Abstract
In this study the first results on evaluation and assessment of grafted bioengineered skin substitutes using an optical Diffuse Reflectance Spectroscopy (DRS) system with a remote optical probe are shown. The proposed system is able to detect early vascularization of skin substitutes expressing the Vascular Endothelial Growth Factor (VEGF) protein compared to normal grafts, even though devitalized skin is used to protect the grafts. Given the particularities of the biological problem, data analysis is performed using two Blind Signal Separation (BSS) methods: Principal Component Analysis (PCA) and Independent Component Analysis (ICA). These preliminary results are the first step towards point-of-care diagnostics for skin implants early assessment.
OCIS codes: (170.6935) Tissue characterization; (170.6510) Spectroscopy, tissue diagnostics
1. Introduction
Bioengineered skin substitutes appeared to solve one of the main problems in the treatment of extensive burns as it is the need for early coverage. This technology overcomes surface limitations of traditional skin grafts and it is an important help reducing the pain and potential complications during the recovery process [1]. Although robust bioengineered skin has been developed, an ideal substitute for human skin has not been achieved yet, and more tests are required to improve its performance and expand its field of application. Researchers from the Regenerative Medicine Unit of the Epithelial Biomedicine Division based at the Centro de Investigaciones Energéticas, Medioambientales y Tecnológicas (CIEMAT), Madrid, Spain have developed a humanized mouse model suitable to the study of bioengineered skin engraftment. This model has probed its validity as a preclinical platform for evaluating tissue regeneration, faithfully reproducing the functional and structural characteristics of the human wound healing process [2].
Skin regeneration tests are performed extracting skin from the back of an immunodeficient mouse and replacing it with the bioengineered skin substitute. Then, the extracted mouse skin is devitalized (by several cycles of freezing and thawing) and placed over the implant to ensure its protection during the engraftment process [3]. However, although in this manner the grafted tissue is protected, engraftment cannot readily be assessed as it is covered by the abovementioned devitalized skin.
Current implementations of optical techniques for noninvasive characterization of tissue cannot be used due to the presence of the protective devitalized skin on top of the engraftment. Besides this, such protection gets dehydrated with the pass of the days changing its optical characteristics. Only after this devitalized skin slough off, approximately three weeks after grafting, the graft recovery state can be confirmed.
In this study the first results on angiogenesis evaluation and assessment of bioengineered skin substitutes using a portable Diffuse Reflectance Spectroscopy (DRS) system [4] with a non-contact optical probe are shown. The analysis of the measured reflectances at different wavelengths is performed using two Blind Signal Separation (BSS) methods: Principal Component Analysis (PCA) and Independent Component Analysis (ICA). The proposed sensor is able to assess early enhanced vascularization of skin grafts expressing the Vascular Endothelial Growth Factor (VEGF) protein compared to normal grafts through a protective devitalized skin with an important change of its optical characteristics during time. These preliminary results are the first step towards a point-of-care diagnostics for skin implants early assessment.
2. Materials and methods
2.1 Experimental protocol
All experimental procedures involving this paper were approved by the Animal Experimental Ethical Committee (IACUC-CEEA) of CIEMAT as part of the project “Molecular, genetic and cellular bases of skin diseases: development of experimental models humanized and innovative therapeutic procedures”. Four skin humanized mice were employed in the study carried out in the Epithelial Biomedicine Division in CIEMAT, Madrid, Spain. Two of the mice were grafted with a normal bioengineered skin for control, while in the other two grafts the keratinocytes of the bioengineered skins were made to overexpress VEGF protein. Vascularization is expected to appear earlier and in higher proportion in the VEGF-expressing grafted mice and the aim of this work is to study the viability of noninvasively and remotely measure this difference. The mice with VEGF-expressing grafts were labelled as VEGF1 and VEGF2 and the control mice as CTL1 and CTL2.
A control point was selected to be exclusively used to quantify the consistency of the measurements of the sensor; this point was located at the nape of the mouse. All the measurements to monitor the evolution of the bioengineered skin were carried out in the centre of the grafts. A photograph taken to a mouse is shown in Fig. 1(a)) where the locations of the control and measurement points are presented. In this picture it is possible to see the devitalized skin avoiding direct optical access to the graft; dehydration, wrinkles, and non-uniform shape are evident several days after engraftment. A detail of the remote optical head performing a measurement on a fresh engraftment (few minutes after surgery) is presented in Fig. 1(b)). It is important to note the appearance of the devitalized skin on top of the graft. Comparison with Fig. 1(a)) shows the clear degradation of the covering and the change on its optical characteristics during the evolution of the experiment.
Fig. 1.
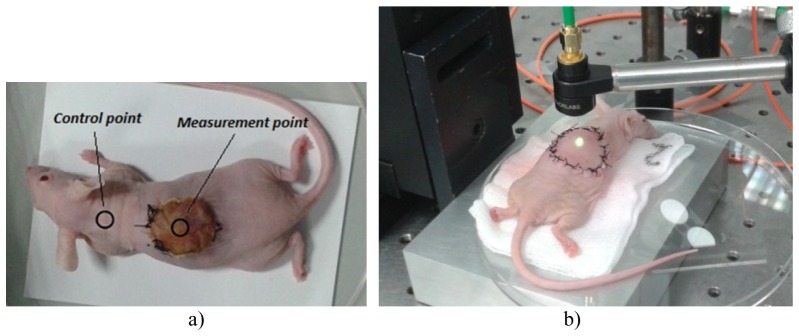
a) Photograph of a mouse several days after engraftment. Note the devitalized skin on top on the engraftment, avoiding direct optical access to it. b) Detail of the optical remote probe performing a measurement on a fresh engraftment.
Measurements were performed on anesthetized mice on days 0, 2 and 6 after grafting and were followed by biopsies on day 7. To ensure the consistency of the data acquisition, three measurements were performed and averaged in each location.
2.2 Sensor design
The block diagram of the sensor is shown in Fig. 2. In the architecture of the instrument it is possible to differentiate between the electronic and optical subsystems. As mentioned in the introduction, as a part of the optical system, a non-contact remote optical probe is to be used in this study. It was decided to cover all the wavelength range of the therapeutic window and the sensor was equipped with four different laser diodes with 532 nm, 635 nm, 850 nm and 1064 nm (PD-LD Inc., New Jersey, USA) wavelengths. The light of the four lasers is combined in a multimode fiber by means of optical couplers to carry the light to a fiber collimator (F220FC-780, Thorlabs Inc., New Jersey, USA) that is used for illumination of the area of interest. The measurement head is completed by a silicon photodiode (FDS100, Thorlabs Inc., New Jersey, USA) and two polarizers (LPVIS050-MP, Thorlabs Inc., New Jersey, USA) attached to the collimator and the photodetector to eliminate the effect of the Fresnell reflection in the surface of the tissue.
Fig. 2.
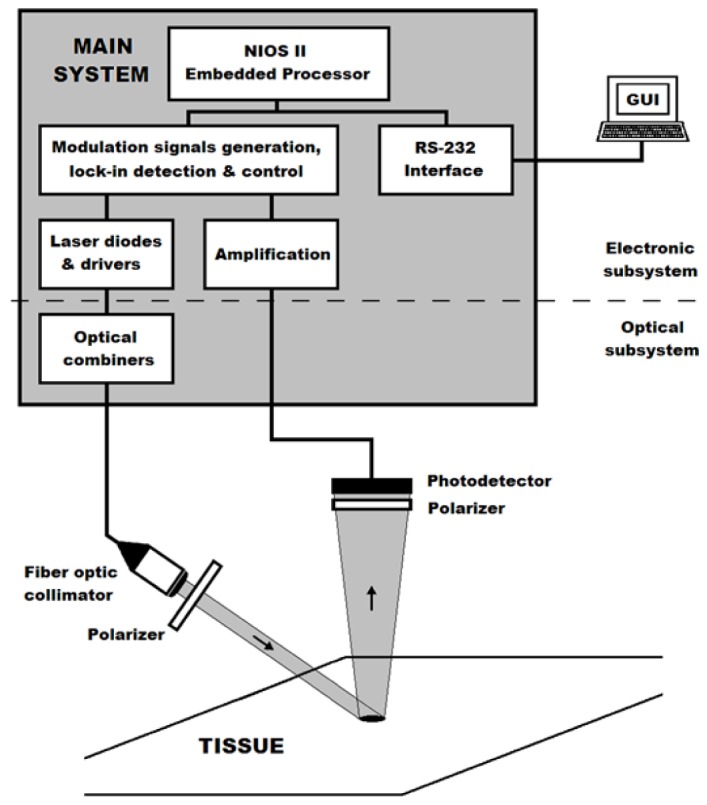
Block diagram of the sensor.
The four lasers are amplitude modulated at four different frequencies (3800, 4500, 5100 and 6000 Hz respectively) and the single photodiode is used to simultaneously measure all the signals. This is done taking advantage of parallel lock-in detection implemented on a multipurpose Field Programmable Gate Array (FPGA) (Cyclone III, Altera Corporation, California, USA) based system that embedded a four channel lock-in amplifier. With the exception of the laser diode drivers and the photodetector amplification stage, all the electronic subsystem is implemented into the FPGA. This multi-channel design was developed in the Electronic Technology Department of the Universidad Carlos III de Madrid (Spain). A Nios II Embedded Processor is used to control the lock-in amplifiers and to generate the modulation and control signals. By means of a RS-232 interface, the instrument is connected to a PC where a Graphic User Interface (GUI) allows the control of the different parameters, the triggering of measurements, and data saving.
2.3 Diffuse reflectance data analysis
The presence of the devitalized skin and the evolution of its optical characteristics (as shown in Fig. 1(a) and 1(b)) make standard reflectance spectrum analysis procedures like analytical models for the propagation of light in tissue or Monte-Carlo simulations difficult to apply. Likewise, it is not possible to extract valuable information from the direct analysis of the values of reflectances at the different wavelengths, given that these values are affected by many factors, like the distance from the optical head to the tissue or the movements of the mouse during the measurements. In this situation, different BSS techniques offer a better performance. In the biological problem addressed by this paper, BSS methods allow us to recover the spectral shape of several contributors (like the devitalized skin or hemoglobin) from the measurement of their mixes, relying only on the assumption of mutual independence between such contributors. There are several BSS methods each having particular characteristics, for this study PCA and ICA have been selected.
PCA has recently been evaluated to classify multispectral noncontact diffuse reflectance data both for imaging reconstruction [5] and real time evaluation of quantitative blood concentrations [6]. This technique linearly transforms data into an orthogonal coordinate system whose axes correspond to the principal components. The new coordinates of the data are the linear combination of the initial data. Each succeeding principal component is calculated in a way in which accounts for the largest possible variance, being possible to reduce the dimensionality of the data by exploiting any existing redundancy of information. Principal components and coordinates have been calculated applying the Singular Value Decomposition algorithm.
On the other hand, ICA [7] can separate the spectra of a series of constituents from the measurements of their mixtures and, besides this, estimate the concentration of the different constituents. This method has also been previously employed to classify spectroscopic data [8] and also in biomedical applications [9]. ICA calculations have been performed using the Fixed Point Algorithm for ICA from Hyvärinen [10].
In this study, PCA and ICA calculations have been made using Matlab (MathWorks Inc, Massachusetts, USA).
3. Results and discussion
3.1 Results of the biopsies
The results of the histology of the tissues extracted from the engraftments on day 7 (after measurements were performed on days 2 and 6) are shown in Fig. 3, two sections of the a) control and b) VEGF-expressing grafts are presented. The analysis confirmed that the amount of vascularization in the VEGF-expressing grafts was bigger than that of the control grafts while the rest of the skin structure is similar.
Fig. 3.
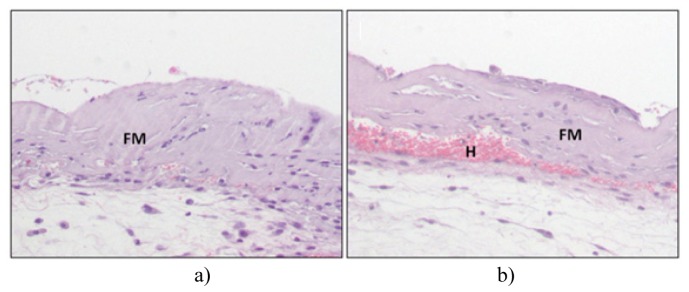
Histological appearance of engrafted bioengineered skins seven days after grafting. a) Control graft. b) VEGF-producing graft. Note the hemorrhagic blood vessels (H) invading the fibrin dermal matrix (FM).
3.2 PCA analysis
PCA has been directly applied to the reflectance values at each wavelength for the four mice on the measurements carried out on days 2 and 6. The high values of variance (92% and 98% on day 2 and 6 respectively) obtained for the first principal component lead to the conclusion that only the coordinates of each mouse for this first component are relevant in the separation between graft types. Besides this, the first principal component was found to be practically equal on both days, with a mean difference of 3.2%. This invariability in the principal components with time was also found by Kanierstorfer et al. [6] in a skin chromophore mapping study.
On day 6 there is a clear difference between coordinates for the first principal component of the mice from each group as shown in Fig. 4(a)). This difference is well in agreement with the histological study previously presented where significant differences in vascularization levels were obtained. As it was said before, due to the small value of variance explained the rest of components, their coordinates are not relevant in the separation. The results of the PCA analysis showed that separation on day 2 is not possible mainly because not enough time has passed for any significant change to appear in the grafts. The evolution of the coordinates for the first principal component from day 2 to day 6 is shown in Fig. 5(a)).
Fig. 4.
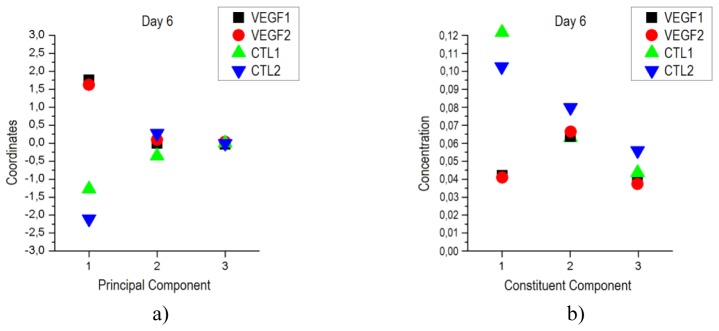
a) PCA coordinates for each engraftment on day 6. Note the clear differentiation between graft types for the first principal component. b) Concentration obtained in the ICA analysis for the three constituent components. It is possible to clearly differentiate between graft types for the constituent component 1.
Fig. 5.
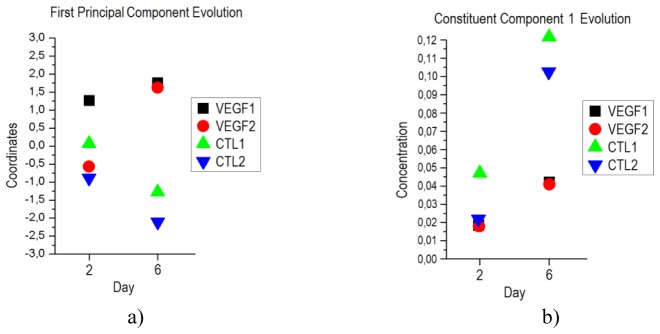
a) Evolution of the coordinates of the first principal component of the PCA analysis for each mouse for days 2 and 6. b) Evolution of the concentration for the constituent component 1 obtained in the ICA analysis for days 2 and 6.
3.3 ICA analysis
The ICA method was, as before, applied to the reflectance values measured at each wavelength for the four mice on days 2 and 6. Three constituent components together with the estimation of their concentrations were obtained for each day. The concentrations of the three constituent components obtained for day 6 are shown in Fig. 4(b)). It is possible to see how the concentrations for the first constituent component (CC1) are clearly separable for the two types of grafts, with values of 0.043 and 0.041 for the VEGF-expressing grafts and 0.123 and 0.102 for the control grafts. The concentrations of the rest of constituent components are not distinguishable for the different groups and hence, given the results of the histology, it is possible to conclude that the concentration of CC1 is related to the level of vascularization. As in the case of the PCA analysis, as shown in Fig. 5(b)), there is no possible differentiation between VEGF-expressing and control grafts on day 2, but in day 6 such differentiation is obvious.
4. Conclusions
In this work the design of a DRS system that uses a remote optical probe to assess and evaluate the evolution of bioengineered skin substitutes covered by a devitalized skin has been described. Since the particularities of the biological problem addressed make difficult the application of standard spectroscopic procedures, the classification of the reflected spectra was done taking advantage of Blind Signal Separation. Spectroscopic data was analyzed using PCA and ICA being possible to differentiate, in both cases, between the early vascularization of skin substitute grafts expressing the VEGF protein and normal grafts. These preliminary results are the first step towards a point-of-care diagnostics for early skin engraftment assessment.
References and links
- 1.MacNeil S., “Progress and opportunities for tissue-engineered skin,” Nature 445(7130), 874–880 (2007). 10.1038/nature05664 [DOI] [PubMed] [Google Scholar]
- 2.Escámez M. J., García M., Larcher F., Meana A., Muñoz E., Jorcano J. L., Del Río M., “An In Vivo Model of Wound Healing in Genetically Modified Skin-Humanized Mice,” J. Invest. Dermatol. 123(6), 1182–1191 (2004). 10.1111/j.0022-202X.2004.23473.x [DOI] [PubMed] [Google Scholar]
- 3.Martínez-Santamaría L., Guerrero-Aspizua S., Del Río M., “Skin bioengineering: preclinical and clinical applications,” Actas Dermosifiliogr. 103(1), 5–11 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Vogel A., Chernomordik V. V., Riley J. D., Hassan M., Amyot F., Dasgeb B., Demos S. G., Pursley R., Little R. F., Yarchoan R., Tao Y., Gandjbakhche A. H., “Using noninvasive multispectral imaging to quantitatively assess tissue vasculature,” J. Biomed. Opt. 12(5), 051604 (2007). 10.1117/1.2801718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eguizabal A., Laughney A. M., García-Allende P. B., Krishnaswamy V., Wells W. A., Paulsen K. D., Pogue B. W., Lopez-Higuera J. M., Conde O. M., “Direct identification of breast cancer pathologies using blind separation of label-free localized reflectance measurements,” Biomed. Opt. Express 4(7), 1104–1118 (2013). 10.1364/BOE.4.001104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kainerstorfer J. M., Ehler M., Amyot F., Hassan M., Demos S. G., Chernomordik V., Hitzenberger C. K., Gandjbakhche A. H., Riley J. D., “Principal component model of multispectral data for near real-time skin chromophore mapping,” J. Biomed. Opt. 15(4), 046007 (2010). 10.1117/1.3463010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hyvärinen A., Oja E., “Independent component analysis: algorithms and applications,” Neural Netw. 13(4-5), 411–430 (2000). 10.1016/S0893-6080(00)00026-5 [DOI] [PubMed] [Google Scholar]
- 8.Chen J., Wang X. Z., “A new approach to near-infrared spectral data analysis using independent component analysis,” J. Chem. Inf. Comput. Sci. 41(4), 992–1001 (2001). 10.1021/ci0004053 [DOI] [PubMed] [Google Scholar]
- 9.A. Croitor Sava, D. M. Sima, M. C. Martinez-Bisbal, B. Celda, and S. Van Huffel, “Non-negative blind source separation techniques for tumor tissue typing using HR-MAS signals,” in Proceedings of IEEE Conference on Engineering in Medicine and Biology Society (EMBC) (IEEE, 2010) pp. 3658–3661. 10.1109/IEMBS.2010.5627436 [DOI] [PubMed] [Google Scholar]
- 10.Hyvärinen A., “Fast and robust fixed-point algorithms for Independent Component Analysis,” IEEE Trans. Neural Netw. 10(3), 626–634 (1999). 10.1109/72.761722 [DOI] [PubMed] [Google Scholar]


