Abstract
Background
Knowledge of tuberculosis incidence and associated factors is required for the development and evaluation of strategies to reduce the burden of HIV-associated tuberculosis.
Methods
Systematic literature review and meta-analysis of tuberculosis incidence rates among HIV-infected individuals taking combination antiretroviral therapy.
Results
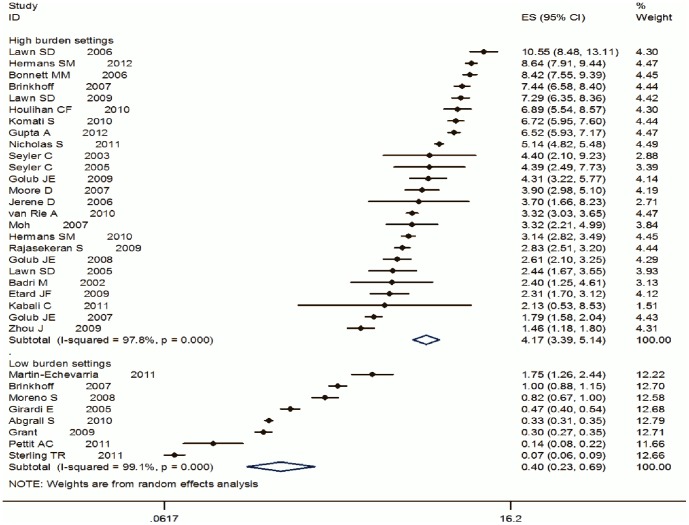
From PubMed, EMBASE and Global Index Medicus databases, 42 papers describing 43 cohorts (32 from high/intermediate and 11 from low tuberculosis burden settings) were included in the qualitative review and 33 in the quantitative review. Cohorts from high/intermediate burden settings were smaller in size, had lower median CD4 cell counts at study entry and fewer person-years of follow up. Tuberculosis incidence rates were higher in studies from Sub-Saharan Africa and from World Bank low/middle income countries. Tuberculosis incidence rates decreased with increasing CD4 count at study entry and duration on combination antiretroviral therapy. Summary estimates of tuberculosis incidence among individuals on combination antiretroviral therapy were higher for cohorts from high/intermediate burden settings compared to those from the low tuberculosis burden settings (4.17 per 100 person-years [95% Confidence Interval (CI) 3.39–5.14 per 100 person-years] vs. 0.4 per 100 person-years [95% CI 0.23–0.69 per 100 person-years]) with significant heterogeneity observed between the studies.
Conclusions
Tuberculosis incidence rates were high among individuals on combination antiretroviral therapy in high/intermediate burden settings. Interventions to prevent tuberculosis in this population should address geographical, socioeconomic and individual factors such as low CD4 counts and prior history of tuberculosis.
Introduction
Human immune deficiency virus (HIV)-associated tuberculosis (TB) is an important public health problem particularly in high HIV prevalence settings. In 2012, the World Health Organisation (WHO) estimated that up to 1.1 million reported TB cases and 320 000 deaths from TB occurred in people living with HIV [1]. In the same year, up to 75% of all HIV-associated TB cases occurred in Sub-Saharan Africa [1]. Combination antiretroviral therapy (cART) or highly active antiretroviral therapy (HAART) reduces the risk of TB by 67% (95% CI 61–73%) among people living with HIV [2]. The risk of TB declines in proportion to the increases in CD4 counts after cART initiation [3]. In the high burden setting of Cape Town, South Africa, the risk of TB while on cART with a CD4 count of >700 cells/ml3 remained four fold higher than in HIV-uninfected persons from the same community [4]. Because cART alone is not sufficient to prevent HIV-associated TB, additional strategies are required. In order to develop additional strategies for preventing HIV-associated TB in people taking cART such as novel TB vaccines, an understanding of the incidence of and risk factors for HIV-associated TB in high/intermediate and low TB burden settings is required. We conducted a systematic review and meta-analysis to summarise and describe trends in the incidence of TB among adults taking cART in high/intermediate and low TB burden settings, stratified by geographical region, CD4 count, previous history of TB and duration on cART. We highlight the disparities in TB incidence rates between high and low TB burden settings and discuss the implications for interventions to further reduce the risk of HIV-associated TB among individuals on cART.
Methods
Search strategy and selection of papers
PubMed, EMBASE and Global Index Medicus databases were searched in parallel using search strings adapted to the requirements of each database (Table S1). For the PubMed search we conducted two separate searches using MeSH terms i) “tuberculosis” AND “incidence” ii) “tuberculosis” AND “HAART” with all the available qualifiers. Both PubMed searches were limited to papers describing studies in humans, published in English between 1st January 2000 and 31st March 2012. For the EMBASE search we used EMTREE terms “tuberculosis” AND “incidence” OR “HAART” with all the available qualifiers and limited the search to papers describing studies in humans, published in English between 1st January 2000 and 31st March 2012. For the Global Index Medicus search, we searched all indexes and all sources (which include AIM, LILACS, IMEMR, IMSEAR, WPRIM, WHOLIS and Medline) using the keywords “tuberculosis” “incidence” “HAART” and limited the search to studies written in the English language. No other limits applied.
The search outputs were imported into a combined file in reference management software and duplicates removed. Two independent reviewers (TM and TK) screened all titles and abstracts to identify papers for full text review. Full texts were then screened by the same reviewers and eligibility criteria applied. Eligibility for inclusion required reporting a TB incidence rate for a cohort of individuals on cART and more than 100 participants included in the cohort. Review papers, papers exclusively reporting multi-drug or extensively drug resistant (MDR/XDR) TB as outcomes, and papers reporting exclusively on children younger than 15 years of age were not eligible. Where discordance occurred in the independent review of papers, the papers were discussed and consensus achieved. References lists included in the eligible papers were hand searched in order to identify additional eligible papers. The search criteria and other methods used in the review were included in a protocol outline agreed upon by the authors before data collection commenced. This protocol outline can be found in the supplementary information (Table S2).
Abstraction of data from eligible papers
Data on study characteristics (including but not limited to: author, date of publication, location, sample size, cohort characteristics and TB incidence rates) were abstracted using a standardized form (Table S3). If a study described two or more distinct cohorts, data was abstracted for each of the cohorts meeting the criteria for inclusion in the review.
Estimates of national TB incidence rates, national adult HIV prevalence rates and World Bank income classification for the country and year each study was published were obtained from the WHO database of TB burden [5], UNAIDS Global HIV estimates [6], [7] and the World Bank classifications respectively [8]. We used the estimates of national HIV prevalence rates last updated in 2009, and the estimates of national TB incidence rates last updated in 2010. Therefore, the HIV estimates from 2009 were used for studies published after 2009 and, the 2010 estimates of national TB incidence were used for studies published after 2010. For multi-country studies, the data on national TB incidence and HIV prevalence rates were not abstracted but were assigned to low or high burden depending on the average national TB incidence rate in the countries the cohorts were from. Cohorts described in the studies were classified as high/intermediate burden if the estimated national TB incidence rate for the country and year were ≥25 per 100 000 population per year, and as low burden if the TB incidence rates were below this threshold. Cohorts were also classified as being from low, middle or high income settings according to the World Bank income classification.
Assessment of study quality
Study quality was assessed using a standardized tool (see Table S4) adapted from the Newcastle-Ottawa Scale (NOS) for cohort studies [9]. The tool was used to assess the following study characteristics: sampling methods, presence of sampling bias, exclusion of TB at cohort entry, outcomes ascertainment during follow up, duration of follow up and loss to follow up rates. Each of these criteria was assigned a score as shown in Box 1 (Table S4). The highest possible score was 6 and studies with scores ≥4 were considered to be of good quality. No studies were excluded from the reviews on the basis of their quality scores.
Data analysis and presentation
In the qualitative part of the review, all eligible cohorts reporting a TB incidence rate among individuals on cART were classified into high/intermediate and low burden settings and described with respect to cohort characteristics. TB incidence rates were summarized according to CD4 cell count strata, duration on cART and by prior history of TB. Where studies only reported number of TB cases and person-years of follow up for the different strata in the cohort, the incidence rates and confidence intervals were computed in Stata 12 (Stata Corporation, College Station, Texas, USA).
Meta-analysis
Meta-analyses (quantitative reviews) were conducted to determine summary estimates of the TB incidence rates among HIV-infected individuals on cART overall and point estimates across different categories or strata of study quality, study designs, national HIV prevalence rates, national TB incidence rates, CD4 count, durations on cART and prior history of TB. To be eligible for inclusion in meta-analyses, studies were required to report both the number of TB cases and person-years of follow up by the different categories listed. These fields were required to enable estimation of incidence rates of TB and associated standard errors using the random effects model. These data were recorded onto the abstraction forms and entered into an Excel 2007 sheet (Microsoft Corporation, Washington, USA) and exported into Stata 12 for analysis. I-squared estimates were used to determine heterogeneity between studies.
Results
Summary of studies
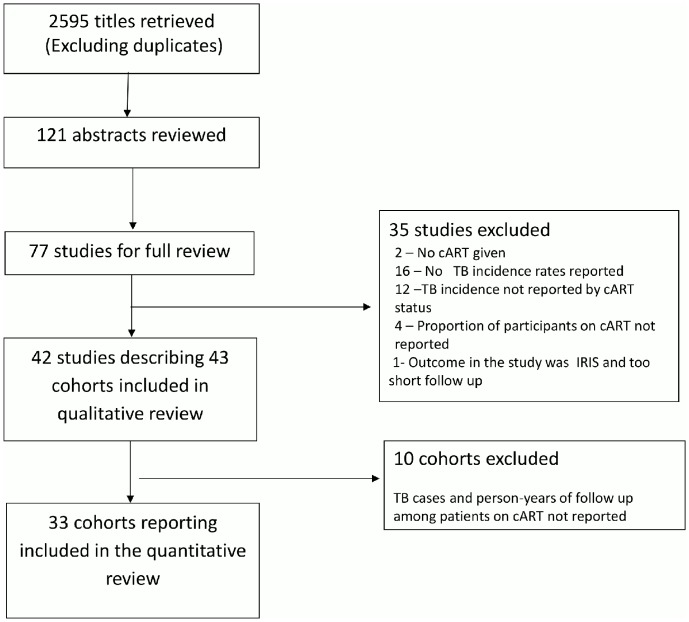
From 2945 unique study titles retrieved, 121 titles were eligible for abstract review and 77 for full text review. From the full texts reviewed, 42 studies [4], [10]–[50] describing 43 cohorts were eligible for inclusion in the qualitative review and a subset of 33 cohorts for inclusion in the quantitative review (meta-analysis - Figure 1). Of the 43 cohorts, 32 (74%) were from high/intermediate burden settings with national TB incidence rates ranging from of 46 to 981 per 100 000 and national HIV prevalence rate ranging from 0.3% to 18.2%. Eleven cohorts (26%) were from low burden settings with national TB incidence rates ranging from 4.1 to 17 per 100 000 population per year and national HIV prevalence rate of 0.2% to 0.6%. The full list and characteristics of papers included in the review are presented in Table S5.
Figure 1. Summary of search findings.
Characteristics of cohorts from high/intermediate and low burden settings are presented in Table 1. When compared to cohorts from low burden settings, cohorts from high/intermediate burden were smaller in size, had lower median CD4 cell counts at study entry and had fewer person-years follow up. TST positivity was reported for a few cohorts in both settings (five cohorts (15.6%) from high/intermediate burden settings and five (45.5%) cohorts from low burden settings).
Table 1. Characteristics of study cohorts from high burden settings and from low burden settings.
| Characteristic | High/intermediate burden (N = 32) | Low burden (N = 11) | ||
| number of cohorts | number of cohorts | |||
| Prospective study design (n, %) | 32 | 16 (50) | 11 | 2 (18.2) |
| Cohort size (range) | 32 | 101–19325 | 11 | 1824–72850 |
| Cohorts from clinical care settings (n, %) | 32 | 24 (75) | 11 | 11 (100) |
| % males in the cohort, (range) | 31 | 21–91 | 10 | 67–77 |
| Median/mean age of cohort, (range) | 28 | 31–38 | 10 | 33–41 |
| % of cohort with prior history of TB, (range) | 20 | 0–100 | 3 | 0–13.9 |
| % of cohort who were MSM, (range) | 4 | 18.7–59 | 6 | 17.3–58.7 |
| % of cohort who were IDU, (range) | 3 | 1.6–14 | 6 | 4.8–59 |
| % of cohort on cART at baseline/by end of study, (range) | 32 | 5.7–100 | 11 | 10.2–100 |
| Cohort with 100% of participants on cART, (n, %) | 32 | 22 (68.8) | 11 | 5 (45.5) |
| % of cohort on NNRTI-based treatment, (range) | 10 | 35–100 | 2 | 26.3–33 |
| % of cohort on PI- based treatment, (range) | 5 | 3.2–73 | 2 | 56–63 |
| Median CD4 count at cohort entry, cells/µl), (range) | 27 | 37–444 | 10 | 207–422 |
| Median CD4 count at cART initiation(cells/µl), (range) | 20 | 97–252 | 5 | 207–297 |
| Median duration of follow up in months, (range) | 24 | 8–73 | 4 | 15.8–56.4 |
| Person-years of follow up, (range) | 27 | 159–18162 | 10 | 14711–427957 |
| Person-years of follow up on cART,(range) | 25 | 65.1–18162 | 8 | 11248–313807 |
| Availability of mycobacterial culture, (n, %) | 29 | 19 (65.3) | 10 | 10 (100%) |
N is total number of cohorts, n is number of cohorts with the characteristic, IQR – interquartile range, MSM- men who have sex with men, IDU- intravenous drug user, NNRTI – non-nucleoside reverse transcriptase inhibitors, PI – protease inhibitors, cART- combination antiretroviral therapy.
Study quality
Table 2 describes the findings of the study quality assessment. The overall quality scores ranged from 2–6 with the majority of the papers (27 of 42 studies, 64%) being considered to be of acceptable quality (score ≥4).
Table 2. Summary of study quality.
| Criteria | high burden (N = 32) | low burden(N = 11) | ||
| n (%) | studies | n (%) | studies | |
| 1. Sampling method | ||||
| Reported sampling method used | 32 (100) | all studies | 11 (100) | all studies |
| 2. Sampling bias | ||||
| Assessed and reported on sampling bias | 18 (56.3) | 4,11, 14, 17, 19, 20, 22, 24, 30, 31, | 2 (18.2) | 29, 32 |
| 33, 34, 36,37, 38, 39, 43,47 | ||||
| 3. Screening of TB at study commencement | ||||
| Reported screening/exclusion of TB at cohort entry | 32 (100) | all studies | 11 (100) | all studies |
| 4. Ascertainment of outcomes | ||||
| Smear + culture + clinical + chest radiograph | 21 (62.5) | 4, 14, 17, 19, 20, 21, 24, 27, 28, 30, | 7 (63.6) | 12, 15, 26, 32, 35, 48, 49 |
| 31, 33,34, 35, 36, 37, 42, 43,46, 47, | ||||
| 49 | ||||
| Smear + clinical + chest radiograph (no culture) | 7 (21.2) | 10, 11, 25,38, 39, 41, 50 | ||
| Clinical + chest radiograph (no smear or culture) | 1 (3.0) | 30 | 0 (0) | |
| Treatment initiation records only | 2 (6.1) | 22, 23, | 1 (9.1) | 18 |
| Not specified | 2 (6.1) | 40, 45 | 3 (27.2) | 16, 29, 44 |
| 5. Median duration of follow up | ||||
| Median follow-up <9 months | 1 (3) | 41 | 0 (0) | |
| Median follow-up ≥9 months | 23 (72.7) | 4, 14, 17, 19, 20, 21, 22, 23, 24, 25 | 5 (45.4) | 15, 18, 29, 32,44 |
| 28, 30, 31, 33, 34, 36, 37,39, 42, | ||||
| 43, 45, 46, 47 | ||||
| Median follow up not reported | 8 (24.2) | 10, 11, 27, 35, 38, 40, 49, 50 | 6 (55.5) | 12, 13, 16, 26, 48, 49 |
| 6. Loss to follow up | ||||
| <20% of participants lost to follow-up | 15 (45.5) | 11,14, 17, 19, 20, 21, 22, 23, 24, 27 | 1 (9.1) | 29 |
| 28, 31, 36, 39, 40 | ||||
| >20% of participants lost to follow-up | 1 (3.0) | 4 | 0 | |
| Loss to follow up not reported | 16 (48.5) | 10, 25, 30, 33, 34, 35, 37, 38, 41, | 10 (90.9) | 12, 13, 15, 16, 18, 26, 32, 44, |
| 42, 43, 45, 46, 47, 49, 50 | 48, 49 | |||
| Overall study quality score (median, range) | 5 (2–6) | 3 (2–5) | ||
| Studies with quality score ≥4 | 24 (72.7) | 4, 11, 14, 17, 19, 20, 21, 22, 23, 24, | 3 (27.3) | 15, 29, 32 |
| 27, 28, 30, 31, 33, 34, 36, 37,38, | ||||
| 39, 42, 43, 46, 47, | ||||
n = number of studies with characteristic.
Qualitative review of TB incidence rates
Table 3 summarises the characteristics of TB cases and the TB incidence rates reported across different CD4 count and duration on cART strata, for both high/intermediate and low burden cohorts. The proportion of TB cases with pulmonary TB was higher in cohorts from high/intermediate burden settings compared to those from low burden settings. The median CD4 counts at study entry among individuals who subsequently developed TB were similar between high/intermediate and low burden cohorts. The TB incidence rates reported among individuals on cART were seven to 30 times higher in cohorts from high/intermediate burden settings compared to those from low TB burden settings.
Table 3. TB cases characteristics and incidence rates reported in high burden cohorts and in low burden cohorts.
| Cohort characteristics | High/intermediate TB burden | Low TB burden | ||
| (N = 32) | (N = 11) | |||
| Number of cohorts reporting | Number of cohorts reporting | |||
| Proportion (%) of TB cases with pulmonary TB (range) | 16 | 43–81 | 6 | 44–65 |
| Proportion (%) of TB cases who are male (range) | 5 | 33.2–67.6 | 4 | 63.3–91 |
| Median CD4 count of TB cases at study entry, cells/µl (range) | 8 | 75–197 | 3 | 80–179 |
| TB incidence rate among those on cART, | 32 | 0.6–10.5 | 11 | 0.02–1.5 |
| cases/100person-years, (range) | ||||
| TB incidences across baseline CD4 count strata | ||||
| cases/100person-years, (range) | ||||
| <100 | 6 | 0.6–6.8 | 1 | 0.84 |
| 101–200 | 2 | 1.7–4.8 | 0 | . |
| 201–350 | 3 | 1.7–3.7 | 1 | 0.47 |
| 351–500 | 3 | 1.8–3 | 1 | 0.19 |
| >500 | 1 | 2 | 1 | 0.17 |
| TB incidences across current CD4 count strata, | ||||
| cases/100person-years,(range) | ||||
| <100 | 4 | 8.9–25.5 | 3 | 0.11–0.83 |
| 101–200 | 4 | 3.6–11.2 | 1 | 0.09 |
| 201–350 | 3 | 1.8–7.8 | 2 | 0.08–0.26 |
| 351–500 | 3 | 0.7–5.0 | 3 | 0.04–0.21 |
| >500 | 2 | 1.5–4.1 | 2 | 0.06–0.1 |
| TB incidences with increasing duration on cART | ||||
| cases/100person-years,(range) | ||||
| 0–3months | 14 | 3.4–23 | 5 | 0.22–1.7 |
| 3–6months | 10 | 2.2–10.7 | 4 | 0.15–1 |
| 6–12months | 10 | 1.2–7.0 | 5 | 0.07–0.62 |
| 12–24 months | 13 | 1.3–6.7 | 3 | 0.07–0.33 |
| 24–36 months | 7 | 1.4–7.4 | 3 | 0.09–0.18 |
| >36 months | 4 | 0.4–5.8 | 1 | 0.05 |
| TB incidence among those with prior history of TB, | 6 | 1.9–11.9 | 1 | 1.19 |
| cases/100person-years, (range) | ||||
| TB incidence among those with no prior history of TB | 6 | 1.8–8.1 | 1 | 1.83 |
| cases/100person-years, (range) |
N is total number of cohorts, n is number of cohorts with the characteristic, IQR – interquartile range, cART- combination antiretroviral therapy.
In cohorts from high/intermediate and low burden settings, TB incidence rates generally increased with decreasing current CD4 counts and CD4 count at cART initiation, more so with CD4 counts less than 200 cells/µl (Table 3). TB incidence rates also increased with durations on cART less than six months. In six cohorts from high/intermediate burden settings, TB incidence rates were higher among those with prior history of TB compared to those with no prior history of TB (1.9–11.9 per 100 person-years compared to 1.8–8.1 per 100 person-years).
TB incidence rates among individuals on cART also varied with geographical location with highest incidence rates found in cohorts from Sub-Saharan Africa (range 0.9–7.82 per 100 person-years, n = 23), followed by those in Asia (range 1.32–2.83 per 100 person-years, n = 2), in South America (0.2–2.6 per 100 person-years, n = 4), and in Europe and North America (range 0.02–1.9 per 100 person-years, n = 9). Rates were much higher among cohorts from low income countries (range 0.9–8.6 per 100 person-years, n = 16) and middle income countries (0.6–10.5 per 100 person-years, n = 16); compared to those from high income countries (0.02–1.9 per 100 person-years, n = 9).
Meta-analysis of TB incidence among HIV-infected adults on cART
Thirty-three cohorts were eligible for inclusion in the meta-analysis. (See Figure 2). Heterogeneity was computed separately for high/intermediate (I2 = 98%, p-value <0.001) and low (I2 = 99.1%, p-value <0.001) burden settings and was large in both settings. As expected, the summary estimate of TB incidence among those on cART was higher for cohorts from high/intermediate burden settings compared to those from the low burden settings–4.17 per 100 person-years (95% CI 3.39–5.14 per 100 person-years) vs. 0.4 per 100 person-years (95% CI 0.23–0.69 per 100 person-years, (Figure 2). In the analyses stratifying summary estimates of TB incidence rates by study quality, study design (retrospective or prospective studies), national TB incidence rates and national HIV prevalence rates (see Table 4), heterogeneity remained high. This implied that these variables did not explain most of the heterogeneity observed in the TB incidence rates.
Figure 2. Forest plot showing summary estimates of TB incidence rates among individuals on cART comparing cohorts from high TB burden settings to low TB burden settings.
Table 4. Summary estimates from meta-analysis of TB incidence rates stratified study level variables.
| Variable | High/intermediate burden | Low burden | ||||||
| N Studies | Summary estimate (per 100 person-years) | I2¥ | p* | N studies | Summary estimate (per 100 person-years) | I2 | p* | |
| Study quality | ||||||||
| low | 10 | 3.7 (2.3–5.6) | 98.8% | <0.001 | 6 | 0.37 (0.18–0.77) | 93.3% | <0.001 |
| high | 14 | 4.5 (3.6–5.5) | 95.5% | <0.001 | 2 | 0.50 (0.19–1.31) | 98.4% | <0.001 |
| Study Design | ||||||||
| Retrospective | 13 | 3.7 (2.7–5.3) | 98.7% | <0.001 | 6 | 0.40 (0.16–1.04) | 99.3% | <0.01 |
| Prospective | 12 | 4.6 (3.6–6.0) | 95.2% | <0.001 | 2 | 0.39 (0.28–0.55) | 943% | <0.01 |
| National TB incidence | ||||||||
| rates (per 100 000 | ||||||||
| population) | ||||||||
| <25 | 6 | 0.34 (0.18–0.64) | 99% | <0.001 | ||||
| 25–200 | 7 | 2.8 (2.1–3.6) | 83.7% | <0.001 | ||||
| 201–800 | 6 | 3.7 (2.1–6.4) | 98% | <0.001 | ||||
| >800 | 8 | 5.6 (4.2–7.4) | 96.7% | <0.001 | ||||
| National HIV prevalence | ||||||||
| rates | ||||||||
| <1% | 4 | 2.3 (1.8–3.0) | 88.8% | <0.001 | 6 | 0.34 (0.18–0.64) | 99% | <0.001 |
| 1–4.9% | 3 | 3.7 (2.7–4.9) | 0 | 0.735 | ||||
| 5–10% | 5 | 4.3 (2.3–8.1) | 98.1% | <0.001 | ||||
| >10% | 9 | 5.2 (3.9–6.9) | 96.3% | <0.001 |
I2- Amount of heterogeneity not explained by the variable, *p-value for heterogeneity not explained by the variable, N – number of cohorts.
Meta-analysis of TB incidence among HIV-infected adults on cART stratified by CD4 counts, duration on cART and prior history of TB
Summary estimates of TB incidence rates stratified by CD4 counts at entry, duration on cART and prior history of TB are shown in Table 5. The summary estimates of the TB incidence rates were higher in cohorts from high/intermediate burden settings compared to those from low burden settings across all baseline CD4 count strata, duration on cART and prior history of TB strata, although inference was limited by number of cohorts. Among cohorts from high/intermediate burden settings, TB incidence rates were higher in the baseline CD4 count <200 cells/µl stratum compared to those in 200–350 cells/µl or >350 cells/µl strata. There was significant heterogeneity in the TB incidence rates across the different strata in the meta-analysis.
Table 5. Summary estimates from meta-analysis of TB incidence rates stratified by baseline CD4 count, duration on cART and previous history of TB.
| Variable | High/intermediate burden | Low burden | ||||||
| N | Summary estimate | I2¥ | p* | N | Summary estimate | I2 | p* | |
| Studies | (per 100 person-years) | Studies | (per 100 person-years) | |||||
| Baseline CD4 count | ||||||||
| <200 | 5 | 4.47 (3.55–5.63) | 89.6% | <0.001 | 1 | 0.84 (0.31–1.62) | - | - |
| 200–350 | 3 | 2.32 (1.54–3.51) | 42.3% | 0.177 | 1 | 0.46 (0.35–0.60) | - | - |
| >350 | 3 | 2.34 (1.78–3.08) | 0.00% | 0.877 | 1 | 0.23 (0.16–0.34) | - | - |
| Duration on cART | ||||||||
| <3months | 6 | 13.67 (10.62–17.60) | 86.9% | <0.001 | 3 | 0.73 (0.27–1.99) | 96.3% | <0.01 |
| 3–6 months | 6 | 6.11 (4.62–8.09) | 76.4% | 0.001 | 3 | 0.46 (0.18–1.19) | 93.6% | <0.01 |
| 6–12 months | 6 | 3.14 (2.20–4.48) | 83.9% | <0.001 | 3 | 0.29 (0.12–0.74) | 94.5% | <0.01 |
| 12–24 months | 4 | 3.94 (1.97–7.88) | 94.5% | <0.001 | 3 | 0.19 (0.08–0.47) | 94.8% | <0.01 |
| >24 months | 1 | 5.87(5.15–6.68) | - | - | 2 | 0.09(0.03–0.23) | 91.3% | <0.01 |
| Previous History TB | ||||||||
| Yes | 4 | 4.74 (2.10–10.73) | 93.1% | <0.001 | 1 | 1.20 (0.39–3.71) | - | - |
| No | 4 | 2.78 (1.36–5.68) | 97.1% | <0.001 | 1 | 1.83 (1.30–2.59) | - | - |
I2- Amount of heterogeneity not explained by the variable, *p-value for heterogeneity not explained by the variable, N – number of cohorts.
Discussion
This review summarises and describes trends in TB incidence rates among HIV-infected adults on cART, comparing cohorts from high/intermediate burden settings with those from low burden settings. In the qualitative review, the incidence rates in cohorts from high/intermediate burden settings were seven to 30 times higher than rates in cohorts those from low burden settings. In the quantitative review the rates in high/intermediate burden settings were 10 times higher than those from low burden settings. Rates were highest in cohorts from low/middle income countries, from Sub-Saharan Africa, especially among individuals with baseline and current CD4 counts less than 200 cells/µl and among those on cART for less than six months.
Geographic and socio-economic factors
Our review suggests that background socio-economic conditions as measured by the World Bank classification may be associated with increased TB incidence among individuals on cART. In a 2009 study, Dye et al found that improvements in sanitation, lower child mortality and a higher human development index were associated with declines in national TB notification rates among 134 countries studied [51]. In this review, Sub-Saharan Africa, a region largely consisting of low income countries had the highest TB incidence rates among HIV-infected individuals on cART and should be a priority region for implementation of public health interventions which address social determinants of TB.
Individual level factors
The high rates of TB among HIV-infected adults on cART in this review, particularly in cohorts from high burden settings confirm that cART alone is not sufficient to prevent TB in populations with HIV [2]. Results reported here indicate that additional strategies at individual level are required to prevent HIV-associated TB particularly for individuals who have low CD4 counts, previous history of TB disease or recently initiated cART. Low CD4 counts during treatment with cART may be a result of i) late presentation for cART initiation, ii) sub-optimal immune reconstitution, or iii) treatment failure on cART [52], [53]. Concerted efforts should be made to ensure initiation of cART at higher CD4 counts, better adherence to cART and better retention in HIV care. The high rates of TB in the first six months after cART initiation observed in this review may be attributed to inadequate TB screening at start of cART [52], or to unmasking of subclinical TB due to restoration of TB specific immune responses or paradoxical immune reconstitution syndrome (IRIS). [52]–[54] IRIS has been associated with low CD4 counts at cART initiation and could be avoided by initiating cART at higher CD4 counts, [52]–[54] or by adequately excluding and treating TB before initiating cART. In settings with both high HIV prevalence and high TB transmission, TB in individuals with HIV can be also prevented through the scaling up of TB preventive therapy and strengthening TB infection control measures at facility and community levels as repeated TB episodes among those on cART have been linked to re-infection [55].
Implications for further research
The findings of this review can inform the development of additional tools - such as new TB vaccines and drug regimens for TB preventive therapy - to prevent TB in HIV-infected populations. Should a vaccine candidate or preventive therapy regimen progress to phase IIb or III trials including HIV-infected subjects and measuring TB disease as an endpoint, large numbers of subjects will be needed to obtain a sufficient number of endpoints in the trials. High/intermediate burden settings should be priority locations for the conduct of such trials to help minimise the required sample size. However, with cART initiation occurring at increasingly higher CD4 count thresholds and use of isoniazid preventive therapy (IPT) being scaled up in most settings, the expected number of endpoints in such HIV-infected populations may be smaller and such trials may become infeasible even in high/intermediate burden settings. Prioritising enrolments of participants with prior history of TB may increase the number of endpoints in such trials.
Strengths and limitations
Summarising the TB incidence rates among individuals taking cART emphasises the magnitude of the TB burden especially in high TB and HIV burden settings. Such data highlight the limitations of cART as a tool for TB prevention in such settings, with challenges remaining due to ongoing transmission, late presentation into care or sub-optimal immune restoration in cART care. This review included 42 studies, the majority of which were of good quality. However this review also had some important limitations. We restricted our search to literature published in English as we did not have resources for translation. We did not search conference abstracts as these were likely to have incomplete follow up data. Twenty-three percent of the cohorts included in the qualitative review were not eligible for inclusion in the meta-analysis because they did not report number of TB cases, or the person – years of follow up stratified by use of cART. There was limited duration of follow up of participants especially in studies from high/intermediate burden settings. The association of longer duration of follow up in the cohort with reduction in TB incidence was evident in the reported rates and from the meta-analysis. This may have contributed to higher rates of TB observed in the cohorts from high/intermediate TB burden settings. There was large heterogeneity in the TB incidence rates in both high/intermediate burden and low TB burden settings which was not fully explained by study variables in stratified analyses. Conducting univariable or multivariable meta-regression analyses would have allowed us to better the determine factors accounting for the heterogeneity observed but the limited number of studies particularly from low TB burden settings precluded this. TB incidence rates in individuals on cART are likely influenced by local TB transmission and the rates of reactivation of latent infection. Data on TST positivity were not available for most studies and any heterogeneity as a result of these two variables could not be accounted for. Another limitation was that the review and meta-analysis included data collected at the individual level in the studies which were then aggregated to give study level variables. This made the review prone to aggregation bias.
Despite these limitations, the study provides valuable information for the evaluation, planning and implementation of preventive strategies for HIV-associated TB. Strategies to further reduce the risk of TB among individuals on cART such as the use of TB preventive therapy regimens, early initiation of cART, better TB screening at initiation of and during cART, and TB infection control can be appropriately targeted based on these data.
Supporting Information
Summary of searches.
(DOCX)
Protocol outline.
(DOCX)
Data abstraction form.
(DOCX)
Modified Ottawa Scale.
(DOC)
List of studies included.
(XLS)
PRISMA checklist.
(DOC)
Acknowledgments
The authors thank Edith Roset-Bahmanyar for her contribution to the design of the systematic literature review, and Sofia Dos Santos Mendes (XPE Pharma&Science on behalf of GlaxoSmithKline Vaccines) for editorial assistance.
Funding Statement
GlaxoSmithKline Biologicals S.A. funded the work disclosed in this manuscript (for data collection for the reported study). Co-author Dominique Rosillon is employed by GlaxoSmithKline Vaccines and co-author Rebecca C. Harris was employed by CROMsource during the course of the study. GlaxoSmithKline provided support in the form of salary for author DR, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of this author is articulated in the ‘author contributions’ section. CROMsource provided support in the form of salary for author RCH, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organisation (2013) Global Tuberculosis Report 2013. Geneva, Switzerland. Available: http://apps.who.int/iris/bitstream/10665/91355/1/9789241564656_eng.pdf. Accessed 2014 Jan 16.
- 2. Lawn SD, Wood R, De Cock KM, Kranzer K, Lewis JJ, et al. (2010) Antiretrovirals and isoniazid preventive therapy in the prevention of HIV-associated tuberculosis in settings with limited health-care resources. Lancet Infect Dis 10: 489–498. [DOI] [PubMed] [Google Scholar]
- 3. Suthar AB, Lawn SD, Del Amo J, Getahun H, Dye C, et al. (2012) Antiretroviral Therapy for Prevention of Tuberculosis in Adults with HIV: A Systematic Review and Meta-Analysis. PLoS Med 9: e1001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gupta A, Wood R, Kaplan R, Bekker LG, Lawn SD (2012) Tuberculosis Incidence Rates during 8 Years of Follow-Up of an Antiretroviral Treatment Cohort in South Africa: Comparison with Rates in the Community. PLoS One 7: e34156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organisation(2010) Tuberculosis Country Profiles. Available: http://www.who.int/tb/country/data/download/en/index.html. Accessed 2012 May 10.
- 6.UNAIDS (2010) HIV estimates with uncertainity bounds 2009. Availble: http://www.unaids.org/globalreport/documents/HIV_Estimates_GR2010_2009_en.xls. Accessed 2012 May 10.
- 7.UNAIDS (2012) Adult (15–49) HIV prevalence percent by country, 1990–2007. Available: data.unaids.org/pub/…/20080813_gr08_prev1549_1990_2007. Accessed 2012 May 10.
- 8.World Bank (2012) World Bank Analytical Classifications 1987–2010. Available: http://data.worldbank.org/about/country-classifications. Accessed 2012 Jul 1.
- 9.Wells GA SB, O'Connell D, Peterson J, Welch V, Losos M, et al. (n.d.) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute. Available: http://www.medicine.mcgill.ca/rtamblyn/Readings/The%20Newcastle%20-%20Scale%20for%20assessing%20the%20quality%20of%20nonrandomised%20studies%20in%20meta-analyses.pdf. Accessed 2012 May 11.
- 10. Hermans S, van Leth F, Manabe Y, Hoepelman A, Lange J, et al. (2012) Earlier initiation of antiretroviral therapy, increased tuberculosis case finding and reduced mortality in a setting of improved HIV care: a retrospective cohort study. HIV Med. [DOI] [PubMed] [Google Scholar]
- 11. Worodria W, Massinga-Loembe M, Mayanja-Kizza H, Namaganda J, Kambugu A, et al. (2011) Antiretroviral treatment-associated tuberculosis in a prospective cohort of HIV-infected patients starting ART. Clin Dev Immunol 2011: 758350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taarnhoj GA, Engsig FN, Ravn P, Johansen IS, Larsen CS, et al. (2011) Incidence, risk factors and mortality of tuberculosis in Danish HIV patients 1995–2007. BMC Pulm Med 11: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pettit AC, Jenkins CA, Stinnette SE, Rebeiro PF, Blackwell RB, et al. (2011) Tuberculosis risk before and after highly active antiretroviral therapy initiation: does HAART increase the short-term TB risk in a low incidence TB setting? J Acquir Immune Defic Syndr 57: 305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nicholas S, Sabapathy K, Ferreyra C, Varaine F, Pujades-Rodriguez M (2011) Incidence of tuberculosis in HIV-infected patients before and after starting combined antiretroviral therapy in 8 sub-Saharan African HIV programs. J Acquir Immune Defic Syndr 57: 311–318. [DOI] [PubMed] [Google Scholar]
- 15. Martin-Echevarria E, Rodriguez-Zapata M, Torralba M, Fernandez JM, Moreno A, et al. (2011) Incidence of tuberculosis in HIV-infected patients receiving HAART: interaction between TST and CD4 count. Int J Tuberc Lung Dis 15: 1347–1352. [DOI] [PubMed] [Google Scholar]
- 16. Kruk A, Bannister W, Podlekareva DN, Chentsova NP, Rakhmanova AG, et al. (2011) Tuberculosis among HIV-positive patients across Europe: changes over time and risk factors. AIDS 25: 1505–1513. [DOI] [PubMed] [Google Scholar]
- 17. Kabali C, von Reyn CF, Brooks DR, Waddell R, Mtei L, et al. (2011) Completion of isoniazid preventive therapy and survival in HIV-infected, TST-positive adults in Tanzania. Int J Tuberc Lung Dis 15: 1515–1521 i.. [DOI] [PubMed] [Google Scholar]
- 18. Sterling TR, Lau B, Zhang J, Freeman A, Bosch RJ, et al. (2011) Risk factors for tuberculosis after highly active antiretroviral therapy initiation in the United States and Canada: implications for tuberculosis screening. J Infect Dis 204: 893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van Rie A, Westreich D, Sanne I (2011) Tuberculosis in patients receiving antiretroviral treatment: incidence, risk factors, and prevention strategies. J Acquir Immune Defic Syndr 56: 349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lawn SD, Kranzer K, Edwards DJ, McNally M, Bekker LG, et al. (2010) Tuberculosis during the first year of antiretroviral therapy in a South African cohort using an intensive pretreatment screening strategy. AIDS 24: 1323–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Komati S, Shaw PA, Stubbs N, Mathibedi MJ, Malan L, et al. (2010) Tuberculosis risk factors and mortality for HIV-infected persons receiving antiretroviral therapy in South Africa. AIDS 24: 1849–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Houlihan CF, Mutevedzi PC, Lessells RJ, Cooke GS, Tanser FC, et al. (2010) The tuberculosis challenge in a rural South African HIV programme. BMC Infect Dis 10: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hermans SM, Kiragga AN, Schaefer P, Kambugu A, Hoepelman AI, et al. (2010) Incident tuberculosis during antiretroviral therapy contributes to suboptimal immune reconstitution in a large urban HIV clinic in sub-Saharan Africa. PLoS One 5: e10527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hanrahan CF, Golub JE, Mohapi L, Tshabangu N, Modisenyane T, et al. (2010) Body mass index and risk of tuberculosis and death. AIDS 24: 1501–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dembele M, Saleri N, Carvalho AC, Saouadogo T, Hien AD, et al. (2010) Incidence of tuberculosis after HAART initiation in a cohort of HIV-positive patients in Burkina Faso. Int J Tuberc Lung Dis 14: 318–323. [PubMed] [Google Scholar]
- 26. Abgrall S, Del Giudice P, Melica G, Costagliola D (2010) HIV-associated tuberculosis and immigration in a high-income country: incidence trends and risk factors in recent years. AIDS 24: 763–771. [DOI] [PubMed] [Google Scholar]
- 27. Zhou J, Elliott J, Li PC, Lim PL, Kiertiburanakul S, et al. (2009) Risk and prognostic significance of tuberculosis in patients from The TREAT Asia HIV Observational Database. BMC Infect Dis 9: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lawn SD, Myer L, Edwards D, Bekker LG, Wood R (2009) Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. AIDS 23: 1717–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grant AD, Bansi L, Ainsworth J, Anderson J, Delpech V, et al. (2009) Tuberculosis among people with HIV infection in the United Kingdom: opportunities for prevention? AIDS 23: 2507–2515. [DOI] [PubMed] [Google Scholar]
- 30. Golub JE, Pronyk P, Mohapi L, Thsabangu N, Moshabela M, et al. (2009) Isoniazid preventive therapy, HAART and tuberculosis risk in HIV-infected adults in South Africa: a prospective cohort. AIDS 23: 631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Etard JF, Diouf A, De Beaudrap P, Akoi K, Ngom-Gueye NF, et al. (2009) Short and Long-Term Incidence of Tuberculosis and CD4-Cell Count Dynamic on HAART in Senegal. Open AIDS J 3: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moreno S, Jarrin I, Iribarren JA, Perez-Elias MJ, Viciana P, et al. (2008) Incidence and risk factors for tuberculosis in HIV-positive subjects by HAART status. Int J Tuberc Lung Dis 12: 1393–1400. [PubMed] [Google Scholar]
- 33. Lannoy LH, Cortez-Escalante JJ, Evangelista Mdo S, Romero GA (2008) Tuberculosis incidence and risk factors among patients living with HIV/AIDS in public health service institutions in Brasilia, Federal District. Rev Soc Bras Med Trop 41: 549–555. [DOI] [PubMed] [Google Scholar]
- 34. Golub JE, Durovni B, King BS, Cavalacante SC, Pacheco AG, et al. (2008) Recurrent tuberculosis in HIV-infected patients in Rio de Janeiro, Brazil. AIDS 22: 2527–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moore D, Liechty C, Ekwaru P, Were W, Mwima G, et al. (2007) Prevalence, incidence and mortality associated with tuberculosis in HIV-infected patients initiating antiretroviral therapy in rural Uganda. AIDS 21: 713–719. [DOI] [PubMed] [Google Scholar]
- 36. Moh R, Danel C, Messou E, Ouassa T, Gabillard D, et al. (2007) Incidence and determinants of mortality and morbidity following early antiretroviral therapy initiation in HIV-infected adults in West Africa. AIDS 21: 2483–2491. [DOI] [PubMed] [Google Scholar]
- 37. Miranda A, Morgan M, Jamal L, Laserson K, Barreira D, et al. (2007) Impact of antiretroviral therapy on the incidence of tuberculosis: the Brazilian experience, 1995–2001. PLoS One 2: e826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Golub JE, Saraceni V, Cavalcante SC, Pacheco AG, Moulton LH, et al. (2007) The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS 21: 1441–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lawn SD, Myer L, Bekker LG, Wood R (2006) Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS 20: 1605–1612. [DOI] [PubMed] [Google Scholar]
- 40. Jerene D, Naess A, Lindtjorn B (2006) Antiretroviral therapy at a district hospital in Ethiopia prevents death and tuberculosis in a cohort of HIV patients. AIDS Res Ther 3: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bonnet MM, Pinoges LL, Varaine FF, Oberhauser BB, O'Brien DD, et al. (2006) Tuberculosis after HAART initiation in HIV-positive patients from five countries with a high tuberculosis burden. AIDS 20: 1275–1279. [DOI] [PubMed] [Google Scholar]
- 42. Seyler C, Toure S, Messou E, Bonard D, Gabillard D, et al. (2005) Risk factors for active tuberculosis after antiretroviral treatment initiation in Abidjan. Am J Respir Crit Care Med 172: 123–127. [DOI] [PubMed] [Google Scholar]
- 43. Lawn SD, Badri M, Wood R (2005) Tuberculosis among HIV-infected patients receiving HAART: long term incidence and risk factors in a South African cohort. AIDS 19: 2109–2116. [DOI] [PubMed] [Google Scholar]
- 44. Girardi E, Sabin CA, d'Arminio Monforte A, Hogg B, Phillips AN, et al. (2005) Incidence of Tuberculosis among HIV-infected patients receiving highly active antiretroviral therapy in Europe and North America. Clin Infect Dis 41: 1772–1782. [DOI] [PubMed] [Google Scholar]
- 45. Seyler C, Anglaret X, Dakoury-Dogbo N, Messou E, Toure S, et al. (2003) Medium-term survival, morbidity and immunovirological evolution in HIV-infected adults receiving antiretroviral therapy, Abidjan, Cote d'Ivoire. Antivir Ther 8: 385–393. [PubMed] [Google Scholar]
- 46. Hung CC, Hsiao CF (2003) Recurrence of tuberculosis in HIV-1-infected adults treated after rifamycin-based treatment and highly active antiretroviral therapy. J Acquir Immune Defic Syndr 34: 437–439. [DOI] [PubMed] [Google Scholar]
- 47. Badri M, Wilson D, Wood R (2002) Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet 359: 2059–2064. [DOI] [PubMed] [Google Scholar]
- 48. Jones JL, Hanson DL, Dworkin MS, DeCock KM (2000) HIV-associated tuberculosis in the era of highly active antiretroviral therapy. The Adult/Adolescent Spectrum of HIV Disease Group. Int J Tuberc Lung Dis 4: 1026–1031. [PubMed] [Google Scholar]
- 49. Brinkhof MW, Egger M, Boulle A, May M, Hosseinipour M, et al. (2007) Tuberculosis after initiation of antiretroviral therapy in low-income and high-income countries. Clin Infect Dis 45: 1518–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rajasekaran S, Raja K, Jeyaseelan L, Vijila S, Priya K, et al. (2009) Post-HAART tuberculosis in adults and adolescents with HIV in India: Incidence, clinical and immunological profile. Indian Journal of Tuberculosis 69–76. [PubMed]
- 51. Dye C, Lonnroth K, Jaramillo E, Williams BG, Raviglione M (2009) Trends in tuberculosis incidence and their determinants in 134 countries. Bull World Health Organ 87: 683–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lawn SD, Wood R (2011) Tuberculosis in antiretroviral treatment services in resource-limited settings: addressing the challenges of screening and diagnosis. J Infect Dis 204 Suppl 4: S1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Laureillard D, Marcy O, Madec Y, Chea S, Chan S, et al. (2013) Paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome after early initiation of antiretroviral therapy in a randomized clinical trial. AIDS 27: 2577–2586. [DOI] [PubMed] [Google Scholar]
- 54. Manabe YC, Breen R, Perti T, Girardi E, Sterling TR (2009) Unmasked tuberculosis and tuberculosis immune reconstitution inflammatory disease: a disease spectrum after initiation of antiretroviral therapy. J Infect Dis 199: 437–444. [DOI] [PubMed] [Google Scholar]
- 55. Middelkoop K, Bekker LG, Shashkina E, Kreiswirth B, Wood R (2012) Retreatment tuberculosis in a South African community: the role of re-infection, HIV and antiretroviral treatment. Int J Tuberc Lung Dis 16: 1510–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of searches.
(DOCX)
Protocol outline.
(DOCX)
Data abstraction form.
(DOCX)
Modified Ottawa Scale.
(DOC)
List of studies included.
(XLS)
PRISMA checklist.
(DOC)