Abstract
Egg masses of the yellow-spotted salamander Ambystoma maculatum form an association with the green alga “Oophila amblystomatis” (Lambert ex Wille), which, in addition to growing within individual egg capsules, has recently been reported to invade embryonic tissues and cells. The binomial O. amblystomatis refers to the algae that occur in A. maculatum egg capsules, but it is unknown whether this population of symbionts constitutes one or several different algal taxa. Moreover, it is unknown whether egg masses across the geographic range of A. maculatum, or other amphibians, associate with one or multiple algal taxa. To address these questions, we conducted a phylogeographic study of algae sampled from egg capsules of A. maculatum, its allopatric congener A. gracile, and two frogs: Lithobates sylvatica and L. aurora. All of these North American amphibians form associations with algae in their egg capsules. We sampled algae from egg capsules of these four amphibians from localities across North America, established representative algal cultures, and amplified and sequenced a region of 18S rDNA for phylogenetic analysis. Our combined analysis shows that symbiotic algae found in egg masses of four North American amphibians are closely related to each other, and form a well-supported clade that also contains three strains of free-living chlamydomonads. We designate this group as the ‘Oophila’ clade, within which the symbiotic algae are further divided into four distinct subclades. Phylogenies of the host amphibians and their algal symbionts are only partially congruent, suggesting that host-switching and co-speciation both play roles in their associations. We also established conditions for isolating and rearing algal symbionts from amphibian egg capsules, which should facilitate further study of these egg mass specialist algae.
Introduction
“Oophila amblystomatis” is a binomial corresponding to the chlamydomonad green alga that lives in association with several species of North American amphibian embryos [1]. These amphibians include the ambystomatid salamanders Ambystoma maculatum (spotted salamander) and A. gracile (Northwestern salamander), and ranid frogs Lithobates sylvatica (wood frog) and L. aurora (red-legged frog) [2]. This alga was provisionally named by Lambert, who collected and preserved samples of the algal cells from A. maculatum embryos in 1905, north of Boston [3]. The use of the informal designation “Oophila amblystomatis” has remained in the scientific literature, with the occasional spelling of “Oophilia” [4]–[10], despite the lack of a formal taxonomic description. Additionally, while many researchers accept the chlamydomonad designation for Oophila, it remains unknown whether the algae consist of a mono- para- or polyphyletic group within and among these different amphibian hosts [2].
Most research on O. amblystomatis has focused on just one of these hosts, the spotted salamander A. maculatum. The A. maculatum-algal association was documented over 125 years ago [11], and the majority of subsequent research on this association focused on the reciprocal benefits to the algae and host [4]–[6], [12]–[16]. The embryo benefits from (i) an increase in the partial pressure of oxygen in its egg capsules provided by the algae during daylight hours [6], (ii) potential removal of nitrogenous waste ([12] although see [15]), and (iii) potential transfer of photosynthate from algae to embryos [16]. Other potential benefits to the embryos may include reduced production of carbonic acid (H2CO3), as a result of reduced CO2 inside the egg capsule, or the direct or indirect exclusion of microbial pathogens by the algae. Benefits for the algae may include the provision of nitrogenous wastes in the form of ammonia by the hosts [12], the increased CO2 within the egg capsule, as well as a protective environment for the algae to flourish.
Past researchers considered this association to be ectosymbiotic (i.e. inside the egg capsule, but outside the body), but Kerney et al. [17] recently showed that algal cells invade host embryonic tissues and cells. This unique example of algal endosymbiosis in a vertebrate host cells raises many immediate research questions. Is O. amblystomatis the sole algal symbiont associated with A. maculatum? Are there signs of co-evolution between symbiont and host? Finally, are the symbionts of other North American amphibians closely related to those found associating with A. maculatum? Answers to these questions will advance our understanding of the nature of this intriguing association.
This study employs a phylogeographic approach to better understand the identity and relationships among algae that form symbiotic associations with North American amphibians. To this end, we chose 18S rDNA as a marker as this gene has been broadly sampled across eukaryotic algae (e.g. the SILVA rRNA database [18]) and hence is suitable for placing taxa of interest to major phylogenetic groups (e.g. [19]–[20]). We inferred an 18S rDNA phylogeny from environmental samples of algae collected from Ambystoma maculatum, A. gracile, Lithobates sylvatica, and L. aurora egg capsules. We also included in our analysis three “Chlamydomonas gloeophila,” (Skuja) strains, which were obtained from A. maculatum egg masses in the Northeastern USA in the early 1950' or as free-living cells from a freshwater body in England. We found algae, which associate with embryos of these four amphibian taxa, form a clade together with three cultured strains of chlamydomonad taxa, but not C. gloeophila. We suggest that the genus Oophila is accurately assigned to this discrete algal lineage. Within this group, amphibian-associated algae fall into four possible subclades that do not strictly correspond to their host species.
Materials and Methods
Collection and preparation of samples
Egg clutches of amphibians were collected from vernal ponds or other types of temporary or permanent freshwater bodies between 2009 and 2013. Collection sites included multiple locations from New Jersey (USA), Tennessee (USA), California (USA), British Columbia (Canada), and Nova Scotia (Canada) (Figure 1, Table 1). None of the amphibian species from whose egg masses algae were collected are endangered or protected. Collections were approved as part of Animal Care Protocols to RK (IACUC#2013F17; Gettysburg College Animal Care Committee) and CB (CCAC#12-007-N; St. Francis-Xavier Animal Care Committee). Most samples were from locations for which no specific permission was required. Samples from the Greenbrook Sanctuary (private land) were collected with permission (Sandra Bonardi, Director) and samples from the University of the South (private land) were collected by Professor David G. Haskell. Nova Scotia and British Columbia Ministry of Natural Resources granted permission for collections. For NS collections, a letter from the relevant authorities, but no permit number is issued. For BC collections, permits #NA11-68662 was issued to RK for A. gracile; #NA12-76509 to CB for L. aurora). Sampling locations, including GPS co-ordinates are listed in Table 1. Typically, clutches were collected when the algal bloom inside each egg was visible, which occurs after Harrison stage 17 [17], [21]. Algal cells were collected by piercing an egg and withdrawing the capsular fluid using an insulin syringe, or by dissecting out the capsular part of the egg with fine forceps. These collection methods constitute environmental samples, since no selection of cells was performed prior to DNA extraction. C. gloeophila strains were obtained from the Experimental Phycology and Culture Collection of Algae at the University of Göttingen (strains SAG 12–4, 12–5) and the Culture Collection of Algae and Protozoa, maintained by the Scottish Association of Marine Science (strain 11/127).
Figure 1. Map of the geographic range and collection sites for egg masses of four amphibian hosts.
Species range maps are plotted on a map of North America (see the Materials and Methods). The dark green color represents a range overlap between L. sylvatica and A. maculatum, and the pink color represents a range overlap between L. aurora and A. gracile. Numbered locations correspond to higher detail panels below. The maps of collection sites for algae corresponding to egg masses from A. maculatum and L. sylvatica in Nova Scotia, Canada (1), A. gracile in California, USA (2), L. aurora and A. gracile in Vancouver Island, British Columbia, Canada, and A. maculatum in New Jersey and Tennessee of USA (4/5).
Table 1. Collection details of egg masses from which algae were sampled.
| Location | Code | Coordinates (dec. degrees) | Date | Sample type | Host | GenBank Acc. | |
| Nova Scotia | lon | lat | |||||
| St. Andrews | StA1 | −61.750 | 45.499 | 01-04-11 | ES* | A. maculatum | KJ711228, KJ711229, KJ711248 |
| St. Andrews | StA2 | −61.750 | 45.499 | 05-05-12 | ES | A. maculatum | KJ711230, KJ711231, KJ711232 |
| East Bay | EB | −60.362 | 46.013 | 25-04-11 | ES | A. maculatum | KJ711198, KJ711238 |
| Graywood (Hwy 8) | GW | −65.426 | 44.622 | 14-05-11 | ES | A. maculatum | KJ711202 |
| Wolfville | WV | −64.297 | 45.001 | 13-05-11 | ES | A. maculatum | KJ711236, KJ711237 |
| Hwy#7 | H7 | −62.055 | 45.318 | 21-04-12 | ES | A. maculatum | KJ711242, KJ711205, KJ711206 |
| Fairmont | FM | −61.922 | 45.684 | 26-04-12 | ES | A. maculatum | KJ711199, KJ711200, KJ711201 |
| Snakepit 2 | SP2 | −63.639 | 44.872 | 28-04-12 | ES | A. maculatum | KJ711226, KJ711227, KJ711246, KJ711247 |
| Trafalgar | TF | −62.566 | 45.121 | 29-04-12 | ES | A. maculatum | KJ711233, KJ711234, KJ711235 |
| Queensville | QV | −61.366 | 45.734 | 29-04-12 | ES | A. maculatum | KJ711215, KJ711216, KJ711217, KJ711218, KJ711219 |
| Rocky Mountain | RM | −62.253 | 45.394 | 02-05-12 | ES | A. maculatum | KJ711220, KJ711221, KJ711222 |
| Mira River | MR | −60.015 | 46.017 | 05-05-12 | ES | A. maculatum | KJ711207, KJ711208, KJ711209 |
| Oxford | O | −63.886 | 45.707 | 11-05-12 | ES | A. maculatum | KJ711210, KJ711211, KJ711243 |
| Point Michaud | PM | −60.668 | 45.593 | 22-05-12 | ES | A. maculatum | KJ711212, KJ711213, KJ711214 |
| Cape George | CG | −61.941 | 45.836 | 28-05-12 | ES | A. maculatum | KJ711196, KJ711197 |
| Halifax | Hb_cul | −63.654 | 44.617 | 2011 | CI** | A. maculatum | KJ711137 |
| Halifax | H | −63.666 | 44.691 | 2009 | ES | A. maculatum | KJ711203, KJ711204, KJ711239, KJ711240, KJ711241 |
| Beaver Bank | BB_cul | −63.680 | 44.818 | 2011 | CI | A. maculatum | KJ711131, KJ711132, KJ711134, KJ711135, KJ711136, KJ711138 |
| Halifax | R | −63.654 | 44.617 | 2011 | ES | L. sylvatica | KJ711139, KJ711140, KJ711141, KJ711142, KJ711143, KJ711144, KJ711145, KJ711146, KJ711147 |
| British Columbia | |||||||
| Bamfield | BF | −125.016 | 48.871 | 05-05-11 | ES | A. gracile | KJ711133, KJ711162, KJ711163, KJ711164, KJ711165, KJ711166, KJ711167, KJ711168, KJ711169, KJ711170, KJ711171, KJ711172, KJ711173 |
| Wood Lake | WL | −125.581 | 48.991 | 24-03-12 | ES | L. aurora | KJ711223 |
| Swan Lake | SL | −125.595 | 49.001 | 21-03-12 | ES | L. aurora | KJ711224, KJ711225, KJ711244, KJ711245 |
| New Jersey, USA | |||||||
| Greenbrook Sanctuary | GSa | −73.924 | 40.914 | 17-03-12 | ES | A. maculatum | KJ711181, KJ711182, KJ711183, KJ711184, KJ711186, KJ711187, KJ711188, KJ711189, KJ711190, KJ711193 |
| Greenbrook Sanctuary | GSb | −73.924 | 40.914 | 25-03-12 | ES | A. maculatum | KJ711174, KJ711175, KJ711176, KJ711177, KJ711194 |
| Greenbrook Sanctuary | GSc | −73.924 | 40.914 | 14-04-12 | ES | A. maculatum | KJ711178, KJ711179, KJ711180, KJ711185 |
| Greenbrook Sanctuary | GSa_cul | −73.924 | 40.914 | 17-03-12 | CI | A. maculatum | KJ711195 |
| Greenbrook Sanctuary | GSb_cul | −73.924 | 40.914 | 25-03-12 | CI | A. maculatum | KJ711191, KJ711192 |
| Tennessee, USA | |||||||
| Sewanee | TN | −85.910 | 35.204 | 02-18-13 | ES | A. maculatum | KJ711249, KJ711250, KJ711251, KJ711252, KJ711253, KJ711254, KJ711255, KJ711256 |
| California, USA | |||||||
| Arcata | CA | −124.082 | 40.867 | 03-07-12 | ES | A. gracile | KJ711148, KJ711149, KJ711150, KJ711151, KJ711152, KJ711153, KJ711154, KJ711155, KJ711156, KJ711157, KJ711158, KJ711159, KJ711160, KJ711161 |
| Culture collections | |||||||
| C. gloeophila | SAG 12-4 | −86.568 | 39.143 | 1953 | CI | A. maculatum | KJ711128 |
| C. gloeophila | SAG 12-5 | −76.475 | 42.447 | 1953 | CI | A. maculatum | KJ711129 |
| C. gloeophila | CCAP 11/127 | −2.935 | 54.381 | 1992 | CI | Free-living | KJ711130 |
*ES = environmental sequence; **CI = cultured isolate.
Name codes correspond to branch names in Figure 2.
Map Construction
Maps were plotted using maps (ver. 2.3−2; http://cran.r-project.org/projects/maps/), mapdata (ver. 2.2−2; http://cran.r-project.org/projects/mapdata/) and maptools (ver. 0.8.23; http://cran.r-project.org/projects/maptools/) packages implemented in R (ver. 3.0.1) [22]. Host species range maps were downloaded from the IUCN Red List of Threatened Species (ver. 2012.2 http://www.iucnredlist.org/) [23]–[26]. Sample collection sites were recorded using GPS or in cases where GPS co-ordinates were not recorded, were estimated using a geographic locator (http://www.findlatitudeandlongitude.com/). Collection locations on the maps were annotated in Adobe Illustrator CS5 (ver.15.1.0).
Culturing and microscopy of algal symbionts
Algal cells associated with A. maculatum egg capsules were isolated using a finely drawn Pasteur pipette. In addition to single cell isolation, cultures of green algae were established by serial dilution techniques using disposable multi-well plates. These were cultured in modified AF6 medium [27]. Whereas we also used 0.5−1% agar solidified AF6 medium for culturing, the symbiotic algae grew very poorly on an agar plate (see the discussion for further details). Cultures were maintained in a plastic tube with vent screw cap and at 15−20°C with a 12-hour light cycle, under broad-spectrum growth lights. Cultures were transferred aseptically every 4−8 weeks. Algae were imaged using an Axiovert 100 microscope (Zeiss, Oberkochen, Germany) and an Olympus DP73 digital camera (Tokyo, Japan). The Olympus cellSens image capture software was used to measure the cells.
Molecular sequencing and phylogenetic analysis
From each individual egg, the capsular algae were processed for DNA extraction using a DNeasy Blood and Tissue kit, or in some cases, a DNeasy Plant tissue kit (Qiagen, Hilden, Germany). The extracted DNA was used as a template for PCR using sets of “universal” eukaryotic 18S rDNA primers, including nu-SSU-0024-5′ (5′-CTGGTTGATCCTGCCAGTAGT-3′), nu-SSU-0033-5′ (5′- CCTGCCAGT AGTCATAYGCTT-3′), nu-SSU-1757-3′ (5′-CAGGTTCACCTACGGAAACCT-3′), and nu-SSU-1768-3′ (5′- TGA TCC TTC YGC AGG TTC ACC-3′) [28]. Amplified products were purified using a QIAquick gel extraction kit (Qiagen) and cloned into a pGEM-T Easy vector (Promega Corp., Madison, Wisconsin, USA). From each cloning reaction, 4−12 colonies were selected for Sanger sequencing on ABI 3730xl DNA Analyzers (Applied Biosystems, Carlsbad, California, USA). Newly obtained sequences have been deposited to the GenBank (Table 1).
Algal 18S rDNA sequences were edited to remove the plasmid and PCR primer regions and were aligned manually to an alignment used in our earlier study [17] using Mesquite ver. 2.75 [29]. All other green algal sequences were obtained from GenBank. Representative chlorophycean algae were chosen as a diverse sampling of available sequences for this group. Ambiguously aligned sequence regions were removed. The final alignment was used for phylogenetic analyses under likelihood and parsimony criteria. Maximum likelihood (ML) analysis was performed using RAxML ver. 8.0.0 [30], under the GTR+gamma+I model, which was selected using Modeltest ver. 3.7 [31]. ML trees were inferred through 100 iterations, each started from different randomized stepwise addition parsimony trees. The sequences were also analyzed by the maximum parsimony method using PAUP* [32]. Bootstrap analyses were based on 1,000 replicates. Uncorrected (“p”) pairwise distances of amphibian green algal 18S rDNA were calculated using PAUP* [32]. Sequence alignments—both masked and unmasked—used in this study are available as Data S1, S2.
Results
18S rDNA analysis of green algal symbionts
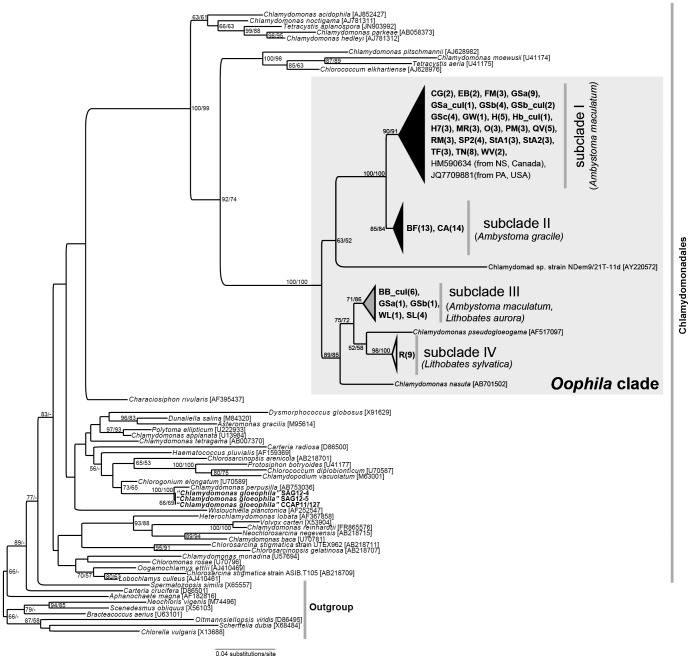
Phylogenetic analyses of 18S rDNA show that all sequences (n = 126) of algae sampled from amphibian egg capsules in this study are clustered together within the Chlamydomonadales, forming a well-supported clade together with Chlamydomonas pseudogloeogama, C. nasuta, and chlamydomonad sp. strain NDem9/21T-11d (Figure 2). Within this clade, four subclades, which we name I−IV, were identified. Sub-clades I, II, IV correspond to symbiotic green algae of A. maculatum, A. gracile, and L. sylvatica, respectively, whereas clade III includes green algal sequences obtained from A. maculatum and the parapatric L. aurora. C. pseudogloeogama is sister to subclade IV and C. nasuta branches with a clade comprising subclades III, IV, and C. pseudogloeogama. The chlamydomonad sp. strain NDem9/21T-11d is sister to subclades I+II. Pairwise distance analyses of 18S rDNA indicate that sequences among different subclades differ by 0.65−4.22% (Table 2).
Figure 2. Maximum likelihood (ML) tree of algal 18S rDNA sequences from egg masses of four amphibian taxa from various North American localities.
The data matrix included 1,653 characters and 180 sequences. Newly obtained sequences are bold-faced. ML and MP bootstrap values greater than 50% are shown at corresponding nodes. Subclades I−IV are collapsed into triangles for visual clarity; an un-collapsed version of the tree can be found as Figure S1. Numbers in parentheses indicates the number of sequences obtained and analyzed for the corresponding sample. See Table 1 for naming conventions and GenBank accession numbers.
Table 2. Percentage pairwise distances (uncorrected) of 18S rDNA among the Oophila subclades I−IV.
| II | III | IV | |
| I | 0.65−1.72% | 3.09−4.16% | 3.27−4.22% |
| II | - | 2.97−3.58% | 3.15−3.62% |
| III | - | - | 1.37−1.88% |
A total of 1,685 nucleotide positions were included for the analysis.
In addition, we obtained 18S rDNA data from three stock algal strains of Chlamydomonas gloeophila, two of which, SAG 12−4 and 12−5, were isolated from A. maculatum egg masses in 1953 by Starr. The other strain, CCAP 11/127, was isolated from a freshwater pond in Cumbria, England in 1992 by Jaworski. Sequences of the three C. gloeophila strains were very similar to each other (Figure 2), but they branched with C. perpusilla, and not with or within the ‘Oophila’ clade.
Culturing and observation of green algal symbionts of A. maculatum
We have isolated and established strains of algae associated with two geographically distinct A. maculatum populations. Note that while the cultures are monotypic in terms of 18S rDNA sequence diversity, they may not necessarily be clonal. The algae grew in AF6 liquid medium [27], although their growth rate was relatively low compared to strains of other chlamydomonad taxa such as C. pseudogloeogama (strain SAG 15.73) and C. nasuta, (strain NIES-2225). In comparison to these same taxa, the A. maculatum-derived algae grew extremely poorly on agar-solidified AF6 media. Despite observing some growth of algae in solid media, the majority of algal colonies did not match the symbiont algal 18S rDNA sequences and instead were matched to other green algae, with 99% or greater sequence similarities, such as Chlamydomonad sp. Tow9/21T-1w (AY220568.1), C. sp. Tow8/18T-1w (AY220567.1), C. debaryana (FR865523.1) and Chlorococcum minutum (JN968585.1). This indicates the presence of non-Oophila symbionts that outcompeted numerically dominant Oophila under solid growth conditions and therefore agar-solidified media should not be used to isolate A. maculatum-associated algae.
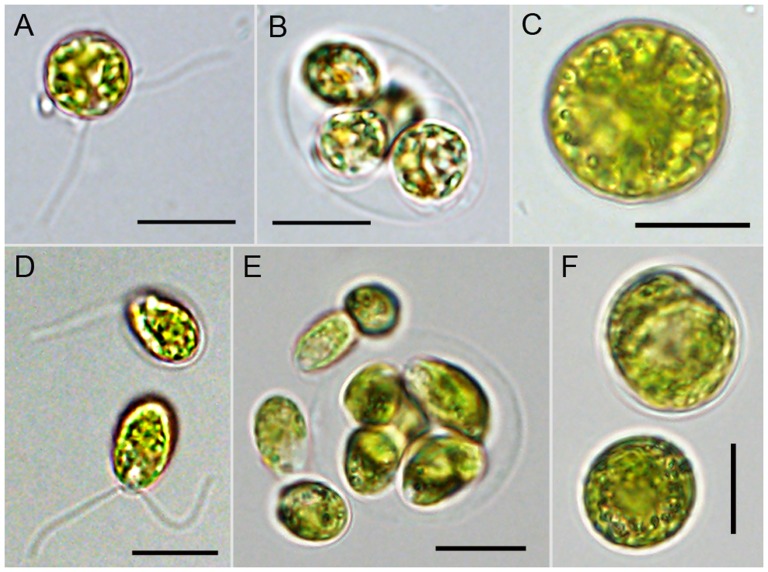
Our cultured strains of A. maculatum algal symbionts fall into two distinct subclades, I and III (Figure 2). Algae from both subclades appear to display the canonical chlamydomonad life cycle [33], consisting of vegetative cells (zoospores or gametes, and zygotes (Figure 3). Older cultures tend to have more putative zygotes, the formation of which may be a result of nutrient depletion, as in C. reinhardtii [34]. A major difference between strains of distinct phylogenetic groups, which were established and maintained using the same culturing method, is the shape of the zoospores (or gametes). Flagellated cells of the strain belonging to the subclade I are spherical and 9−11 µm in diameter (n = 10), whereas those of the clade II are oblong in shape and measure 10−11 µm in length and 6−7 µm in width (n = 10). Both types of strain are characterized by zoospores having two flagella, which are each nearly twice the length of the cell (Figure 3). Vegetative cell growth of these algae occurs within a parental cell wall, each containing up to eight daughter cells, which are released freely into the media.
Figure 3. Light microscopic images of cultured strains of A. maculatum algae.
The Oophila strains Hb_cul-rk (A−C) and BB_cul-B (D−F) belong to subclades I and III, respectively. Monotypic cultures displayed at least three different cell types, which include 1) free-swimming biflagellates (A, D), which correspond to zoospores or gametes, 2) cells enclosed within a mother cell wall (B, E), likely representing asexually dividing zoospores, and 3) larger non-motile zygotes (C, F). Scale bars: 10 µm (A−F).
Discussion
We have used sequences derived from DNA amplified using “universal” eukaryotic primers for 18S rDNA to infer the genetic diversity of single celled eukaryotic algal taxa associated with egg masses of four North America amphibian taxa. We acknowledge that a single gene approach can be limited in terms of resolving detailed relationships among closely related samples [35]. However, our 18S rDNA phylogeny reveals that: (i) symbiotic green algae sampled from egg capsules from four amphibians from two different coasts of North America form a distinct, well-supported phylogenetic clade together with three other chlamydomonad taxa within the Chlamydomonadales, (ii) there is a partial relationship between host taxonomic identity and symbiont genotype, and (iii) there are at least two distinct morphological types of algal symbiont found in these amphibian egg masses, and these correspond to phylogenetically distinct lineages. We discuss each of these findings in turn.
Designation of the ‘Oophila’ clade
Our study shows that algal symbiont sequences obtained from broadly distributed populations of North American amphibians are similar to each other and form a well-supported clade together with three chlamydomonad taxa (Figure 2). We designate this group as the ‘Oophila’ clade based on the fact that the majority of sequences belonging to the group correspond to symbionts found in amphibian egg masses. Within the ‘Oophila’ clade, amphibian algae were further divided into four distinct subclades; some of these subclades display substantial genetic divergence within the 18S rDNA region (e.g.>3% between the subclades I and IV). In the broader phylogenetic context of chlamydomonads, the Oophila clade is nested within the Moewusinia clade [36].
Three free-living chlamydomonad taxa are included in the ‘Oophila’ clade. These include 1) Chlamydomonas pseudogloeogama, which is sister to subclade IV (100% bootstrap support, ML), 2) C. nasuta, which branches with subclades III+IV, and 3) the chlamydomonad strain NDem9/21T-11d, which is sister to subclades I+II (Figure 2). The C. pseudogloeogama isolate reported in Hoham et al. [37] was SAG 15.73, originally isolated from “snow detritus” in the High Tatra mountains of Slovakia in 1963 by F. Hindák. C. nasuta, whose identity has recently been verified by 18S rDNA sequence [38] was isolated from soil samples from Connecticut, USA. Finally, the chlamydomonad sp. NDem9/21T-11d was isolated from a freshwater body located in Itasca State Park of Minnesota, USA by Fawley and colleagues (the sequence has been deposited in GenBank without an associated journal publication). It is currently not clear whether these free-living taxa could form symbioses with amphibian embryos. If so, that would suggest the presence of lineage-specific traits that may allow this algal association. Alternatively, association with amphibian embryos may have arisen independently within the ‘Oophila’ clade, or was lost in these free living “species.”
The suggestion that Oophila is environmentally acquired as an egg mass symbiont [5], [39], [40], requires that it be a cold tolerant species capable of overwintering in peri- or sub-zero temperatures and then emerging in a planktonic form in the early spring. This is consistent with observed temperature tolerances of Oophila in our cultures, which showed growth even under refrigerated conditions with the temperature as low as 6°C. In this sense it is notable that C. pseudogloeogama, which is closely related to symbiotic algae of amphibian egg masses (Figure 2), is a cold adapted species. Thus, whereas the original collection site for C. pseudogloeogama is geographically distant from those for algae associated with embryos of North American amphibians, their close genetic distance may reflect similarities in their capacity to survive cold climates. The life cycles of neither O. amblystomatis nor C. pseudogloeogama have been carefully verified, but appear comparable to that of C. moeuwsii [33], which is closely related to the ‘Oophila’ clade (Figure 2).
Oophila is numerically dominant in A. maculatum egg caspules
Environmental sequencing using universal PCR primers for eukaryotic 18S rDNA [28] should amplify DNA sequences from a diverse array of eukaryotes in a sample, thus allowing a relatively unbiased sampling of taxa that would otherwise be difficult to detect and quantify using microscopy. All sequences returned from an environmental sequencing approach fell into the ‘Oophila’ clade (Figure 2), which includes an Oophila 18S rDNA sequence from our earlier study [17]. Although Oophila appears to be the most abundant green alga in A. maculatum egg capsules, our sampling strategy was not likely (nor was it meant) to detect rare taxa, and thus we cannot conclude that Oophila is the sole algal symbiont. Specifically, we chose a sampling strategy in which we sequenced 18S rDNA from a range of samples from geographically distant locales and distantly related hosts (i.e. frogs vs. salamanders), rather than sequence deeply from fewer samples. For example, if a non-Oophila taxon were present in a particular egg capsule at an incidence of 1% ( = 0.01) of the population of O. amblystomatis, one hundred clones, on average, would need to be sequenced in order to detect this taxon for a given sample. However, we sampled numerous egg capsules from different egg masses, yielding a total of 126 sequences. Assuming equal incidence of non-Oophila taxa among all samples and no amplification bias during PCR, the cumulative probability (
= 0.01) of the population of O. amblystomatis, one hundred clones, on average, would need to be sequenced in order to detect this taxon for a given sample. However, we sampled numerous egg capsules from different egg masses, yielding a total of 126 sequences. Assuming equal incidence of non-Oophila taxa among all samples and no amplification bias during PCR, the cumulative probability (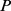 ) of detecting one non-Oophila sequence can be estimated by:
) of detecting one non-Oophila sequence can be estimated by:
| (1) |
where  = the incidence (proportion) of non-Oophila taxa in an egg capsule and
= the incidence (proportion) of non-Oophila taxa in an egg capsule and 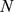 = the number of sequences. Expressing with respect to
= the number of sequences. Expressing with respect to  :
:
| (2) |
based on our sampling regime, the mean incidence of non-Oophila taxa among A. maculatum capsules sample would need to be  0.068 (6.8%) in order to observe at least one non-Oophila sequence with a probability>0.99 (Table 3).
0.068 (6.8%) in order to observe at least one non-Oophila sequence with a probability>0.99 (Table 3).
Table 3. Maximum incidence of non-Oophila taxa in egg capsules, based on a>0.99 cumulative probability of detecting sequences and the actual number of sequences obtained (see equation 2 in Discussion).
| Maximum incidence per host | Combined maximum incidence | |||
| A. maculatum | A. gracile | L. aurora | L. sylvatica | - |
| 0.068 | 0.156 | 0.601 | 0.318 | 0.036 |
We thus conclude that Oophila is numerically the most abundant algal taxon in A. maculatum egg capsules. Although Oophila is also the only taxon detected from A. gracile, L. sylvatica and L. aurora egg capsules, our sample size from these taxa does not permit the same strength of conclusion as with A. maculatum (Table 3). Using different sampling techniques Kerney et al. [17] reported that the community in egg capsules of A. maculatum is not monotypic with respect to green algae. Gilbert [4] and Graham et al. [16] noted spherical and ovoid cells in a single clutch, raising the possibility of either phenotypic plasticity within the population of Oophila in the capsule, the co-existence of multiple, distinct green algal taxa, or the presence morphologically distinct subclades of Oophila within a single A. maculatum egg capsule or egg mass. The latter is supported by our data; in two A. maculatum egg capsule samples (GSa & GSb), both the subclade I and III sequence types were identified (Figure 2). In addition, our culturing work, especially the experiment that involved the use of agar solidified media, suggested the presence of non-Oophila green algae, albeit numerically not dominant, in A. maculatum egg masses. The abundance of other algal taxa relative to Oophila as well as the full extent of their diversity, however, remains unknown. Additional sequencing in combination with microscopic cell counting will be necessary to quantify the relative abundance of Oophila to other taxa that co-exist in single egg capsules.
Two possibilities can explain the result that the Oophila complex is the most common algal taxon among these North American amphibian hosts. One possibility is that algae belonging to the Oophila species complex are common and abundant in North American amphibian breeding habitat. DNA sequences that fall into ‘Oophila’ clade I have been identified from two A. maculatum breeding pools before and during the breeding season (CDB and YL, unpublished data), but the abundance and commonality of free-living Oophila in breeding habitats remains to be assessed. A second possibility is that taxa of the Oophila clade have evolved into specialist colonizers of amphibian egg masses, such that their distribution patterns coincide with host ranges. Further characterization of host specificity, or identification of Oophila from vernal pools that do not contain these amphibian egg masses could help resolve these two possibilities.
Chlamydomonas gloeophila is not Oophila
In addition to gathering sequence data from amphibian egg masses collected in this study, three culture strains of “C. gloeophila” were obtained and processed for 18S rDNA sequencing. Two of these were isolated more than 60 years ago from A. maculatum egg clutches collected in New York State (USA) and Connecticut (USA). Whereas sequences from these strains match well to the strain of C. gloeophila collected from a freshwater sample in England, none of the C. gloeophila strains are closely related to the ‘Oophila’ clade (Figure 2), but rather are likely members of the Chlorogonia clade [36]. Interestingly, these cultures were established and maintained on agar-solidified growth media, which our culturing experiments showed are not suitable for growing symbiotic green algae of A. maculatum. We, thus, suggest that these C. gloeophila strains may not correspond to numerically dominant algal symbionts of A. maculatum. Rather, these likely represent low abundance green algae that occurred in A. maculatum egg masses, which nevertheless might have outcompeted Oophila under non-liquid growth conditions.
Relationship between host identity and symbiont genotype
18S rDNA sequence evolution is too slow to permit correlations between symbiont genotype and geography within a host species. However, it is interesting that the sequences most divergent to those from A. maculatum algae were derived from algae from the wood frog L. sylvatica, a host with whom both the geographic range and even breeding habit of A. maculatum overlaps. With two exceptions (e.g. some algae from the Greenbrook Sanctuary, NJ, USA and from Beaver Bank, NS, Canada, see Table 1), all sequences from A. maculatum form a sister group to those from A. gracile, an allopatric congener (Figure 2) [41], [42]. This raises the question as to whether the symbionts of A. gracile also enter embryonic tissue and cells, in a manner similar to the symbionts of A. maculatum. Finally, it is currently unknown whether Oophila belonging to subclade III can invade A. maculatum embryonic cells as has been shown for Oophila belonging to subclade I [17]. Other algal symbioses have shown both repeated convergent origins (e.g. algal symbionts of the ciliate Paramecium bursaria [43], or a single origin with subsequent phylogenetic congruence, indicating co-speciation (e.g. Chlorella sp. symbionts of the green hydra [44]. A multiple origins model of North American amphibian-algal symbioses is consistent with finding presumably non-symbiotic chlamydomonad green algae within the Oophila clade, and a lack of strict host-symbiont phylogenetic congruence. However further analyses of population-level host-symbiont congruence using additional gene markers [45], environmental sampling from vernal pools, and the diversity cell-cell interactions is needed to further clarify how these fascinating ecological, evolutionary, and developmental associations are established and maintained.
Supporting Information
18S rDNA sequence alignments—masked—used in this study.
(FAS)
18S rDNA sequence alignments—unmasked—used in this study.
(FAS)
Acknowledgments
The authors thank Sandra Bonardi, Marlena Dlutek, Sharyn Marks, Barb Beasley, Ainslie Cogswell, David Haskell, Jantina Toxopeus and Katelyn MacNeil for assistance with sample collections, O. Roger Anderson for providing access to a growth chamber, and Ashley Yang and Saul Scheinbach for assistance with molecular sequencing work.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All new Oophila sequences are available from GenBank under accession numbers found in the article (Table 2) and in the Supporting Information files.
Funding Statement
This study was supported by the Natural Sciences and Engineering Research Council of Canada - Discovery Grant to CB (http://www.nserc-crsng.gc.ca/professors-professeurs/grants-subs/dgigp-psigp_eng.asp). EK and RK were funded by startup grants by their respective institutions, which are intramural by definition. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wille N (1909) Abteiling. Chlorophyceae. In: Syllabus der pflanzenfamilien 6. (Engler, A. Eds) Berlin.
- 2. Kerney R (2011) Symbioses between salamander embryos and green algae. Symbiosis 54: 107–119. [Google Scholar]
- 3. Collins FS, Holden I, Setchell WA (1905) Phycotheca Boreali-Americana. A collection of dried specimens of the algae of North America. Fascile XXVI: 1267. [Google Scholar]
- 4. Gilbert PW (1942) Observations on the eggs of Ambystoma maculatum with especial reference to the green algae found within the egg envelopes. Ecology 23: 215–227. [Google Scholar]
- 5. Gilbert PW (1944) The alga-egg relationship in Ambystoma maculatum. A case of symbiosis. Ecology 25: 366–369. [Google Scholar]
- 6. Pinder A, Friet S (1994) Oxygen transport in egg masses of the amphibians Rana sylvatica and Ambystoma maculatum: convection, diffusion and oxygen production by algae. J Exp Biol 197: 17–30. [DOI] [PubMed] [Google Scholar]
- 7. Valls JH, Mills NE (2007) Intermittent hypoxia in eggs of Ambystoma maculatum: embryonic development and egg capsule conductance. J Exp Biol 210: 2430–2435. [DOI] [PubMed] [Google Scholar]
- 8. Hardy LM, Lucas C (1991) A crystalline protein is responsible for dimorphic egg jellies in the spotted salamander Ambystoma maculatum (Shaw) (Caudata Ambystomatidae). Comp Biochem Physiol 100: 653–660. [Google Scholar]
- 9. Ruth B (1993) A molecular and functional evaluation of the egg mass color polymorphism of the spotted salamander Ambystoma maculatum . J Herpetology 27: 306–314. [Google Scholar]
- 10. Sacerdote A, King R (2009) Dissolved oxygen requirements for hatching success of two ambystomatid salamanders in restored ephemeral ponds. Wetlands 29: 1202–1213. [Google Scholar]
- 11. Orr H (1888) Note on the development of amphibians, chiefly concerning the central nervous system; with additional observations on the hypophysis, mouth, and the appendages and skeleton of the head. Quart J Micro Sci NS 115: 483–489. [Google Scholar]
- 12. Goff L, Stein JR (1978) Ammonia: basis for algal symbiosis in salamander egg masses. Life Sci 22: 1463–1468. [DOI] [PubMed] [Google Scholar]
- 13. Bachmann MD, Carlton RG, Burkholder JM, Wetzel GW (1985) Symbiosis between salamander eggs and green algae: microelectrode measurements inside eggs demonstrate effect of photosynthesis on oxygen concentration. Can J Zool 64: 1586–1588. [Google Scholar]
- 14. Tattersall G, Spiegelaar N (2008) Embryonic motility and hatching success of Ambystoma maculatum are influenced by a symbiotic alga. Can J Zool 86: 1289–1298. [Google Scholar]
- 15. Bianchini K, Tattersall GJ, Sashaw J (2012) Acid water interferes with salamander-green algae symbiosis during early embryonic development. Physiol Biochem Zool 85: 470–480. [DOI] [PubMed] [Google Scholar]
- 16. Graham ER, Fay SA, Davey A, Sanders RW (2013) Intracapsular algae provide fixed carbon to developing embryos of the salamander Ambystoma maculatum . J Exp Biol 216: 452–459. [DOI] [PubMed] [Google Scholar]
- 17. Kerney R, Kim E, Hangarter RP, Heiss AA, Bishop CD, et al. (2011) Intracellular invasion of green algae in a salamander host. Proc NatAcad of Sci USA 108: 6497–6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, et al. (2013) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucl. Acids Res. 41 (D1): D590–D596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gontcharov AA, Marin BA, Melkonian MA (2003) Molecular phylogeny of conjugating green algae (Zygnemophyceae, Streptophyta) inferred from SSU rDNA sequence comparisons. J Mol Evol 56: 89–104. [DOI] [PubMed] [Google Scholar]
- 20. Kim E, Archibald JM (2013) Ultrastructure and molecular phylogeny of the cryptomonad Goniomonas avonlea sp. nov. Protist 164: 160–82. [DOI] [PubMed] [Google Scholar]
- 21.Harrison RG (1969) Harrison stages and description of the normal development of the spotted salamander, Amblystoma punctatum. in Organization and Development of the Embryo. New Haven: Yale University Press. pp.44−66.
- 22.R Development Core Team (2008) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, Available: http://www.R-project.org.
- 23.NatureServe and IUCN (International Union for Conservation of Nature) (2007) Ambystoma maculatum In: IUCN 2012. IUCN Red List of Threatened Species. Version 2012.1 Available: http://www.iucnredlist.org. Downloaded on 14 July 2012.
- 24.NatureServe and IUCN (International Union for Conservation of Nature) (2007) Lithobates aurora In: IUCN 2012. IUCN Red List of Threatened Species. Version 2012.1 Available: http://www.iucnredlist.org. Downloaded on 14 July 2012
- 25.NatureServe and IUCN (International Union for Conservation of Nature) (2007) Ambystoma gracile In: IUCN 2012. IUCN Red List of Threatened Species. Version 2012.1 Available: http://www.iucnredlist.org. Downloaded on 14 July 2012
- 26.NatureServe and IUCN (International Union for Conservation of Nature) (2007) Lithobates sylvatica In: IUCN 2012. IUCN Red List of Threatened Species. Version 2012.1 Available: http://www.iucnredlist.org. Downloaded on 14 July 2012.
- 27.Wantanabe MM, Kawachi M, Hiroki M, Kasai F (2000) NIES Collection List of Strains 6 ed., Microalgae and Protozoa. Tsukuba, Japan: Microbial Culture Collections, National Insitute for Enviornmental Studies.
- 28. Kim E, Simpson AG, Graham LE (2006) Evolutionary relationships of apusomonads inferred from taxon-rich analyses of 6 nuclear encoded genes. Mol. Biol. Evol 23: 2455–2466. [DOI] [PubMed] [Google Scholar]
- 29.Maddison WP, Maddison DR (2011) Mesquite: a modular system for evolutionary analysis. Available: http://mesquiteproject.org.
- 30. Stamatakis A (2006) RAxML-VI-HPC: Maximum Likelihood-based Phylogenetic Analyses with Thousands of Taxa and Mixed Models. Bioinformatics 22: 2688–2690. [DOI] [PubMed] [Google Scholar]
- 31. Posada D, Crandall KA (1998) MODELTEST: testing the model of DNA substitution. Bioinformatics 14: 817–818. [DOI] [PubMed] [Google Scholar]
- 32.Swofford DL (2003) PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). V4.0 Sunderland, MA: Sinauer Associates.
- 33.Lee RE (2008) Phycology. Cambridge University Press, NY USA.
- 34. Sager R, Granick S (1954) Nutritional control of sexuality in Chlamydomonas reinhardtii . J Gen Physiol 37: 729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gontcharov AA, Marin B, Melkonian M (2004) Are combined analyses better than single gene phylogenies? A case study using SSU rDNA and rbcL sequence comparisons in the Zygnematophyceae (Streptophyta). Mol Biol Evol 21: 612–24. [DOI] [PubMed] [Google Scholar]
- 36. Nakada T, Kazuharu M, Hisayoshi N (2008) Molecular systematics of Volvocales (Chlorophyceae, Chlorophyta) based on exhaustive 18S rDNA phylogenetic analyses. Mol Phyl and Evol 48: 281–291. [DOI] [PubMed] [Google Scholar]
- 37. Hoham RW, Bonome TA, Martin CW, Leebens-Mack JH (2002) A combined 18S rDNA and rbcL phylogenetic analysis of Chloromonas and Chlamydomonas (Chlorophyceae, Volvocales) emphasizing snow and other cold-temperature habitats. J. Phycol 38: 1051–1064. [Google Scholar]
- 38. Yumoto K, Kasai F, Kawachi M (2013) Taxonomic re-examination of Chlamydomonas strains maintained in the NIES-Collection. J Microbiol Cult Collect 29: 1–12. [Google Scholar]
- 39. Gatz J (1973) Algal entry into the eggs of Ambystoma maculatum . J Herpetol 7: 137–138. [Google Scholar]
- 40.Gilbert SF, Epel D (2008) Ecological Developmental Biology: Integrating Epigenetics, Medicine, and Evolution. Sunderland, MA: Sinauer Associates.
- 41. Shaffer HB, Clark JM, Kraus F (1991) When molecules and morphology clash: A phylogenetic analysis of the North American ambystomatid salamanders (Caudata: Ambystomatidae). Syst Biol 40: 284–303. [Google Scholar]
- 42. Jones TR, Kluge AG, Wolf AJ (1993) When theories and methodologies clash: A phylogenetic reanalysis of the North American Ambystomatid salamanders (Caudata: Ambystomatidae). Syst Biol 42: 92–102. [Google Scholar]
- 43. Hoshina R, Imamura N (2008) Multiple origins of the symbioses in Paramecium bursaria . Protist 159: 53–63. [DOI] [PubMed] [Google Scholar]
- 44. Kawaida H, Ohba K, Koutake Y, Shimizu H, Tachida H, et al. (2013) Symbiosis between hydra and chlorella: Molecular phylogenetic analysis and experimental study provide insight into its origin and evolution. Mol Phylog Evol 66: 906–914. [DOI] [PubMed] [Google Scholar]
- 45. Zamudio KR, Savage WK (2003) Historical isolation, range expansion, and secondary contact of two highly divergent mitochondrial lineages in spotted salamanders (Ambystoma maculatum). Evolution 57: 1631–1652. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
18S rDNA sequence alignments—masked—used in this study.
(FAS)
18S rDNA sequence alignments—unmasked—used in this study.
(FAS)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All new Oophila sequences are available from GenBank under accession numbers found in the article (Table 2) and in the Supporting Information files.