Abstract
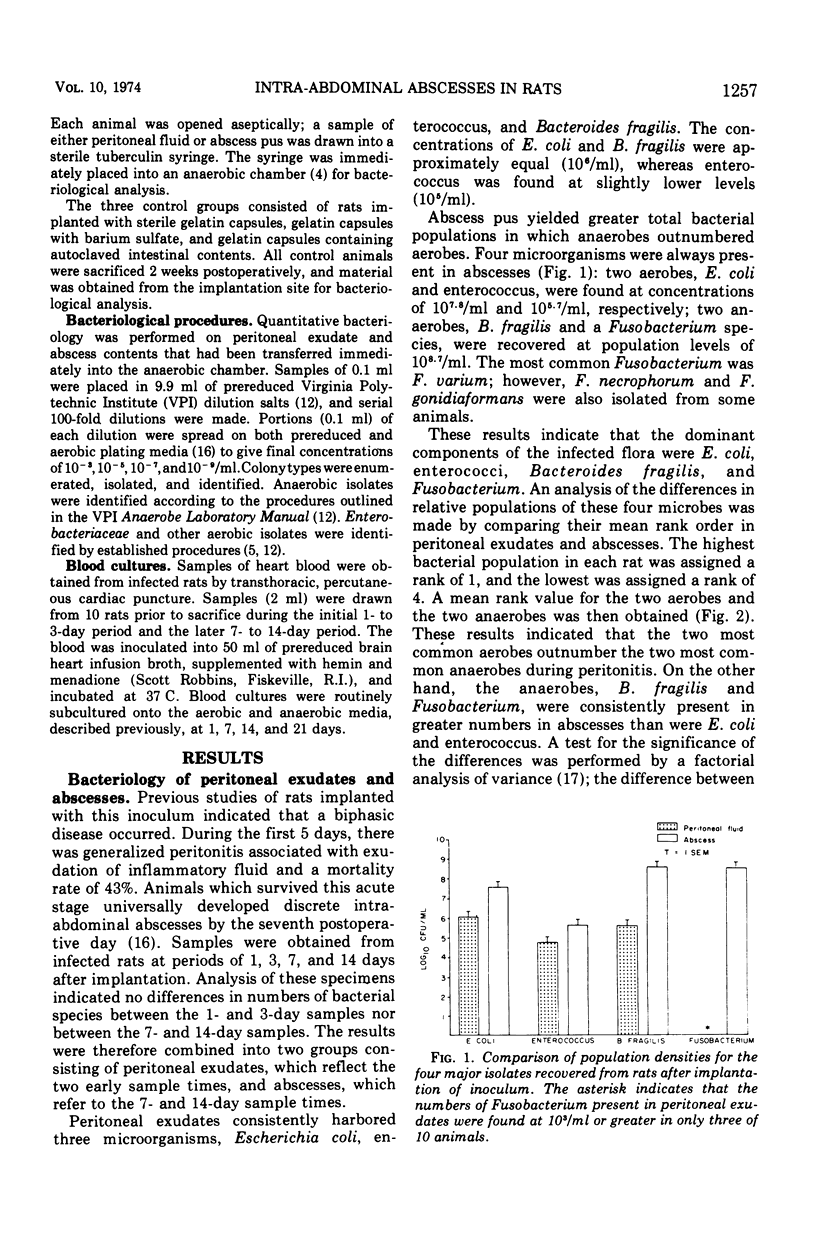
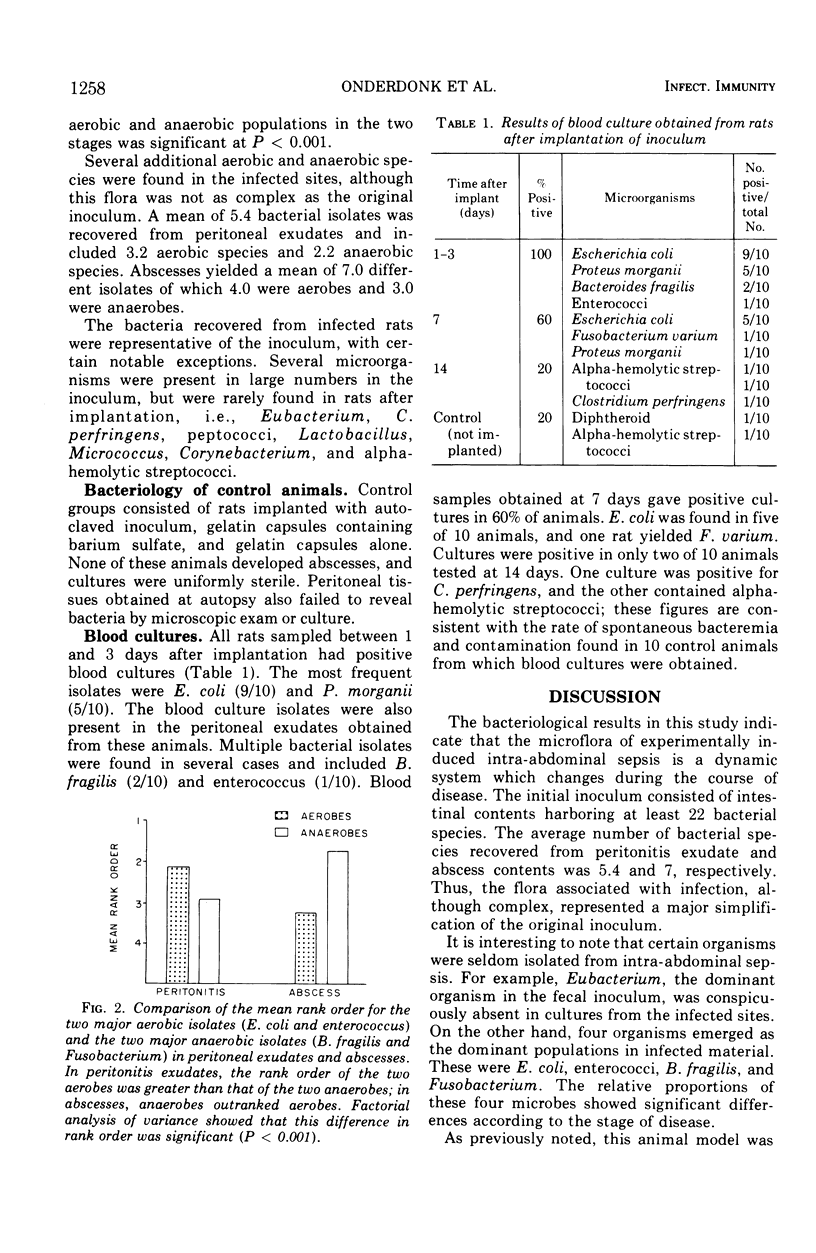
An animal model simulating intra-abdominal sepsis was produced by implanting large bowel contents into the pelvic region of rats. Bacteriological analysis of infected sites showed quantitative differences according to the stage of disease. During the initial, often lethal, peritonitis stage, Escherichia coli (mean concentration, 106/ml), enterococci (105) and Bacterioides fragilis (106) were always present. Blood cultures obtained during this phase were uniformly positive, with E. coli being the principal isolate. Animals that survived this early acute peritonitis stage developed indolent intra-abdominal abscesses. The major isolates in abscess contents were B. fragilis (108.7) and Fusobacterium (108.6); E. coli (107.8) and enterococci (105.7) were also present but in lesser concentrations. Rank order analysis of these four species in peritoneal exudates and abscess pus showed that the two aerobes outranked the two anaerobes during the early stage of the disease, whereas the reverse was true in abscesses. These experiments also illustrated that a major simplification of the original fecal inoculum occurred, even though the subsequent infection remained bacteriologically complex.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altemeier W. A. THE BACTERIAL FLORA OF ACUTE PERFORATED APPENDICITIS WITH PERITONITIS: A BACTERIOLOGIC STUDY BASED UPON ONE HUNDRED CASES. Ann Surg. 1938 Apr;107(4):517–528. doi: 10.1097/00000658-193804000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arank A., Syed S. A., Kenney E. B., Freter R. Isolation of anaerobic bacteria from human gingiva and mouse cecum by means of a simplified glove box procedure. Appl Microbiol. 1969 Apr;17(4):568–576. doi: 10.1128/am.17.4.568-576.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbach S. L., Bartlett J. G. Anaerobic infections. 1. N Engl J Med. 1974 May 23;290(21):1177–1184. doi: 10.1056/NEJM197405232902106. [DOI] [PubMed] [Google Scholar]
- Gossling J., Slack J. M. Predominant gram-positive bacteria in human feces: numbers, variety, and persistence. Infect Immun. 1974 Apr;9(4):719–729. doi: 10.1128/iai.9.4.719-729.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill G. B., Osterhout S., Pratt P. C. Liver abscess production by non-spore-forming anaerobic bacteria in a mouse model. Infect Immun. 1974 Mar;9(3):599–603. doi: 10.1128/iai.9.3.599-603.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Gross W. B. Liver granulomas of turkeys--causative agents and mechanism of infection. Avian Dis. 1968 Aug;12(3):417–422. [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl Microbiol. 1974 May;27(5):961–979. doi: 10.1128/am.27.5.961-979.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein W. M., Onderdonk A. B., Bartlett J. G., Gorbach S. L. Experimental intra-abdominal abscesses in rats: development of an experimental model. Infect Immun. 1974 Dec;10(6):1250–1255. doi: 10.1128/iai.10.6.1250-1255.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


