Abstract
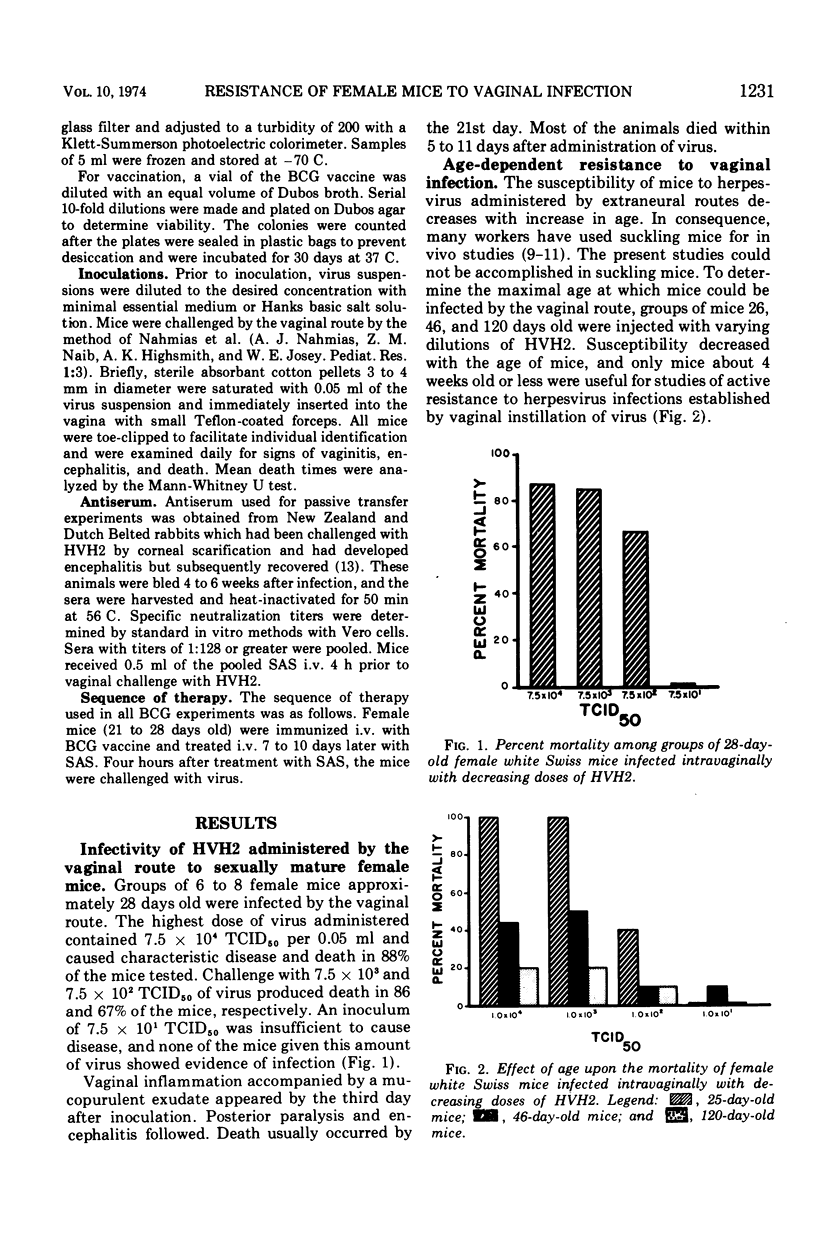
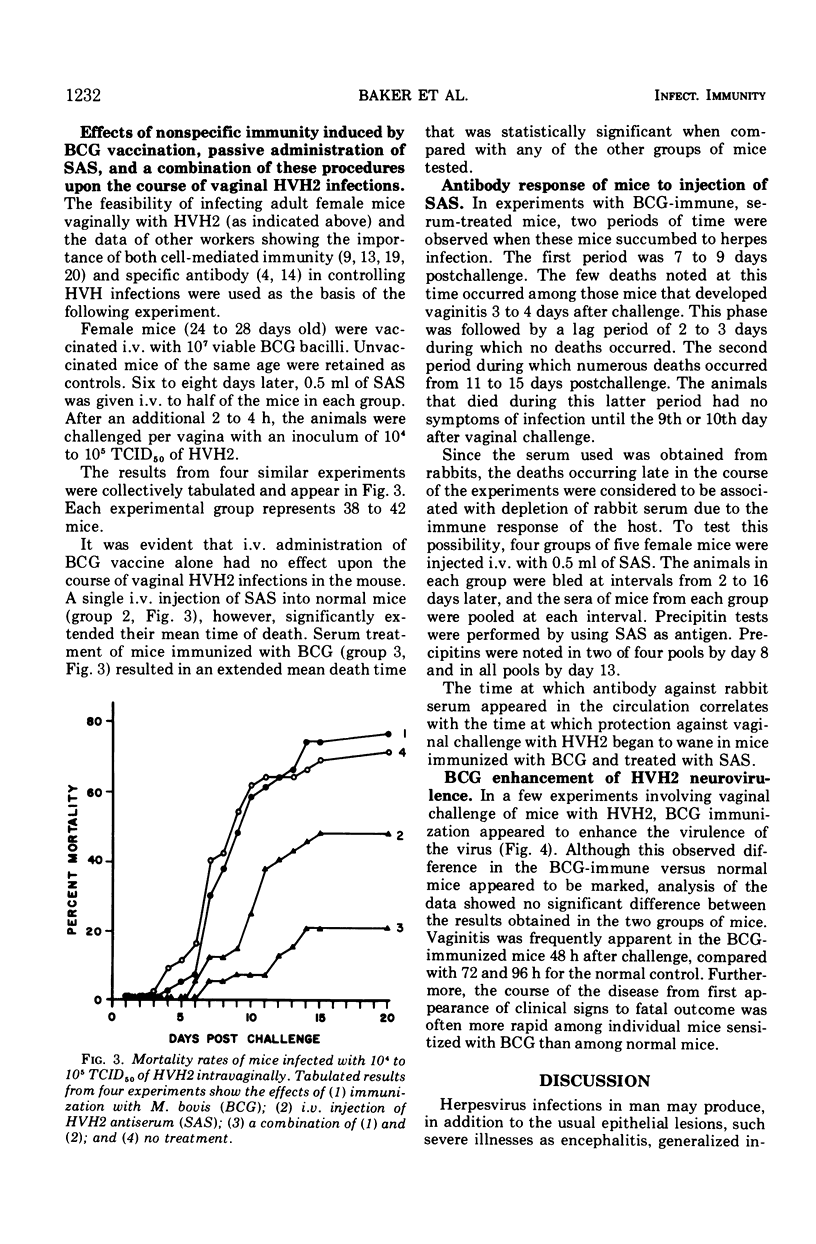
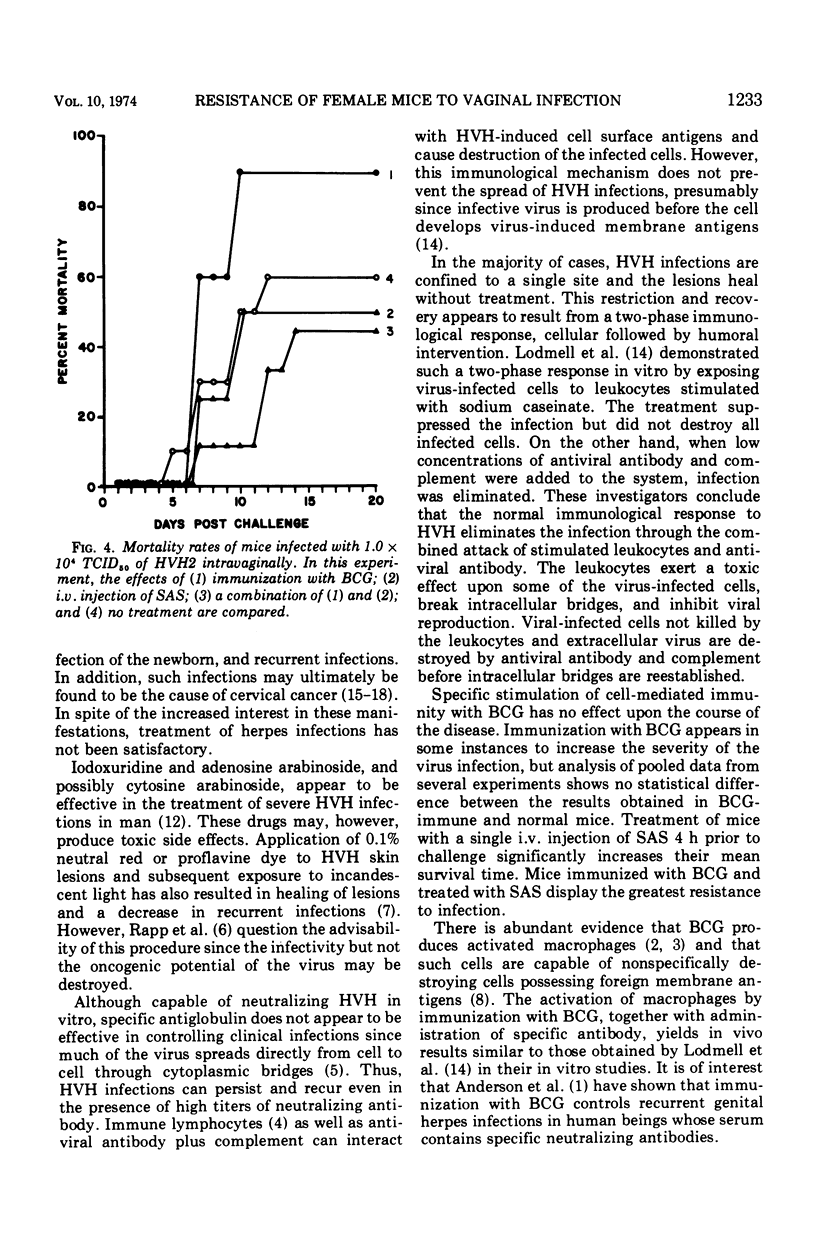
The susceptibility of 4- to 6-week-old female white Swiss mice to intravaginal inoculation with Herpesvirus hominis type 2 (HVH2) and the effect of prior intravenous immunization with Mycobacterium bovis (BCG) and/or treatment with specific HVH2 antiserum (SAS) were investigated. Mice inoculated intravaginally developed vaginitis, posterior paralysis, encephalitis, and death. Prior immunization with BCG either had no effect or appeared in some cases to enhance the course of the disease, whereas a single 0.5-ml intravenous injection of SAS provided significant protection. However, synergistic interaction of BCG immunization and treatment with SAS produced the greatest degree of protection in mice challenged intravaginally with HVH2.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIOZZI G., BENACERRAF B., GRUMBACH F., HALPERN B. N., LEVADITI J., RIST N. Etude de l'activité granulopexique du système réticulo-endothélial au cours de l'infection tuberculeuse expérimentale de la souris. Ann Inst Pasteur (Paris) 1954 Sep;87(3):291–300. [PubMed] [Google Scholar]
- Blanden R. V., Lefford M. J., Mackaness G. B. The host response to Calmette-Guérin bacillus infection in mice. J Exp Med. 1969 May 1;129(5):1079–1107. doi: 10.1084/jem.129.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian R. T., Ludovici P. P., Jeter W. S. Cell-to-cell transmission of herpes simplex virus in primary human amnion cells. Proc Soc Exp Biol Med. 1971 Dec;138(3):1109–1115. doi: 10.3181/00379727-138-36061. [DOI] [PubMed] [Google Scholar]
- Duff R., Rapp F. Properties of hamster embryo fibroblasts transformed in vitro after exposure to ultraviolet-irradiated herpes simplex virus type 2. J Virol. 1971 Oct;8(4):469–477. doi: 10.1128/jvi.8.4.469-477.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs J. B., Jr Macrophage nonimmunologic recognition: target cell factors related to contact inhibition. Science. 1973 May 25;180(4088):868–870. doi: 10.1126/science.180.4088.868. [DOI] [PubMed] [Google Scholar]
- Hirsch M. S., Zisman B., Allison A. C. Macrophages and age-dependent resistance to Herpes simplex virus in mice. J Immunol. 1970 May;104(5):1160–1165. [PubMed] [Google Scholar]
- JOHNSON R. T. THE PATHOGENESIS OF HERPES VIRUS ENCEPHALITIS. I. VIRUS PATHWAYS TO THE NERVOUS SYSTEM OF SUCKLING MICE DEMONSTRATED BY FLUORESCENT ANTIBODY STAINING. J Exp Med. 1964 Feb 1;119:343–356. doi: 10.1084/jem.119.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON R. T. THE PATHOGENESIS OF HERPES VIRUS ENCEPHALITIS. II. A CELLULAR BASIS FOR THE DEVELOPMENT OF RESISTANCE WITH AGE. J Exp Med. 1964 Sep 1;120:359–374. doi: 10.1084/jem.120.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson C. L., Ushijima R. N., Karim R., Baker M. B., Baker R. E. Herpesvirus hominis type 2 infections in rabbits: effect of prior immunization with attenuated Mycobacterium bovis (BCG) cells. Infect Immun. 1972 Oct;6(4):465–468. doi: 10.1128/iai.6.4.465-468.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodmell D. L., Niwa A., Hayashi K., Notkins A. L. Prevention of cell-to-cell spread of herpes simplex virus by leukocytes. J Exp Med. 1973 Mar 1;137(3):706–720. doi: 10.1084/jem.137.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp F. Question: do herpesviruses cause cancer? Answer: of course they do. J Natl Cancer Inst. 1973 Apr;50(4):825–832. doi: 10.1093/jnci/50.4.825. [DOI] [PubMed] [Google Scholar]
- Rawls W. E., Adam E., Melnick J. L. An analysis of seroepidemiological studies of herpesvirus type 2 and carcinoma of the cervix. Cancer Res. 1973 Jun;33(6):1477–1482. [PubMed] [Google Scholar]
- Roizman B., Frenkel N. The transcription and state of herpes simplex virus DNA in productive infection and in human cervical cancer tissue. Cancer Res. 1973 Jun;33(6):1402–1416. [PubMed] [Google Scholar]
- Royston I., Aurelian L. The association of genital herpesvirus with cervical atypia and carcinoma in situ. Am J Epidemiol. 1970 Jun;91(6):531–538. doi: 10.1093/oxfordjournals.aje.a121164. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Restriction of herpes simplex virus by macrophages. An analysis of the cell-virus interaction. J Exp Med. 1971 Jan 1;133(1):19–38. doi: 10.1084/jem.133.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zisman B., Hirsch M. S., Allison A. C. Selective effects of anti-macrophage serum, silica and anti-lymphocyte serum on pathogenesis of herpes virus infection of young adult mice. J Immunol. 1970 May;104(5):1155–1159. [PubMed] [Google Scholar]


