Abstract
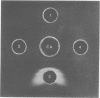
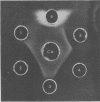
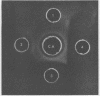
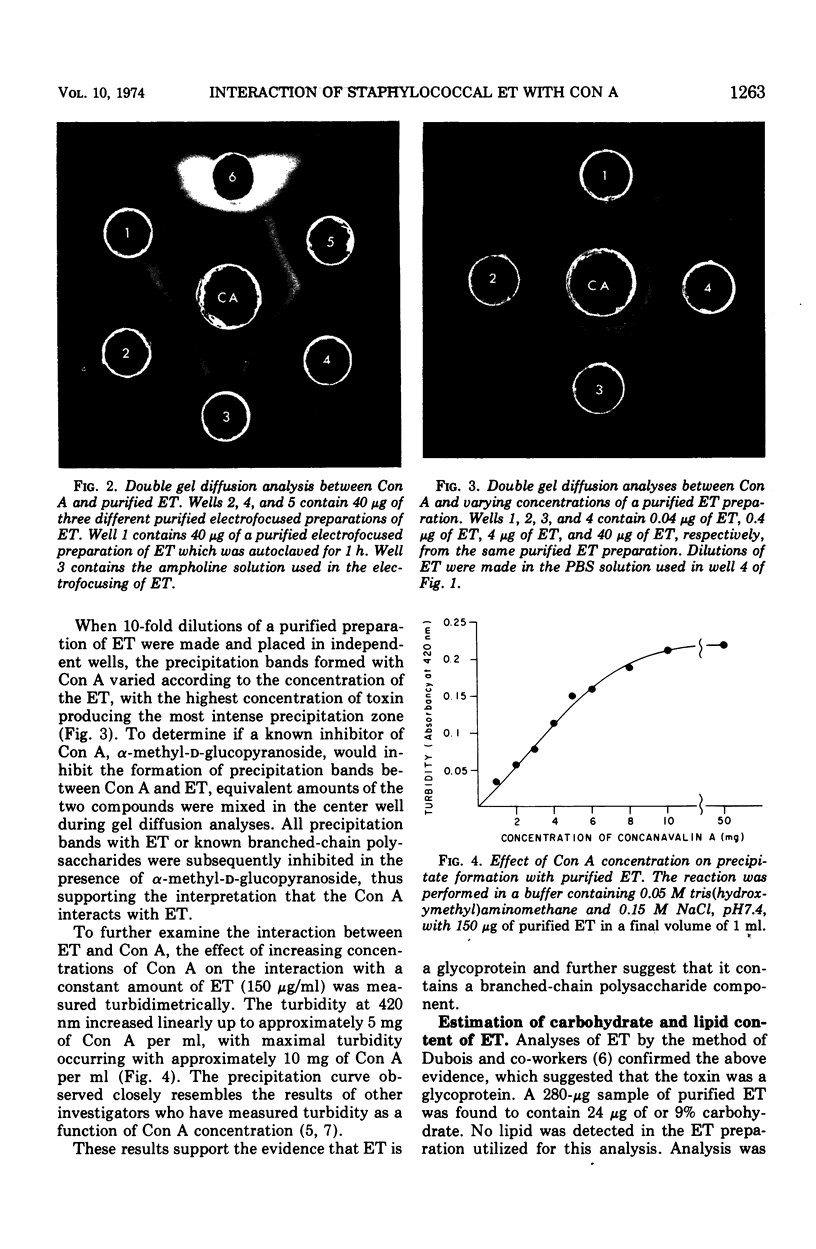
Treatment of mouse embryo fibroblasts (MEF) grown in vitro with purified staphylococcal exfoliative toxin (ET) increased the concanavalin A (Con A) agglutinability of MEF 3.5-fold over control cells. Possible explanations for this phenomenon were investigated. ET lacked proteolytic activity on denatured casein. Con A, however, was found to interact directly with ET as evidenced by the formation of precipitation in an agar gel diffusion plate and in increased turbidity in solution. This interaction was inhibited by α-methyl-d-glucopyranoside. The ability of Con A to precipitate with ET suggests that the toxin contains a carbohydrate component and that the carbohydrate associated with ET is branched rather than linear. An analysis of a purified preparation of ET indicated the presence of 9% carbohydrate and no lipid.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burger M. M. Surface changes in transformed cells detected by lectins. Fed Proc. 1973 Jan;32(1):91–101. [PubMed] [Google Scholar]
- Cuatrecasas P. Interaction of Vibrio cholerae enterotoxin with cell membranes. Biochemistry. 1973 Aug 28;12(18):3547–3558. doi: 10.1021/bi00742a031. [DOI] [PubMed] [Google Scholar]
- Doyle R. J., Birdsell D. C. Interaction of concanavalin A with the cell wall of Bacillus subtilis. J Bacteriol. 1972 Feb;109(2):652–658. doi: 10.1128/jb.109.2.652-658.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle R. J. Modification of bacteriophage phi 25 adsorption to Bacillus subtilis by concanavalin A. J Bacteriol. 1973 Jan;113(1):198–202. doi: 10.1128/jb.113.1.198-202.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDSTEIN I. J., HOLLERMAN C. E., SMITH E. E. PROTEIN-CARBOHYDRATE INTERACTION. II. INHIBITION STUDIES ON THE INTERACTION OF CONCANAVALIN A WITH POLYSACCHARIDES. Biochemistry. 1965 May;4:876–883. doi: 10.1021/bi00881a013. [DOI] [PubMed] [Google Scholar]
- Inbar M., Sachs L. Interaction of the carbohydrate-binding protein concanavalin A with normal and transformed cells. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1418–1425. doi: 10.1073/pnas.63.4.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetten A. M., Vogels G. D. Nature and properties of a Staphylococcus epidermidis bacteriocin. J Bacteriol. 1972 Oct;112(1):243–250. doi: 10.1128/jb.112.1.243-250.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapral F. A., Miller M. M. Product of Staphylococcus aureus responsible for the scalded-skin syndrome. Infect Immun. 1971 Nov;4(5):541–545. doi: 10.1128/iai.4.5.541-545.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo I., Sakurai S., Sarai Y. Purification of exfoliatin produced by Staphylococcus aureus of bacteriophage group 2 and its physicochemical properties. Infect Immun. 1973 Aug;8(2):156–164. doi: 10.1128/iai.8.2.156-164.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillibridge C. B., Melish M. E., Glasgow L. A. Site of action of exfoliative toxin in the staphylococcal scaled-skin syndrome. Pediatrics. 1972 Nov;50(5):728–738. [PubMed] [Google Scholar]
- Melish M. E., Glasgow L. A. Staphylococcal scalded skin syndrome: the expanded clinical syndrome. J Pediatr. 1971 Jun;78(6):958–967. doi: 10.1016/s0022-3476(71)80425-0. [DOI] [PubMed] [Google Scholar]
- Melish M. E., Glasgow L. A. The staphylococcal scalded-skin syndrome. N Engl J Med. 1970 May 14;282(20):1114–1119. doi: 10.1056/NEJM197005142822002. [DOI] [PubMed] [Google Scholar]
- Melish M. E., Glasgow L. A., Turner M. D. The staphylococcal scalded-skin syndrome: isolation and partial characterization of the exfoliative toxin. J Infect Dis. 1972 Feb;125(2):129–140. doi: 10.1093/infdis/125.2.129. [DOI] [PubMed] [Google Scholar]
- Millet J. Characterization of proteinases excreted by Bacillus subtilis Marburg strain during sporulation. J Appl Bacteriol. 1970 Mar;33(1):207–219. doi: 10.1111/j.1365-2672.1970.tb05245.x. [DOI] [PubMed] [Google Scholar]
- Reeder W. J., Ekstedt R. D. Study of the interaction of concanavalin A with staphylocccal teichoic acids. J Immunol. 1971 Feb;106(2):334–340. [PubMed] [Google Scholar]
- Rogolsky M., Warren R., Wiley B. B., Nakamura H. T., Glasgow L. A. Nature of the genetic determinant controlling exfoliative toxin production in Staphylococcus aureus. J Bacteriol. 1974 Jan;117(1):157–165. doi: 10.1128/jb.117.1.157-165.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit J. A., de Klerk H. C., Coetzee J. N. Properties of a Proteus morganii bacteriocin. J Gen Microbiol. 1968 Nov;54(1):67–75. doi: 10.1099/00221287-54-1-67. [DOI] [PubMed] [Google Scholar]