Abstract
Scanning laser ophthalmoscopes (SLOs) are able to achieve superior contrast and axial sectioning capability compared to fundus photography. However, SLOs typically use monochromatic illumination and are thus unable to extract color information of the retina. Previous color SLO imaging techniques utilized multiple lasers or narrow band sources for illumination, which allowed for multiple color but not “true color” imaging as done in fundus photography. We describe the first “true color” SLO, handheld color SLO, and combined color SLO integrated with a spectral domain optical coherence tomography (OCT) system. To achieve accurate color imaging, the SLO was calibrated with a color test target and utilized an achromatizing lens when imaging the retina to correct for the eye’s longitudinal chromatic aberration. Color SLO and OCT images from volunteers were then acquired simultaneously with a combined power under the ANSI limit. Images from this system were then compared with those from commercially available SLOs featuring multiple narrow-band color imaging.
OCIS codes: (170.4460) Ophthalmic optics and devices; (330.1710) Color, measurement; (170.0110) Imaging systems; (170.5755) Retina scanning; (170.4470) Ophthalmology; (110.4500) Optical coherence tomography
1. Introduction
Scanning laser ophthalmoscopy (SLO) and optical coherence tomography (OCT) are retinal imaging modalities commonly utilized in the clinic to diagnose retinal diseases. SLO is a confocal imaging technique similar to confocal microscopy that can acquire high contrast 2-D en-face retinal images in real time [1]. OCT is an interferometric imaging technique that allows for high resolution depth sectioning and has the capability of producing 2-D cross sectional images (B-scans) of the retina with high axial resolution at and exceeding video rate [2]. The combination of monochromatic SLO and OCT was first explored by Podoleanu et al. [3] and further developed by various groups [4–11] in either simultaneous or sequential SLO-OCT. While conventional SLO and OCT have demonstrated strong clinical success in retinal imaging, they lack color information, which is critical in fundus photography and indirect ophthalmoscopy for evaluation of retinal pathologies such as neovascular age-related macular degeneration [12]. Fundus cameras, while able to image the retina in color, lack the contrast and depth sectioning capability of SLO and OCT systems.
The introduction of color imaging to the SLO was first demonstrated by Manivannan et al. [13] by using red, green, and blue lasers sequentially to acquire red, green, and blue reflectance images of the retina on a single detector. Simultaneous color imaging with three color lasers and three detectors [14, 15] and quasi-simultaneous color imaging using pulsed red, green, and blue lasers and a single detector [16, 17] were also demonstrated and shown to obtain color fundus images that resembled color fundus photography but with higher contrast. Recently, an SLO employing adaptive optics has demonstrated color imaging using narrowband red, green, and blue filters on a supercontinuum source with each color obtained sequentially [18]. All of these techniques utilized multiple lasers or narrowband colors for illumination and are therefore not capable of generating “true color” fundus images [19]. As Bartsch et al. explains in [19], the combination of red, green, and blue lasers can appear to an observer as white light, but the reflectance of objects illuminated by such light consists of only 3 discrete wavelengths. Conversely, in fundus photography, the illumination spans the full visible spectrum. Therefore, the majority of the spectral information used in fundus cameras to determine color is lost in a 3 narrowband source illumination approach. Since the retina is known to have continually varying spectral reflectivity that spans the visible spectrum [20], sampling only 3 narrow bands of wavelengths does not provide enough information to reconstruct the “true” color of the retina and may even miss certain features of the retina detectable with the full visible spectrum [19]. Also, the previous color SLO techniques (except for [18]) did not compensate for the longitudinal chromatic aberration (LCA) of the human eye, which is known to introduce a chromatic difference of refraction of ~2 diopters (D) over the visible spectrum [21]. As a result, without an achromatizing lens, images from each color channel are acquired at different depth sections of the retina, thus introducing errors in the combined color image.
In this paper, we present a spectral domain (SD)-OCT and “true color” SLO handheld probe design with a spectrally reshaped supercontinuum white light source for the SLO illumination and an achromatizing lens to correct for the LCA of the eye. This is the first “true color” SLO, handheld color SLO, and color SLO with OCT system. Color calibration was achieved by imaging a 30 patch color test target with calibrated color values and applying a least-squares, polynomial color transformation to match the raw acquired colors from the SLO to the known color values of the test target. With this probe, we demonstrate “true color” SLO images taken simultaneously with OCT B-scans at 5° and 20° fields-of-view (FOV) with patient exposures safely below the ANSI limit [22]. We then compare the imaging results taken by our system with commercial SLOs that image with multiple colors.
2. Methods
2.1 System design
The “true color” SLO-OCT handheld probe was built from a modification of our prior handheld SLO-OCT design [10]. A schematic layout of the system is shown in Fig. 1 below.
Fig. 1.
Side view schematic of the color SLO-OCT handheld probe design. All optical components are labeled and described in the legend. The illumination beam diameter at the eye’s pupil for SLO and OCT was 2.0 and 2.5 mm, respectively. The collection beam diameter for SLO was between 2.0 and 7.0 mm depending on the dilation of the eye’s pupil because backscattered light from the retina fills the pupil in the return path and the pupil is the limiting aperture of the SLO collection path. The collection beam diameter for OCT was the same as the OCT illumination beam diameter because the OCT illumination/collection fiber’s numerical aperture is the limiting aperture of the OCT collection path. The multimode fiber shown in the schematic is used to transfer the collected light from the SLO arm into an RGB color separation module consisting of 2 dichroic filters and 3 photomultiplier tubes (PMTs). A filter is placed in front of the red channel’s PMT to remove any contribution due to the OCT light source that is returned through the SLO collection path.
The SLO source was a supercontinuum laser (NKT Photonics A/S, Birkerød, Denmark) that was filtered to transmit light between 430 to 700 nm. To make the output illumination spectrum more uniform and allow more even light collection among the 3 color channels, the spectrum was reshaped with 2 wavelength division multiplexers (WDMs) and 3 variable optical attenuators (VOAs) as shown in Fig. 1. The illumination spectrum before and after reshaping is shown in Fig. 2. Deviations in the reshaped spectrum from a perfectly uniform spectrum resulted in color accuracy errors that were minimized by the color calibration procedure described in Section 2.3.
Fig. 2.
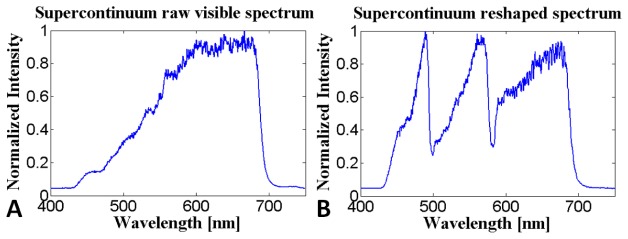
The SLO illumination spectrum before (A) and after (B) reshaping the visible portion of the supercontinuum laser spectrum with 2 WDMs and 3 VOAs.
The OCT source was a superluminescent diode (SLD) operating at 840 ± 35 nm (Superlum, Moscow, Russia). An 80:20 fiber coupler was used for the OCT interferometer and a commercial spectrometer (SD800, Bioptigen Inc, Durham, NC) was used as the OCT detector. The OCT axial resolution and 6 dB falloff range were measured to be 7 µm (in air) and 1.1 mm, respectively. OCT B-scans consisted of 2048 × 500 pixels and were acquired at 36.4 frames per second.
Sample reflectance signal from the SLO arm was collected confocally using a multimode fiber with a 50 µm diameter. Given a 3 mm collection pupil (undilated) and a 50 mm focal length collection lens, the multimode fiber diameter was approximately 1.9, 2.3, and 2.7 times the Airy disc diameter for the red, green, and blue channels, respectively. This range of confocal pinhole sizes have been demonstrated to give a good balance between image sharpness and throughput [23, 24]. The wavelength-dependent confocality of the SLO resulted in relatively tighter confocal gates for the higher wavelength channels, which affected the amount of light collected and the optical section thickness imaged by each color channel. Differences in light collection from each color channel were accounted for by the color calibration procedure, while differences in optical section thickness seemed to be relatively minimal. The theoretical full width at half maximum (FWHM) optical section thicknesses were calculated for this system according to equations described in [25] to be 317, 312, and 309 µm for the red, green, and blue channels, respectively.
After the multimode fiber, the collected light was split into three different color channels (430-495, 495-580, and 580-700nm) for photomultiplier tube (PMT) detection (H10721-20, Hamamatsu, Shizuoka-ken, Japan). Wavelength-dependent sensitivity of the PMTs and other wavelength-dependent components of the SLO were also accounted for via the color calibration procedure. SLO images consisted of 530 × 580 pixels and were acquired at 16 frames per second (fps).
2.2 Optical design
The optical design for the OCT arm was unchanged from our previous handheld SLO-OCT design but the SLO arm had two major modifications to reduce chromatic aberration: 1) the collimating aspheric lens was replaced by a parabolic mirror collimator and 2) two identical custom achromatizing lenses were implemented with one placed after the illumination fiber collimator and the other before the detection fiber collimator at equal distances away from the eye. Two achromatizing lenses were used instead of one in a common path in order to prevent back reflections of the achromatizing lens from entering the SLO’s detection fiber. The idea of using an achromatizing lens to correct vision or improve retinal image quality was first presented in [21, 26–30], and the strategy of designing an achromatizing lens placed directly after a collimation lens was first demonstrated by Zawadzki et al. [31]. Similar to Zawadzki et al. [31], our design strategy involved optimizing the performance of the achromatizing lens in the Zemax model of the SLO imaging system together with a model eye. The model eye we used was created based on parameters of a wide-field eye model with a simplified gradient-index crystalline lens of 30 year olds as determined in a study by Goncharov and Dainty [32]. The dispersion functions for different ocular media were not given in [32] so were modeled as suggested by Atchison and Smith [33] in the same form in Zemax as described by Jaeken et al. [34]. The achromatizing lens design was optimized to produce a zero-power symmetric triplet made from readily available stock glasses with surface curvatures, thicknesses and optical material as variables. The lens was manufactured by Optimax Systems, Inc. using S-FPL51 (Ohara catalogue) and N-ZK7 (Schott catalogue) glasses, and is shown in the schematic on the left part of Fig. 3. The LCA of the model eye (given in terms of chromatic focal shift) at the retina was calculated before and after implementation of the achromatizing lens and is shown on the right part of Fig. 3. The achromatizing lens was designed to correct LCA from 400 – 900 nm instead of just the visible spectrum (400 – 700 nm) to allow LCA correction for broadband NIR sources also with the same lens.
Fig. 3.
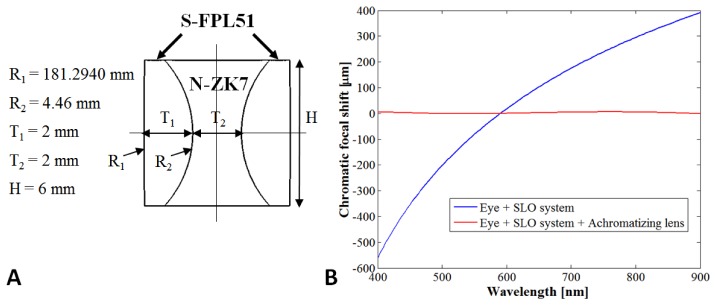
A) Achromatizing lens (AL) design. Dimensions are given to the left of the schematic. B) Chromatic focal shift at the retina of the eye model from 400 – 900 nm after passing through all SLO optics (shown by blue line) and after correction with the AL (shown by red line). The maximum focal shift range is 950 and 7 μm for the blue and red lines, respectively.
The optical design performance of both the color SLO and OCT is given in the form of spot diagram plots in Fig. 4. Although the achromatizing lens corrects for the LCA of the human eye, the transverse chromatic aberration (TCA) of the eye is left uncorrected and can be visualized by the spot diagrams in Fig. 4(A)-4(B) without and with the achromatizing lens, respectively. However, since the three color channels used for SLO collection each detect a much smaller portion of the SLO illumination spectrum, TCA can be partially corrected in post-processing by warping the images from two of the color channels to match the image from a single color channel. We demonstrate this in Fig. 4(C), in which the green and blue channels are warped computationally via an affine transform to match the red channel, thus reducing the contribution of TCA to image blurring. Following this procedure, the design resolution of the color SLO with the achromatizing lens and correction of TCA in post-processing was aberration-limited at ~7.8 μm. The OCT design resolution was nearly diffraction limited at ~7.5 μm as can be visualized by the spot diagrams in Fig. 4(D).
Fig. 4.
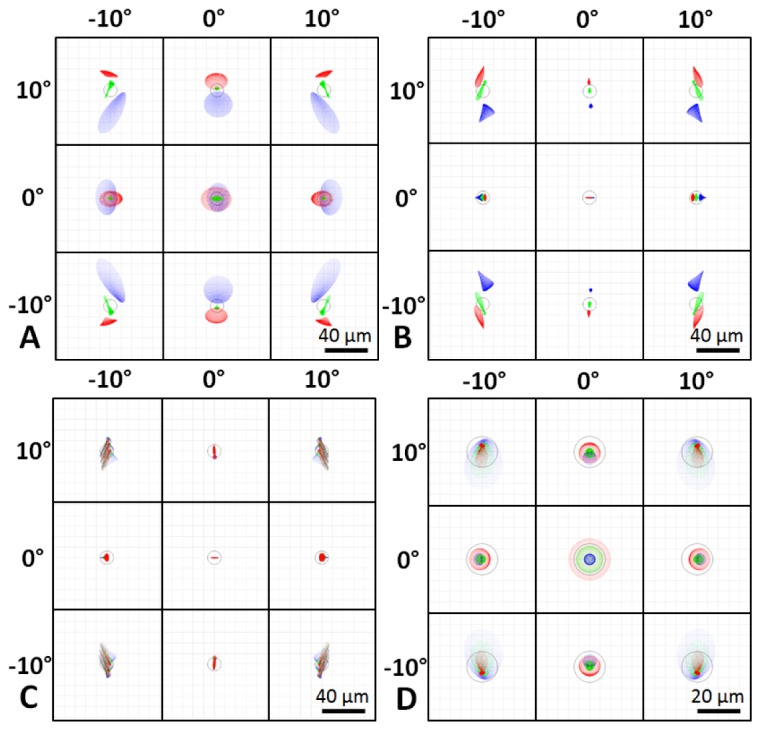
Optical design spot diagrams for the illumination on the retina of a model eye spanning a 20° FOV for the “true color” SLO without (A) and with (B, C) the achromatizing lens and for the OCT system (D). The green and blue color SLO channels were warped to match the red channel in (C), to obtain an aberration-limited resolution of 7.8 μm. OCT was nearly diffraction limited at 7.5 µm. Spot diagrams are color coded for 3 wavelengths spanning the bandwidth of the respective sources and Airy disks are shown by black circles.
2.3 Color calibration
The color calibration method we applied on the raw color SLO images is similar to that performed in color photography and is described by the flowchart in Fig. 5 below. The equations for the various transforms shown in Fig. 5 are given in [35].
Fig. 5.

Color calibration flowchart with the raw, un-calibrated color SLO red, green, and blue (RGB) images as the input and calibrated sRGB color SLO images as the output.
The first step in this method is to apply a spectrum-to-XYZ transform to the raw SLO color image (SLO RGB) in order to transform the image into the Commission Internationale de l'Eclairage (CIE) XYZ color space. After this transform, the SLO image is in the XYZ color space but the reference white point of this space is given by the X, Y, Z coordinates of the SLO illumination source spectrum. To make the reference white point for the SLO image one of the standard illuminants used in color spaces, such as D50 (the white point of a 5000 K blackbody source), we applied a chromatic adaptation transform using the Bradford method, which was found to give excellent results during the development of the sRGB color space standard [36]. The SLO image was then transformed into the L*a*b* color space, which is a color space designed to appear more perceptually uniform [37]. This space is ideal for color calibration, because in this space, minimized error corresponds to minimized perceptual color difference. After color calibration in L*a*b* space, the image was transformed into sRGB space, which has a D65 white point (the white point of a 6500 K blackbody source), for viewing purposes.
For color calibration, images of a 30 patch color test target with known L*a*b* (D50) color values (ColorGauge Nano Target, Image Science Associates, Williamson, NY) were acquired by the color SLO. This was done after translating the last lens in the color SLO to create an image plane ~17 cm after the last lens. After image acquisition, lens reflections and other stray light artifacts were removed by background subtraction and 100 images were averaged to increase the signal-to-noise ratio (SNR). The averaged image was then transformed into L*a*b* space prior to applying a least-squares, polynomial calibration matrix. A single L*a*b* value was determined for each color patch on the test target image by taking the median L*a*b* value of the center 40 × 40 pixels of each patch. Median values were taken in order to remove the effect of outliers due to occasional specular reflections within patches. The error of the color calibration is given in terms of the CIE76 color difference (), which is the Euclidean distance between the known and measured L*a*b* value for a given color patch and is given by the formula
| (1) |
where ,, andare the known L*a*b* values for each patch and,, andare the measured L*a*b* values for each patch. A single error value is determined for a given color calibration by taking the average CIE76 color difference over all 30 color patches. Results from the color calibration matrix on color SLO and Nikon D3100 digital SLR images are shown in Fig. 6(A) along with the equation for the color calibration matrix in Fig. 6(B). Only up to 2nd order polynomial color calibration is shown, since higher order calibrations did not significantly improve the color accuracy while adding computational complexity.
Fig. 6.

Color calibration on color SLO and Nikon D3100 digital SLR images of a 30 patch color test target with known L*a*b* (D50) color values (A) using a least squares, polynomial calibration matrix,, described in (B). is a 3 × 30 matrix with the known L*a*b* (D50) values for each of the patches of the color test target. is a (3n + 1) × 30 matrix containing the L*a*b* (D50) values of a single pixel in the color image, where is the order of the polynomial used for fitting. “Poly1” and “poly2” correspond to first and second order polynomial fits, respectively. The mean CIE76 color differences across all 30 patches are given for each calibration order under the images in A).
2.4 Experimental setup
Color SLO and OCT images were acquired simultaneously spanning either a 5° or 20° FOV at 16 and 36.4 fps, respectively. Different FOVs were obtained by varying the voltages supplied to the scanners of both the SLO and OCT systems. Background measurements taken prior to imaging were subtracted from the color SLO and OCT images to remove static artifacts including lens reflections and scratches or dust on optical elements. The SNR of the OCT system was measured to be 100 dB for 50 µs integration time and 300 µW illumination power at the sample. The irradiance incident on the eye for the color SLO and OCT were under the ANSI limit at 50 µW and 300 µW, which comprised 46% and 41%, respectively, of the ANSI maximum permissible exposure (MPE) limit [22]. The ANSI MPE limit calculation was done using the equations in [22] for an 8 hour exposure duration of a point source after correcting for the pupil constriction assumption embedded in the standard as described in [38]. No correction was done for the eye movement assumption embedded in the standard because the maximum assumed motion spans a visual angle of 5° [38], which is the minimum FOV used in this system. All SLO images were averaged after motion correction, which involved preprocessing using LoG/Gabor filtering and registration via cross correlation as described previously in [10].
2.5 Ethical considerations
The use of our experimental setup for in vivo measurements in humans was approved by the local Institutional Review Board and adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from each subject. Subjects had no known ocular pathology and consisted of a 25-year-old male myope (−3 D) and a 30-year-old male emmetrope.
3. Results
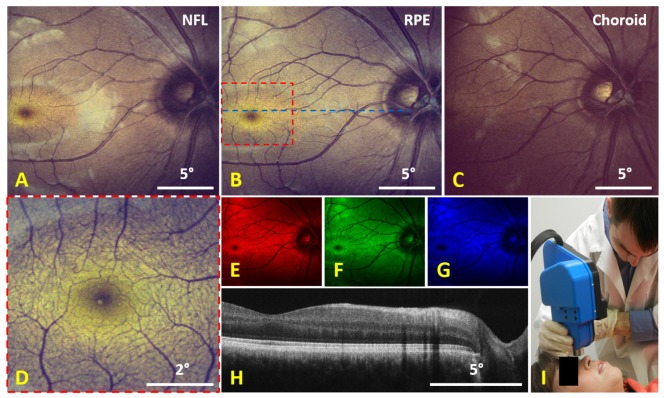
Imaging results from a human volunteer are shown in Fig. 7 below. Due to the confocality of the color SLO, color images could be obtained at different depth sections as shown in Fig. 7(A)-7(C) by shifting the focus of the SLO arm. At smaller FOVs, the color SLO was able to visualize much of the microvasculature structure near the fovea (Fig. 7(D)). The raw, un-calibrated red, green, and blue channel images used to create the color SLO image in Fig. 7(B) are depicted in the color of their respective channels in Fig. 7(E)-7(G). Simultaneously acquired OCT images were averaged as shown in Fig. 7(H) and an image of the color SLO and OCT handheld probe is shown in Fig. 7(I).
Fig. 7.
Imaging results from a human volunteer taken with the handheld color SLO and OCT system. A-C) 100 frame average of calibrated (2nd order polynomial) 20° FOV color SLO images taken with the focus at different depth sections with A) focused on the nerve fiber layer (NFL), B) focused on the retinal pigment epithelium (RPE), and C) focused on the choroid. D) 90 frame average of calibrated 5° FOV color SLO images at the location indicated by the dotted red square in B). The raw, un-calibrated red, green, and blue (RGB) color channels of B) are shown by E), F), and G), respectively. H) 20 frame average OCT B-scan taken at the location indicated by the dotted blue line in B). I) Image of the handheld color SLO and OCT system operated in handheld mode.
Imaging results from a different human volunteer are shown in Fig. 8. Images were acquired without (Fig. 8(A)-8(D)) and with (Fig. 8(E)-8(F)) the achromatizing lens to evaluate the effect of LCA and how well it was corrected by the achromatizing lens. With the achromatizing lens implemented, 5° and 20° FOV color SLO images were acquired near the fovea and optic disc, respectively, and were averaged to get Fig. 8(I) and Fig. 8(J).
Fig. 8.
Color SLO imaging results taken from a human volunteer (different subject from Fig. 7). Images (100 frame averages) were taken without (A-D) and with (E-H) the achromatizing lens (AL). A) and E) are color calibrated (2nd order polynomial) color images, B) and F) are the red channel images, C) and G) are the green channel images, and D) and H) are the blue channel images. Note the reduction of the specular reflections around vessels in H) as compared to D). I) 100 frame average of calibrated 5° FOV color SLO images at the location indicated by the dotted red square in E) visualizing parafoveal cones. J) 100 frame average of calibrated 20° FOV color SLO images taken of the optic disc.
A volunteer was also imaged with two commercially available multiple narrowband color SLOs and the results are shown in Fig. 9. Images from the multiple narrowband color SLOs were digitally cropped to match the size of the averaged “true color” SLO image.
Fig. 9.

Comparison between two commercial multiple color SLOs and the “true color” SLO. A) Image acquired by the Optos 200 TX ultra-widefield SLO (Optos, Dunfermline, Scotland). B) Image acquired by the Spectralis HRA + OCT (6 mode) system (Heidelberg Engineering, Heidelberg, Germany). C) 100 frame average from the “true color” SLO system. The images in A) and B) have been cropped to match the field of view of the “true color” SLO.
4. Discussion
In this paper we have described the development of a “true color” SLO and OCT handheld system and used it to acquire both 5° and 20° FOV images of the human retina in vivo. Similar to other multiple color SLO systems, this device can image different depth sections of the retina but it also has the advantage of “true color” imaging as used in fundus photography and indirect ophthalmoscopy.
Images acquired without the achromatizing lens (Fig. 8(A)-8(D)) demonstrated the effects of LCA on color SLO acquisition. While the red channel image (Fig. 8(B)) had a deeper focus at the retinal pigment epithelium, the blue channel image (Fig. 8(D)) had a shallower focus at the nerve fiber layer and thus contained more specular reflections. The focal separation between the different color channel images resulted in the combined color image (Fig. 8(A)) having specular reflections with a blue tint and a different overall color of the retina when compared to the color image taken with the achromatizing lens (Fig. 8(E)). Images taken with the achromatizing lens (Fig. 8(E)-8(H)) had a more uniform appearance between the color channels, and did not have a specular reflection that varied noticeably between the color channels. We believe these two factors indicate a significant improvement in the reduction of LCA by the achromatizing lens.
A comparison between the different color SLO imaging systems in Fig. 9 reveals several important differences in the appearance of the retina. Some notable differences include the overall color of the image, the appearance of the foveal and optic disc regions, and the presence of specular reflections. The overall and foveal color differences between the images were expected because neither the Optos nor the Spectralis instruments were designed to image in “true color”. The appearance of the dark ring within the optic disc of the retina in both the Spectralis and “true color” SLO is in stark contrast to the yellow optic disc from the Optos system. A potential cause of this dark ring is a higher depth resolution in these two systems compared to the Optos system as a result of a tighter confocal gate that better rejects light reflecting from the center of the optic disc when the focus is elsewhere. Finally, the Spectralis image features a large specular reflection surrounding the fovea, which is less present in either the Optos or “true color” SLO images. Similar specular reflections have been seen in the “true color” SLO either without the achromatizing lens (Fig. 8(A)) or when the focus was set to the nerve fiber layer of the retina (Fig. 7(A)).
One notable advantage of the “true color” SLO compared to other color fundus imaging systems is that the macula luteal pigment is readily visible and appears yellow as expected from anatomy [39] (see yellow oval region surrounding the foveola in the “true color” SLO image in Fig. 7, Fig. 8, and Fig. 9). This visualization of the macula lutea is not expected to be as noticeable in fundus photography because in the “true color” SLO the confocal gate can reject out of focus light that reaches the choroidal region and thus enhance the color visualization of the superior sections of the retina. With this higher sensitivity to color variations in the retina, the “true color” SLO could potentially provide earlier detection of age-related macular degeneration (AMD) by earlier visualization of drusen, which appears as yellow-white spots in the retina. In addition, since the “true color” SLO offers improved visualization of the macula luteal pigment it may be possible, with proper calibration, to objectively assess macular pigment density, which can be used to evaluate the effects of various drugs or dietary supplements such as Lutein and Zeaxanthin on the macular pigment density [39].
5. Conclusion
We have demonstrated a “true color” SLO and OCT handheld probe that implements a custom achromatizing lens and images both 5° and 20° fields-of-view of the retina. “True color” images of the retina were obtained with an SLO for the first time and at different depth sections of the retina. In addition, this is the first demonstration of simultaneous true color SLO and OCT image acquisition. Retinal images including the foveal region of the retina clearly depicted the yellow pigment of the macula, which is less visible with other commercial color SLO systems or fundus photography. The use of this technology may provide a compact solution for confocal color imaging of the retina combined with OCT and may be used to further study and evaluate treatments that affect the macula pigment density and distribution.
Acknowledgments
This research was supported by NIH grants R21 EY021321, R01 EY023039, the North Carolina Biotechnology Center IDG 2012-1015, the Hartwell Foundation, and the Fitzpatrick Foundation Scholars Fellowship (DN). Portions of these results were presented at the 2014 SPIE Photonics West Annual Meeting, San Francisco CA, February 2014 and at the 2014 ARVO Annual Meeting, Orlando FL, May 2014. Dr. Izatt is co-founder and Chief Science Advisor for Bioptigen, Inc. and has corporate, equity, and intellectual property interests (including royalties) in this company. Duke University also equity and intellectual property interests (including royalties) in Bioptigen, Inc.
References and links
- 1.Webb R. H., Hughes G. W., Delori F. C., “Confocal scanning laser ophthalmoscope,” Appl. Opt. 26(8), 1492–1499 (1987). 10.1364/AO.26.001492 [DOI] [PubMed] [Google Scholar]
- 2.Huang D., Swanson E. A., Lin C. P., Schuman J. S., Stinson W. G., Chang W., Hee M. R., Flotte T., Gregory K., Puliafito C. A., Fujimoto J. G., “Optical coherence tomography,” Science 254(5035), 1178–1181 (1991). 10.1126/science.1957169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Podoleanu A. G., Jackson D. A., “Combined optical coherence tomograph and scanning laser ophthalmoscope,” Electron. Lett. 34(11), 1088–1090 (1998). 10.1049/el:19980793 [DOI] [Google Scholar]
- 4.Hammer D. X., Iftimia N. V., Ustun T. E., Magill J. C., Ferguson R. D., “Dual OCT/SLO imager with three-dimensional tracker,” Proc. SPIE 5688, 33–44 (2005). 10.1117/12.592762 [DOI] [Google Scholar]
- 5.Pircher M., Götzinger E., Sattmann H., Leitgeb R. A., Hitzenberger C. K., “In vivo investigation of human cone photoreceptors with SLO/OCT in combination with 3D motion correction on a cellular level,” Opt. Express 18(13), 13935–13944 (2010). 10.1364/OE.18.013935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tao Y. K., Farsiu S., Izatt J. A., “Interlaced spectrally encoded confocal scanning laser ophthalmoscopy and spectral domain optical coherence tomography,” Biomed. Opt. Express 1(2), 431–440 (2010). 10.1364/BOE.1.000431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zawadzki R. J., Jones S. M., Pilli S., Balderas-Mata S., Kim D. Y., Olivier S. S., Werner J. S., “Integrated adaptive optics optical coherence tomography and adaptive optics scanning laser ophthalmoscope system for simultaneous cellular resolution in vivo retinal imaging,” Biomed. Opt. Express 2(6), 1674–1686 (2011). 10.1364/BOE.2.001674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vienola K. V., Braaf B., Sheehy C. K., Yang Q., Tiruveedhula P., Arathorn D. W., de Boer J. F., Roorda A., “Real-time eye motion compensation for OCT imaging with tracking SLO,” Biomed. Opt. Express 3(11), 2950–2963 (2012). 10.1364/BOE.3.002950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braaf B., Vienola K. V., Sheehy C. K., Yang Q., Vermeer K. A., Tiruveedhula P., Arathorn D. W., Roorda A., de Boer J. F., “Real-time eye motion correction in phase-resolved OCT angiography with tracking SLO,” Biomed. Opt. Express 4(1), 51–65 (2013). 10.1364/BOE.4.000051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LaRocca F., Nankivil D., Farsiu S., Izatt J. A., “Handheld simultaneous scanning laser ophthalmoscopy and optical coherence tomography system,” Biomed. Opt. Express 4(11), 2307–2321 (2013). 10.1364/BOE.4.002307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marques M. J., Bradu A., Podoleanu A. G., “Towards simultaneous Talbot bands based optical coherence tomography and scanning laser ophthalmoscopy imaging,” Biomed. Opt. Express 5(5), 1428–1444 (2014). 10.1364/BOE.5.001428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hibbs S. P., Smith A., Chow L. P., Downes S. M., “Colour photographs for screening in neovascular age-related macular degeneration: are they necessary?” Eye (Lond.) 25(7), 918–921 (2011). 10.1038/eye.2011.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manivannan A., Kirkpatrick J. N. P., Sharp P. F., Forrester J. V., “Novel approach towards colour imaging using a scanning laser ophthalmoscope,” Br. J. Ophthalmol. 82(4), 342–345 (1998). 10.1136/bjo.82.4.342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reinholz F., Ashman R. A., Eikelboom R. H., “Simultaneous three wavelength imaging with a scanning laser ophthalmoscope,” Cytometry 37(3), 165–170 (1999). [DOI] [PubMed] [Google Scholar]
- 15.Ashman R. A., Reinholz F., Eikelboom R. H., “Improvements in Colour Fundus Imaging Using Scanning Laser Ophthalmoscopy,” Lasers Med. Sci. 16(1), 52–59 (2001). 10.1007/PL00011337 [DOI] [PubMed] [Google Scholar]
- 16.Manivannan A., Van der Hoek J., Vieira P., Farrow A., Olson J., Sharp P. F., Forrester J. V., “Clinical investigation of a true color scanning laser ophthalmoscope,” Arch. Ophthalmol. 119(6), 819–824 (2001). 10.1001/archopht.119.6.819 [DOI] [PubMed] [Google Scholar]
- 17.Vieira P., Manivannan A., Sharp P. F., Forrester J. V., “True colour imaging of the fundus using a scanning laser ophthalmoscope,” Physiol. Meas. 23(1), 1–10 (2002). 10.1088/0967-3334/23/1/301 [DOI] [PubMed] [Google Scholar]
- 18.Y. N. Sulai, D. H. Scoles, and A. Dubra, “Imaging the retina with a confocal red-green-blue AOSLO,” ARVO/ISIE Meeting Abstracts 54(2013). [Google Scholar]
- 19.Bartsch D. U., Freeman W. R., Lopez A. M., “A false use of “true color”,” Arch. Ophthalmol. 120(5), 675–676 (2002). [PubMed] [Google Scholar]
- 20.Delori F. C., Pflibsen K. P., “Spectral reflectance of the human ocular fundus,” Appl. Opt. 28(6), 1061–1077 (1989). 10.1364/AO.28.001061 [DOI] [PubMed] [Google Scholar]
- 21.Benny Y., Manzanera S., Prieto P. M., Ribak E. N., Artal P., “Wide-angle chromatic aberration corrector for the human eye,” J. Opt. Soc. Am. A 24(6), 1538–1544 (2007). 10.1364/JOSAA.24.001538 [DOI] [PubMed] [Google Scholar]
- 22.Laser Institute of America, American National Standard for Safe Use of Lasers ANSI Z136.1–2007 (American National Standards Institute, Inc., 2007). [Google Scholar]
- 23.LaRocca F., Dhalla A. H., Kelly M. P., Farsiu S., Izatt J. A., “Optimization of confocal scanning laser ophthalmoscope design,” J. Biomed. Opt. 18(7), 076015 (2013). 10.1117/1.JBO.18.7.076015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y., Poonja S., Roorda A., “MEMS-based adaptive optics scanning laser ophthalmoscopy,” Opt. Lett. 31(9), 1268–1270 (2006). 10.1364/OL.31.001268 [DOI] [PubMed] [Google Scholar]
- 25.Wilson T., “Resolution and optical sectioning in the confocal microscope,” J. Microsc. 244(2), 113–121 (2011). 10.1111/j.1365-2818.2011.03549.x [DOI] [PubMed] [Google Scholar]
- 26.Van Heel A. C. S., “Correcting the Spherical and Chromatic Aberrations of the Eye,” J. Opt. Soc. Am. 36, 237–239 (1946). [DOI] [PubMed] [Google Scholar]
- 27.Bedford R. E., Wyszecki G., “Axial Chromatic Aberration of the Human Eye,” J. Opt. Soc. Am. 47(6), 564–565 (1957). [DOI] [PubMed] [Google Scholar]
- 28.Powell I., “Lenses for correcting chromatic aberration of the eye,” Appl. Opt. 20(24), 4152–4155 (1981). 10.1364/AO.20.004152 [DOI] [PubMed] [Google Scholar]
- 29.Lewis A. L., Katz M., Oehrlein C., “A modified achromatizing lens,” Am. J. Optom. Physiol. Opt. 59(11), 909–911 (1982). 10.1097/00006324-198211000-00011 [DOI] [PubMed] [Google Scholar]
- 30.Fernández E. J., Unterhuber A., Považay B., Hermann B., Artal P., Drexler W., “Chromatic aberration correction of the human eye for retinal imaging in the near infrared,” Opt. Express 14(13), 6213–6225 (2006). 10.1364/OE.14.006213 [DOI] [PubMed] [Google Scholar]
- 31.Zawadzki R. J., Cense B., Zhang Y., Choi S. S., Miller D. T., Werner J. S., “Ultrahigh-resolution optical coherence tomography with monochromatic and chromatic aberration correction,” Opt. Express 16(11), 8126–8143 (2008). 10.1364/OE.16.008126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goncharov A. V., Dainty C., “Wide-field schematic eye models with gradient-index lens,” J. Opt. Soc. Am. A 24(8), 2157–2174 (2007). 10.1364/JOSAA.24.002157 [DOI] [PubMed] [Google Scholar]
- 33.Atchison D. A., Smith G., “Chromatic dispersions of the ocular media of human eyes,” J. Opt. Soc. Am. A 22(1), 29–37 (2005). 10.1364/JOSAA.22.000029 [DOI] [PubMed] [Google Scholar]
- 34.Jaeken B., Lundström L., Artal P., “Peripheral aberrations in the human eye for different wavelengths: off-axis chromatic aberration,” J. Opt. Soc. Am. A 28(9), 1871–1879 (2011). 10.1364/JOSAA.28.001871 [DOI] [Google Scholar]
- 35.B. J. Lindbloom, “Useful color equations”, retrieved http://www.brucelindbloom.com/.
- 36.Nielsen M., Stokes M., “The Creation of the sRGB ICC Profile,” Color and Imaging Conference 1998, 253–257 (1998). [Google Scholar]
- 37.McLaren K., “XIII—The Development of the CIE 1976 (L* a* b*) Uniform Colour Space and Colour-difference Formula,” J. Soc. Dyers Colourists 92(9), 338–341 (1976). 10.1111/j.1478-4408.1976.tb03301.x [DOI] [Google Scholar]
- 38.Delori F. C., Webb R. H., Sliney D. H., American National Standards Institute , “Maximum permissible exposures for ocular safety (ANSI 2000), with emphasis on ophthalmic devices,” J. Opt. Soc. Am. A 24(5), 1250–1265 (2007). 10.1364/JOSAA.24.001250 [DOI] [PubMed] [Google Scholar]
- 39.Ahmed S. S., Lott M. N., Marcus D. M., “The macular xanthophylls,” Surv. Ophthalmol. 50(2), 183–193 (2005). 10.1016/j.survophthal.2004.12.009 [DOI] [PubMed] [Google Scholar]