Rituximab (RTX) therapy is used increasingly to manage myasthenia gravis patients, and case series suggest particular benefit for those patients who have antibodies to muscle specific kinase (MuSK-MG).1
We report a MuSK-MG patient with persistent and severe B cell depletion 3 years after receiving RTX.
A 62 year old woman developed ptosis, bulbar symptoms, and weight loss in 2000. In 2005 she developed hypercarbic respiratory failure and was eventually diagnosed with MuSK-MG. She had 3 additional hospitalizations for exacerbations, including one for crisis, and required BiPAP at night. An initial excellent response to therapeutic plasma exchange waned over time. Intravenous immunoglobulin, azathioprine, and prednisone did not provide significant improvement. In 2010, she was treated with 6 doses of 1000mg RTX over approximately 8 weeks (578 mg/m2 per infusion) and remained on prednisone and azathioprine. Following the RTX infusions she had a tenuous course and later developed progressive respiratory insufficiency and required nocturnal BiPAP. A chest CT showed no evidence of thymoma.
Blood biomarker samples were drawn 34 months after RTX treatment, at which time she required supplemental oxygen. Clinical evaluation demonstrated persistent oculobulbar and facial weakness and disability (MG-composite: 23, MG-Manual Muscle Testing: 23, MG-Quality of Life-15: 30).2–5 We measured B cells by flow cytometry after staining for CD19 and identified helper and cytotoxic T cells by CD4 and CD8 expression, respectively.
Polychromatic flow cytometry demonstrated 49.5% T helper and 44.7% cytotoxic T cells. Only 0.06% of lymphocytes were CD19+ B cells; naïve, memory, and plasma cell subpopulations were undetectable (Figure). Repeat B cell markers performed over 36 months after her initial RTX treatment continued to show profound B cell depletion with <1% CD19+ B cells. Creatine kinase and thyroid profile were normal. She had had no serious infections.
Figure.
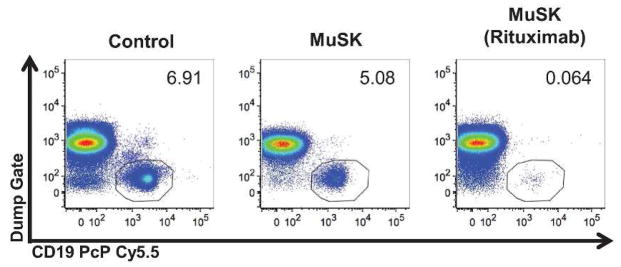
Prolonged B cell depletion 34 months after rituximab treatment. Peripheral blood mononuclear cells from a healthy control, a patient with MuSK-MG, and our patient with MuSK-MG treated with rituximab were surface stained with CD19 PcP Cy5.5 conjugate. In order to isolate lymphocytes, a dump channel stained for CD3, CD14, and CD16 Pacific-Blue along with LIVE/DEAD dye conjugated to the same fluorophore was used to gate out macrophages, neutrophils, NK cells, dendritic cells, and dead cells. B cell populations are indicated by the circles. The logarithmic scales on the X and Y axes represent the fluorescence intensity, and the numbers in the flow plot represent the frequency of CD19+ B cells among all lymphocytes in the peripheral blood.
Following depletion with RTX, B cell populations typically recover within 12 months.6 This MuSK-MG patient had profound, prolonged B cell depletion 3 years after receiving RTX. It has been observed that recovery of B cell populations begins with naïve B cells, and memory B cell regeneration may be delayed.7 However, in patients with autoimmune disease, such prolonged B cell depletion after RTX has only been reported in 2 systemic lupus erythematosus patients, both of whom were given RTX in combination with cyclophosphamide.8 In these cases, B cells remained low 5–7 years after RTX therapy. The underlying mechanism and the effect of concomitant cyclophosphamide therapy on the risk of developing prolonged B cell depletion with RTX are uncertain. In our case, it is also unclear how the unconventional RTX dosing regimen, combined with other immunosuppressive drugs, may have affected B cell recovery. Due to poor disease control, our patient continued to receive azathioprine and varying doses of prednisone, raising the risk for serious infections.9 As the use of RTX for neurologic diseases increases, clinicians need to be aware of the possibility of prolonged B cell depletion, particularly when it is combined with other immunosuppressives.
Acknowledgments
This study was supported by a clinician-scientist development award sponsored by the American Academy of Neurology Foundation and the Myasthenia Gravis Foundation of America (Dr. Guptill) and a pilot grant from the Duke Translational Research Institute (CTSA grant UL1RR024128). This publication was made possible with the help from the Duke University Center for AIDS Research (CFAR), a NIH funded program (P30 AI 64518).
Abbreviations
- CT
computed tomography
- MuSK MG
muscle specific kinase antibody positive myasthenia gravis
- RTX
Rituximab
References
- 1.Diaz-Manera J, Martinez-Hernandez E, Querol L, Klooster R, Rojas-Garcia R, Suarez-Calvet X, Munoz-Blanco JL, Mazia C, Straasheijm KR, Gallardo E, Juarez C, Verschuuren JJ, Illa I. Long-lasting treatment effect of rituximab in MuSK myasthenia. Neurology. 2012;78(3):189–193. doi: 10.1212/WNL.0b013e3182407982. [DOI] [PubMed] [Google Scholar]
- 2.Jaretzki A, 3rd, Barohn RJ, Ernstoff RM, Kaminski HJ, Keesey JC, Penn AS, Sanders DB. Myasthenia gravis: recommendations for clinical research standards. Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America. Neurology. 2000;55(1):16–23. doi: 10.1212/wnl.55.1.16. [DOI] [PubMed] [Google Scholar]
- 3.Burns TM, Conaway M, Sanders DB, Composite MG, Group M-QS. The MG Composite: A valid and reliable outcome measure for myasthenia gravis. Neurology. 2010;74(18):1434–1440. doi: 10.1212/WNL.0b013e3181dc1b1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns TM, Conaway MR, Cutter GR, Sanders DB. Less is more, or almost as much: a 15-item quality-of-life instrument for myasthenia gravis. Muscle Nerve. 2008;38(2):957–963. doi: 10.1002/mus.21053. [DOI] [PubMed] [Google Scholar]
- 5.Sanders DB, Tucker-Lipscomb B, Massey JM. A simple manual muscle test for myasthenia gravis: validation and comparison with the QMG score. Annals of the New York Academy of Sciences. 2003;998:440–444. doi: 10.1196/annals.1254.057. [DOI] [PubMed] [Google Scholar]
- 6.Roll P, Palanichamy A, Kneitz C, Dorner T, Tony HP. Regeneration of B cell subsets after transient B cell depletion using anti-CD20 antibodies in rheumatoid arthritis. Arthritis and rheumatism. 2006;54(8):2377–2386. doi: 10.1002/art.22019. [DOI] [PubMed] [Google Scholar]
- 7.Anolik JH, Barnard J, Owen T, Zheng B, Kemshetti S, Looney RJ, Sanz I. Delayed memory B cell recovery in peripheral blood and lymphoid tissue in systemic lupus erythematosus after B cell depletion therapy. Arthritis and rheumatism. 2007;56(9):3044–3056. doi: 10.1002/art.22810. [DOI] [PubMed] [Google Scholar]
- 8.Lu TY, Jonsdottir T, van Vollenhoven RF, Isenberg DA. Prolonged B-cell depletion following rituximab therapy in systemic lupus erythematosus: a report of two cases. Annals of the rheumatic diseases. 2008;67(10):1493–1494. doi: 10.1136/ard.2008.091124. [DOI] [PubMed] [Google Scholar]
- 9.Clifford DB, Ances B, Costello C, Rosen-Schmidt S, Andersson M, Parks D, Perry A, Yerra R, Schmidt R, Alvarez E, Tyler KL. Rituximab-associated progressive multifocal leukoencephalopathy in rheumatoid arthritis. Archives of neurology. 2011;68(9):1156–1164. doi: 10.1001/archneurol.2011.103. [DOI] [PMC free article] [PubMed] [Google Scholar]


