed using Kruskal-Wallis or generalized Fisher exact tests [38]. Unadjusted probabilities of event times were estimated using the method of Kaplan and Meier (KM) [39]. The log-rank test [40] was used to compare unadjusted OS, NRRS, EFS and RFS between subgroups. Bayesian log-normal regression models were used to assess the joint effects of patient covariates and treatments on OS, and similarly on each of the other outcomes NRRS, EFS and RFS. The covariates included cytogenetics (Bad versus Other), disease status at BMT (In CR versus not in CR), donor type (Sibling or Other related versus unrelated donor), age, whether any blasts were present in the patient’s peripheral blood (PB), and PB platelet count. The lognormal model was selected after assessing goodness-of-fit for several parametric models, including the exponential, Weibull, and log-logistic, using the Bayesian Information Criterion (BIC) and the Bayesian chi-squared method [41]. The log-normal model assumes a normal distribution for the log-transformed event time, denoted log(T) ~ N(μ, r), where μ is a linear combination of covariate effects and treatment effects, and r is the precision parameter, equal to the inverse of the variance, σ2, of log(T).
For each model fit, we assumed that each parameter in μ followed a non-informative normal prior with mean 0 and variance 1000, and a non-informative inverse-Gamma prior for σ2, with mean 1 and variance 1000. All statistical analyses were carried out in Splus 6.1 [42] or, for the Bayesian model fits, in WinBugs 1.4 [43].
Bayesian Method for Comparing Bu-Cy vs. Bu-Flu
Patients were not randomized between Bu-Flu and BuCy2 with all BuCy2 patients enrolled prior to t* = 4/18/2001 and all Bu-Flu patients enrolled after this date. Consequently, the Bu-Flu vs. BuCy2 (“treatment”) effect was confounded with the post t* vs. pre t* (“period”) effect in the data from the 215 patients, and this treatment effect cannot be estimated from these data. To address this problem, we first fit a Bayesian log normal regression model to the data from the 215 BuCy2 and Bu-Flu patients, including patient prognostic covariates and a parameter θ accounting for the confounded treatment-period effect by including an indicator [Bu-Flu] in the linear term μ. We assumed that this parameter was the sum of the actual Bu-Flu-versus-BuCy2 (treatment) effect, θTRT, and a period effect,θPERIOD, formally, θ = θTRT + θPERIOD. We also fit a similar Bayesian regression model, including the same covariates and an indicator [PERIOD] = 1 if the patient was enrolled after t* and 0 if before t*, to the data on the 78 MF patients treated over a period of time spanning the two periods both before and after t*. Since this provided a posterior estimate of θPERIOD, we obtained the treatment effect of interest as θTRT = θ − θPERIOD. That is, under the above additivity assumption that the confounded effect of Bu-Flu after t* versus BuCy2 before t* was equal to the sum of the Bu-Flu vs. BuCy2 treatment effect plus the post t* vs. pre t* period effect, the period effect was estimated separately, and the IV Bu-Flu versus IV BuCy2 effect was obtained by subtraction [2, 30]. All treatment-covariate interactions were included initially in the model, and interaction terms for which the posterior probability of a beneficial effect was not either > 0.90 or < 0.10 were dropped.
Results
Patients Treated with BuCy2 vs. Bu-Flu (N=215)
Table 1a summarizes characteristics of the 215 BuCy2 and Bu-Flu patients. The only significant covariate imbalances were that the Bu-Flu patients were on average 7 years older and had a lower percentage of Sib/Related donors (52.7% versus 79.1%). In terms of age and donor type, therefore, since patients were not randomized between the two regimens, the Bu-Flu group would be at a disadvantage in any statistical comparison that does not account for these covariates. Only a minority of patients were transplanted while in any CR (47.8% of BuCy2 patients and 46.6% of the Bu-Flu patients).
Table 1.
| Table 1a. Patient Characteristics by Treatment group in patients treated with BuCy2 or Bu-Flu. | |||
|---|---|---|---|
| Variable | BuCy2 (N=67) | Bu-Flu (N=148) | P-value |
| Cytogenetics | 1.00 | ||
| Others | 47 (70.1) | 104 (70.3) | |
| Bad | 20 (29.9) | 44 (29.7) | |
| Disease Status | 0.88 | ||
| Others | 35 (52.2) | 79 (53.4) | |
| CR | 32 (47.8) | 69 (46.6) | |
| CR1 | 12 (18) | 36 (24) | |
| Allo type | 0.0003 | ||
| Others | 14 (20.9) | 70 (47.3) | |
| Sib/Related | 53 (79.1) | 78 (52.7) | |
| PB blast | 0.11 | ||
| >0 | 24 (35.8) | 37 (25.0) | |
| 0 | 43 (64.2) | 111 (75.0) | |
| Age | 39 (13 – 64) | 46 (19 – 66) | 0.01 |
| PB PLT | 86 (2 – 330) | 89 (3 – 463) | 0.41 |
| Table 1b. Patient Characteristics by Treatment group in patients treated with MF. | |||
|---|---|---|---|
| Variable | Before 4/18/2001 (N=50) | After 4/18/2001 (N=28) | P-value |
| Cytogenetics | 0.16 | ||
| Others | 23 (46.0) | 18 (64.3) | |
| Bad | 27 (54.0) | 10 (35.7) | |
| Disease Status | 0.02 | ||
| Others | 44 (88.0) | 18 (64.3) | |
| CR | 6 (12.0) | 10 (35.7) | |
| Allo type | 0.24 | ||
| Others | 32 (64.0) | 14 (50.0) | |
| Sib/Related | 18 (36.0) | 14 (50.0) | |
| PB blast | 0.08 | ||
| >0 | 19 (38.0) | 5 (17.9) | |
| 0 | 31 (62.0) | 23 (82.1) | |
| Age | 54 (23 – 66) | 54 (22 – 74) | 0.69 |
| PB PLT | 46 (2 – 284) | 54 (9 – 377) | 0.16 |
Number in each cell is N (%) for categorical variable and median (range) for continuous variable.
Abbreviations: Cyto, cytogenetics; PB, peripheral blood; Allo, allogeneic; Sib, sibling; Rel, related; PLT, platelet; CR, complete remission)
Patients Treated with MF (N=78)
Table 1b summarizes patient characteristics of the 78 MF patients. The only significant imbalance within the group was that the MF patients treated after April 18, 2001 had a higher fraction of patients transplanted in CR, compared with those treated before April 18, 2001 (35.7% vs. 12.0%).
Unadjusted Analyses
Overall Survival (OS)
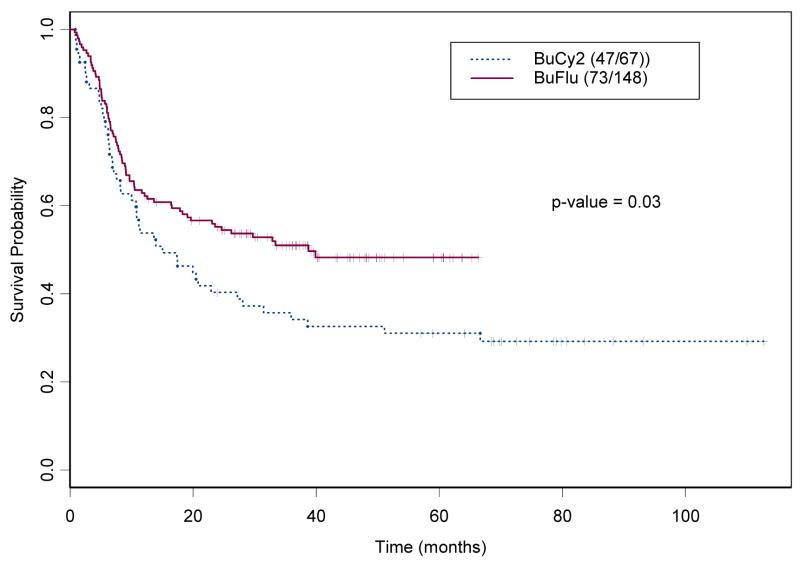
For this analysis, the event was defined as death from any cause. Among the 215 IV Bu patients, 120 (55.8%) died (47 [70%] in the BuCy2 group and 73 [49%] in the Bu-Flu group). The median OS time was 24.6 months (95% confidence interval, CI, 16.6 to 51.1 months). The median follow-up time was 74.6 months (95% CI 69.8 – 83.6 months) for the BuCy2 group and 39.1 months (95% CI 36.7 – 45.4 months) for the Bu-Flu group. Figure 1a shows the Kaplan-Meier estimates for OS in these two groups, indicating that the Bu-Flu patients survived significantly longer, but had a shorter follow up time.
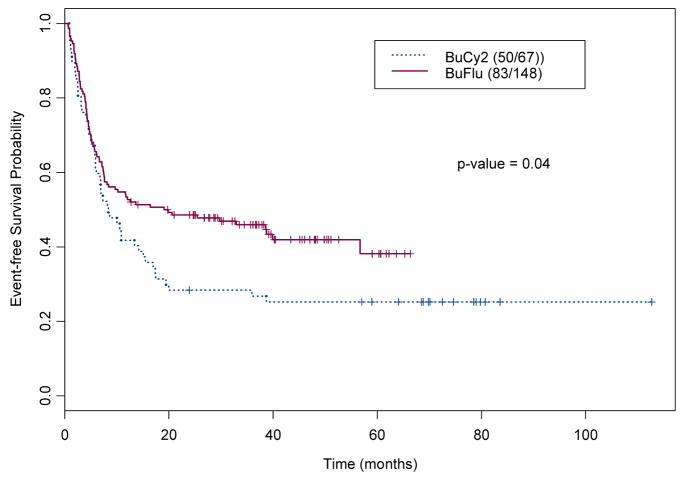
Figure 1.
Kaplan-Meier Estimates for the probabilities of (a) Overall Survival, and (b) Event-Free Survival by treatment Group in 215 patients treated with BuCy2 (----) and Bu-Flu (––) (the numbers within parenthesis indicate number of events and cohort size).
Event-free Survival (EFS)
For this analysis, the event was defined as progression or death from any cause. Among the 215 IV Bu patients, 133 (61.9%) progressed or died, (50 BuCy2 patients and 83 Bu-Flu patients). The median EFS time was 11.8 months for the whole group (95% CI, 7.6 to 20 months). Figure 1b shows the Kaplan-Meier estimates for the EFS in these two groups, indicating that the Bu-Flu patients had a longer EFS, compared with the BuCy2 group (19.1 months vs. 8.4 months respectively).
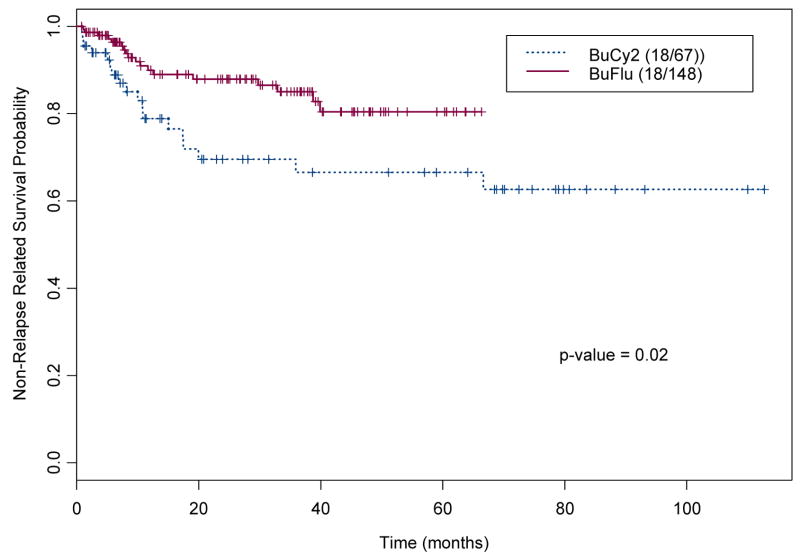
Non-relapse Related Survival (NRRS)
For this analysis, the event was defined as death without disease recurrence. Among the 215 Bu-treated patients, 36 (16.7%) died of treatment-related causes and without recurrent disease (18, 26.9%, of the BuCy2 and 18, 12%, of the Bu-Flu patients). The median NRRS time has not been reached in either group. Figure 2a shows the Kaplan-Meier estimates for the NRRS in these two groups, indicating that the Bu-Flu patients had a longer NRRS time, compared to BuCy2 group; the estimated NRRS at 3 years was 86% in the Bu-Flu group and 66% in the BuCy2 group.
Figure 2.
Kaplan-Meier Estimates for the probabilities of (a) Non-Relapse Related Survival, and (b) Relapse-Free Survival, by treatment Group in 215 patients treated with BuCy2 (----) and Bu-Flu (––) (the numbers within parenthesis indicate number of events and cohort size).
Relapse-Free Survival (RFS)
For this analysis, the event was defined as death due to persistent or recurrent disease, with all other terminating events considered to be right (administrative) censoring. Among the 215 IV Bu patients, 84 (39.1%) died of persistent or recurrent disease, 55 (37%) of the Bu-Flu patients and 29 (43%) of the BuCy2 patients. The median RFS time has not been reached (95% confidence interval, CI, 38.6 – NA). Figure 2b shows the Kaplan-Meier estimates for the RFS in these two groups, indicating that there was no significant difference between the IV Bu-Flu and IV BuCy2 patients in terms of RFS. Bearing in mind, again, that patients were not randomized, and moreover the KM curves ignore covariate effects, it is notable that the plateau for the Bu-Flu RFS curve is about 0.10 higher than that of the BuCy2 RFS curve.
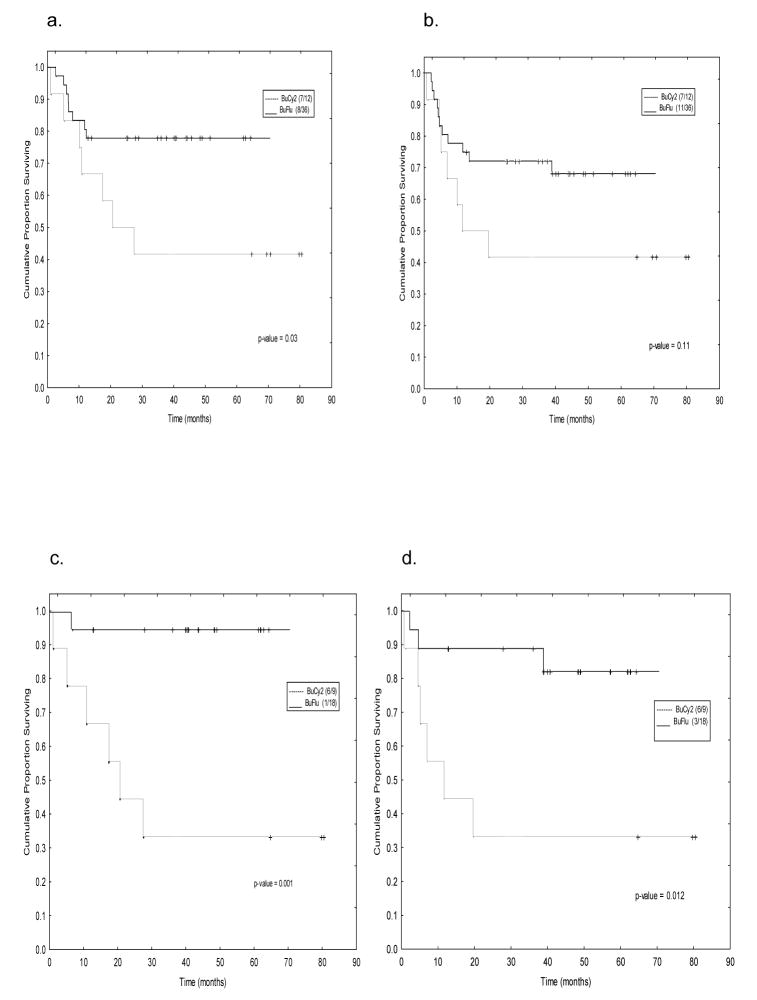
Early Disease, CR1 Patients
Patients transplanted in CR1 constituted only a small subgroup (Table 1a), 12 (18%) in the BuCy2 group and 36 (24%) of the Bu-Flu patients. However, the most striking differences in outcome were encountered when comparing these subgroups; the 3-year overall survival was 78% after Bu-Flu and 42% after BuCy2, and the 3-year EFS percentages were 74% and 42%, respectively (Figs 3a and 3b). Further, there were no differences in outcome related to the use of matched related vs. unrelated donors (data not shown). Young patients (up to and including age 40), fared even better with Bu-Flu, their 3-year OS and EFS were 94% and 83% respectively (Figs. 3c and 3d). Finally, the one-year-TRM for patients transplanted in CR using the Bu-Flu program was 6%, significantly better than that achieved with the BuCy2 regimen (21%).
Figure 3.
Survival of patients transplanted in CR1, “early disease” with BuCy2 (----) and Bu-Flu (––); (a) Overall Survival, and (b) Event-Free Survival in all CR1 patients. In graphs (c) Overall Survival, and (d) Event-Free Survival is depicted for patients up to and including 40 years of age (the numbers within parenthesis indicate number of events and cohort size).
Graft vs. Host Disease
The overall incidence of acute graft vs host disease (aGVHD) grades II–IV was 33.3% after BuCy2, 26.1% after Bu-Flu and 42.1% after the MF regimen. Among patients who had a fully HLA-matched related donor the incidence of aGVHD grades II–IV was 32.7% after BuCy2, 15.8% after Bu-Flu and 25% after MF. The corresponding incidence of extensive cGVHD was 36.1% after BuCy2, 34.1% after Bu-Flu and 39.4% after MF.
Covariate Adjusted Analyses
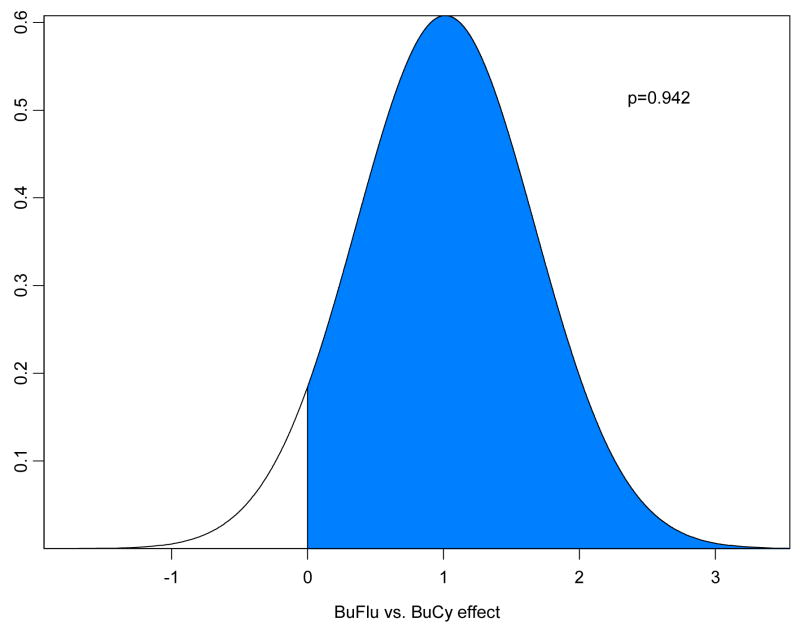
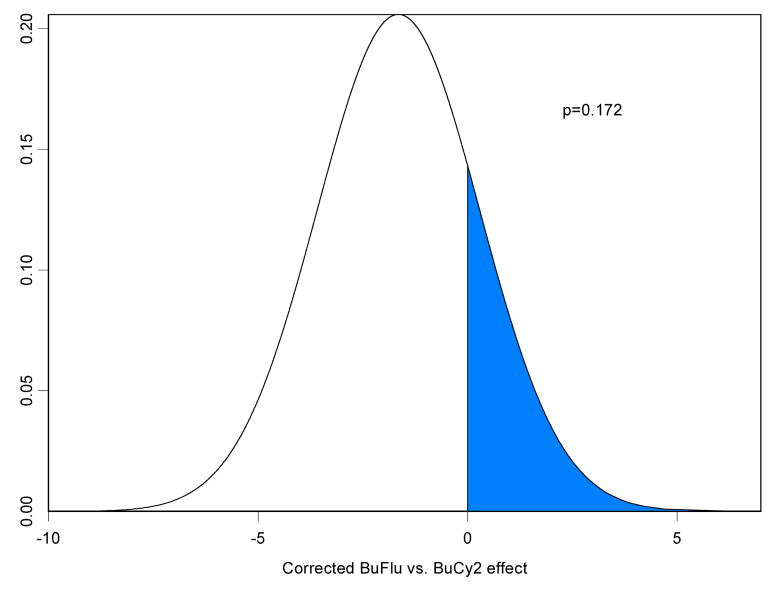
Table 2a summarizes a fitted Bayesian log-normal survival model for OS for the 215 IV Bu patients, including 67 treated with BuCy2 and 148 who received Bu-Flu. Table 2b summarizes a similar model fit to the data from the 78 MF patients, with a period effect in place of the confounded Bu-Flu-versus-BuCy2 treatment effect. Assuming, as described above, that this time period effect was distributed in a similar fashion in the FM and BuCy2-Bu-Flu data sets, the posterior distribution of the treatment effect obtained by subtracting the period effect is summarized in Figure 4a, which indicates that, after accounting for the period effect, IV Bu-Flu was greatly superior to IV BuCy2 in terms of OS. Remarkably, even the uncorrected Bu-Flu-vs-BuCy2 effect favored Bu-Flu over BuCy2 (Table 2a), despite the fact that the MF data showed a detrimental effect of the later period 2001–2004 when most of the Bu-Flu trial was conducted. Similar analyses are given in Table 3 and Figure 4b for EFS, and Tables 4 and Figure 5a for NRRS. Table 5 and Figure 5b examines the relationship between treatment arm (BuCy2 vs. Bu-Flu) and relapse-free survival (RFS). After accounting for covariates and subtracting the period effect in the Bayesian analyses, the posterior probability that Bu-Flu is superior to BuCy2 in terms of NRRS is > 0.99 and in terms of RFS is only 0.17. It may be argued that, since non-disease-related and disease-related deaths are competing risks, Bu-Flu has a much lower overall death rate but may result in a slightly higher death rate due to recurrent disease.
Table 2.
| Table 2a. Fitted Bayesian log-normal survival model for Overall Survival of 215 patients, including 67 patients treated with BuCy2 and 148 with treated Bu-Flu. | |||||
|---|---|---|---|---|---|
| Variable | Mean | SD | Posterior 95% Credible Interval | Probability of a Beneficial Effect | |
|
| |||||
| 2.50% | 97.50% | ||||
| Intercept | 2.372 | 0.563 | 1.263 | 3.474 | - |
| Cyto = bad (vs. Other) | −0.114 | 0.277 | −0.657 | 0.433 | 0.339 |
| Disease status = CR (vs. not in CR) | 0.241 | 0.339 | −0.431 | 0.903 | 0.764 |
| Allo type = Sib/Other Rel (vs. Unrelated) | 0.647 | 0.273 | 0.109 | 1.195 | 0.991 |
| Age | −0.023 | 0.011 | −0.045 | −0.001 | 0.020 |
| PB Blast = 0 (vs. > 0) | 1.129 | 0.319 | 0.494 | 1.778 | 1.000 |
| PB PLT | 0.003 | 0.001 | 0.000 | 0.006 | 0.990 |
| Confounded Bu-Flu (vs. BuCy2) Effect | 0.591 | 0.292 | 0.015 | 1.164 | 0.977 |
| R | 0.363 | 0.054 | 0.268 | 0.478 | |
| Table 2b. Fitted Bayesian log-normal survival model for Overall Survival of 78 patients treated with MF. | |||||
|---|---|---|---|---|---|
| Variable | Mean | SD | Posterior 95% Credible Interval | Probability of a Beneficial Effect | |
|
| |||||
| 2.50% | 97.50% | ||||
| Intercept | 3.370 | 1.437 | 0.523 | 6.237 | - |
| Cyto = bad (vs. Other) | −0.878 | 0.500 | −1.875 | 0.087 | 0.038 |
| Disease status = CR (vs. not in CR) | 1.377 | 0.708 | 0.012 | 2.798 | 0.976 |
| Allo type = Sib/Other Rel (vs. Unrelated) | 0.550 | 0.506 | −0.451 | 1.558 | 0.865 |
| Age | −0.023 | 0.025 | −0.072 | 0.026 | 0.169 |
| PB Blast = 0 (vs. > 0) | 0.770 | 0.559 | −0.323 | 1.875 | 0.919 |
| PB PLT | 0.001 | 0.002 | −0.003 | 0.004 | 0.679 |
| After 04/01 (vs. Before 04/01) | −0.811 | 0.573 | −1.945 | 0.313 | 0.078 |
| R | 0.250 | 0.056 | 0.151 | 0.371 | - |
Abbreviations: Cyto, cytogenetics; PB, peripheral blood; Allo, allogeneic; Sib, sibling; Rel, related; PLT, platelet; CR, complete remission)
Figure 4.
Posterior distribution under the lognormal regression model for (a) Overall Survival, and (b) Event-Free Survival of the corrected Bu-Flu vs. BuCy2 treatment effect. In these plots, p denotes the probability of a beneficial effect of Bu-Flu versus Bu Cy2, and is represented by the area of the shaded region in the respective figure.
Table 3.
Fitted Bayesian log-normal survival model for Event-Free Survival in 215 patients, including 67 patients treated with BuCy2 and 148 with treated Bu-Flu.
| Variable | Mean | SD | Posterior 95% Credible Interval | Probability of a Beneficial Effect | |
|---|---|---|---|---|---|
|
| |||||
| 2.50% | 97.50% | ||||
| Intercept | 1.622 | 0.583 | 0.491 | 2.752 | - |
| Cyto = bad (vs. Other) | −0.090 | 0.287 | −0.660 | 0.473 | 0.386 |
| Disease status = CR (vs. not in CR) | 0.501 | 0.341 | −0.158 | 1.162 | 0.932 |
| Allo type = Sib/Other Rel (vs. Unrelated) | 0.230 | 0.279 | −0.320 | 0.781 | 0.799 |
| Age | −0.011 | 0.011 | −0.033 | 0.012 | 0.171 |
| PB Blast = 0 (vs. > 0) | 1.307 | 0.336 | 0.650 | 1.965 | 1.000 |
| PB PLT | 0.002 | 0.001 | 0.000 | 0.004 | 0.961 |
| Confounded Bu-Flu (vs.BuCy2) Effect | 0.416 | 0.295 | −0.141 | 1.011 | 0.922 |
| R | 0.327 | 0.045 | 0.248 | 0.424 | - |
Abbreviations: Cyto, cytogenetics; PB, peripheral blood, Allo, allogeneic; Sib, sibling; Rel, related; PLT, platelet; CR, complete remission)
Table 4.
Fitted Bayesian log-normal survival model for Non-relapse related survival (NRRS) in 215 patients, including 67 patients treated with BuCy2 and 148 with treated Bu-Flu.
| Variable | Mean | SD | Posterior 95% Credible Interval | Probability of a Beneficial Effect | |
|---|---|---|---|---|---|
|
| |||||
| 2.50% | 97.50% | ||||
| Intercept | 5.131 | 1.343 | 2.671 | 7.944 | - |
| Cyto = bad (vs. Other) | 0.996 | 0.681 | −0.294 | 2.371 | 0.934 |
| Disease status = CR (vs. not in CR) | −0.092 | 0.712 | −1.488 | 1.313 | 0.448 |
| Allo type = Sib/Other Rel (vs. Unrelated) | 1.396 | 0.602 | 0.277 | 2.684 | 0.994 |
| Age | −0.055 | 0.025 | −0.109 | −0.009 | 0.009 |
| PB Blast = 0 (vs. > 0) | 0.922 | 0.709 | −0.466 | 2.334 | 0.909 |
| PB PLT | 0.002 | 0.002 | −0.002 | 0.007 | 0.755 |
| Confounded Bu-Flu (vs. BuCy2) Effect | 1.880 | 0.651 | 0.664 | 3.258 | 0.999 |
| R | 0.166 | 0.049 | 0.087 | 0.277 | - |
Abbreviations: Cyto, cytogenetics; PB, peripheral blood, Allo, allogeneic; Sib, sibling; Rel, related; PLT, platelet; CR, complete remission)
Figure 5.
Posterior distributions under the lognormal regression model for (a) Non-Relapse Related Survival, and (b) Relapse-Free Survival of the corrected Bu-Flu vs. BuCy2 treatment effect within the 215 Bu-treated patients. In the plot, p denotes the probability of beneficial effect of Bu-Flu versus BuCy2, and is represented by the area of the shaded region in the respective figure.
Table 5.
Fitted Bayesian log-normal survival model for Relapse-free survival (RFS) in 215 patients, including 67 patients treated with BuCy2 and 148 with treated Bu-Flu.
| Variable | Mean | SD | Posterior 95% Credible Interval | Probability of a Beneficial Effect | |
|---|---|---|---|---|---|
|
| |||||
| 2.50% | 97.50% | ||||
| Intercept | 2.856 | 0.659 | 1.575 | 4.145 | - |
| Cyto = bad (vs. Other) | −0.549 | 0.325 | −1.189 | 0.088 | 0.046 |
| Disease status = CR (vs. not in CR) | 0.219 | 0.450 | −0.663 | 1.096 | 0.688 |
| Allo type = Sib/Other Rel (vs. Unrelated) | 0.398 | 0.331 | −0.248 | 1.046 | 0.889 |
| Age | −0.012 | 0.014 | −0.038 | 0.014 | 0.181 |
| PB Blast = 0 (vs. > 0) | 1.322 | 0.394 | 0.560 | 2.109 | 1.000 |
| PB PLT | 0.005 | 0.002 | 0.001 | 0.009 | 0.998 |
| Confounded Bu-Flu (vs. BuCy2) Effect | 0.042 | 0.356 | −0.652 | 0.760 | 0.547 |
| R | 0.309 | 0.056 | 0.208 | 0.429 | - |
Abbreviations: Cyto, cytogenetics; PB, peripheral blood, Allo, allogeneic; Sib, sibling; Rel, related; PLT, platelet; CR, complete remission)
Discussion
Several investigators have reported a dose-response relationship between the pretransplant conditioning regimen and long-term outcome after allo-HSCT in acute leukemia [3, 4, 10, 12, 28]. In this context, IV Bu provides a valuable tool for safe and reproducible delivery of intensive conditioning treatment; the intra- and inter-individual variability in systemic Bu exposure is considerably lower than what is typically obtained with oral Bu [17, 20, 21, 44]. Non-randomized comparisons between patients conditioned with IV Bu-based [7, 19] and those receiving oral BuCy2 [6] - or TBI-based therapy [27, 28] appeared favorable for the IV Bu-based combinations relative to a lower TRM/increased safety. The main benefit was seen in terms of increased safety/lower TRM at 100 days and at one year post-transplantation [6, 7, 17, 19, 27]. The experience of both our group [17, 45], and that of Russell et al [18, 19], suggested that (minor) variants of this Bu-Flu±ATG combination would be well tolerated and safe, reduced toxicity myeloablative conditioning treatments. There was some concern however, that the “favorable” comparison between IV Bu-Flu and IV BuCy2 constituted a non-randomized assessment of sequential conditioning programs during a time when supportive care had improved in a way that greatly favored the more recent program. A similar quandary was highlighted by Chae et al, who reported on a favorable outcome when comparing Bu-flu to BuCy2 in a mixed patient population transplanted for a variety of hematologic malignancies (46). Although these authors reported a greatly improved outcome after Bu-Flu, their analysis was complicated by reporting observations made in a mixed patient population with varying ages, performed as two sequential programs, and further by changing from oral to IV Bu and then changing cyclophosphamide to Fludarabine, all of which may unpredictably contribute to the final clinical observations. The classical approach to comparison of different treatment programs is to perform a prospective randomized trial to obtain an unbiased estimate of the difference in treatment effects. While this ideal route is frequently used, a large body of data results from single arm trials, as was the present case. Any comparison of such single-arm, consecutive trials will suffer from the confounding effect(s) of unknown factors, here including possible changes in referral patterns, improved supportive care routines with introduction of new antifungal- antiviral- and antibacterial agents as well as use of rabbit-ATG, and increasing experience of the nursing and medical staff. We have argued that a Bayesian sensitivity analysis can provide a basis for a comparative evaluation of different treatment programs in the presence of such confounding effects [2, 29]. In the present investigation, we exploited the data from the separate MF trial, a program that remained unaltered from 1997–2004 [26], by estimating the combined confounding factors as a “period” effect using the MF trial data. We assumed that the “period” related changes that influenced treatment outcome for the MF patients would similarly influence outcome for patients allocated to the BuCy2 and Bu-Flu regimens over time, since there was no systematic bias in allocating patients to the MF regimen versus that of the Bu-based regimens during this time period. Moreover, all three trials were conducted in the same institution. It may be argued on fundamental grounds that patients treated more recently should have benefited from more advanced supportive care routines, more highly skilled medical staff etc. After accounting for known patient characteristics in the analysis of the MF data, however, both overall survival and the chance for relapse-free survival worsened over time, indicating that the remaining period effect favored the earlier period (1997–2001) over the later time period. Thus, improvements in supportive care were, apparently, more than balanced out by a changing referral pattern, an increasing median patient age, increasing use of alternative donors and possibly other, unknown factors. Given that the estimated period effect greatly favored the earlier period, when the BuCy2 trial was conducted, it is quite remarkable that even the uncorrected, confounded Bu-Flu-vs-BuCy2 effect (Table 2 and Figs 1a, 2b, 3a, and 3b) showed Bu-Flu to be a superior regimen.
Our Bayesian analyses lead to the conclusion that the observed differences in 100-day and one year mortality rates between the BuCy2- and Bu-Flu-regimens are likely attributable to a superior safety and tolerance profile of the latter program in patients with AML/MDS. In reference to a low TRM, this conclusion is further supported by Russell et al who reported similar findings both in patients with advanced hematologic malignancies [18], and more recently in better-prognosis patients with AML and ALL [19]. The lingering concern that a replacement of Cy with Flu would represent an overall decreased treatment intensity that translated into less side effects at the price of inferior antileukemic effect, especially in subjects with active leukemia at time of transplant [4] also appears largely unfounded, our results indicate that patients receiving Bu-Flu would not be at any disadvantage in this respect as depicted in Fig 5b; It is important to remember, that both Cy and Flu were primarily used because of their immunosuppressive rather than antileukemic properties, i.e. to enhance engraftment. It is true, however, that while a high relapse rate exists in patients transplanted with chemotherapy-refractory leukemia and a clinically high tumor load the search should continue for ways to further improve the Bu-Flu regimen. This program should be considered primarily as a therapeutic platform, to which other, both pre- and post-grafting components may be added safely to improve tumor control. Overall, it is tempting to conclude that Bu-Flu±ATG represents a significant improvement for patient safety, at least in the first (few) year(s) after allogeneic HSCT, since the outcome of patients treated with Bu-Flu was significantly better than what would be expected based on our past experience with the BuCy2 regimen and from comparisons with data using other conditioning programs in patients with AML/MDS [26, 47–50]. Although it was not a primary objective to compare GVHD rates after the different patient populations due to their disparity in age, proportion of donors other than fully matched related donors, etc. it was noteworthy that the incidence of GVHD among patients transplanted after Bu-Flu (~16%) with a fully matched related donor was only half of that observed after BuCy2 (~33%).
The favorable outcome of the Bu-Flu patients, in view of a relative long follow-up time (a median of 30 months) and a comparatively large number of patients with a high median age (46 years) appears to challenge the concept that an age above 50 or 55 years necessitates a reduced-intensity regimen for allogeneic HSCT in AML/MDS. Finally, the Bu-Flu data suggest that it may be time for a prospective evaluation of allogeneic HSCT vs. conventional induction and consolidation chemotherapy for AML/MDS for all patients who do not have APL or core binding factor leukemia, regardless of cytogenetic risk pattern. Such a study should, as a minimum, cover the population up to age 40. Previous comparisons of allogeneic HSCT and conventional maintenance chemotherapy mostly have relied on TBI-based conditioning therapy, which yielded excessive TRM without corresponding patient benefit [51, 52].
In summary, the consistent and reproducible systemic Bu exposure that was achieved with a parenteral Bu formulation, when paired with Flu±rabbit-ATG, is likely to continue having a significant impact on (early) post-transplant safety and survival in the studied patient population(s).
Acknowledgments
Supported by National Institute of Health grants 2PO1 CA55164 and 2P30CA16672–26, and the Stephen L. and Lavinia P. Boyd Fund for Leukemia Research. The authors are greatly indebted to the nursing staff of the in-patient and outpatient transplant care centers and to the members of the stem cell transplant coordinator staff.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bhagwatwar HP, Phadungpojna S, Chow DS, Andersson BS. Formulation and stability of busulfan for intravenous administration in high-dose chemotherapy. Cancer Chemother Pharmacol. 1996;37:401–408. doi: 10.1007/s002800050404. [DOI] [PubMed] [Google Scholar]
- 2.Thall PF, Champlin RE, Andersson BS. Comparison of 100-day mortality rates associated with i.v. busulfan and cyclophosphamide vs other preparative regimens in allogeneic bone marrow transplantation for chronic myelogenous leukemia: Bayesian sensitivity analyses of confounded treatment and center effects. Bone Marrow Transplant. 2004;33:1191–1199. doi: 10.1038/sj.bmt.1704461. [DOI] [PubMed] [Google Scholar]
- 3.de Lima M, Anagnostopoulous A, Munsell M, et al. Non-Ablative versus Reduced Intensity Conditioning Regimens in the treatment of Acute Myeloid Leukemia and High-Risk Myelodysplastic Syndrome. Dose is Relevant for Long-Term Disease Control after Allogeneic Hematopoietic Stem Cell Transplantation. Blood. 2004;104 :865–872. doi: 10.1182/blood-2003-11-3750. [DOI] [PubMed] [Google Scholar]
- 4.Shimoni A, Hardan I, Shem-Tov N, Yeshurun M, Yerushalmi R, Avigdor A, Ben-Bassat I, Nagler A. Allogeneic hematopoietic stem-cell transplantation in AML and MDS using myeloablative versus reduced-intensity conditioning: the role of dose intensity. Leukemia. 2006;20:322–328. doi: 10.1038/sj.leu.2404037. [DOI] [PubMed] [Google Scholar]
- 5.Shimoni A, Hardan I, Shem-Tov N, et al. Comparison between two fludarabine-based reduced-intensity conditioning regimens before allogeneic hematopoietic stem-cell transplantation: fludarabine/Melphalan is associated with higher incidence of acute graft-versus-host disease and non-relapse mortality and lower incidence of relapse than fludarabine/busulfan. Leukemia. 2007;21:2109–2116. doi: 10.1038/sj.leu.2404886. [DOI] [PubMed] [Google Scholar]
- 6.Dix SP, Wingard JR, Mullins RE, et al. Association of busulfan area under the curve with veno-occlusive disease following BMT. Bone Marrow Transplant. 1996;17:225–230. [PubMed] [Google Scholar]
- 7.Kashyap A, Wingard J, Cagnoni P, et al. Intravenous vs oral busulfan as part of a busulfan/cyclophosphamide preparative regimen for allogeneic hematopoietic stem cell transplantation: decreased incidence of hepatic venoocclusive disease (HVOD), HVOD related mortality and overall 100 Day mortality. Biol Blood Marrow Transplant. 2002;8:493–500. doi: 10.1053/bbmt.2002.v8.pm12374454. [DOI] [PubMed] [Google Scholar]
- 8.Jones RJ, Lee KS, Beschorner WE, et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987;44:778–783. doi: 10.1097/00007890-198712000-00011. [DOI] [PubMed] [Google Scholar]
- 9.McDonald GB, Slatter JT, Bouvier ME, et al. Cyclophosphamide metabolism, liver toxicity, and mortality following hematopoietic stem cell transplantation. Blood. 2003;101:2043–2048. doi: 10.1182/blood-2002-06-1860. [DOI] [PubMed] [Google Scholar]
- 10.Slatter JT, Sanders JE, Buckner CD, et al. Graft-rejection and toxicity following bone marrow transplantation in relation to busulfan pharmacokinetics. Bone Marrow Transplant. 1995;16:31–42. [PubMed] [Google Scholar]
- 11.Andersson B, Couriel D, Madden T, et al. Busulfan systemic exposure relative to regimen-related toxicity and acute graft vs. host disease; defining a therapeutic window for IV BuCy2 in chronic myelogenous leukemia. Biol Blood Marrow Transplant. 2002;8:477–485. doi: 10.1053/bbmt.2002.v8.pm12374452. [DOI] [PubMed] [Google Scholar]
- 12.Hassan M, Ljungman P, Ringden O, et al. The effect of busulphan and its 4-hydroxy metabolite: time interval influence on therapeutic efficacy and therapy-related toxicity. Bone Marrow Transplant. 2000;25:915–924. doi: 10.1038/sj.bmt.1702377. [DOI] [PubMed] [Google Scholar]
- 13.Williams CB, Day SD, Reed MD, et al. Dose modification protocol using intravenous busulfan (Busulfex) and cyclophosphamide followed by autologous or allogeneic peripheral stem cell transplantation in patients with hematologic malignancies. Biol Blood Marrow Transplant. 2004;10:614–623. doi: 10.1016/j.bbmt.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Mamlouk K, Saracino G, Berryman RB, et al. Modification of the Bu/Cy myeloablative regimen using daily parenteral busulfan: reduced toxicity without the need for pharmacokinetic monitoring. Bone Marrow Transplant. 2005;35:747–754. doi: 10.1038/sj.bmt.1704871. [DOI] [PubMed] [Google Scholar]
- 15.Terenzi A, Aristei C, Aversa F, et al. Efficacy of fludarabine as an immunosuppressor for bone marrow transplantation conditioning: Preliminary results. Transplantation Proc. 1996;28:3101. [PubMed] [Google Scholar]
- 16.Gandhi V, Plunkett W. Clinical pharmacology of fludarabine. Clin Pharmacokinet. 2002;41(2):93–103. doi: 10.2165/00003088-200241020-00002. [DOI] [PubMed] [Google Scholar]
- 17.de Lima M, Couriel D, Thall PF, et al. Once daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004;104:857–864. doi: 10.1182/blood-2004-02-0414. [DOI] [PubMed] [Google Scholar]
- 18.Russell JA, Tran HT, Quinlan BN, et al. Once daily intravenous busulfan given with fludarabine as conditioning for allogeneic stem cell transplantation: study of pharmacokinetics and early clinical outcomes. Biol Blood Marrow Transpl. 2002;8:468–476. doi: 10.1053/bbmt.2002.v8.pm12374451. [DOI] [PubMed] [Google Scholar]
- 19.Russell JA, Savoie ML, Balogh A, et al. Allogeneic transplantation for adult acute leukemia in first and second remission with a novel regimen incorporating daily intravenous busulfan, fludarabine, 400 cGy total-body irradiation, and thymoglobulin. Biol Blood Marrow Transplant. 2007;13:814–821. doi: 10.1016/j.bbmt.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen L, Leger F, Lennon S, Puozzo C. Intravenous busulfan in adults prior to haematopoietic stem cell transplantation: a population pharmacokinetic study. Cancer Chemother Pharmacol. 2006;57:191–198. doi: 10.1007/s00280-005-0029-0. [DOI] [PubMed] [Google Scholar]
- 21.Madden T, de Lima M, Thapar N, et al. Pharmacokinetics of once daily IV busulfan as part of pretransplant preparative regimens; a comparison with an every 6 hour dosing schedule. Biol Blood Marrow Transpl. 2007;13:56–64. doi: 10.1016/j.bbmt.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 22.Geddes M, Kangarloo SB, Naveed F, et al. High busulfan exposure is associated with worse outcomes in a daily IV busulfan and fludarabine allogeneic transplant regimen. Biol Blood Marrow Transplant. 2008;14:220–228. doi: 10.1016/j.bbmt.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 23.Yau JC, LeMaistre CF, Andersson BS, et al. Allogeneic bone marrow transplantation for hematological malignancies following Etoposide, Cyclophosphamide and fractionated total body Irradiation. Am J Hematology. 1992;41:40–44. doi: 10.1002/ajh.2830410108. [DOI] [PubMed] [Google Scholar]
- 24.Przepiorka D, Ippoliti C, Giralt S, et al. A phase I–II study of high dose thiotepa, busulfan and cyclophosphamide as a preparative regimen for allogeneic marrow transplantation. Bone Marrow Transplantation. 1994;14:449–453. [PubMed] [Google Scholar]
- 25.Przepiorka D, Khouri I, Thall P, et al. Thiotepa, busulfan and cyclophosphamide as a preparative regimen for allogeneic transplantation for advanced chronic myelogenous leukemia. Bone Marrow Transplant. 1999;23:977–981. doi: 10.1038/sj.bmt.1701764. [DOI] [PubMed] [Google Scholar]
- 26.Giralt S, Thall PF, Khouri I, et al. Melphalan and purine analog-containing preparative regimens: reduced-intensity conditioning for patients with hematologic malignancies undergoing allogeneic progenitor cell transplantation. Blood. 2001;97:631–637. doi: 10.1182/blood.v97.3.631. [DOI] [PubMed] [Google Scholar]
- 27.Kebriaei P, Saliba RM, Ma C, et al. Allogeneic hematopoietic stem cell transplantation after rituximab-containing myeloablative preparative regimen for acute lymphoblastic leukemia. Bone Marrow Transplant. 2006;38:203–209. doi: 10.1038/sj.bmt.1705425. [DOI] [PubMed] [Google Scholar]
- 28.Marks DI, Forman SJ, Blume KG, et al. A comparison of cyclophosphamide and total body irradiation with etoposide and total body irradiation as conditioning regimens for patients undergoing sibling allografting for acute lymphoblastic leukemia in first or second complete remission. Biol Blood Marrow Transplant. 2006;12:438–453. doi: 10.1016/j.bbmt.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 29.Estey E, Thall PF, Giles F, et al. Gemtuzumab ozogamycin with or without interleukin 2 in patients 65 years of age or older with untreated AML and high-risk MDS: comparison with idarubicin + continuous-infusion high-dose cytosine arabinoside. Blood. 2002;99:4343–4349. doi: 10.1182/blood.v99.12.4343. [DOI] [PubMed] [Google Scholar]
- 30.Thall PF, Wang X. Bayesian sensitivity analyses of confounded treatment effects. In: Crowley JC, Pauler DP, editors. Handbook of Statistics in Clinical Oncology. 2 Revised and Expanded. Boca Raton: Chapman & Hall/CRC Taylor Francis Group; 2006. pp. 523–540. [Google Scholar]
- 31.Keating MJ, Smith TL, Gehan EA, et al. Factors related to length of complete remission in adult acute leukemia. Cancer. 1980;45:2017–2029. doi: 10.1002/1097-0142(19800415)45:8<2017::aid-cncr2820450806>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 32.Greenberg P, Cox C, LeBeau M, et al. International scoring system for evaluating prognosis in myelodysplastic Syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 33.Andersson BS, Kashyap A, Gian V, et al. Conditioning therapy with intravenous busulfan and cyclophosphamide (IV BuCy2) for hematologic malignancies prior to allogeneic stem cell transplantation: A phase II Study. Biol Blood Marrow Transplant. 2002;8:145–154. doi: 10.1053/bbmt.2002.v8.pm11939604. [DOI] [PubMed] [Google Scholar]
- 34.De Lima M, Champlin RE, Thall PF, et al. Phase I/II study of gemtuzumab ozogamicin added to fludarabine, melphalan and allogeneic hematopoietic stem cell transplantation for high-risk CD33 positive myeloid leukemias and Myelodysplastic syndrome. Leukemia. 2007 Nov 8; doi: 10.1038/sj.leu.2405014. E-pub. [DOI] [PubMed] [Google Scholar]
- 35.Przepiorka D, Khouri I, Ippoliti C, et al. Tacrolimus and minidose methotrexate for prevention of acute graft-versus-host disease after HLA-mismatched marrow or blood stem cell transplantation. Bone Marrow Transplant. 1999;24:763–768. doi: 10.1038/sj.bmt.1701983. [DOI] [PubMed] [Google Scholar]
- 36.Körbling M, Huh YO, Durett A, et al. Allogeneic blood stem cell transplantation: peripheralization and yield of donor-derived primitive hematopoietic progenitor cells (CD34+ Thy-1dim) and lymphoid subsets, and possible predictors of engraftment and graft-versus-host disease. Blood. 1995;86:2842–2848. [PubMed] [Google Scholar]
- 37.Przepiorka D, Smith TL, Folloder J, et al. Risk factors for acute graft-versus-host disease after allogeneic blood stem cell transplantation. Blood. 1999;94:1465–1470. [PubMed] [Google Scholar]
- 38.Snedecor GW, Cochoran WG. Statistical Methods. 7. The Iowa State University Press; Ames, Iowa: 1980. [Google Scholar]
- 39.Kaplan EL, Meier P. Nonparametric estimator from incomplete observations. J Am Statistical Assoc. 1958;53:457–481. [Google Scholar]
- 40.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rept. 1966;60:163–170. [PubMed] [Google Scholar]
- 41.Johnson V. A Bayesian X2 test for goodness-of-fit. Annals of Statistics. 2004;32:2361–2384. [Google Scholar]
- 42.Venables WN, Ripley BD. Modern Applied Statistics With Splus. 3. New York: Springer; 1999. [Google Scholar]
- 43.WinBugs Version 1.4. Imperial College & Medical Research Council (MRC); UK: [Google Scholar]
- 44.Vassal G. Pharmacologically-guided dose adjustment of busulfan in high-dose chemotherapy regimens: rationale and pitfalls (review) Anticancer Res. 1994;14:2363–2370. [PubMed] [Google Scholar]
- 45.de Lima M, Wang X, Thall PF, et al. Long-Term Follow-Up of IV Busulfan (Bu) with Fludarabine (FLU) vs IV Bu with Cyclophosphamide (Cy) as Pre (Allogeneic) Transplant Conditioning Therapy for AML/MDS. Blood. 2006;108:322. (Abstr) [Google Scholar]
- 46.Chae YS, Sohn SK, Kim JG, et al. New myeloablative conditioning regimen with fludarabine and busulfan for allogeneic stem cell transplantation : comparison with BuCy2. BMT. 2007;40:541–547. doi: 10.1038/sj.bmt.1705770. [DOI] [PubMed] [Google Scholar]
- 47.McCune JS, Batchelder A, Deeg HJ, et al. Cyclophosphamide following targeted oral busulfan as conditioning for hematopoietic cell transplantation: pharmacokinetics, liver toxicity, and mortality. Biol Blood Marrow Transplant. 2007;13:853–862. doi: 10.1016/j.bbmt.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 48.Chang C, Sorer BE, Scott BL, et al. Hematopoietic cell transplantation in patients with myelodysplastic syndrome or acute myeloid leukemia arising from myelodysplastic syndrome: similar outcomes in patients with de novo disease and disease following prior therapy or antecedent hematologic disorder. Blood. 2007;110:1379–1387. doi: 10.1182/blood-2007-02-076307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hallemeier C, Girgis M, Blum W, et al. Outcomes of adults with acute myelogenous leukemia in remission given 550 cGy of single-exposure total body irradiation, cyclophosphamide, and unrelated donor bone marrow transplants. Biol Blood Marrow Transplant. 2004;10:310–319. doi: 10.1016/j.bbmt.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 50.Alyea EP, Kim HT, Ho V, et al. Impact of conditioning regimen intensity on outcome of allogeneic hematopoietic cell transplantation for advanced acute myelogenous leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant. 2006;12:1047–1055. doi: 10.1016/j.bbmt.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 51.Burnett AK, Wheatley K, Goldstone AH, et al. The value of allogeneic bone marrow transplant in patients with acute myeloid leukaemia at differing risk of relapse: results of the UK MRC AML 10 trial. Br J Haematol. 2002;118:385–400. doi: 10.1046/j.1365-2141.2002.03724.x. [DOI] [PubMed] [Google Scholar]
- 52.Burnett AK. Evaluating the contribution of allogeneic and autologous transplantation to the management of acute myeloid leukemia in adults . Cancer Chemother Pharmacol. 2001;48(Suppl 1):S53–58. doi: 10.1007/s002800100306. [DOI] [PubMed] [Google Scholar]