Abstract
Vortioxetine, a novel antidepressant with multimodal action, is a serotonin (5-HT)3, 5-HT7 and 5-HT1D receptor antagonist, a 5-HT1B receptor partial agonist, a 5-HT1A receptor agonist and a 5-HT transporter (SERT) inhibitor. Vortioxetine has been shown to improve cognitive performance in several preclinical rat models and in patients with major depressive disorder. Here we investigated the mechanistic basis for these effects by studying the effect of vortioxetine on synaptic transmission, long-term potentiation (LTP), a cellular correlate of learning and memory, and theta oscillations in the rat hippocampus and frontal cortex. Vortioxetine was found to prevent the 5-HT-induced increase in inhibitory post-synaptic potentials recorded from CA1 pyramidal cells, most likely by 5-HT3 receptor antagonism. Vortioxetine also enhanced LTP in the CA1 region of the hippocampus. Finally, vortioxetine increased fronto-cortical theta power during active wake in whole animal electroencephalographic recordings. In comparison, the selective SERT inhibitor escitalopram showed no effect on any of these measures. Taken together, our results indicate that vortioxetine can increase pyramidal cell output, which leads to enhanced synaptic plasticity in the hippocampus. Given the central role of the hippocampus in cognition, these findings may provide a cellular correlate to the observed preclinical and clinical cognition-enhancing effects of vortioxetine.
Keywords: Serotonin, long-term potentiation, electroencephalography, cognition, CA1, 5-hydroxytryptamine 3 receptor
Introduction
Psychiatric disorders are often accompanied by symptoms of cognitive impairment that contribute significantly to the degree of disability and further impair the quality of life associated with these conditions. In patients suffering from major depressive disorder (MDD), several symptoms of cognitive dysfunction are frequently reported (Millan et al., 2012). Cognitive dysfunction ranges across a broad spectrum of domains, such as attention, memory (several subdomains, including working, episodic, and semantic memory), psychomotor speed, and executive function (Millan et al., 2012). Moreover, these symptoms often persist as residual symptoms in remitted patients (Conradi et al., 2011). Since cognitive dysfunction is one of the most common residual symptoms of MDD, it follows that currently used antidepressants do not offer adequate therapeutic efficacy and that there is a need for new treatment options (McClintock et al., 2011).
The biological substrate for the modulation of cognitive function is complex and involves multiple neuromodulators and neurotransmitters (Millan et al., 2012). Whereas the role of serotonin (5-HT) in MDD has been extensively studied and is generally accepted, there is less understanding with regard to how 5-HT interacts with cognitive processing (Cowen and Sherwood, 2013). The preclinical literature provides support for the notion that serotonergic modulation of glutamate neurotransmission, which is essential for cognitive processing, may be important for the beneficial effects of 5-HT on cognition (Pehrson and Sanchez, 2013).
5-HT exerts its neuromodulatory actions by activating various 5-HT receptor subtypes. However, not all serotonergic receptors have enhancing effects on cognition. The activation of 5-HT2A, 5-HT4 and 5-HT1A receptors has positive effects on memory and cognitive functioning in animal studies (Buhot et al., 2000; Meneses, 2003). In contrast, the activation of 5-HT3 receptors has been shown to impair memory retention in rats (Hong and Meneses, 1996). Furthermore, 5-HT3 receptor antagonists exhibit memory-enhancing properties in a number of preclinical cognitive models (Arnsten et al., 1997; Brambilla et al., 1993; Fontana et al., 1995; Pitsikas and Borsini, 1996; Pitsikas et al., 1994). Similarly, antagonism of 5-HT7 receptors has been shown to be beneficial for memory function (Horiguchi et al., 2011; Horisawa et al., 2011; McLean et al., 2009; Meneses, 2003). Thus, accumulating evidence from animal models suggests that 5-HT receptors are involved in both the enhancement and inhibition of cognitive processes.
Vortioxetine, an antidepressant with a multimodal mechanism of action (Adell, 2010; Alvarez et al., 2012), has recently been approved for the treatment of MDD. In vitro studies using cell lines expressing cloned human receptors or the 5-HT transporter (SERT) have demonstrated that vortioxetine is a 5-HT3A (Ki=3.7 nM), 5-HT7 (Ki=19 nM) and 5-HT1D (Ki=54 nM) receptor antagonist, 5-HT1B receptor partial agonist (Ki=33 nM; IA 55%), 5-HT1A receptor agonist (Ki=15 nM) and inhibitor of the 5-HT transporter (SERT; Ki=1.6 nM (Bang-Andersen et al., 2011; Westrich et al., 2012). In rodent studies, vortioxetine has shown positive effects against cognitive dysfunction (Du Jardin et al., 2013; Jensen et al., 2013; Mørk et al., 2013). For example, vortioxetine improves acquisition and retention of contextual fear memory and object recognition memory in rats (Mørk et al., 2013). More important, in clinical studies of MDD patients, vortioxetine has shown positive effects in several cognitive domains compared to placebo (Katona et al., 2012; McIntyre et al., 2013). Since the individual 5-HT receptor activities of vortioxetine have the potential to modulate glutamate neurotransmission and cognition, we have hypothesized that vortioxetine’s multimodal action will result in enhanced glutamate transmission and thus improve cognitive function (Pehrson and Sanchez, 2013). The aim of the present study was to test this hypothesis by studying the effects of vortioxetine on the function of glutamatergic pyramidal cells and on synaptic plasticity in rat hippocampal slices, and on the strength of theta oscillations in awake rats.
In the hippocampus, 5-HT exerts net inhibitory effects on pyramidal cells by hyperpolarizing their membrane potential and potentiating gamma-aminobutyric acid (GABA) transmission measured as an increase in spontaneous inhibitory post-synaptic currents (sIPSCs) (Passani et al., 1994; Ropert and Guy, 1991; Shen and Andrade, 1998; Turner et al., 2004). Furthermore, the enhancing effect of 5-HT on sIPSCs largely depends on the activation of 5-HT3 receptors on GABAergic inhibitory interneurons (McMahon and Kauer, 1997; Turner et al., 2004). Since vortioxetine is a potent 5-HT3 receptor antagonist, we hypothesized that vortioxetine would counteract the 5-HT-induced increase of sIPSCs and disinhibit pyramidal cell output. Enhancement of pyramidal cell activity would translate into increased synaptic plasticity and stronger theta firing of pyramidal cells. Furthermore, we hypothesized that such effects would not be observed with selective 5-HT reuptake inhibitors (SSRIs).
Materials and methods
Hippocampal brain slice preparation
Experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by Lundbeck’s Institutional Care and Use Committee (IACUC). All efforts were made to minimize the number of animals used and their suffering. Hippocampal slices were prepared from male Sprague Dawley rats (2–3 weeks old for patch-clamp recordings and 4–5 weeks old for LTP recordings). Animals were decapitated and their brains were quickly removed and placed in ice-cold high Mg2+/low Ca2+ artificial cerebral spinal fluid (aCSF) containing (in mM): 119 NaCl, 2.5 KCl, 1 Na2HPO4, 26.2 NaHCO3, 0.5 CaCl2, 7 MgCl2, and 11 glucose, aerated with 95% O2 / 5% CO2, pH 7.25–7.35. The brains were blocked and glued onto the stage of a vibratome (VT1200S, Leica Microsystems Inc., Bannockburn, Illinois, USA). Horizontal or transverse hippocampal slices (300–350 μm in thickness) were then cut and incubated in the regular oxygenated aCSF containing (in mM): 119 NaCl, 2.5 KCl, 1 Na2HPO4, 26 NaHCO3, 2.5 CaCl2, 1.3 MgCl2, and 11 glucose at 35oC for the first 60 min and then transferred to room temperature prior to recordings.
Whole cell patch-clamp recordings
sIPSCs and miniature inhibitory post-synaptic currents (mIPSCs) were recorded from CA1 pyramidal cells in the voltage-clamp mode. Patch pipettes (3–4 MΩ) were pulled from thick-walled borosilicate glass tubing (outer diameter: 1.5 mm, inner diameter: 0.75 mm; Sutter Instrument, Novato, California, USA) and filled with a solution containing (in mM): 110 CsCl2, 10 NaCl, 5 MgCl2 0.6 ethylene glycol tetraacetic acid (EGTA), 40 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 5 QX-314·Cl, 2 MgATP, and 0.2 Na2GTP, pH adjusted to 7.3 with CsOH. The osmolarity was adjusted to 290 mOsm with sucrose. sIPSCs were pharmacologically isolated by adding the N-methyl-D-aspartate (NMDA) receptor antagonist D-(-)-2-amino-5-phosphonopentanoic acid (D-AP5, 50 µM) and the α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) / kainate receptor antagonist 6,7-dinitroquinoxaline-2,3-dione (DNQX, 10 µM) to aCSF. mIPSCs were further isolated by adding tetrodotoxin (TTX) to aCSF. Recordings were acquired using a Multiclamp amplifier 700B and the pClamp 10 software package (Molecular Devices, Sunnyvale, California, USA). Signals were digitized at 5 kHz and filtered at 2 kHz. CA1 pyramidal cells were voltage-clamped at −70 mV. Access resistance was monitored throughout the experiments. Neurons whose series resistance changed by >15% were excluded from the analyses.
Following drug treatments, frequency and amplitude of sIPCSs were normalized to pre-drug baseline levels using the following formula:
Statistical analyses were performed using either Student’s t-test, one-way analysis of variance (ANOVA) or Kolmogorov-Smirnov (K-S) test. Results were considered significant at p<0.05. Figures were made using GraphPad Prism (GraphPad Software, San Diego, California, USA) and Adobe Illustrator software (Adobe Systems Incorporated, San Jose, California, USA) packages.
LTP recordings
LTP was recorded in hippocampal slices using extracellular field potential recordings. The stimulating electrode consisting of a bipolar concentric tungsten electrode (FHC Inc., Bowdoin, Maine, USA) was placed at the level of the Schaeffer collateral fibers. The recording electrode consisting of a glass pipette filled with aCSF was placed at the level of the CA1 stratum radiatum. The aCSF was similar in composition to the one used in whole-cell patch recordings except that Ca2+ and Mg2+ concentrations were 2 mM. A 20 min baseline was recorded once every minute at an intensity that evoked a response of ∼35% of the maximum evoked response. LTP was induced using one theta-burst stimulation (TBS) consisting of four trains of four pulses at 100 Hz separated by 200 ms. LTP was recorded for one hour after induction and expressed as percentage of baseline responses. Data were acquired with pClamp software (Molecular Devices, Sunnyvale, California, USA) at a sampling rate of 10 kHz and low-pass filtered at 1 kHz. Data were analyzed with pClamp software (Molecular Devices, Sunnyvale, California, USA). Statistical analysis was performed using a two-way ANOVA with repeated measures. Results were considered significant at p<0.05.
In vivo electroencephalography (EEG) recordings
Theta rhythms were recorded in freely-moving Sprague Dawley male rats (250–300 g) with EEG electrodes. Animals were individually housed under a 12 h light/dark (06:00–18:00) cycle and temperature (21±2°C) and humidity (60±10%) control with chow and water ad libitum. The drugs were dosed three hours into the light cycle (09:00–10:00). A multi-day Latin square, cross-over design was used (n=9 rats per condition). Stainless-steel screw electrodes for EEG recordings and wire electrodes for electromyographic (EMG) recordings of dorsal neck muscles were implanted in each animal under anesthesia as previously described (Bastlund et al., 2004; Vogel et al., 2002). Bipolar (differential) EEG ground screw electrodes (size #00-80) were placed in each hemisphere supradural, approximately 2.0 mm anterior and 2.0 mm lateral to Bregma for frontal cortical EEG recordings. Electrodes were connected to a sterile multi-channel telemetric device (TL10M3-F50-EEE; Data Sciences International (DSI), St Paul, Minnesota, USA) that was implanted subcutaneously on the flank. These transmitters also digitally monitored locomotor activity counts that were used in sleep staging. Locomotor activity counts were calculated using Dataquest A.R.T software (DSI, St. Paul, Minnesota, USA) as a function of alterations in transmitter signal strength as the animal moved through the receptive field of the receiver located beneath the animals’ home cage. EEG and EMG signals were recorded using Dataquest A.R.T software (DSI, St. Paul, Minnesota, USA) at a sampling rate of 500 Hz. Artifacts were removed from the data and sleep stages assigned manually for every 10-second epoch using EEG, EMG, and locomotor activity counts by conventional methods. Data were classified as active wake, quiet wake, slow wave sleep, and paradoxical or rapid eye movement (REM) sleep (Parmentier-Batteur et al., 2012). All data were scored into these stages; however, only active wake data is shown in this paper, since we believe this is the only relevant stage in which cognitive load occurs (Maire et al., 2013). Active wake data were further analyzed using Microsoft Excel (Microsoft Corporation, Seattle, WA). The relative theta power (4–8 Hz) in each 15 min bin per treatment group was averaged and plotted against time from 0 to 180 min post dose. The percent change in theta power from baseline was calculated from the pre- (15–75 min before) and post- (45–90 min after) dose averages using the formula:
where each animal served as its own control. An ANOVA with Fisher’s least significant difference (LSD) post-hoc test (Statistica, Cary, North Carolina, USA) was used to determine differences. Results were considered significant at p<0.05.
Chemicals and drugs
All chemicals and drugs were obtained from Sigma (St. Louis, Missouri, USA) unless otherwise noted. Stock solutions were made in either water or DMSO at 1000-fold their final concentration and stored at −20°C. D-AP5, DNQX, QX-314 bromide and m-chlorophenylbiguanide hydrochloride (m-CPBG) were purchased from Tocris Bioscience (Ellisville, Missouri, USA). Vortioxetine and escitalopram were synthesized at H Lundbeck A/S (Copenhagen, Denmark). For whole-cell patch recordings, 5-HT (100 µM) and m-CPBG (20 µM) were focally applied to the brain slice surface via a fast speed perfusion system (ALA Scientific Instruments, Farmingdale, New York, USA). 5-HT solution was made fresh before each experiment and co-applied with 50 µM ascorbic acid to decrease oxidization. Vortioxetine (20 µM) and escitalopram (10 µM) were added directly to aCSF and applied via a bath perfusion system. For in vivo EEG recordings, vehicle, vortioxetine, escitalopram were dissolved in 20% aqueous ß-cyclodextrin and administered subcutaneously (s.c.) in a volume of 2.0 mL/kg.
Results
5-HT enhances spontaneous GABAergic transmission in hippocampal slices
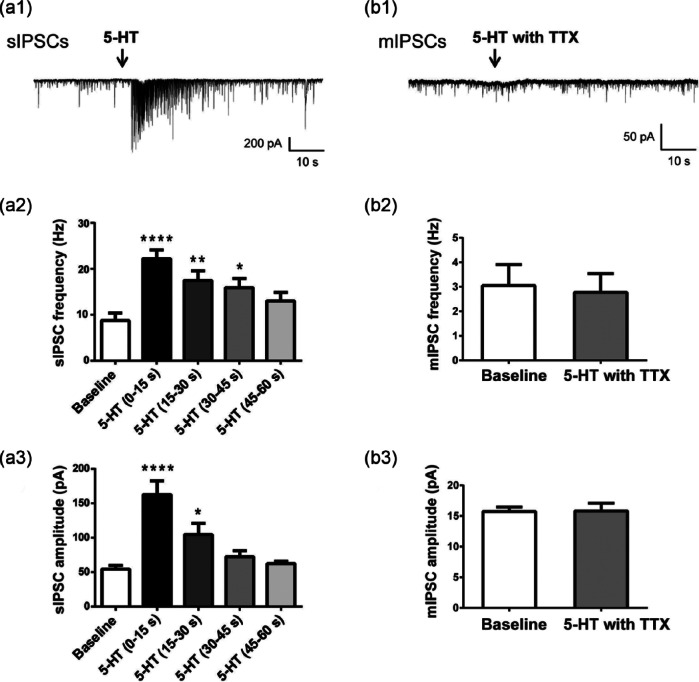
Consistent with published results, local application of 5-HT significantly increased sIPSCs recorded from CA1 pyramidal cells (Figure 1(a1)). Approximately 85% of cells responded to 5-HT with a burst-like enhancement in sIPSC frequency and amplitude. Only those cells were included in the analysis of this study (n=24 cells). The effect of 5-HT was always transient and lasted for ~60 s (Figures 1(a1)–(a3)). Because of rapid desensitization, 5-HT was applied focally onto the surface of the slice via a fast speed perfusion system. Although fast desensitizing, 5-HT responses were repeatable after a 3–5 min washout (data not shown). Frequency and amplitude analyses for 60 s of recordings for 10 representative cells following 5-HT application are shown in Figures 1(a2) and 1(a3). Peak 5-HT response was observed within the first 15 s, during which sIPSC frequency and amplitude were increased by 301±34% and 261±26%, respectively (n=24 cells).
Figure 1.
Serotonin (5-HT) increased frequency and amplitude of spontaneous inhibitory post-synaptic currents (sIPSCs), but not miniature inhibitory post-synaptic currents (mIPSCs), recorded from hippocampal CA1 pyramidal cells. (a1), (b1) Representative traces of sIPSCs (a1) and mIPSCs (b1) recorded from a CA1 pyramidal cell before and after local application of 5-HT (100 µM for 500 ms). mIPSCs were recorded in the presence of 1 µM tetrodotoxin (TTX). (a2), (a3) Serotonin (5-HT) transiently increased the frequency ((a2), ****p<0.001, **p=0.0078, *p=0.0361, one-way analysis of variance (ANOVA) with Dunnett’s multiple comparisons test vs baseline) and amplitude ((a3), ****p<0.001, *p=0.0235, one-way ANOVA with Dunnett’s multiple comparisons test vs baseline) of sIPSCs. The largest increase was observed in the first 15 s after 5-HT application and the response to 5-HT was largely desensitized after 60 s. Bars represent the mean±standard error of the mean (SEM) of recordings from 10 cells. (b2), (b3) 5-HT had no effect on mIPSC frequency (b2) or amplitude (b3) (p>0.05, paired Student’s t-test). Bars represent the mean±SEM of 60 s recordings from four cells.
In contrast to the prominent effect noted with sIPSCs, 5-HT had no effect on mIPSCs recorded from CA1 pyramidal neurons in the presence of 1 µM TTX (Figure 1(b1)). The responses elicited from 5-HT on sIPSCs and mIPSCs recorded from the same cell are shown in Figures 1(a1) and 1(b1). Note that despite a strong enhancement of sIPSCs, there was no change in mIPSCs after 5-HT application. The lack of effect of 5-HT on mIPSCs was observed for all recorded neurons (n=4, Figures 1(b2) and 1(b3)).
Vortioxetine, but not escitalopram, blocks the 5-HT-induced increase in sIPSC frequency and amplitude
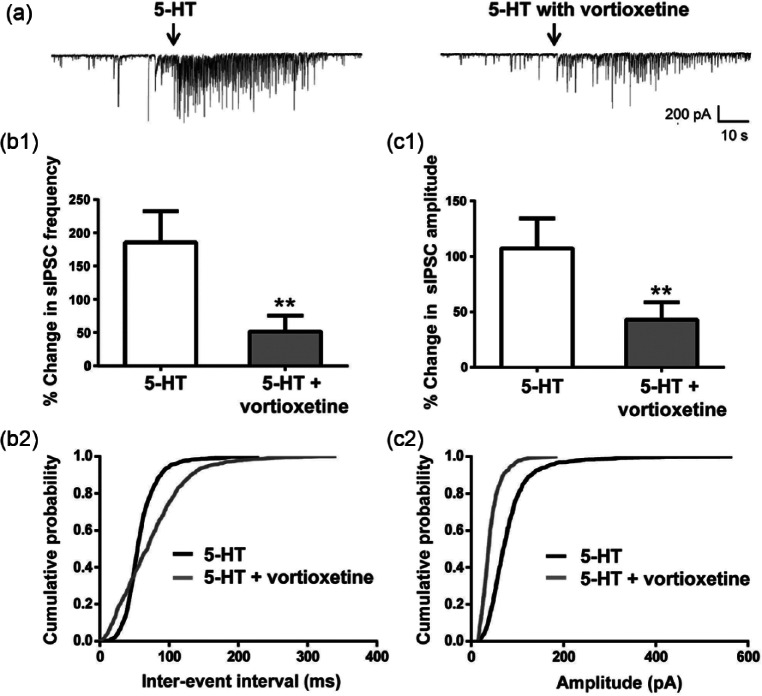
The effect of vortioxetine was only studied in cells that responded to 5-HT. In these cells, bath application of 20 µM vortioxetine did not affect baseline sIPSCs (n=15, data not shown), but largely suppressed the 5-HT-mediated increase in sIPSC frequency and amplitude (Figure 2, p<0.05 for both, paired Student’s t-test). Vortioxetine required at least 15 min of application to achieve its maximal effect, which lasted for >30 min. We never observed a complete wash-out of the vortioxetine response, most likely due to the fact that vortioxetine is >99% protein bound in rat brain tissue (Sanchez et al., 2014) and thus is probably difficult to wash-out. The inhibitory effect of vortioxetine on sIPSCs was observed in 14 of the 15 cells tested (Figures 2(b1) and 2(c1)). To control for a possible desensitization of 5-HT response, vortioxetine was applied after the third application of 5-HT to a subset of cells. Under these conditions, vortioxetine still fully inhibited 5-HT responses, indicating that desensitization could not explain the inhibitory effect seen with vortioxetine (n=3, data not shown).
Figure 2.
Vortioxetine blocked the serotonin (5-HT)-induced increase in spontaneous inhibitory post-synaptic currents (sIPSCs).
(a) Representative sIPSCs recorded from a CA1 pyramidal neuron in response to 5-HT before (left) and after 15 min application of 20 µM vortioxetine (right). (b1), (c1) Vortioxetine blocked the 5-HT induced increase in sIPSC frequency ((b1), **p=0.0073, paired Student’s t-test) and amplitude ((c1), **p=0.0066, paired Student’s t-test). Frequency and amplitude were normalized to the mean value during the 30 s of recordings prior to 5-HT application. Bars represent the mean±standard error of the mean (SEM) of sIPSCs from 15 cells. (b2), (c2) Averaged cumulative probability plots of sIPSC inter-event interval (b2) and amplitude (c2) for 5-HT alone and 5-HT+vortioxetine. Data for these graphs were derived from 60 s of continuous recordings. Each data point represents the mean from 15 cells. Vortioxetine significantly increased the inter-event interval time in 14/15 cells and shifted amplitudes of sIPSCs to lower values in 13/15 cells after 5-HT application (p<0.05, Kolmogorov-Smirnov (K-S) test).
Averaged cumulative probability distributions for inter-event interval and amplitude of sIPSCs for 5-HT alone and 5-HT+vortioxetine conditions are shown in Figures 2(b2) and 2(c2). In 14 of 15 cells, there was a significant shift to lower frequencies in the distribution of sIPSCs (i.e. longer inter-event intervals), and in 13 of 15 cells, there was a significant shift of sIPSC amplitudes to smaller values in the presence of vortioxetine (p<0.05, K-S test). Thus, vortioxetine had a potent blocking effect on the 5-HT-induced increase in sIPSCs.
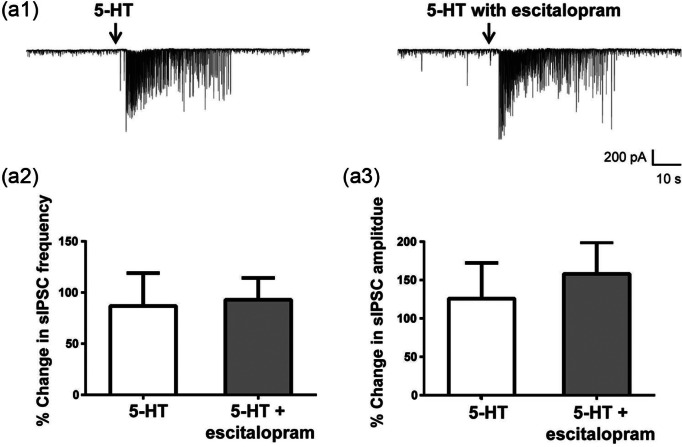
In contrast to vortioxetine, escitalopram did not inhibit the 5-HT-mediated increase in sIPSCs in any of the cells tested (Figure 3, n=6). In two of six cells, escitalopram slightly enhanced 5-HT-induced sIPSCs, but in the remaining four cells, there was no change in 5-HT response. Overall, escitalopram had no significant effect on the frequency and amplitude of 5-HT responses (Figures 3(a2) and 3(a3), p>0.05, paired Student’s t-test).
Figure 3.
Escitalopram had no effect on serotonin (5-HT) increase of spontaneous inhibitory post-synaptic currents (sIPSCs). (a1) Representative sIPSCs recorded from a CA1 pyramidal neuron in response to 5-HT before (left) and after 15 min perfusion with 10 µM escitalopram (right). (a2), (a3) Escitalopram did not change the 5-HT response on sIPSC frequency (a2) or amplitude (a3) (p>0.05, paired Student’s t-test). Frequency and amplitude were normalized to the mean value during the 30 s of recordings prior to 5-HT application. Bar graphs represent the mean±standard error of the mean (SEM) from six cells.
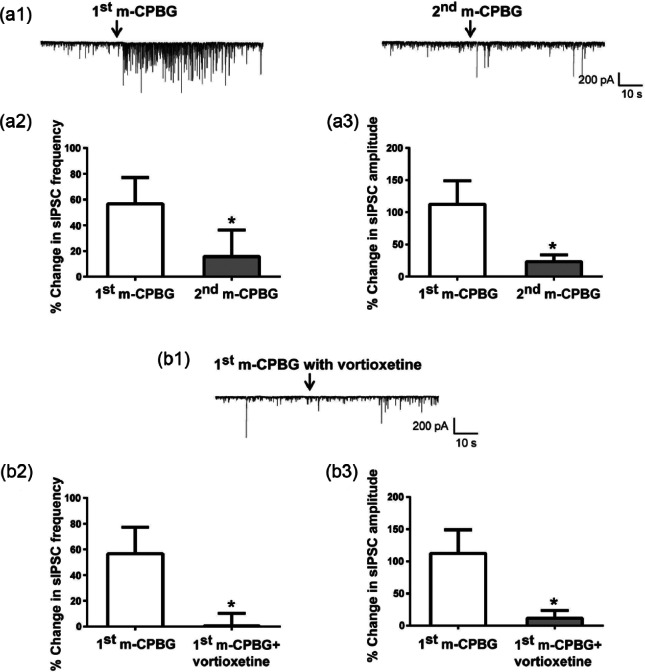
Finally, we investigated the interaction between vortioxetine and the 5-HT3 receptor agonist m-CPBG (Figure 4). In 68% (11/16) of examined cells, local application of m-CPBG (20 µM) produced a transient increase in sIPCSs in a manner similar to that of 5-HT, suggesting that 5-HT3 receptors were involved in the 5-HT response (Figure 4(a1)). However, in most of the responding cells (9/11) the effect of m-CPBG was not repeatable after various (3–25 min) application intervals (Figure 4(a1), compare left and right panels). Even after a 25 min wash-out, there was a significant difference in response between the first and second applications of m-CPBG (Figures 4(a2) and 4(a3)). Due to this long-lasting desensitization of m-CPBG responses, the effect of vortioxetine was tested in a separate set of cells (Figures 4(b1)–4(b3), n=9). A 20–30 min pretreatment with vortioxetine (20 µM) blocked m-CPBG response in all recorded neurons. In the presence of vortioxetine, the effect of the first application of m-CPBG on both sIPSC frequency and amplitude was significantly attenuated (Figures 4(b2) and 4(b3), p<0.05 for both, unpaired Student’s t-test). Thus, vortioxetine blocked 5-HT and m-CPBG responses in a similar manner, suggesting that 5-HT3 receptor antagonism contributed to its inhibitory effect.
Figure 4.
The serotonin (5-HT)3 receptor agonist m-chlorophenylbiguanide hydrochloride (m-CPBG) transiently increased spontaneous inhibitory post-synaptic currents (sIPSCs) and vortioxetine blocked the m-CPBG effect. (a1) Representative sIPSCs recorded from a CA1 pyramidal neuron in response to two applications of the 5-HT3 receptor agonist m-CPBG. In most of the responding cells, the effect of m-CPBG was not repeatable after various application intervals (compare right and left traces). (a2), (a3) The second application of m-CPBG did not induce the same increase in sIPSC frequency ((a2), *p=0.031, paired Student’s t-test) or amplitude ((a3), *p=0.024, paired Student’s t-test) as the first application. Bar graphs represent the mean±standard error of the mean (SEM) from 11 cells. Because of strong desensitization of m-CPBG responses, the effect of vortioxetine was tested in a separate set of cells. (b1) Representative sIPSCs recorded from a CA1 pyramidal neuron in response to m-CPBG in the presence of 20 µM vortioxetine. (b2), (b3) Pre-treatment with vortioxetine blocked the m-CPBG increase in sIPSC frequency ((b2), *p=0.034, unpaired Student’s t-test) and amplitude ((b3), *p=0.028, unpaired Student’s t-test). Frequency and amplitude were normalized to the mean value during the 30 s of recordings prior to 5-HT application. Bar graphs represent the mean±SEM from 11 cells for the m-CPBG only condition and from nine cells for the m-CPBG + vortioxetine condition.
Vortioxetine, but not escitalopram, potentiates theta burst LTP in hippocampal slices
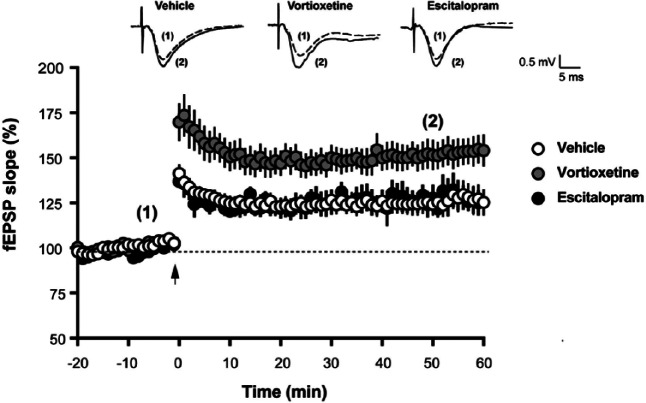
LTP was induced by stimulating the Schaeffer collateral fibers with a single train of theta-burst stimulation (TBS), as described in the Materials and Methods section. Following TBS, there was a long-lasting increase in the slope of the field excitatory post-synaptic potentials (fEPSPs) (Figure 5). The average LTP in vehicle-treated slices 60 min after TBS was 125±7% (n=17 slices from 12 animals). Perfusion of hippocampal slices with 20 µM vortioxetine for 30 min prior to TBS significantly increased the magnitude of LTP without affecting baseline transmission (Figure 5, p=0.0017, two-way ANOVA F(2,38)=7.548). The average LTP in vortioxetine-treated slices 60 min after TBS was 154±9% (n=14 slices from nine rats). In contrast, 10 µM escitalopram had no effect on LTP (Figure 5). The average LTP in escitalopram-treated slices 60 min after TBS was 127±5% (n=5 slices from four animals), which was very similar to the magnitude of LTP in vehicle-treated slices.
Figure 5.
Vortioxetine enhanced theta burst long-term potentiation (LTP) in hippocampal slices.
Representative traces of field excitatory post-synaptic potential (fEPSP) recordings from vehicle-treated, vortioxetine-treated and escitalopram-treated slices are shown on top of the graph. Each trace is the mean of five sweeps taken either immediately before (dashed line marked with (1) or 50 min after (solid line marked with (2)) theta-burst stimulation (TBS). TBS, marked with an arrow in the time course graph, induced a long-lasting increase in the slopes of fEPSPs. For each time point, fEPSP slopes were calculated from either vehicle-treated (n=17 slices from 12 animals), vortioxetine-treated (n=14 from nine animals) or escitalopram-treated slices (n=5 slices from four animals) and expressed as % of baseline. Data are shown as the mean±standard error of the mean (SEM). Perfusion of hippocampal slices with vortioxetine for 30 min prior to TBS increased LTP without affecting baseline transmission (p=0.0017 vortioxetine vs vehicle, two-way analysis of variance (ANOVA) F(2,38)=7.548)). Escitalopram had no effect on LTP (p>0.05).
Vortioxetine, but not escitalopram, increases frontal cortical theta power in in vivo EEG recordings
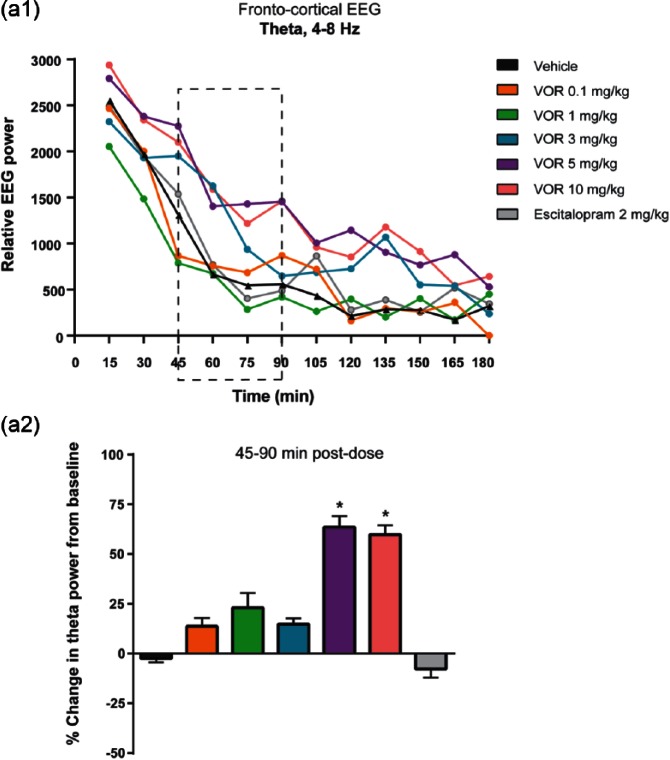
Vortioxetine (tested at 0.1, 1.0, 3.0, 5.0 and 10 mg/kg, s.c.) produced a significant dose-response effect on frontal cortical theta power during the active wake state in EEG recordings (Figure 6; Leiser et al., 2014). The effect was observed in the relative theta power following treatment (Figure 6(a1)). To quantify this effect, theta power was compared pre- (15–75 min before) and post- (45–90 min after) dose using each rat as its own control and plotted as a percent change. The 45–90 min time bin was chosen to ensure that sample EEG data were taken when the drug was onboard and the data were not confound by behavioral change due to dosing. There was a significant treatment effect in theta power during this time (Figure 6(a2), p=0.00062, one-way ANOVA F(14,113)=2.9964). At 5.0 and 10 mg/kg, vortioxetine significantly increased theta (p<0.05, post-dose vs vehicle, LSD post-hoc comparison). In contrast, escitalopram (2.0 mg/kg, s.c.) had no effect on theta power (Figure 6(a2)).
Figure 6.
Vortioxetine increased frontal cortical theta power in in vivo electroencephalography (EEG) recordings. (a1) Total relative theta power are shown for each treatment for every 15 min from 0–180 min postdose. A treatment effect was observed for vortioxetine (VOR), but not escitalopram (p=0.0226, one-way analysis of variance (ANOVA) F(65,497)=1.4180). (a2) Pharmacologically-induced changes in EEG recordings were quantified by averaging the 10 s bins constituting 15–75 min before (baseline) and 45–90 min post-drug treatment. Theta power was expressed as the percentage change from baseline. Bar graphs represent the mean±standard error of the mean (SEM) (n=9 rats per condition). A negative value indicates a decrease in EEG power from baseline. Vortioxetine at 5.0 and 10 mg/kg significantly increased theta power (*p<0.05, post-dose versus vehicle, one-way analysis of variance (ANOVA) with least significant difference (LSD) post-hoc comparison). Escitalopram at 2.0 mg/kg had no effect on theta power.
Hippocampal theta power and frequency can increase with locomotion. Therefore, the Pearson correlation coefficient (r) was used to measure the association of theta power to locomotor activity counts. The r-values were calculated from 0–180 min post dose from every 10 s bin comparing relative power vs activity counts for each recording. The mean and standard deviation (SD) of the r-values for every treatment are shown in Table 1. There was no correlation between theta power and locomotor activity counts suggesting that the increase of theta by vortioxetine was not driven by an increase locomotion.
Table 1.
The association of theta power to locomotor activity counts.
| Pearson r | Mean | SD |
|---|---|---|
| Vehicle | 0.2348 | 0.0943 |
| VOR 0.1 | 0.2913 | 0.0821 |
| VOR 1 | 0.2411 | 0.1079 |
| VOR 3 | 0.1712 | 0.1106 |
| VOR 5 | 0.1488 | 0.0967 |
| VOR 10 | 0.0746 | 0.0804 |
| ESC | 0.1834 | 0.0744 |
ESC: escitalopram; VOR: vortioxetine.
There was no correlation between theta power and locomotor activity counts in electroencephalography (EEG) recordings for any treatment. The mean and standard deviation (SD) of Pearson correlation coefficient (r) are shown. The r-values were calculated comparing relative theta power vs activity count from 0–180 min for each recording.
Discussion
In the present study, we used electrophysiological techniques to examine the effects of vortioxetine in the hippocampus and frontal cortex. We confirmed that local application of 5-HT increased the amplitude and frequency of sIPSCs recorded from CA1 pyramidal cells. This effect was reproduced by the 5-HT3 receptor agonist m-CPBG, suggesting that the effect involved the 5-HT3 receptor stimulation of GABAergic interneurons. Vortioxetine blocked the 5-HT- and mCPBG-induced increases in GABA transmission. Furthermore, vortioxetine enhanced theta-burst LTP in hippocampal slices and increased theta rhythms during active wake, most likely through antagonism of 5-HT3 receptors. By comparison, the SSRI escitalopram had no effect on the 5-HT-mediated increase in GABA transmission, LTP, or in vivo theta oscillations.
Vortioxetine blocks 5-HT enhancement of GABA transmission
5-HT is known to modulate the activity of interneurons and to increase GABA transmission across multiple brain regions, including the hippocampus, cerebral cortex, amygdala, thalamus, and striatum (Chen et al., 2008; Deng and Lei, 2008; Koyama et al., 2000; Monckton and McCormick, 2002; Passani et al., 1994; Ropert and Guy, 1991; Shen and Andrade, 1998; Tan et al., 2004; Turner et al., 2004; West et al., 2009; Zhou and Hablitz, 1999). In the hippocampus, 5-HT exerts inhibitory effects on pyramidal cells’ function by hyperpolarizing their membrane potential and increasing GABA transmission (Passani et al., 1994; Ropert and Guy, 1991; Shen and Andrade, 1998; Turner et al., 2004). Our results confirmed the enhancing effect of 5-HT on spontaneous GABA release. Application of 5-HT in our experiments produced a burst-like increase in the frequency and amplitude of sIPSCs recorded from CA1 pyramidal cells. The goal of this study was to investigate the effect of 5-HT and vortioxetine on GABA transmission and we therefore only focused on the effect of 5-HT on GABA release. We did not observe hyperpolarization of pyramidal cells in response to 5-HT. This was expected, because the experiments were done in the voltage-clamp mode with a cesium-based patch solution. The hyperpolarizing effect of 5-HT is mainly mediated by 5-HT1A and 5-HT1B receptors and occurs through an increase in potassium conductance (Andrade and Nicoll, 1987; Beck et al., 1985; Chaput et al., 1990; Monckton and McCormick, 2002). Since our internal patch-solution contained cesium which blocks potassium channels, it precluded hyperpolarization of pyramidal cells by 5-HT.
Vortioxetine blocked the 5-HT-induced increase in sIPSCs. Vortioxetine was tested at 20 µM, which in the rat is several fold higher than the Ki(s) for each of its receptors, to ensure that vortioxetine binding to all its targets in brain slices was saturated. This includes binding to rat 5-HT1A receptors to which vortioxetine binds with a Ki of 230 nM compared to a Ki of 15 nM at the human 5-HT1A receptor (Mørk et al., 2012). Given vortioxetine’s multimodal mechanism of action, we next investigated which receptor subtypes were responsible for the blocking effect of vortioxetine on 5-HT responses.
Based on findings reported in the literature (Passani et al., 1994; Piguet and Galvan, 1994; Ropert and Guy, 1991; Turner et al., 2004) and fast desensitization of 5-HT responses observed in our experiments, we hypothesized that 5-HT3 receptor antagonism played a significant role in vortioxetine’s effect. 5-HT3 receptor agonists have been shown to depolarize hippocampal interneurons (Kawa, 1994; McMahon and Kauer, 1997). Furthermore, the enhancing effect of 5-HT on GABA transmission can be blocked by the 5-HT3 receptor antagonists DAU 6215 (Passani et al., 1994) and tropisetron (Dorostkar and Boehm, 2007; Ropert and Guy, 1991; Turner et al., 2004) and mimicked by the 5-HT3 receptor agonists 2-methyl-5-HT (although it also activates 5-HT2 receptors (Passani et al., 1994; Ropert and Guy, 1991)) and m-CPBG (Turner et al., 2004; Zhou and Hablitz, 1999). In our experiments, local application of m-CPBG produced an increase in sIPSCs that was very similar to the effect of 5-HT. Vortioxetine blocked the m-CPBG-induced enhancement of sIPSCs in all cells tested, suggesting that 5-HT3 receptor antagonism is important in mediating the effect of vortioxetine.
Although we have shown that the 5-HT3 receptor antagonism is involved in the response of vortioxetine, a contribution from vortioxetine’s other receptor activities cannot be ruled out. Vortioxetine’s 5-HT7 receptor antagonism is of particular interest here, because the activation of 5-HT7 receptors can enhance GABA release in the CA1 area of the hippocampus (Tokarski et al., 2011). At the same time, it is unlikely that SERT inhibition had a role in the vortioxetine response. An equivalent concentration of the SSRI, escitalopram (10 µM for escitalopram and 20 µM for vortioxetine) had no effect on the 5-HT-mediated increase in GABA transmission. Escitalopram was tested at half the concentration of vortioxetine since it has a higher affinity for the SERT (Ki=1.1nM for escitalopram vs 1.6 nM for vortioxetine, (Mørk et al., 2012; Owens et al., 2001). In conclusion, additional studies are needed to elucidate which targets of vortioxetine apart from 5-HT3 receptors might be involved in its inhibitory effect.
Interestingly, the blocking effect of vortioxetine was more complete on m-CPBG responses than on 5-HT responses. This could be explained by the fact that although 5-HT3 receptors are largely involved in the action of 5-HT on GABA release, they are not the only receptor subtype engaged in this effect. Activation of 5-HT6, 5-HT7, and in particular 5-HT2A/5-HT2C receptors has been shown to result in increased GABA transmission in hippocampal slices (Piguet and Galvan, 1994; Shen and Andrade, 1998; Tokarski et al., 2011; West et al., 2009). Since vortioxetine has low affinity for 5-HT2A/2C and 5-HT6 receptors (Bang-Andersen et al., 2011), it is expected that its block of 5-HT responses would not be complete. However, vortioxetine still significantly attenuated the effect of 5-HT on sIPSCs, suggesting that its multimodal profile was sufficient to counteract the 5-HT enhancement of GABA transmission.
Vortioxetine enhances LTP in hippocampal slices
5-HT is known to decrease hippocampal LTP, in part by prolonging the GABAB receptor-mediated inhibition of pyramidal cells during the LTP induction phase and thereby decreasing the depolarization of pyramidal cells (Corradetti et al., 1992; Passani et al., 1994; Staubli and Otaky, 1994). 5-HT3 receptor antagonists can reverse this inhibitory effect and potentiate LTP in brain slices and in vivo (Corradetti et al., 1992; Maeda et al., 1994; Passani et al., 1994; Staubli and Otaky, 1994; Staubli and Xu, 1995). In the present study vortioxetine significantly increased the magnitude of theta-burst LTP in the CA1 area of the hippocampus, which is consistent with vortioxetine reversing the 5-HT inhibition of pyramidal cells and the fact that the strength of LTP is controlled by the degree of excitation of pyramidal cells during theta burst stimulation (Arai and Lynch, 1992; Wigstrom and Gustafsson, 1983).
It is interesting to note that although SSRIs have been shown to inhibit hippocampal LTP in vivo (Mnie-Filali et al., 2006), escitalopram had no effect on LTP in hippocampal slices in our study. This discrepancy might be explained by the low endogenous tone of 5-HT in hippocampal brain slices which may not be sufficient to observe an effect of SERT blockade. However, our LTP result with vortioxetine and the study by Maeda et al. (1994) showing facilitating effects of 5-HT3 antagonists on synaptic plasticity in hippocampal slices suggest that the amount of 5-HT released in hippocampal brain slices during high-frequency stimulation is sufficient to at least activate 5-HT3 receptors.
Vortioxetine increases theta power in in vivo EEG recordings
Theta oscillations are predominantly driven via hippocampal entorhinal-cortical projections (Young and McNaughton, 2009) and have been linked to cognitive functions in preclinical as well as clinical studies (Basar et al., 2000; Caplan et al., 2001; Klimesch, 1999; Raghavachari et al., 2001, 2006). Moreover, pyramidal cells tend to fire in theta bursts during learning (Berger et al., 1983; Otto et al., 1991). Interestingly, the 5-HT3 receptor antagonist ondansetron has been shown to increase theta oscillations in vivo in freely moving rats (Staubli and Xu, 1995).
Our EEG data obtained using implantable telemetry devices showed that vortioxetine at 5.0 and 10 mg/kg significantly increased theta power during active wake. In contrast, escitalopram did not change theta power. This further differentiates vortioxetine from SSRIs and highlights a significant role of one or more of vortioxetine’s receptor activities. It is important to note that vortioxetine did not have an effect on theta oscillations at the lower doses (0.1, 1.0 and 3.0 mg/kg). At these doses, vortioxetine has been reported to have full occupancy at 5-HT3 receptors, but little to no occupancy at 5-HT1A and 5-HT7 receptors (Pehrson and Sanchez, 2013). At 5.0 and 10 mg/kg vortioxetine is expected to have ~30–40% occupancy at 5HT1A and 5-HT7 receptors, ~50–80% occupancy at 5-HT1B receptors and full occupancy at 5-HT3 receptors and the SERT (Pehrson and Sanchez, 2013). Thus, a synergistic effect of 5-HT3 and 5-HT7 receptor antagonism, 5-HT1A receptor agonism and 5HT1B receptor partial agonism may be required for the enhancing effect of vortioxetine on theta oscillations in vivo. Further studies are needed to understand the complex functional interaction between vortioxetine’s multiple receptor activities.
Preclinical and clinical data suggest that theta rhythms tend to increase during memory tasks, especially during encoding (Basar et al., 2000; Klimesch, 1999). Furthermore, an increase in theta is correlated with improved performance in working memory tests in humans (Caplan et al., 2001; Raghavachari et al., 2001, 2006). Therefore, the facilitating effect of vortioxetine on theta power may contribute to its memory-enhancing properties observed in pre-clinical rat models (Du Jardin et al., 2013; Jensen et al., 2013; Mørk et al., 2013) and in the clinic (Katona et al., 2012; McIntyre et al., 2013).
Conclusions
We have shown that vortioxetine decreased the 5-HT-induced inhibitory drive of pyramidal cells and enhanced theta rhythms and hippocampal LTP, at least in part by antagonism at 5-HT3 receptors. The data also indicate that vortioxetine activity at other receptors is likely to be important. Vortioxetine distinguished itself from the SSRI escitalopram, which showed no effect on any of these measures. Given the central role of the hippocampus in the regulation of cognitive functions and the enhancing effects of vortioxetine on synaptic activity in the hippocampus, our results may provide a cellular correlate to the observed preclinical and clinical effects of vortioxetine on cognition.
Footnotes
Conflict of interest: E Dale, H Zhang, SC Leiser, N Plath and C Sanchez are full-time employees of Lundbeck. The work of Y Xiao, D Lu and C Yang was sponsored by H Lundbeck A/S.
Funding: The study was funded by H Lundbeck A/S.
References
- Adell A. (2010) Lu-AA21004, a multimodal serotonergic agent, for the potential treatment of depression and anxiety. IDrugs 13: 900–910. [PubMed] [Google Scholar]
- Alvarez E, Perez V, Dragheim M, et al. (2012) A double-blind, randomized, placebo-controlled, active reference study of Lu AA21004 in patients with major depressive disorder. Int J Neuropsychopharmacol 15: 589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade R, Nicoll RA. (1987) Pharmacologically distinct actions of serotonin on single pyramidal neurones of the rat hippocampus recorded in vitro. J Physiol 394: 99–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai A, Lynch G. (1992) Factors regulating the magnitude of long-term potentiation induced by theta pattern stimulation. Brain Res 598: 173–184. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Lin CH, Van Dyck CH, et al. (1997) The effects of 5-HT3 receptor antagonists on cognitive performance in aged monkeys. Neurobiol Aging 18: 21–28. [DOI] [PubMed] [Google Scholar]
- Bang-Andersen B, Ruhland T, Jorgensen M, et al. (2011) Discovery of 1-[2-(2,4-dimethylphenylsulfanyl)phenyl]piperazine (Lu AA21004): A novel multimodal compound for the treatment of major depressive disorder. J Med Chem 54: 3206–3221. [DOI] [PubMed] [Google Scholar]
- Basar E, Basar-Eroglu C, Karakas S, et al. (2000) Brain oscillations in perception and memory. Int J Psychophysiol 35: 95–124. [DOI] [PubMed] [Google Scholar]
- Bastlund JF, Jennum P, Mohapel P, et al. (2004) Measurement of cortical and hippocampal epileptiform activity in freely moving rats by means of implantable radiotelemetry. J Neurosci Methods 138: 65–72. [DOI] [PubMed] [Google Scholar]
- Beck SG, Clarke WP, Goldfarb J. (1985) Spiperone differentiates multiple 5-hydroxytryptamine responses in rat hippocampal slices in vitro. Eur J Pharmacol 116: 195–197. [DOI] [PubMed] [Google Scholar]
- Berger TW, Rinaldi PC, Weisz DJ, et al. (1983) Single-unit analysis of different hippocampal cell types during classical conditioning of rabbit nictitating membrane response. J Neurophysiol 50: 1197–1219. [DOI] [PubMed] [Google Scholar]
- Brambilla A, Ghiorzi A, Pitsikas N, et al. (1993) DAU 6215, a novel 5-HT3-receptor antagonist, selectively antagonizes scopolamine-induced deficit in a passive-avoidance task, but not scopolamine-induced hypermotility in rats. J Pharm Pharmacol 45: 841–843. [DOI] [PubMed] [Google Scholar]
- Buhot MC, Martin S, Segu L. (2000) Role of serotonin in memory impairment. Ann Med 32: 210–221. [DOI] [PubMed] [Google Scholar]
- Caplan JB, Madsen JR, Raghavachari S, et al. (2001) Distinct patterns of brain oscillations underlie two basic parameters of human maze learning. J Neurophysiol 86: 368–380. [DOI] [PubMed] [Google Scholar]
- Chaput Y, Araneda RC, Andrade R. (1990) Pharmacological and functional analysis of a novel serotonin receptor in the rat hippocampus. Eur J Pharmacol 182: 441–456. [DOI] [PubMed] [Google Scholar]
- Chen L, Yung KK, Chan YS, et al. (2008) 5-HT excites globus pallidus neurons by multiple receptor mechanisms. Neuroscience 151: 439–451. [DOI] [PubMed] [Google Scholar]
- Conradi HJ, Ormel J, de Jonge P. (2011) Presence of individual (residual) symptoms during depressive episodes and periods of remission: A 3-year prospective study. Psychol Med 41: 1165–1174. [DOI] [PubMed] [Google Scholar]
- Corradetti R, Ballerini L, Pugliese AM, et al. (1992) Serotonin blocks the long-term potentiation induced by primed burst stimulation in the CA1 region of rat hippocampal slices. Neuroscience 46: 511–518. [DOI] [PubMed] [Google Scholar]
- Cowen P, Sherwood AC. (2013) The role of serotonin in cognitive function: Evidence from recent studies and implications for understanding depression. J Psychopharmacol 27: 575–583. [DOI] [PubMed] [Google Scholar]
- Deng PY, Lei S. (2008) Serotonin increases GABA release in rat entorhinal cortex by inhibiting interneuron TASK-3 K+ channels. Mol Cell Neurosci 39: 273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorostkar MM, Boehm S. (2007) Opposite effects of presynaptic 5-HT3 receptor activation on spontaneous and action potential-evoked GABA release at hippocampal synapses. J Neurochem 100: 395–405. [DOI] [PubMed] [Google Scholar]
- Du Jardin KG, Jensen JB, Sanchez C, et al. (2013) Vortioxetine dose-dependently reverses 5-HT depletion-induced deficits in spatial working and object recognition memory: A potential role for 5-HT receptor agonism and 5-HT receptor antagonism. Eur Neuropsychopharmacol 24: 160–171. [DOI] [PubMed] [Google Scholar]
- Fontana DJ, Daniels SE, Henderson C, et al. (1995) Ondansetron improves cognitive performance in the Morris water maze spatial navigation task. Psychopharmacology (Berl) 120: 409–417. [DOI] [PubMed] [Google Scholar]
- Hong E, Meneses A. (1996) Systemic injection of p-chloroamphetamine eliminates the effect of the 5-HT3 compounds on learning. Pharmacol Biochem Behav 53: 765–769. [DOI] [PubMed] [Google Scholar]
- Horiguchi M, Huang M, Meltzer HY. (2011) The role of 5-hydroxytryptamine 7 receptors in the phencyclidine-induced novel object recognition deficit in rats. J Pharmacol Exp Ther 338: 605–614. [DOI] [PubMed] [Google Scholar]
- Horisawa T, Ishibashi T, Nishikawa H, et al. (2011) The effects of selective antagonists of serotonin 5-HT7 and 5-HT1A receptors on MK-801-induced impairment of learning and memory in the passive avoidance and Morris water maze tests in rats: Mechanistic implications for the beneficial effects of the novel atypical antipsychotic lurasidone. Behav Brain Res 220: 83–90. [DOI] [PubMed] [Google Scholar]
- Jensen JB, du Jardin KG, Song D, et al. (2013) Vortioxetine, but not escitalopram or duloxetine, reverses memory impairment induced by central 5-HT depletion in rats: Evidence for direct 5-HT receptor modulation. Eur Neuropsychopharmacol 24: 148–159. [DOI] [PubMed] [Google Scholar]
- Katona C, Hansen T, Olsen CK. (2012) A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. Int Clin Psychopharmacol 27: 215–223. [DOI] [PubMed] [Google Scholar]
- Kawa K. (1994) Distribution and functional properties of 5-HT3 receptors in the rat hippocampal dentate gyrus: A patch-clamp study. J Neurophysiol 71: 1935–1947. [DOI] [PubMed] [Google Scholar]
- Klimesch W. (1999) EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res Brain Res Rev 29: 169–195. [DOI] [PubMed] [Google Scholar]
- Koyama S, Matsumoto N, Kubo C, et al. (2000) Presynaptic 5-HT3 receptor-mediated modulation of synaptic GABA release in the mechanically dissociated rat amygdala neurons. J Physiol 529: 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiser SC, Pehrson AL, Robichaud PJ, et al. The multimodal antidepressant vortioxetine increases frontal cortical oscillations unlike escitalopram and duloxetine – a quantitative electroencephalographic study in the rat. Br J Pharmacol 2014. May 21. doi: 10.1111/bph.12782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock SM, Husain MM, Wisniewski SR, et al. (2011) Residual symptoms in depressed outpatients who respond by 50% but do not remit to antidepressant medication. J Clin Psychopharmacol 31: 180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre RS, Lophaven S, Olsen CK. (2013) Randomized, double-blind, placebo-controlled study of the efficacy of vortioxetine on cognitive dysfunction in adult patients with major depressive disorder (MDD) (abstract T160). Neuropsychopharmacology 38: S380-S381. [Google Scholar]
- McLean SL, Woolley ML, Thomas D, et al. (2009) Role of 5-HT receptor mechanisms in sub-chronic PCP-induced reversal learning deficits in the rat. Psychopharmacology (Berl) 206: 403–414. [DOI] [PubMed] [Google Scholar]
- McMahon LL, Kauer JA. (1997) Hippocampal interneurons are excited via serotonin-gated ion channels. J Neurophysiol 78: 2493–2502. [DOI] [PubMed] [Google Scholar]
- Maeda T, Kaneko S, Satoh M. (1994) Inhibitory influence via 5-HT3 receptors on the induction of LTP in mossy fiber-CA3 system of guinea-pig hippocampal slices. Neurosci Res 18: 277–282. [DOI] [PubMed] [Google Scholar]
- Maire M, Reichert CF, Schmidt C. (2013) Sleep-wake rhythms and cognition. J Cogn Behav Psychother 13: 133–170. [Google Scholar]
- Meneses A. (2003) A pharmacological analysis of an associative learning task: 5-HT(1) to 5-HT(7) receptor subtypes function on a Pavlovian/instrumental autoshaped memory. Learn Mem 10: 363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ, Agid Y, Brune M, et al. (2012) Cognitive dysfunction in psychiatric disorders: Characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov 11: 141–168. [DOI] [PubMed] [Google Scholar]
- Mnie-Filali O, El Mansari M, Espana A, et al. (2006) Allosteric modulation of the effects of the 5-HT reuptake inhibitor escitalopram on the rat hippocampal synaptic plasticity. Neurosci Lett 395: 23–27. [DOI] [PubMed] [Google Scholar]
- Monckton JE, McCormick DA. (2002) Neuromodulatory role of serotonin in the ferret thalamus. J Neurophysiol 87: 2124–2136. [DOI] [PubMed] [Google Scholar]
- Mørk A, Montezinho LP, Miller S, et al. (2013) Vortioxetine (Lu AA21004), a novel multimodal antidepressant, enhances memory in rats. Pharmacol Biochem Behav 105: 41–50. [DOI] [PubMed] [Google Scholar]
- Mørk A, Pehrson A, Brennum LT, et al. (2012) Pharmacological effects of Lu AA21004: A novel multimodal compound for the treatment of major depressive disorder. J Pharmacol Exp Ther 340: 666–675. [DOI] [PubMed] [Google Scholar]
- Otto T, Eichenbaum H, Wiener SI, et al. (1991) Learning-related patterns of CA1 spike trains parallel stimulation parameters optimal for inducing hippocampal long-term potentiation. Hippocampus 1: 181–192. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Knight DL, Nemeroff CB. (2001) Second-generation SSRIs: Human monoamine transporter binding profile of escitalopram and R-fluoxetine. Biol Psychiatry 50: 345–350. [DOI] [PubMed] [Google Scholar]
- Parmentier-Batteur S, O’Brien JA, Doran S, et al. (2012) Differential effects of the mGluR5 positive allosteric modulator CDPPB in the cortex and striatum following repeated administration. Neuropharmacology 62: 1453–1460. [DOI] [PubMed] [Google Scholar]
- Passani MB, Pugliese AM, Azzurrini M, et al. (1994) Effects of DAU 6215, a novel 5-hydroxytryptamine 3 (5-HT3) antagonist on electrophysiological properties of the rat hippocampus. Br J Pharmacol 112: 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehrson AL, Sanchez C. (2013) Serotonergic modulation of glutamate neurotransmission as a strategy for treating depression and cognitive dysfunction. CNS Spectr 19: 121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet P, Galvan M. (1994) Transient and long-lasting actions of 5-HT on rat dentate gyrus neurones in vitro. J Physiol 481: 629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitsikas N, Borsini F. (1996) Itasetron (DAU 6215) prevents age-related memory deficits in the rat in a multiple choice avoidance task. Eur J Pharmacol 311: 115–119. [DOI] [PubMed] [Google Scholar]
- Pitsikas N, Brambilla A, Borsini F. (1994) Effect of DAU 6215, a novel 5-HT3 receptor antagonist, on scopolamine-induced amnesia in the rat in a spatial learning task. Pharmacol Biochem Behav 47: 95–99. [DOI] [PubMed] [Google Scholar]
- Raghavachari S, Kahana MJ, Rizzuto DS, et al. (2001) Gating of human theta oscillations by a working memory task. J Neurosci 21: 3175–3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavachari S, Lisman JE, Tully M, et al. (2006) Theta oscillations in human cortex during a working-memory task: Evidence for local generators. J Neurophysiol 95: 1630–1638. [DOI] [PubMed] [Google Scholar]
- Ropert N, Guy N. (1991) Serotonin facilitates GABAergic transmission in the CA1 region of rat hippocampus in vitro. J Physiol 441: 121–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez C, Assin KE, Artigas F. Vortioxetine, a novel antidepressant with multimodal activity: Review of preclinical and clinical data. Pharm & Ther. DOI: 10.1016/j.pharmthera.2014.07.001 [DOI] [PubMed] [Google Scholar]
- Shen RY, Andrade R. (1998) 5-Hydroxytryptamine 2 receptor facilitates GABAergic neurotransmission in rat hippocampus. J Pharmacol Exp Ther 285: 805–812. [PubMed] [Google Scholar]
- Staubli U, Otaky N. (1994) Serotonin controls the magnitude of LTP induced by theta bursts via an action on NMDA-receptor-mediated responses. Brain Res 643: 10–16. [DOI] [PubMed] [Google Scholar]
- Staubli U, Xu FB. (1995) Effects of 5-HT3 receptor antagonism on hippocampal theta rhythm, memory, and LTP induction in the freely moving rat. J Neurosci 15: 2445–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H, Zhong P, Yan Z. (2004) Corticotropin-releasing factor and acute stress prolongs serotonergic regulation of GABA transmission in prefrontal cortical pyramidal neurons. J Neurosci 24: 5000–5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokarski K, Kusek M, Hess G. (2011) 5-HT7 receptors modulate GABAergic transmission in rat hippocampal CA1 area. J Physiol Pharmacol 62: 535–540. [PubMed] [Google Scholar]
- Turner TJ, Mokler DJ, Luebke JI. (2004) Calcium influx through presynaptic 5-HT3 receptors facilitates GABA release in the hippocampus: In vitro slice and synaptosome studies. Neuroscience 129: 703–718. [DOI] [PubMed] [Google Scholar]
- Vogel V, Sanchez C, Jennum P. (2002) EEG measurements by means of radiotelemetry after intracerebroventricular (ICV) cannulation in rodents. J Neurosci Methods 118: 89–96. [DOI] [PubMed] [Google Scholar]
- West PJ, Marcy VR, Marino MJ, et al. (2009) Activation of the 5-HT(6) receptor attenuates long-term potentiation and facilitates GABAergic neurotransmission in rat hippocampus. Neuroscience 164: 692–701. [DOI] [PubMed] [Google Scholar]
- Westrich L, Pehrson A, Zhong H, et al. (2012) In vitro and in vivo effects of the multimodal antidepressant vortioxetine (Lu AA21004) at human and rat targets (abstract). Int J Psychiatry Clin Pract 16 Suppl 1: 47. [Google Scholar]
- Wigstrom H, Gustafsson B. (1983) Facilitated induction of hippocampal long-lasting potentiation during blockade of inhibition. Nature 301: 603–604. [DOI] [PubMed] [Google Scholar]
- Young CK, McNaughton N. (2009) Coupling of theta oscillations between anterior and posterior midline cortex and with the hippocampus in freely behaving rats. Cereb Cortex 19: 24–40. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Hablitz JJ. (1999) Activation of serotonin receptors modulates synaptic transmission in rat cerebral cortex. J Neurophysiol 82: 2989–2999. [DOI] [PubMed] [Google Scholar]