Abstract
Background
The control of neglected tropical diseases (NTDs) has primarily focused on preventive chemotherapy and case management. Less attention has been placed on the role of ensuring access to adequate water, sanitation, and hygiene and personal preventive measures in reducing exposure to infection. Our aim was to assess whether footwear use was associated with a lower risk of selected NTDs.
Methodology
We conducted a systematic review and meta-analysis to assess the association between footwear use and infection or disease for those NTDs for which the route of transmission or occurrence may be through the feet. We included Buruli ulcer, cutaneous larva migrans (CLM), leptospirosis, mycetoma, myiasis, podoconiosis, snakebite, tungiasis, and soil-transmitted helminth (STH) infections, particularly hookworm infection and strongyloidiasis. We searched Medline, Embase, Cochrane, Web of Science, CINAHL Plus, and Popline databases, contacted experts, and hand-searched reference lists for eligible studies. The search was conducted in English without language, publication status, or date restrictions up to January 2014. Studies were eligible for inclusion if they reported a measure of the association between footwear use and the risk of each NTD. Publication bias was assessed using funnel plots. Descriptive study characteristics and methodological quality of the included studies were summarized. For each study outcome, both outcome and exposure data were abstracted and crude and adjusted effect estimates presented. Individual and summary odds ratio (OR) estimates and corresponding 95% confidence intervals (CIs) were calculated as a measure of intervention effect, using random effects meta-analyses.
Principal Findings
Among the 427 studies screened, 53 met our inclusion criteria. Footwear use was significantly associated with a lower odds of infection of Buruli ulcer (OR = 0.15; 95% CI: 0.08–0.29), CLM (OR = 0.24; 95% CI: 0.06–0.96), tungiasis (OR = 0.42; 95% CI: 0.26–0.70), hookworm infection (OR = 0.48; 95% CI: 0.37–0.61), any STH infection (OR = 0.57; 95% CI: 0.39–0.84), strongyloidiasis (OR = 0.56; 95% CI: 0.38–0.83), and leptospirosis (OR = 0.59; 95% CI: 0.37–0.94). No significant association between footwear use and podoconiosis (OR = 0.63; 95% CI: 0.38–1.05) was found and no data were available for mycetoma, myiasis, and snakebite. The main limitations were evidence of heterogeneity and poor study quality inherent to the observational studies included.
Conclusions/Significance
Our results show that footwear use was associated with a lower odds of several different NTDs. Access to footwear should be prioritized alongside existing NTD interventions to ensure a lasting reduction of multiple NTDs and to accelerate their control and elimination.
Protocol Registration
PROSPERO International prospective register of systematic reviews CRD42012003338
Author Summary
Consistent use of footwear may help in preventing or slowing down the progression of many neglected tropical diseases (NTDs). We conducted a systematic review and meta-analysis to assess the association between footwear use and infection or disease for those NTDs for which the route of transmission or occurrence may be through the feet. We found that footwear use reduces the risk of Buruli ulcer, tungiasis, hookworm, any STH infection, strongyloidiasis, and leptospirosis. No significant association between footwear use and podoconiosis was found and no data were available for mycetoma, myiasis or snakebite. We recommend that access to footwear should be prioritized alongside existing NTD interventions to ensure a lasting reduction of multiple NTDs and to accelerate their control and elimination.
Introduction
Neglected tropical diseases (NTDs) are caused by a variety of pathogens, such as parasites (e.g., ectoparasites, helminths, and protozoa), fungi, bacteria, and viruses, primarily found in the tropical and subtropical regions of the world [1]. NTDs mainly occur in rural and deprived urban areas of low- and middle-income countries, where they may exacerbate poverty by contributing to significant morbidity and mortality, impairing development, and limiting productivity [1], [2]. They have multiple routes of transmission and a single intervention alone is unlikely to have major sustained impact. Population-based chemotherapy is currently the mainstay of the control of various NTDs caused by helminths (e.g., lymphatic filariasis, schistosomiasis, and soil-transmitted helminth (STH) infections) and some bacterial infections (e.g., trachoma) [3], [4]. More recently, attention has been given to water, sanitation, and hygiene (WASH) as an effective and sustainable measure for NTD control [5]–[7]. WASH interventions such as face washing to prevent trachoma, or hand washing to prevent diarrheal diseases and STH infection have been well-studied [8]–[10]. However, less attention has focused on other personal preventive measures to reduce exposure to infection, such as the use of footwear. Some NTDs may be transmitted or occur through the feet, and hence, footwear could prevent this exposure. To our knowledge, there has not yet been a systematic review of the evidence to assess the role of footwear use among these NTDs [8]–[12].
There is continued debate over the role of footwear use as an additional measure of NTD control [13]–[15]. Some studies have highlighted that footwear use could reduce infection with hookworm caused by Necatoramericanus and/or Ancylostoma duodenale (which is also orally infective), but such studies are often cross-sectional and should be interpreted with caution [15]–[17]. Other experts argue that decreases in the burden of hookworm disease are based on large-scale administration of deworming drugs (a strategy termed preventive chemotherapy), socioeconomic development, and improved access to WASH rather than widespread footwear use, while newer evidence indicates that the burden from hookworm disease has not changed significantly over the past 20 years [13], [18]. Furthermore, the lack of adequate change in hookworm disease burden might be due to the overwhelming focus on preventive chemotherapy over the last few decades and less emphasis on other interventions [5]. In the case of podoconiosis (non-filarial elephantiasis), footwear use is currently promoted as a prevention tool, since current evidence suggests that it is caused by barefoot exposure to red clay soil from volcanic rocks [19]. Other studies and anecdotal evidence have additionally suggested that footwear use may prevent Buruli ulcer, cutaneous larva migrans (CLM), leptospirosis, mycetoma (fungal eumycetoma and bacterial actinomycetoma), myiasis, snakebite, strongyloidiasis, and tungiasis [20], [21].
Here, we first identified those NTDs for which the use of footwear might have a potential impact on the risk of infection and disease, based on an understanding of disease etiology and transmission. We next conducted a systematic review and series of meta-analyses of the association between footwear use and the risk of a range of NTDs.
Methods
NTDs were selected to be included in the study based on disease etiology and potential for infection through the feet and thus prevention using footwear (Table 1). A systematic literature review protocol strategy was developed based on the ‘Preferred Reporting Items for Systematic reviews and Meta-Analyses' (PRISMA) checklist (e.g., protocol and registration, eligibility criteria, information sources, searching, study selection, data collection process, data items, risk of bias in individual studies, summary measures, synthesis of results, risk of bias across studies, and additional analyses (see: Checklist S1). This protocol is available at the National Institute for Health Research PROSPERO International prospective register of systematic reviews (identifier: CRD42012003338) (see Protocol S1).
Table 1. Overview of included neglected tropical diseases (NTDs) in the current systematic review and meta-analysis.
| # | Disease | Aetiology | Search Terms+ |
| 1 | Buruli ulcer | Mycobacterium ulcerans: precise transmission unknown but may be associated with insect bites to exposed skin such as feet | exp Buruli Ulcer OR exp, Mycobacterium ulcerans OR exp, mycobacterium infections, nontuberculous OR buruli ulcer* OR mycobacterium ulceran* OR Bairnsdale ulcer OR Daintree ulcer |
| 2 | Podoconiosis | Geochemical non-filarial elephantiasis, Transmission associated with long term barefoot exposure to red clay soil | Podoconiosis OR non-filarial elephantiasis OR mossy foot |
| 3 | Any soil-transmitted helminth (STH) infection, including hookworm | Ascaris lumbricoides: Trichuris trichiura and hookworm, intestinal parasites which produce eggs passed in feces, transmission by ingestion from contaminated hands or utensils or penetration of skin by larvae (i.e., if feet are exposed to contaminated soil) | Soil-transmitted helminth* OR soil transmitted helminth* OR intestinal worm* OR exphelminth OR expHelminthiasis |
| 4 | Hookworm infection | Necatoramericanus and Ancylostoma duodenale, transmission by penetration of skin by larvae (i.e., if feet are exposed to contaminated soil) | Exp hookworm infections OR expancylostomatoidea OR expancylostoma OR necator |
| 5 | Strongyloidiasis | Strongyloides stercoralis: type of STH, which produces eggs that hatch into larvae passed in feces and transmission by penetration of skin by larvae(i.e., if feet are exposed to contaminated soil) | Strongyloid* OR exp strongyloides stercoralis OR exp Stronyloidiasis OR exp strongyloides OR round?worm |
| 6 | Cutaneous larva migrans | Ancylostoma braziliense, A. ceylanicum and other zoonotic hookworms: zoonotic intestinal parasite living in cats and dogs, which produce eggs passed in their feces, transmission by penetration of skin by larvae (i.e., if feet are exposed to contaminated soil) | Exp larva migrans OR cutaneous larva migran* OR creeping eruption OR ground itch OR sandworm* OR plumber's itch OR zoonotic hookworm OR ancylostoma braziliense OR uncinaria stenocephala OR ancylostoma caninum OR exp ancylostoma |
| 7 | Leptospirosis | Leptospira interrogans: bacteria passed in urine, transmission by direct contact through the mucous membranes of the mouth, nose, and eyes, or through cuts and abrasions on the skin (i.e., if feet are exposed to contaminated soil) | Exp leptospirosis OR weil's syndrome OR weil disease OR canicola fever OR canefield fever OR nanukayami fever OR 7-day fever OR Rat Catcher's Yellows OR Fort Bragg Fever OR black jaundice OR Pretibial fever OR Leptospira OR Icterohemorrhagic fever OR Swineherd's disease OR Rice-field fever OR Cane-cutter fever OR Swamp fever OR Mud fever OR Hemorrhagic jaundice OR Stuttgart disease |
| 8 | Tungiasis | Tunga penetrans: ectoparasite on the sand flea, transmission by penetration of skin by sand fleas (i.e., if feet are exposed to contaminated sand) | Exptunga OR Tungapenetrans OR jigger* OR sandflea OR expTungiasis OR Pico OR chigoe flea OR suthi |
| 9 | Myiasis | Dermatobiahominis, Cordylobiaanthropophaga and others: parasite transmitted on a fly larva (and potentially through blood-sucking vectors such as mosquitos), transmission by penetration of skin by larvae(i.e., if feet are exposed to contaminated soil) | Exp myiasis OR dermatobiahominis OR chrysomabezziana OR cordylobiaanthropophaga OR flystrike OR blowfly strike OR fly-blown |
| 10 | Snakebite | Venomous snakes: envenoming, transmission associated with snake bites to exposed skin (i.e., on feet) | Exp snake bite OR exp antivenins OR snakebite* OR exp venoms OR envenoming OR snake poison |
| 11 | Mycetoma | Eumycetoma: Madurellamycetomatis, Pseudallescheriaboydii (and other fungi), Actinomycetoma: Nocardia spp., Streptomyces spp., Actinomadura spp. (and other aerobic actinomycetes), certain fungi or bacteria, transmission probably by entering the body into the subcutaneous tissue through minor trauma, often through the foot | Madura Foot OR expmycetoma OR eumycetoma* OR mycetomapedis OR actinomycetoma* |
+Additional search terms for intervention (exp shoes OR shoe* OR footwear* OR boots OR sandals OR footgear OR exp primary prevention) and all NTDs (exp neglected diseases OR neglected tropical disease* OR NTD* OR exp tropical disease).
A total of 92 known medical and colloquial disease names (see Table 1) were included in a comprehensive list of key search terms. Six terms related to footwear were also included: shoe, footwear, boot, sandal, footgear, or primary prevention. Relevant databases were searched from using these terms, including Medline (coverage from 1950), Embase (coverage from 1947), Cochrane (coverage from 2003), Web of Science (coverage from 1900), CINAHL Plus (coverage from 1937), Popline (coverage from 1970), British Library for Development Studies (coverage from 1987), ELDIS (coverage date unavailable), EPPI-Centre (coverage from 2004), WHO Library (coverage from 1948), and PAHO Library Catalogue (coverage from 1902). The search was conducted from January 1, 2013 to December 31, 2014. Experts in selected NTD areas were contacted for further citation recommendations relevant to the research question. The Brighton and Sussex Medical School (BSMS) Library was consulted for assistance with article retrieval through online databases or manual journal searching. The reference lists of all identified manuscripts were also reviewed for additional citations. Manuscripts in foreign languages (namely, French, Spanish, and Russian) were translated by investigators. No other foreign language articles were identified through this search. When potentially eligible studies did not provide sufficient data in the manuscript, authors were contacted and asked if they would be willing to provide additional data. To this end, additional data were received from authors of five studies [22]–[26].
Pre-defined eligibility criteria included: (i) all intervention and observational study designs; (ii) all study settings; (iii) all ages; (iv) all types of footwear exposures; (v) prevalence or incidence estimates of infection and/or disease outcomes; (vi) all published manuscripts and grey literature; (vii) all publication dates; and (viii) all languages. Observational studies were included because it was hypothesized that few randomized controlled trials (RCTs) had been conducted to answer the research question. Abstracts of identified studies were reviewed before appraisal of full manuscripts when possible. If a study did not explicitly investigate the association between footwear use and any of the target NTDs or did not meet the eligibility criteria, it was excluded. Decisions on inclusion were reached by the consensus of independent screenings conducted by two investigators (ST and KD).
A standardized Excel data extraction form was developed based on the PRISMA statement [27] and used to record the following information: study ID, author, title, journal, publication year, type of literature, research question, study design, study setting, outcome, follow-up, sample size, number of cases, descriptive case data (e.g., age, sex, and proportion wearing footwear), descriptive control data (e.g., age, sex, and proportion wearing footwear), crude and adjusted effect estimates of footwear use on disease, including 95% confidence intervals (CIs) and p-values, and study quality ratings. The methodological quality of studies was assessed by the same investigators. According to a pre-defined scale, the following question was assessed, stating “Were the following items reported?”: (i) study population (e.g., social-ecological characteristics); (ii) selection of participants (i.e., random or convenience); (iii) sample size calculation; (iv) method of measuring footwear use and presence of NTDs; and (v) estimates adjusted for confounding. These questions were scored on a yes/no basis and proportions answering yes to each question were described to assess study quality and risk of bias in and across individual studies. None of the investigators on this review assessed the study quality of their own primary studies.
All primary data and quality ratings were extracted from identified manuscripts. STATA version 12.0 (College Station, TX, United States of America) was used to summarize the data descriptively. RevMan version 5.2 and its generic variance format was used to generate individual forest plots according to primary NTD outcomes [28].We entered odds ratio (OR) estimates of footwear use on a logarithmic scale and standard errors (calculated from 95% CIs). An adjusted OR was used if provided in the manuscript. A few studies only provided raw outcome and exposure data, so we calculated a crude OR in these cases. All calculations and data used are detailed in the footnotes of each figure. A random-effects model in RevMan was then utilized to produce individual study ORs and 95% CIs and to consider a pooled summary effect estimate (using random effects to address potential heterogeneity). Heterogeneity was assessed by the I 2 test with values greater than 50% representing moderate-to-severe heterogeneity.
Results
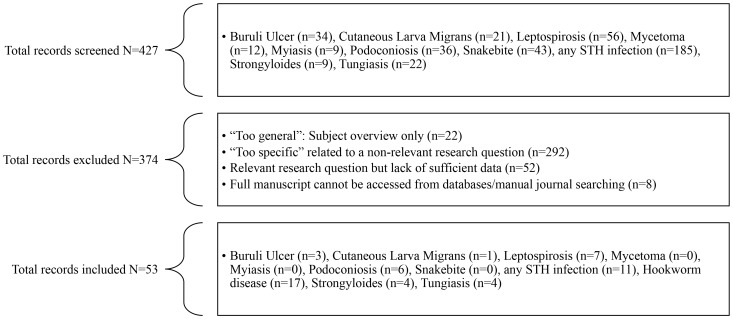
The electronic searches generated 427 citations and abstracts. These were screened and 374 were excluded for a range of reasons (Figure 1). We included 53 sources: Buruli ulcer (n = 3), CLM (n = 1), leptospirosis (n = 7), podoconiosis (n = 6), any STH infections (n = 11), hookworm infection (n = 17), strongyloidiasis (n = 4), and tungiasis (n = 4). No data were found to quantify the association between footwear use and mycetoma, myiasis, and snakebite. Type of source included 50 journal manuscripts (94.3%), two unpublished pieces of work (3.8%), and one book excerpt (1.9%). Information describing the studies included are summarized in Table 2, including study design, publication year, country and outcome. We identified a total of 40 cross-sectional studies (75.4%), eight case-control studies (15.1%), three cohort studies (5.7%), and two RCTs (3.8%). The median publication year was 2003 (range: 1950–2014). Geographically, 29 studies were conducted in Africa (54.7%), 12 in Asia (22.6%), 11 in the Americas (20.8%), and one in Europe (1.9%). The median sample size was 366 individuals (range: 59–129,959). Among the 11 studies with known follow-up periods, the median follow-up time was 12 months (range: 2.5 months to 7 years). Descriptive results by individual studies including sample size, median age, and proportion of females are shown in Table 2. A summary of descriptive results by outcome, including study quality results, are provided in Table 3.
Figure 1. Flow diagram of eligibility and inclusion/exclusion procedures.
Table 2. Included studies: Time, place, design of study, and descriptive results in the current systematic review and meta-analysis.
| With disease | Without disease | ||||||||||
| ID | Author | Pub. Year | Study design | Country | Outcome | N | Age median | Female n (%) | N | Age median | Female n (%) |
| 1 | Landier et al. [44] | 2011 | Case-control | Cameroon | Buruli Ulcer | 77 | 14.0 | 37 (48) | 153 | 13.0 | 74 (48) |
| 2 | Marston et al. [84] | 1995 | Case-control | Côte d'Ivoire | Buruli Ulcer | 46 | 16.0* | 26 (57) | 90 | 21.4* | 45 (50) |
| 3 | Raghunathan et al. [45] | 2005 | Case-control | Ghana | Buruli Ulcer | 116 | 12.0 | 62 (53) | 116 | 11.5 | 57 (49) |
| 4 | Trembley et al. [20] | 2000 | Cross-sec. | Barbados | CLM | 32 | 36.9* | + | 94 | 41.2* | + |
| 5 | Bovet et al. [51] | 1999 | Case-control | Seychelles | Leptospirosis | 125 | 39.0* | 20 (16) | 125 | 39.5* | 20 (16) |
| 6 | Douglin et al. [85] | 1997 | Case-control | Barbados | Leptospirosis | 22 | 30.8* | 8 (36) | 38 | 31.3* | 23 (61) |
| 7 | Johnson et al. [46] | 2004 | Cross-sec. | Peru | Leptospirosis | 182 | 29.0 | 105 (58) | 466 | 29.0 | 251 (54) |
| 8 | Lacerda et al. [52] | 2008 | Cross-sec. | Brazil | Leptospirosis | 44 | 26.0* | 19 (43) | 246 | 28.7* | 120 (49) |
| 9 | Leal-Castellanos et al. [47] | 2003 | Cross-sec. | Mexico | Leptospirosis | 441 | 40.8* | 341 (77) | 728 | 40.2* | 605 (83) |
| 10 | Phraisuwan et al. [48] | 2002 | Case-control | Thailand | Leptospirosis | 43 | 35.0 | 16 (37) | 61 | 40.0 | 33 (54) |
| 11 | Sulong et al. [49] | 2011 | Cross-sec. | Malaysia | Leptospirosis | 73 | 41.7* | 0 (0) | 223 | 42.2* | 0 (0) |
| 12 | Sanchez et al. [55] | 2001 | Cohort | Spain | Strongyloidiasis | 20 | 68.8* | 1 (5) | 132 | 66.0* | 31 (23) |
| 13 | Steinmann et al. [54] | 2007 | Cross-sec. | China | Strongyloidiasis | 21 | 29.0* | 6 (29) | 159 | 25.5* | 92 (58) |
| 14 | Knopp et al. [22] | 2010 | Cross-sec. | Tanzania | Strongyloidiasis | 49 | 22.0 | 22 (45) | 375 | 22.0 | 252 (67) |
| 15 | Yori et al. [53] | 2006 | Cross-sec. | Peru | Strongyloidiasis | 69 | 23.0* | + | 423 | 23.0* | + |
| 16 | Aimpun et al. [59] | 2004 | Cross-sec. | Belize | Any STH infection | 418 | 19.7* | 177 (42) | 135 | 14.1* | 119 (88) |
| 17 | Lello et al. [60] | 2013 | Cross-sec. | Zanzibar | Any STH infection | 132 | + | + | 198 | + | + |
| 18 | Ali et al. [78] | 1999 | Cross-sec. | Ethiopia | Any STH infection | 243 | + | 112 (46) | 39 | + | 9 (23) |
| 19 | Gunawardena et al. [23] | 2011 | Cross-sec. | Sri Lanka | Any STH infection | 549 | 11.1 | 238 (43) | 1341 | 11.2 | 639 (48) |
| 20 | Gamboa et al. [24] | 2009 | Cross-sec. | Argentina | Any STH infection | 152 | + | + | 42 | + | + |
| 21 | Kurup et al. [58] | 2010 | Cohort | Saint Lucia | Any STH infection | 253 | + | 193 (76) | 301 | + | 120 (40) |
| 22 | Khan et al. [63] | 1979 | Cross-sec. | India | Any STH infection | 27 | 11.9* | + | 32 | 39.0 | + |
| 23 | Modjarrad et al. [25] | 2005 | Cross-sec. | Zambia | Any STH infection | 78 | 29.0 | 50 (64) | 228 | 31.0 | 159 (70) |
| 24 | Mihrshahi et al. [57] | 2009 | Cross-sec. | Vietnam | Any STH infection | 70 | 29.7* | 70 (100) | 296 | 29.7* | 296 (100) |
| 25 | Martinez et al. [61] | 1961 | Cross-sec. | Cuba | Any STH infection | 934 | 4.0* | + | 66 | 4.0* | + |
| 26 | Liabsuetrakul et al. [62] | 2009 | Cross-sec. | Thailand | Any STH infection | 190 | 27.3* | 190 (100) | 873 | 27.3* | 873 (100) |
| 27 | Phiri et al. [56] | 2000 | Cross-sec. | Malawi | Any STH infection | 43 | 7.2* | + | 230 | 7.2* | |
| 28 | Woodburn et al. [64] | 2009 | RCT | Uganda | Hookworm infection | 1112 | 23.0 | 1112 (100) | 1386 | 23.0 | 1386 (100) |
| 29 | Traub et al. [65] | 2004 | Cross-sec. | India | Hookworm infection | 141 | + | + | 187 | + | + |
| 30 | Tadesse et al. [75] | 2005 | Cross-sec. | Ethiopia | Hookworm infection | 28 | 11.2* | 9 (32) | 387 | 11.2* | 135 (35) |
| 31 | Pullan et al. [66] | 2010 | Cross-sec. | Uganda | Hookworm infection | 709 | + | + | 1094 | + | + |
| 32 | Nmor et al. [67] | 2009 | Cross-sec. | Nigeria | Hookworm infection | 534 | 8.4* | 184 (34) | 71 | 8.8* | 278 (63) |
| 33 | Lee et al. [68] | 2007 | Case-control | Brunei | Hookworm infection | 18 | + | + | 100 | + | + |
| 34 | Jiraanankul et al. [72] | 2011 | Cohort | Thailand | Hookworm infection | 33 | + | 19 (58) | 319 | + | 191 (60) |
| 35 | Ilechukwu et al. [76] | 2010 | Cross-sec. | Nigeria | Hookworm infection | 150 | + | + | 310 | + | + |
| 36 | Humphries et al. [73] | 2011 | Cross-sec. | Ghana | Hookworm infection | 116 | + | 58 (50) | 142 | + | 75 (53) |
| 37 | Gutman et al. [69] | 2010 | Cross-sec. | Nigeria | Hookworm infection | 223 | 12.0* | + | 314 | 12.0* | + |
| 38 | Erosie et al. [74] | 2002 | Cross-sec. | Ethiopia | Hookworm infection | 113 | 10.9* | 28 (25) | 308 | 10.9 | 102 (33) |
| 39 | Behnke et al. [71] | 2000 | Cross-sec. | Mali | Hookworm infection | 151 | + | + | 134 | + | + |
| 40 | Alemu et al. [70] | 2011 | Cross-sec. | Ethiopia | Hookworm infection | 61 | 11.0 | 30 (49) | 258 | 11.0 | 132 (51) |
| 41 | Mukerji et al. [77] | 1950 | Cross-sec. | India | Hookworm infection | 2166 | 27.0* | 595 (27) | 3125 | 24.9* | 681 (22) |
| 42 | Chongsuvivatwong et al. [16] | 1996 | Cross-sec. | Thailand | Hookworm infection | 100 | 30.5* | + | 92 | 30.5* | + |
| 43 | Bethony et al. [14] | 2002 | Cross-sec. | China | Hookworm infection | 285 | 31.3* | + | 224 | 31.3* | + |
| 44 | Davey et al. [26] | 2006 | Cross-sec. | Ethiopia | Podoconiosis | 248 | 38.0 | 122 (49) | 1152 | 38.0 | 478 (41) |
| 45 | Price et al. [82] | 1974 | Cross-sec. | Ethiopia | Podoconiosis | 15977 | + | 6781 (42) | 27; 596 | + | 6240 (23) |
| 46 | Kloos et al. [35] | 1992 | Cross-sec. | Ethiopia | Podoconiosis | 31 | + | + | 385 | + | + |
| 47 | Molla et al. [38] | 2013 | Case-control | Ethiopia | Podoconiosis | 460 | 51.5* | 243 (53) | 707 | 4.4* | 270 (38) |
| 48 | Yakob et al. [83] | 2008 | Cross-sec. | Ethiopia | Podoconiosis | 73 | + | + | + | 365 | + |
| 49 | Deribe et al. [81] | 2013 | Cross-sec. | Ethiopia | Podoconiosis | 5253 | 45 | 3,045 (58) | 124; 706 | 33 | 62,056 (50) |
| 50 | Muehlen et al. [30] | 2006 | Cross-sec. | Brazil | Tungiasis | 253 | 16.9* | 137 (54) | 243 | 19.2* | 147 (60) |
| 51 | Njau et al. [80] | 2012 | Cross-sec. | Kenya | Tungiasis | 218 | + | + | 167 | + | + |
| 52 | Ugbomoiko et al. [79] | 2007 | Cross-sec. | Nigeria | Tungiasis | 252 | 18.4* | 111 (44) | 305 | 21.1* | 147 (48) |
| 53 | Thielecke et al. [34] | 2013 | RCT | Madagascar | Tungiasis | 77 | 26.7 | 56 (73) | 70 | 25.5 | 61 (87) |
*Age mean reported.
+Missing raw data.
Abrevation: CLM: Cutaneous larva migrans, Cross-sec.: cross-sectional study; RCT: randomized controlled trial.
Table 3. A summary of descriptive information ofand study quality by outcome.
| Buruli Ulcer n = 3 | CLM n = 1 | Leptospirosis n = 7 | Strongyloidiasis n = 4 | Any STH infection n = 11 | Hookworm infection n = 17 | Podoconiosis n = 6 | Tungiasis n = 4 | ||
| Descriptive information | |||||||||
| Median sample size (range) | 230 (136–232) | 126 | 276 (60–1169) | 30 (152–492) | 330 (59–1890) | 1324 (118–5291) | 1284 (416–129959) | 441 (147–557) | |
| Median % of cases | 33 | 17 | 36 | 12 | 46 | 10 | 28 | 53 | |
| Median age | Cases | 12 | 37 | 32 | 22 | 15 | 17 | 38 | 27 |
| Without disease | 13 | 41 | 35 | 22 | 31 | 11 | 38 | 26 | |
| Median % of females | Cases | 50 | + | 40 | 30 | 70 | 30 | 50 | 50 |
| Without disease | 50 | + | 50 | 60 | 80 | 50 | 40 | 60 | |
| Study quality | |||||||||
| Number with cross-sectional survey design (%) | 0 (0) | 1 (100) | 4 (57) | 3 (75) | 10 (91) | 14 (82) | 5 (83) | 3 (75) | |
| Footwear measured | By self-report | 3 (100) | 1 (100) | 7 (100) | 4 (100) | 10 (91) | 14 (82) | 5 (83) | 3 (75) |
| By observation | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (9) | 3 (18) | 1 (17) | 1 (25) | |
| Were the following items reported? (%) | Study population | 3 (100) | 1 (100) | 7 (100) | 4 (100) | 11 (100) | 17 (100) | 6 (100) | 4 (100) |
| Selection of participants | 2 (67) | 1 (100) | 5 (71) | 3 (75) | 5 (45) | 14 (82) | 4 (67) | 4 (100) | |
| Sample size/power calculation | 1 (33) | 1 (100) | 2 (29) | 2 (50) | 4 (36) | 4 (24) | 2 (33) | 3 (75) | |
| Outcome and exposure measurement | 3 (100) | 1 (100) | 7 (100) | 4 (100) | 9 (82) | 15 (88) | 5 (83) | 3 (75) | |
| Adjusted estimates for confounding | 3 (100) | 0 (0) | 5 (71) | 3 (75) | 4 (36) | 11 (65) | 3 (50) | 3 (75) | |
+Missing data.
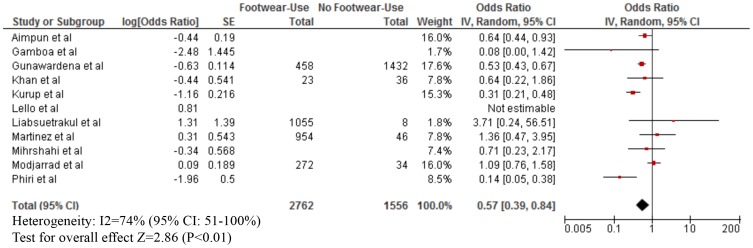
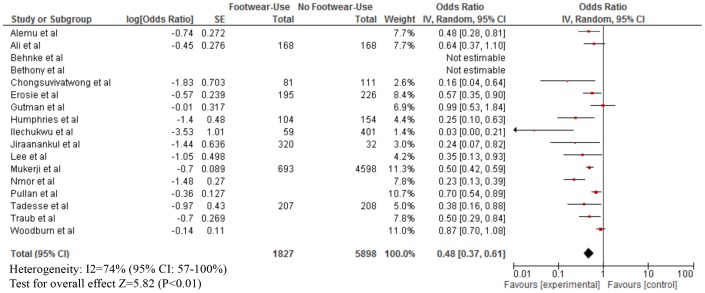
As shown in Table 3, footwear use was mostly measured by self-report. The median proportion of footwear use was: Buruli ulcer (80% for both cases and those without infection), leptospirosis (cases: 40%; without infection: 50%), strongyloidiasis (cases: 25%; without infection: 40%), any STH infection (cases; 60%; without infection: 97%), hookworm infection (cases: 30%; without infection: 50%), podoconiosis (cases: 55%; without disease: 50%), and tungiasis (cases: 30%; without disease: 60%). Our meta-analyses showed that footwear use was significantly associated with a lower odds of Buruli ulcer (OR = 0.15; 95% CI: 0.08–0.29), CLM (OR = 0.24; 95% CI: 0.06–0.96), leptospirosis (OR = 0.59; 95% CI: 0.37–0.94), strongyloidiasis (OR = 0.56; 95% CI: 0.38–0.83), any STH infection (OR = 0.57; 95% CI: 0.39–0.84), hookworm infection (OR = 0.48; 95% CI: 0.37–0.61), and tungiasis (OR = 0.42; 95% CI: 0.26–0.70) (Figures 2–7). On the other hand, footwear use was not significantly associated with the occurrence of podoconiosis (OR: 0.63; 95% CI: 0.38–1.05), as seen in the forest plot of Figure 8. Estimates of I2 varied, including low heterogeneity: strongyloidiasis 0% (95% CI: 0–100%), Buruli ulcer 26% (95% CI: 0–100%); and moderate-to-high heterogeneity: tungiasis 63% (95% CI: 0–100%), leptospirosis 69% (95% CI: 33–100%), any STH infection 74% (95% CI: 51–100%), hookworm infection 74% (95% CI: 57–100%), and podoconiosis 96% (95% CI: 94–96%).
Figure 2. Forest plot of studies showing the association between footwear use and the risk of Buruli ulcer.*.
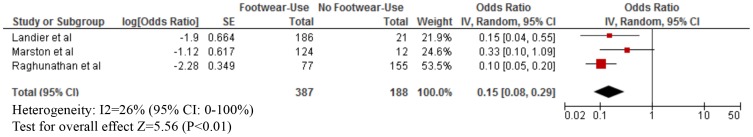
*Inverted Log [odds ratio] and standard error (SE) from effect estimate of barefoot exposure: Landier et al [44]. *Log [odds ratio] and SE calculated from raw data: Marston et al [84] and Raghunathan et al [45].
Figure 7. Forest plot of studies showing the association between footwear use and tungiasis.*.
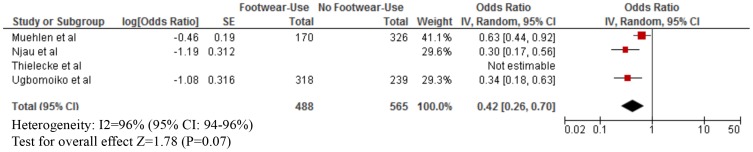
*Adjusted effect estimate: Ugbomoiko et al. [79]. *Stratified exposure totals were not given for the following studies: Njau et al. [80] (N = 385) and Thielecke et al. [34] (N = 147). *The magnitude of the odds ratio and the 95% confidence interval/standard error (SE) were not available so it was not included in the forest plot: Thielecke et al. [34] (marginal decrease in intensity of infection with footwear use).
Figure 8. Forest plot of studies showing the association between footwear use and podoconiosis.*.
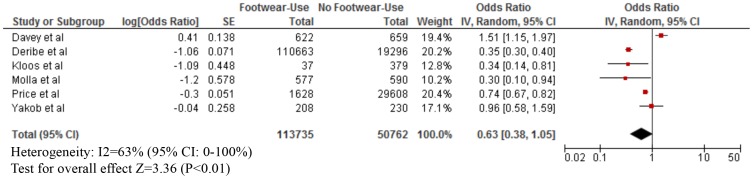
*Adjusted effect estimate: Molla et al. [38]. *Inverted Log [odds ratio] and standard error (SE) from effect estimate of barefoot exposure: Deribe et al. [81] and Molla et al. [38]. *Log [odds ratio] and SE calculated from raw data: Price et al. [82], Kloos et al. [35], and Yakob et al. [83].
Figure 3. Forest plot of studies showing the association between footwear use and leptospirosis.*.
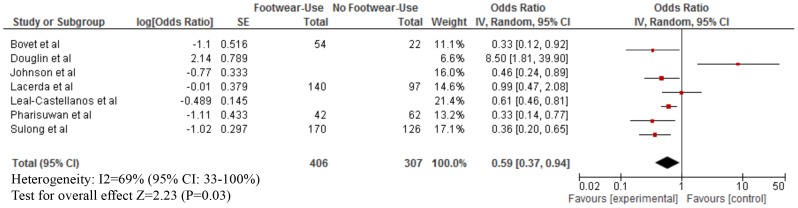
*Stratified exposure totals were not given for the following studies: Johnson et al. (N = 648) [46], Leal-Castellanos et al. (N = 1169) [47], Pharisuwan et al. (N = 104) [48], and Sulong et al. (N = 296) [49]. *A 95% confidence interval/standard error (SE) was not available so it was not included in the forest plot: Alarcon et al. (odds ratio: 0.54) [50] *Adjusted effect estimate: Johnson et al. [46] and Sulong et al. [49]. *Inverted Log [odds ratio] and SE from effect estimate of barefoot exposure: Bovet et al. [51], Johnson et al. [46], and Leal-Castellanos et al. [47]. *Log [odds ratio] and SE calculated from raw data: Lacerda et al. [52].
Figure 4. A forest plot of studies showing the association between footwear use and strongyloidiasis.*.
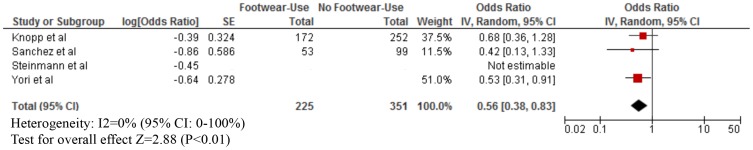
*Stratified exposure totals were not given for the following studies: Yori et al. (N = 492) [53] and Steinmann et al. (N = 180) [54]. * A 95% confidence interval/standard error (SE) was not available so it was not included in the forest plot: Steinmann et al. (odds ratio: 0.64) [54]. *Adjusted effect estimate: Yori et al. [53]. *Inverted Log [odds ratio] and SE from effect estimate of barefoot exposure: Yori et al. [53]. *Log [odds ratio] and SE calculated from raw data (comparing severe form of illness to chronic infection): Sanchez et al. [55].
Figure 5. Forest plot of studies showing the association between footwear use and any soil-transmitted helminth infection.+*.
+Note different x-axis. *Stratified exposure totals were not given for the following studies: Phiri et al. (N = 273) [56], Mihrshahi et al. (N = 366) [57], Kurup et al. (N = 554) [58], and Gamboa et al. (N = 194) [24], Aimpun et al. (N = 553) [59], and Lello et al. (N = 330) [60]. *A 95% confidence interval/standard error (SE) was not available so it was not included in the forest plot: Lello et al. (odds ratio: 0.81) [60]. *Adjusted effect estimate: Phiri et al. [56]and Mihrshahi et al. [57]. *Inverted Log [odds ratio] and SE from effect estimate of barefoot exposure: Phiri et al. [56], Modjarrad et al. [25], Mihrshahi et al. [57], Kurup et al. [58], Gunawardena et al. [23], Gamboa et al. [24]. *Log [odds ratio] and SE calculated from raw data: Martinez et al. [61], Liabsuetrakul et al. [62], and Khan et al. [63].
Figure 6. Forest plot of studies showing the association between footwear use and hookworm infection.+*.
+Note different x-axis. *Stratified exposure totals were not given for the following studies: Woodburn et al. (N = 2498) [64], Traub et al. (N = 328) [65], Pullan et al. (N = 1803) [66], Nmor et al. (N = 978) [67], Lee et al. (N = 118) [68], Gutman et al. (N = 537) [69], Alemu et al. (N = 319) [70] and Behnke et al. (N = 285) [71]. *The magnitude of the odds ratio and the 95% confidence interval/standard error (SE) were not available so it was not included in the forest plot: Behnke et al. [71] (odds ratio for footwear use not significant) and Bethony et al. [14] (footwear use was not significantly associated with hookworm infection). *Adjusted effect estimate: Woodburn et al. [64], Traub et al. [65], Pullan et al. [66], Nmor et al. [67], Lee et al. [68], Jiraanankul et al. [72], Humphries et al. [73], and Gutman et al. [69]. *Inverted Log [odds ratio] and SE from effect estimate of barefoot exposure: Woodburn et al. [64], Traub et al. [65], Pullan et al. [66], Nmor et al. [67], Lee et al. [68], Jiraanankul et al. [72], Humphries et al. [73], Gutman et al. [69], Erosie et al. [74], Alemu et al. [70]. *Log [odds ratio] and SE calculated from raw data: Tadesse et al. [75], Ilechukwu et al. [76], Mukerji et al. [77], Ali et al. [78].
Discussion
We found that footwear use was significantly associated with a lower odds of Buruli ulcer, CLM, leptospirosis, strongyloidiasis, any STH infection, hookworm infection, and tungiasis, highlighting the important role of footwear use in the prevention of NTDs. No significant association was found between footwear use and podoconiosis. We found no data regarding the use footwear and mycetoma, myiasis, and snakebite. The results presented here have important implications for both policy and practice. Promotion of footwear use should be an important part of selected NTD control strategies.
The significant association between footwear use and the lower odds of Buruli ulcer, CLM, hookworm infection, leptospirosis, and tungiasis are consistent with the mode of transmission of these diseases [16], [20], [29]–[34].The risk factors include presence of skin cuts or abrasions and contact with water, soil, or mud during work or recreational activities if the water is contaminated with human and animal excreta, including rodent urine [33]. Our findings are also consistent with a recent meta-analysis on WASH interventions which included some results regarding footwear use and hookworm infection or any STH infection [7]. This review found that footwear use was significantly associated with a lower odds of hookworm infection (OR 0.29; 95% CI 0.18–0.47) and any STH infection (OR = 0.30; 95% CI 0.11–0.83) [7], as compared to the findings on hookworm infection (OR = 0.48; 95% CI: 0.37–0.61) and strongyloidiasis (OR = 0.56, 95% CI 0.38–0.83) in the current analysis.
We did not find a significant association between footwear use and the risk of podoconiosis. Two issues may explain this: first, podoconiosis is a chronic disease primarily affecting the feet, so reverse causality is likely (when an individual first notices foot or leg swelling, he or she starts wearing shoes), and second, podoconiosis requires a long period of exposure, but assessment of current use of footwear does not reflect previous exposure [35]. Studies comparing podoconiosis patients with healthy controls have found that patients tend to wear footwear more than healthy controls to protect their legs from injury or to conceal the swelling in fear of stigma and discrimination [36], [37]. Other studies have suggested that age at first footwear use would be a more precise indicator of protection than current footwear use [38].
A number of our findings support integrated control strategies of NTDs. Footwear use appears to have a protective effect across multiple NTDs and thus may become an important integrated NTD control measure and should be considered by researchers, program planners, and policy makers. Footwear use interventions also have the potential to enhance sustainability of NTD control programs, similar to improved access to clean water, sanitation, and altered hygiene behavior [1], [5], [7], [39]–[43].Advocacy could be integrated into current efforts such as school health services, and indicators on type and frequency of footwear use could be included in NTD monitoring and evaluation. Initial investments may only be needed to create awareness and demonstrate the practical benefits of footwear use and promote it as a continued behavior. A public-private partnership model similar to that of pharmaceutical companies for population-based chemotherapy could be seen as example to leverage resources with footwear companies. However, future cost-effectiveness studies are needed to fully explore the feasibility and sustainability of these interventions.
We aimed to adhere to the PRISMA statement for the reporting of meta-analysis of observational studies. However, there were several limitations in this systematic review. First, only six out of the 56 included studies specified the type of footwear. Thus, we were unable to explore how the type of footwear may have affected the results. Type and frequency of footwear use may vary regionally due to differences in seasonality, socioeconomic conditions, occupation, and cultural practices. These differences could affect the effectiveness of footwear interventions and practical implementation of related interventions. Only one study was identified for CLM which limited our ability to conduct meta-analysis for this outcome.
Second, there was marked heterogeneity with wide CIs between some studies which may have led to imprecise summary estimates. I-squared estimates varied, including low heterogeneity (strongyloidiasis 0% and Buruli ulcer 26%) and moderate-to-high heterogeneity (tungiasis 63%, leptospirosis 69%, any STH infection 74%, hookworm infection 74%, and podoconiosis 96%). This may have been due to the different definitions (e.g., many studies used a questionnaire design without clarifying the type of footwear or consistency of use) or diagnostic methods employed to determine infection or disease across studies. However, we used a random-effects model to calculate summary measures in an attempt to address this heterogeneity. The results using a fixed-effects model did not substantially differ from the random-effects model, indicating only small study biases.
Lastly, most of the studies were observational in nature (e.g., cross-sectional surveys and case-control studies), giving rise to concerns regarding study quality. With cross-sectional surveys, we are unable to reach conclusions about the effect of shoes on the incidence of infection or disease over time and estimates may be confounded by other variables. Only a limited number of studies provided adjusted estimates, often controlling for just a few sociodemographic variables, with potential residual confounding. Case-control studies may be affected by recall bias, depending on how cases recall footwear exposure compared to those without disease. Details on the measurement of footwear use and the presence of NTDs were not always reported which also may have led to biased estimates. Prospective studies specifically designed to look at the effect of footwear use on selected NTDs are needed to answer this research question. RCTs may provide more robust evidence but can be ethically and financially challenging. A recent cluster randomized trial failed to show any association between hookworm infection and footwear use due to contamination [12]. Approaches such as a stepped wedge trial design or a robust cohort study may offer more feasible solutions.
Conclusions
NTDs have multiple routes of transmission and a single intervention alone is unlikely to completely interrupt transmission. Little attention has focused on personal preventive measures to reduce exposure to infection, such as the use of footwear. Our findings provide evidence that footwear use could help prevent a range of different NTDs, including Buruli ulcer, leptospirosis, CLM, tungiasis, any STH infection, strongyloidiasis, and hookworm infection. Although prospective data are still needed to explore the effect of footwear use on the incidence of NTDs over time, these findings support the integrated control strategies of NTDs that include footwear use. Initial investments are required to create awareness and demonstrate the practical benefits of footwear use and promote it as a continued behavior. There may also be a need to provide footwear to particular at-risk groups (e.g., school-aged children for STH infections), and a similar public-private partnership model to that used with pharmaceutical companies for large-scale preventive chemotherapy might be applied to leverage resources with footwear companies. However, future cost-effectiveness studies are needed to fully explore the feasibility and sustainability of these interventions.
Supporting Information
PRISMA checklist.
(DOC)
Study protocol.
(DOC)
Acknowledgments
We would like to thank Dr. Ines Gamboa, Prof. Peter J. Hotez, and Dr. Nilanthi de Silva for comments on an earlier vision of the manuscript and provision of data.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
KD is supported by a Wellcome Trust Fellowship in Public Health and Tropical Medicine [grant number 099876]. SJB is supported by a Wellcome Trust Senior Fellowship in Basic Biomedical Science [grant number 098045]. GD is supported by a Wellcome Trust University award [grant number 091956] to do work in podoconiosis. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Utzinger J, Becker SL, Knopp S, Blum J, Neumayr AL, et al. (2012) Neglected tropical diseases: diagnosis, clinical management, treatment and control. Swiss Med Wkly 142: w13727. [DOI] [PubMed] [Google Scholar]
- 2. Hotez PJ, Molyneux DH, Fenwick A, Kumaresan J, Ehrlich Sachs S, et al. (2007) Control of neglected tropical diseases. N Engl J Med 357: 1018–1027. [DOI] [PubMed] [Google Scholar]
- 3. Anderson R, Hollingsworth TD, Truscott J, Brooker S (2012) Optimisation of mass chemotherapy to control soil-transmitted helminth infection. Lancet 379: 289–290. [DOI] [PubMed] [Google Scholar]
- 4.WHO (2010) Working to overcome the global impact of neglected tropical diseases: first WHO report on neglected tropical diseases. Geneva: World Health Organization (available at: http://whqlibdoc.who.int/publications/2010/9789241564090_eng.pdf; accessed on 25 July 2012).
- 5. Freeman MC, Ogden S, Jacobson J, Abbott D, Addiss DG, et al. (2013) Integration of water, sanitation, and hygiene for the prevention and control of neglected tropical diseases: a rationale for inter-sectoral collaboration. PLoS Negl Trop Dis 7: e2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cairncross S, Hunt C, Boisson S, Bostoen K, Curtis V, et al. (2010) Water, sanitation and hygiene for the prevention of diarrhoea. Int J Epidemiol (Suppl 1): 193–205. [DOI] [PMC free article] [PubMed]
- 7. Strunz EC, Addiss DG, Stocks ME, Ogden S, Utzinger J, et al. (2014) Water, sanitation, hygiene, and soil-transmitted helminth infection: a systematic review and meta-analysis. PLoS Med 11: e1001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ejemot RI, Ehiri JE, Meremikwu MM, Critchley JA (2008) Hand washing for preventing diarrhoea. Cochrane Database Syst Rev 1: CD004265. [DOI] [PubMed] [Google Scholar]
- 9. Ejere HO, Alhassan MB, Rabiu M (2012) Face washing promotion for preventing active trachoma. Cochrane Database Syst Rev 4: CD003659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Emerson PM, Cairncross S, Bailey RL, Mabey DC (2000) Review of the evidence base for the 'F' and 'E' components of the SAFE strategy for trachoma control. Trop Med Int Health 8: 515–527. [DOI] [PubMed] [Google Scholar]
- 11. Smillie WG (1924) Control of hookworm disease in South Alabama. Southern Med J 17: 494–499. [Google Scholar]
- 12. Bird C, Ame S, Albonico M, Bickle Q (2014) Do shoes reduce hookworm infection in school-aged children on Pemba Island, Zanzibar? A pragmatic trial. Trans R Soc Trop Med Hyg 108: 297–304. [DOI] [PubMed] [Google Scholar]
- 13. Hotez P (2008) Hookworm and poverty. Ann N Y Acad Sci 1136: 38–44. [DOI] [PubMed] [Google Scholar]
- 14. Bethony J, Chen J, Lin S, Xiao S, Zhan B, et al. (2002) Emerging patterns of hookworm infection: influence of aging on the intensity of Necator infection in Hainan province, People's Republic of China. Clin Infect Dis 35: 1336–1344. [DOI] [PubMed] [Google Scholar]
- 15. Albonico M, Crompton DWT, Savioli L (1999) Control strategies for human intestinal nematode infections. Adv Parasitol 42: 277–341. [DOI] [PubMed] [Google Scholar]
- 16. Chongsuvivatwong V, Pas-Ong S, McNeil D, Geater A, Duerawee M (1996) Predictors for the risk of hookworm infection: experience from endemic villages in southern Thailand. Trans R Soc Trop Med Hyg 90: 630–633. [DOI] [PubMed] [Google Scholar]
- 17. Killewo JZ, Cairncross S, Smet JE, Maikwano LF, van Asten H (1991) Patterns of hookworm and Ascaris infection in Dar es Salaam. Acta Trop 48: 247–249. [DOI] [PubMed] [Google Scholar]
- 18. Hotez PJ, Alvarado M, Basáñez MG, Bolliger I, Bourne R, et al. (2014) The Global Burden of Disease Study 2010: interpretation and implications for the neglected tropical diseases. PLoS Negl Trop Dis 8: e2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Davey G, Tekola F, Newport MJ (2007) Podoconiosis: non-infectious geochemical elephantiasis. Trans R Soc Trop Med Hyg 101: 1175–1180. [DOI] [PubMed] [Google Scholar]
- 20. Tremblay A, MacLean JD, Gyorkos T, Macpherson DW (2000) Outbreak of cutaneous larva migrans in a group of travellers. Trop Med Int Health 5: 330–340. [DOI] [PubMed] [Google Scholar]
- 21. Feldmeier H, Heukelbach J (2009) Epidermal parasitic skin diseases: a neglected category of poverty-associated plagues. Bull World Health Organ 87: 152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Knopp S, Mohammed KA, Stothard JR, Khamis IS, Rollinson D, et al. (2010) Patterns and risk factors of helminthiasis and anemia in a rural and a peri-urban community in Zanzibar, in the context of helminth control programs. PLoS Negl Trop Dis 4: e681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gunawardena K, Kumarendran B, Ebenezer R, Gunasingha MS, Pathmeswaran A, et al. (2011) Soil-transmitted helminth infections among plantation sector schoolchildren in Sri Lanka: prevalence after ten years of preventive chemotherapy. PLoS Negl Trop Dis 5: e1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gamboa MI, Kozubsky LE, Costas ME, Garraza M, Cardozo MI, et al. (2009) [Associations between geohelminths and socioenvironmental conditions among different human populations in Argentina]. Rev Panam Salud Publica 26: 1–8 (in Spanish). [DOI] [PubMed] [Google Scholar]
- 25. Modjarrad K, Zulu I, Redden D, Nuobvy L, Freedman D, et al. (2005) Prevalnce and predictors of intestinal helminth infections among human imunodeficiency virus type-1-infected adults in an urban African setting Am J Trop Med Hyg. 73: 777–782. [PMC free article] [PubMed] [Google Scholar]
- 26. Davey G, Gebrehanna E, Adeyemo A, Rotimi C, Newport M, et al. (2006) Podoconiosis: a tropical model for gene-environment interactions? Trans R Soc Trop Med Hyg 101: 91–96. [DOI] [PubMed] [Google Scholar]
- 27. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, et al. (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.RevMan (2012) Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration.
- 29. Marion E, Eyangoh S, Yeramian E, Doannio J, Landier J, et al. (2010) Seasonal and regional dynamics of M. ulcerans transmission in environmental context: deciphering the role of water bugs as hosts and vectors. PLoS Negl Trop Dis 4: e731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Muehlen M, Feldmeier H, Wilcke T, Winter B, Heukelbach J (2006) Identifying risk factors for tungiasis and heavy infestation in a resource-poor community in northeast Brazil. Trans R Soc Trop Med Hyg 100: 371–380. [DOI] [PubMed] [Google Scholar]
- 31. Nuti M, Amaddeo D, Autorino GL, Crovatto M, Crucil C, et al. (1992) Seroprevalence of antibodies to hantaviruses and leptospires in selected Italian population groups. Eur J Epidemiol 8: 98–102. [DOI] [PubMed] [Google Scholar]
- 32. Portaels F, Elsen P, Guimaraes-Peres A, Fonteyne PA, Meyers WM (1999) Insects in the transmission of Mycobacterium ulcerans infection. Lancet 353: 986. [DOI] [PubMed] [Google Scholar]
- 33. Sasaki DM, Pang L, Minette HP, Wakida CK, Fujimoto WJ, et al. (1993) Active surveillance and risk factors for leptospirosis in Hawaii. Am J Trop Med Hyg 48: 35–43. [DOI] [PubMed] [Google Scholar]
- 34. Thielecke M, Raharimanga V, Rogier C, Stauss-Grabo M, Richard V, et al. (2013) Prevention of tungiasis and tungiasis-associated morbidity using the plant-based repellent Zanzarin: a randomized, controlled field study in rural Madagascar. PLoS Negl Trop Dis 7: e2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kloos H, Bedri Kello A, Addus A (1992) Podoconiosis (endemic non-filarial elephantiasis) in two resettlement schemes in western Ethiopia. Trop Doct 22: 109–112. [DOI] [PubMed] [Google Scholar]
- 36. Molla YB, Tomczyk S, Amberbir T, Tamiru A, Davey G (2012) Podoconiosis in east and west Gojam zones, Northern Ethiopia. PLoS Negl Trop Dis 6: e1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ayode D, McBride CM, de Heer HD, Watanabe E, Gebreyesus T, et al. (2013) A qualitative study exploring barriers related to use of footwear in rural highland ethiopia: implications for neglected tropical disease control. PLoS Negl Trop Dis 7: e2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Molla YB, Le Blond JS, Wardrop N, Baxter P, Atkinson PM, et al. (2013) Individual correlates of podoconiosis in areas of varying endemicity: a case-control study. PLoS Negl Trop Dis 7: e2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bartram J, Cairncross S (2010) Hygiene, sanitation, and water: forgotten foundations of health. PLoS Med 7: e1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Singer BH, de Castro MC (2007) Bridges to sustainable tropical health. Proc Natl Acad Sci U S A 104: 16038–16043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stocks ME, Ogden S, Haddad D, Addiss DG, McGuire C, et al. (2014) Effect of water, sanitation, and hygiene on the prevention of trachoma: a systematic review and meta-analysis. PLoS Med 11: e1001605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Utzinger J, Raso G, Brooker S, de Savigny D, Tanner M, et al. (2009) Schistosomiasis and neglected tropical diseases: towards integrated and sustainable control and a word of caution. Parasitology 136: 1859–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ziegelbauer K, Speich B, Mäusezahl D, Bos R, Keiser J, et al. (2012) Effect of sanitation on soil-transmitted helminth infection: systematic review and meta-analysis. PLoS Med 9: e1001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Landier J, Boisier P, Fotso Piam F, Noumen-Djeunga B, Sime J, et al. (2011) Adequate wound care and use of bed nets as protective factors against Buruli ulcer: results from a case control study in Cameroon. PLoS Negl Trop Dis 5: e1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Raghunathan PL, Whitney EAS, Asamoa K, Stienstra Y, Taylor TH Jr, et al. (2005) Risk factors for Buruli ulcer disease (Mycobacterium ulcerans infection): results from a case-control study in Ghana. Clin Infect Dis 40: 1445–1453. [DOI] [PubMed] [Google Scholar]
- 46. Johnson MA, Smith H, Joseph P, Gilman RH, Bautista CT, et al. (2004) Environmental exposure and leptospirosis, Peru. Emerg Infect Dis 10: 1016–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Leal-Castellanos CB, García-Suárez R, González-Figueroa E, Fuentes-Allen JL, Escobedo-de la Peñal J (2003) Risk factors and the prevalence of leptospirosis infection in a rural community of Chiapas, Mexico. Epidemiol Infect 131: 1149–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Phraisuwan P, Whitney EAS, Tharmaphornpilas P, Guharat S, Thongkamsamut S, et al. (2002) Leptospirosis: skin wounds and control strategies, Thailand, 1999. Emerg Infect Dis 8: 1455–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sulong MR, Shafei MN, Yaacob NA, Hassan H, Daud A, et al. (2011) Risk factors associated with leptospirosis among town service workers. International Med J 18: 83–88. [Google Scholar]
- 50. Alarcon JO, Romani FR, Tejada RA, Wang PA, Cespedes MJ (2011) Prevalence and risk factors of leptospirosis among rice farmers of endemic area in a tropical region of peru. Am J Trop Med Hyg 85: 331. [Google Scholar]
- 51. Bovet P, Yersin C, Merien F, Davis CE, Perolat P (1999) Factors associated with clinical leptospirosis: a population-based case-control study in the Seychelles (Indian Ocean). Int J Epidemiol 28: 583–590. [DOI] [PubMed] [Google Scholar]
- 52. Lacerda HG, Monteiro GR, Oliveira CCG, Suassuna FB, Queiroz JW, et al. (2008) Leptospirosis in a subsistence farming community in Brazil. Trans R Soc Trop Med Hyg 102: 1233–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yori PP, Kosek M, Gilman RH, Cordova J, Bern C, et al. (2006) Seroepidemiology of strongyloidiasis in the Peruvian Amazon. Am J Trop Med Hyg 74: 97–102. [PMC free article] [PubMed] [Google Scholar]
- 54. Steinmann P, Zhou X-N, Du Z-W, Jiang J-Y, Wang L-B, et al. (2007) Occurrence of Strongyloides stercoralis in Yunnan province, China, and comparison of diagnostic methods. PLoS Negl Trop Dis 1: e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sanchez PR, Guzman AP, Guillen SM, Adell RI, Estruch AM, et al. (2001) Endemic strongyloidiasis on the Spanish Mediterranean coast. QJM 94: 357–363. [DOI] [PubMed] [Google Scholar]
- 56. Phiri K, Whitty CJM, Graham SM, Ssembatya-Lule G (2000) Urban/rural differences in prevalence and risk factors for intestinal helminth infection in southern Malawi. Ann Trop Med Parasitol 94: 381–387. [DOI] [PubMed] [Google Scholar]
- 57. Mihrshahi S, Casey GJ, Montresor A, Phuc TQ, Thach DTC, et al. (2009) The effectiveness of 4 monthly albendazole treatment in the reduction of soil-transmitted helminth infections in women of reproductive age in Viet Nam. Int J Parasitol 39: 1037–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kurup R, Hunjan GS (2010) Epidemiology and control of schistosomiasis and other intestinal parasitic infections among school children in three rural villages of south Saint Lucia. J Vector Borne Dis 47: 228–234. [PubMed] [Google Scholar]
- 59. Aimpun P, Hshieh P (2004) Survey for intestinal parasites in Belize, Central America. Southeast Asian J Trop Med Public Health 35: 506–511. [PubMed] [Google Scholar]
- 60. Lello J, Knopp S, Mohammed KA, Khamis IS, Utzinger J, et al. (2013) The relative contribution of co-infection to focal infection risk in children. Proc Biol Sci 280: 20122813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Martinez Cardenas A, Betancourt Rodriguez Z (1961) [Medical study of 1,000 children. helminthiasis]. Revista Cubana de Pediatria (Impresa) 33: 115–124 (in Spanish). [PubMed] [Google Scholar]
- 62. Liabsuetrakul T, Chaikongkeit P, Korviwattanagarn S, Petrueng C, Chaiya S, et al. (2009) Epidemiology and the effect of treatment of soil-transmitted helminthiasis in pregnant women in southern Thailand. Southeast Asian J Trop Med Public Health 40: 211–222. [PubMed] [Google Scholar]
- 63. Yunus Khan M (1979) An analytical study of factors related to infestation by intestinal parasites in rural school children (report of a pilot study). Public Health 93: 82–88. [DOI] [PubMed] [Google Scholar]
- 64. Woodburn PW, Muhangi L, Hillier S, Ndibazza J, Namujju PB, et al. (2009) Risk factors for helminth, malaria, and HIV infection in pregnancy in Entebbe, Uganda. PLoS Negl Trop Dis 3: e473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Traub RJ, Robertson ID, Irwin P, Mencke N, Andrew Thompson RC (2004) The prevalence, intensities and risk factors associated with geohelminth infection in tea-growing communities of Assam, India. Trop Med Int Health 9: 688–701. [DOI] [PubMed] [Google Scholar]
- 66. Pullan RL, Kabatereine NB, Quinnell RJ, Brooker S (2010) Spatial and genetic epidemiology of hookworm in a rural community in Uganda. PLoS Negl Trop Dis 4: e713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nmor JC, Onojafe JO, Omu BA (2009) Anthropogenic indices of soil-transmitted helminthiasis among children in Delta state, southern Nigeria. Iranian J Public Health 38: 31–38. [Google Scholar]
- 68. Lee VJ, Ong A, Lee NG, Lee WT, Fong KL, et al. (2007) Hookworm infections in Singaporean soldiers after jungle training in Brunei Darussalam. Trans R Soc Trop Med Hyg 101: 1214–1218. [DOI] [PubMed] [Google Scholar]
- 69. Gutman J, Emukah E, Okpala N, Okoro C, Obasi A, et al. (2010) Effects of annual mass treatment with ivermectin for onchocerciasis on the prevalence of intestinal helminths. Am J Trop Med Hyg 83: 534–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Alemu A, Atnafu A, Addis Z, Shiferaw Y, Teklu T, et al. (2011) Soil transmitted helminths and Schistosoma mansoni infections among school children in Zarima town, northwest Ethiopia. BMC Infect Dis 11: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Behnke JM, De Clercq D, Sacko M, Gilbert FS, Ouattara DB, et al. (2000) The epidemiology of human hookworm infections in the southern region of Mali. Trop Med Int Health 5: 343–354. [DOI] [PubMed] [Google Scholar]
- 72. Jiraanankul V, Aphijirawat W, Mungthin M, Khositnithikul R, Rangsin R, et al. (2011) Incidence and risk factors of hookworm infection in a rural community of central Thailand. Am J Trop Med Hyg 84: 594–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Humphries D, Mosites E, Otchere J, Twum WA, Woo L, et al. (2011) Epidemiology of hookworm infection in Kintampo North municipality, Ghana: patterns of malaria coinfection, anemia, and albendazole treatment failure. Am J Trop Med Hyg 84: 792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Erosie L, Merid Y, Ashiko A, Ayine M, Balihu A, et al. (2002) Prevalence of hookworm infection and hemoglobin status among rural elementary school children in southern Ethiopia. Ethiop J Health Dev 16: 113–115. [Google Scholar]
- 75. Tadesse G (2005) The prevalence of intestinal helminthis infections and associated risk factors among school children in Babile town, eastern Ethiopia. Ethiop J Health Dev 19: 140–147. [Google Scholar]
- 76. Ilechukwu GC, Ilechukwu CGA, Ozumba AN, Ojinnaka NC, Ibe BC, et al. (2010) Some behavioural risk factors for intestinal helminthiasis in nursery and primary school children in Enugu, south eastern Nigeria. Niger J Clin Pract 13: 288–293. [PubMed] [Google Scholar]
- 77. Mukerji AK, Mathen KK (1950) Survey of hookworm infection in the Jharia coalfields settlement area. Indian J Med Res 38: 95–118. [PubMed] [Google Scholar]
- 78. Ali I, Mekete G, Wodajo N (1999) Intestinal parasitism and related risk factors among students of Asendabo Elementary and Junior Secondary school, South Western Ethiopia. Ethiop J Health Dev 13: 157–161. [Google Scholar]
- 79. Ugbomoiko US, Ariza L, Ofoezie IE, Heukelbach J (2007) Risk factors for tungiasis in Nigeria: identification of targets for effective intervention. PLoS Negl Trop Dis 1: e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Njau N, Wanzala P, Mutugi M, Ariza L, Heukelback J (2012) Tungiasis (jigger infestation) in rural Kenya, an emerging infectious disease. Retrovirology 9: P37. [Google Scholar]
- 81. Deribe K, Brooker SJ, Pullan RL, Sime H, Gebretsadik A, et al. (2014) Epidemiology and individual, household and geographical risk factors of podoconiosis in Ethiopia: results from the first nationwide mapping. Am J Trop Med Hyg (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Price E (1974) The relationship between endemic elephantiasis of the lower legs and the local soils and climate: a study in Wollamo district, southern Ethiopia. Trop Geog Med 26: 225–230. [PubMed] [Google Scholar]
- 83. Yakob B, Deribe K, Davey G (2008) High levels of misconceptions and stigma in a community highly endemic for podoconiosis in southern Ethiopia. Trans R Soc Trop Med Hyg 102: 439–444. [DOI] [PubMed] [Google Scholar]
- 84. Marston BJ, Diallo MO, Horsburgh CR Jr, Diomande I, Saki MZ, et al. (1995) Emergence of Buruli ulcer disease in the Daloa region of Côte d'Ivoire. Am J Trop Med Hyg 52: 219–224. [DOI] [PubMed] [Google Scholar]
- 85. Douglin CP, Jordan C, Rock R, Hurley A, Levett PN (1997) Risk factors for severe leptospirosis in the parish of St. Andrew, Barbados. Emerg Infect Dis 3: 78–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA checklist.
(DOC)
Study protocol.
(DOC)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.