Abstract
The effects of obesity on asthma diagnosis, control, and exacerbation severity are increasingly recognized; however, the underlying pathophysiology of this association is poorly understood. Mainstream clinical practice has yet to adopt aggressive management of obesity as a modifiable risk factor in asthma care, as is the case with a risk factor like tobacco or allergen exposure. This review summarizes existing data that support the pathophysiologic mechanisms underlying the association between obesity and asthma, as well as the current and future state of treatment for the obese patient with asthma. Our review suggests that evidence of chronic inflammatory response linking obesity and asthma indicates a need to address obesity during asthma management, possibly using patient-centered approaches such as shared decision making. There is a need for research to better understand the mechanisms of asthma in the obese patient and to develop new therapies specifically targeted to this unique patient population.
Keywords: Asthma, obesity, primary care, phenotypes
Introduction
Asthma prevalence
Asthma is classically described as reversible inflammation of airways, characterized by recurrent attacks of shortness of breath, cough, and wheeze, affecting people of all ages. While airway obstruction and bronchial hyper-responsiveness are typically associated with asthma, these symptoms are pathologically related to other factors, such as atopy, obesity, reflux, stressors, and obstructive sleep apnea.1 Asthma prevalence is increasing worldwide, affecting over 300 million people.2 Over the period 1980–1996, asthma prevalence increased among all ages, genders, and racial groups, especially in more urbanized nations such as the US.3 Currently, 24.6 million people living in the US have been diagnosed with asthma.4
Disparities in asthma
Although asthma affects people of all ages, it disproportionately affects children.5–7 Currently, in the US, over 10 million children and adolescents have been diagnosed with asthma, making it the leading chronic childhood illness.8 Since 1999, children 5–17 years of age have demonstrated the highest prevalence rates with 109.3 per 1000 diagnosed with asthma, compared with 76.8 per 1000 in those over 18 years of age.9
Marked disparities in asthma outcomes exist for vulnerable populations such as low income, Hispanic, and African American populations.10 Significant racial inequalities exist, especially in more industrialized countries with the highest numbers of asthma prevalence. For example, in the US, asthma prevalence is 43% higher for non-Hispanic blacks compared with non-Hispanic whites.9
Even among children, these racial differences are evident.7–9 Results from the National Health Interview Survey 1997–2003 found that asthma prevalence was consistently greater among non-Hispanic black children (15.7%) compared with non-Hispanic white children (11.5%) across all levels of income.11 In addition, non-Hispanic black children are 3.6 times more likely to use the emergency department (ED) for asthma-related issues than non-Hispanic white children.12 Multiple asthma-related ED visits are considered risk factors for fatal asthma, which is reflected in the rates of asthma mortality seen among minority groups, especially African Americans.13 In 2006, non-Hispanic blacks had a rate of asthma mortality over 200% higher than non-Hispanic whites.14 Furthermore, from 2003 to 2005, the Centers for Disease Control and Prevention reported that African American children had a rate of asthma mortality 7 times higher than non-Hispanic white children.15 Reasons for poor outcomes are considered multifactorial and include: lack of continuity care, poverty, lack of transportation, limited access to care and health insurance, and not having an asthma action plan.16–18
Asthma costs
Health care costs increase in patients with more severe asthma, but the effect of asthma exacerbations on costs among patients with more severe asthma is difficult to quantify. Comparison of direct health care costs between patients with moderate/severe persistent asthma with and without exacerbations showed patients with moderate/severe persistent asthma who had exacerbations had higher total and asthma-related health care costs than those without exacerbations.19 Moreover, asthma controller medication use was higher in patients with exacerbations. Hospitalization and medications were found to be the most important cost driver of direct costs. Work and school loss accounted for the greatest percentage of indirect costs. The cost of asthma was correlated with co-morbidities, age, and disease severity. Despite the availability of effective preventive therapy, costs associated with asthma are increasing. Strategies including education of patients and physicians, and regular follow-up are required to reduce the economic burden of asthma.19
In a study of Kaiser Permanente’s Southern California System, there was a $3499 difference in direct costs for those with uncontrolled versus controlled asthma – $3298 versus $6797, respectively.20 A national study found a difference of $421221. From these numbers we can assume asthma control saves >$4000 per patient. For a typical large healthcare system with 60,000 asthma patients, if we assume control could be improved for between 2 and 10% of patients, then cost savings vary between $4.8 and $24 million dollars. When lost productivity and premature death are considered, the resultant financial burden of asthma is even higher ($56 billion).22 Because of the high burden of suffering and cost, the Institute of Medicine and Agency for Healthcare Research and Quality (AHRQ) identified asthma as a high priority condition requiring further research.23–25 Research is encouraged around patient-centered initiatives especially those targeting vulnerable, low-income and minority ethnicity patients.
Obesity and asthma
The prevalence of obesity in the US is increasing at an alarming rate. Body mass index (BMI), defined as the weight in kilograms divided by the square of the height in meters, is commonly used to classify overweight and obesity.26 In adults, a BMI between 25 and 29.9 is defined as overweight and a BMI of 30 or higher is considered obese. For children, overweight is defined as a BMI between the 85th and 94th percentile for age and gender, and obese is defined at a BMI at or above the 95th percentile for age and gender.26 The increased prevalence of obesity in adults has been accompanied by a similar increase in the prevalence of obesity in children.27 Similar to asthma, racial and ethnic disparities exist with obesity prevalence. In the US, non-Hispanic blacks have a 51% higher rate of obesity, and Hispanics have a 21% higher rate of obesity compared with non-Hispanic whites.28 Similar to adults, the combined prevalence of obesity and overweight is also higher in non-Hispanic black children (35.4%) compared with non-Hispanic white children (28.2%).29 Hispanic boys ages 6–11 have the highest combined obesity and overweight prevalence (43.9%).
The parallel increase in asthma and obesity prevalence has led to several studies examining the possible relationship between these two conditions. A study examining the trends in obesity among adults, using data from the NHANES I (1971–1975), II (1976–1980), and III (1988–1994), found that BMI increased universally among adults with asthma and those without; however, the prevalence of obesity rose more in the asthma group (21.3–32.8%) compared with the non-asthma group (14.6–22.8%).30 A retrospective study of 143 adults found a similar association between obesity prevalence and asthma severity.31 Obese asthmatics show disparities in response to therapy and have a nearly fivefold risk of hospitalizations due to exacerbations.32 Identifying new mechanisms that improve the delivery of asthma care is an important step toward advancing patient outcomes, avoiding preventable ED visits and hospitalizations, while simultaneously reducing overall healthcare costs.33,34
This relationship between asthma and obesity has also been replicated in the pediatric population. A cross-sectional study using data from the Third National Health and Nutrition Examination Survey 1988–1994 showed that one of the highest risk groups for developing asthma were children over the age of 10 with a BMI greater than or equal to the 85th percentile.35 A European study found that obesity among children 4–11 years of age was associated with asthma regardless of ethnicity, especially among girls.36 Findings from the National Longitudinal Survey of Youth, which followed more than 4000 asthma-free children for 14 years, discovered a BMI at or greater than the 85th percentile at age 2–3 years was a risk factor for subsequent asthma development in boys.37
Obesity is a risk factor for asthma in multiple demographic groups.7,38 Female gender is significantly associated with asthma and obesity.39 In addition, obese asthmatics have a decreased quality of life and increased utilization of resources compared to their non-obese counterparts.40 Factors that could contribute to the pathogenesis of asthma in the obese include both mechanical factors and altered inflammation and immune responses related to the obese state.
Obese asthma phenotypes
Pathophysiology of asthma has been described in detail.41 The disease is considered an inflammatory disease in the airway, leading to airway hyper-responsiveness, obstruction, mucus hyper-production, and airway wall remodeling. Studies in immunology and molecular biology have resulted in an extensive evaluation of inflammatory cells and mediators involved in the pathophysiology of asthma. It is recognized that airway remodeling, characterized by thickening of the airway wall, can contribute to the chronic progression of the disease. Epithelial to mesenchymal cell transitions cause persistence of the inflammatory infiltration and induce histological changes in the airway wall, increasing thickness of the basement membrane, collagen deposition, and smooth muscle hypertrophy and hyperplasia. Resulting airway inflammation and remodeling leads to the airway wall thickening and induces increased airway smooth muscle mass, which generates asthmatic symptoms. Asthma has been considered a classic T helper 2 (TH2) cell-associated inflammatory disease, with TH2-type cytokines, such as interleukin-4 (IL-4), IL-5, and IL-13, driving the disease pathology in patients. Although atopic asthma has a substantial TH2 cell component, the disease is notoriously heterogeneous, and recent evidence has suggested that other T cells also contribute to the development of asthma.42
Recent studies suggest that there are at least two distinct phenotypes of asthma in obesity (Figure 1, Table 1).43,44 The obese state alters both early onset allergic asthma and also leads to the development of a novel form of late onset asthma, in part due to obesity.45–47 Patients with the atopic phenotype are likely to have pathophysiology consistent with early onset allergic asthma that is complicated by the development of obesity. Early onset allergic asthma is characterized by TH2 driven lymphocytic inflammation with increases in cytokines such as IL-4 and IL-5 that promote airway eosinophilia and IL-13 leading to mucus hypersecretion.42,43 Atopic asthma is an inflammatory disorder characterized by accumulation of eosinophils, mast cells, and CD4+ T lymphocytes, and with remodeling of the airway.
Figure 1.
Image of asthma phenotypes for early and late onset obesity-associated asthma (A color version of this figure is available in the online journal)
Table 1.
Asthma–obesity phenotypes
| Phenotype characteristic | Early onset | Late onset | References |
|---|---|---|---|
| Increased atopy/allergen | X | Farzan44, Sideleva and Dixon45, Sideleva et al.46,47 | |
| Younger age onset | X | Farzan44, Sideleva and Dixon45, Sideleva et al.46,47 | |
| Cytokines produced by airway epithelium includes Th2 | X | Wenzel43 | |
| Tidal volumes mechanically restricted | X | Jensen49 | |
| Exacerbations reduced with corticosteroids | X | Desai et al.93 | |
| Metabolic inflammation markers increased | X | Farzan44, Sideleva and Dixon45, Sideleva et al.46,47 | |
| Airway closure improved by weight loss | X | Mahadev53 | |
| Weight gain increases asthma severity | X | Beuther and Sutherland48 and Desai et al.93 | |
| Exacerbations reduced stable weight or weight loss | X | X | Nystad et al.61 |
| Lower markers of Th2/eosinophilia | X | Farzan44, Sideleva and Dixon45, Sideleva et al.46,47 |
Adipose tissue produces a number of cytokines and adipokines which may have a synergistic adverse effect on the airways. Cytokines produced by adipose tissue include plasminogen activator inhibitor-1, monocyte chemotactic factor-1, interleukins 6 and which may affect the airway such as plasminogen activator inhibitor-1, monocyte chemotactic factor-1, IL-6 and 8, and adipokines such as leptin and adiponectin. The precise role of many of these mediators in the pathogenesis of allergic airway disease is not well known; however, a number of studies have shown the potential role of adiponectin (which is decreased in obesity) and leptin (which increases in obesity) in allergic asthma.45–47
The second phenotype relates to obese patients with later onset of asthma and a much lower prevalence of allergic disease. These individuals have late onset asthma, in which obesity plays a role. Meta-analyses demonstrate a direct correlation between obesity and increased risk of the developing non-atopic asthma characterized by this phenotype.48 This type of asthma is currently poorly understood but appears to be characterized by lower markers of airway eosinophilia and TH2 inflammation than are typical of early onset allergic asthma. Additionally, late-onset, non-atopic form of asthma is more common in women than men. A number of factors, both molecular and mechanical, could contribute to innate increases in airway reactivity and lung function with obesity. In addition to the airway hyper-responsiveness caused by the biochemical effects of adipose related cytokines and adipokines described earlier, mechanical changes in lung function and airways likely play an important role. This mechanical linkage between obesity and asthma is thought to be related to restrictive physiology.49 In obese patients, tidal volumes are decreased due to a reduction in chest expansion that is caused both by the weight on the chest itself and the effect of abdominal obesity on flattening the diaphragms. Patients with asthma have an intrinsic impairment of the ability for inspiration to stretch airway smooth muscle. Breathing at the low lung volumes seen with a restrictive lung pattern leads to increased airway hyper-responsiveness.50 It has been hypothesized that breathing at low lung volumes may lead to increased actin–myosin cross-linking in airway smooth muscle, effectively making the muscle stiffer.51 This stretch of smooth muscle is a critical determinant of airway reactivity in vivo; furthermore, in vitro studies have demonstrated that smooth muscle stretch modulates the expression of proteins in airway smooth muscle. Supporting this theory, others have shown that continuous positive airway pressure leads to decreased airway reactivity in both animal models and in humans with asthma.52 Thus, one plausible explanation for the obese asthma phenotype is the effect that breathing at low lung volumes has on smooth muscle remodeling and resultant smooth muscle function and hyper-reactivity.
Expiratory flow limitations can be caused by both a reduction in operating lung volume, as occurs in obesity, and bronchoconstriction, as occurs in asthma. Obese individuals breathe close to the closing volume of the airways, which may promote reduction in operating lung volume. Bronchoconstriction, as occurs in asthma, can also increase expiratory flow limitation during tidal breathing.53 This premature airway closure seen in obesity may have direct effects on airway caliber and airway function in the setting of obesity. Mediators produced by adipose tissue may be important in the pathogenesis of late onset asthma in obesity. Markers of adipose tissue metabolic inflammation are increased particularly in visceral fat of patients with this form of asthma.46 This was not related to enhanced airway inflammation, suggesting that these metabolic mediators could be having a direct effect on the airway.
Many studies have reported elevated serum leptin to be associated with asthma in obesity.45,54 Leptin was significantly increased in visceral adipose tissue of obese asthmatics, and this was related to airway reactivity. Leptin may have multiple effects on lung development; leptin deficient mice show decreased lung volume and alveolar surface area55 and decreased proliferation of tracheal epithelium.56 Further study should be performed to elucidate the role of leptin in the pathogenesis of asthma using conditional leptin knockout mice.
The exact mechanism creating the dose effect seen between obesity and asthma is not fully known. Proposed theories for obesity causing asthma include mechanical, dietary, genetic, and hormonal factors.57 One main theory that has generated the most discussion is the role pro-inflammatory cytokines such as leptin play in the process because adipose tissue is known as a primary source of these systemic immunomodulating agents and could be contributing to the chronic inflammation seen in asthma, creating more symptoms of the disease.57 One theory proposed, which supports the less common view that asthma causes obesity, is that individuals with asthma restrict their levels of activity for fear of inducing an asthma exacerbation, which then leads to a more sedentary lifestyle and an increased risk of obesity.58
Asthma severity – dose response
Obesity is not only a risk factor for the development of asthma in adults and children, but is also associated with worse asthma-related health outcomes as indicated by Manion,7 Guerra et al.,59 and Hjellvik et al.60 A large Norwegian study of more than 135,000 men and women found a 10% increase in asthma prevalence per unit of increase in BMI in men and a 7% increase in prevalence per unit increase in BMI in women.60 In men, the risk of asthma increased by 10% with each unit of increased BMI between 25 and 30. The similar value for women was 7%. Overweight or obese persons reported asthma more often than did normal BMI persons after adjustment for smoking, education, and physical activity.61
Diagnosis
Initially, asthma is diagnosed by physical exam and symptomatology. Signs of respiratory distress, rhinitis, nasal irritation and swelling, prolonged expiratory phases, and triggers of symptoms assist in the diagnosis. Laboratory evaluation is minimal, occasionally involving assessment for eosinophilia or an elevated IgE. Objective diagnosis is obtained by spirometry or pulmonary function tests. A reduced Forced Expiratory Volume1/Forced Vital Capacity (FEV1/FVC) ratio, with reversibility after using bronchodilators, verifies the diagnosis of asthma. Limits occur within this objective testing, primarily in obtaining reproducible flow/volume curves in young children.62 Relatively few data in this age group exist, and performing spirometry in young children requires specific expertise. Spirometry is often not feasible outside the research arena or more specialized clinical settings. Challenge testing, with methacholine, histamine, or exercise has increased specificity for diagnosing asthma, but also is rarely used in clinical settings due to expertise required to administer tests, access to testing, and other factors.63
International guidelines advise that asthma diagnosis should be based on both the presence of symptoms and objective measurements of variable airflow obstruction or bronchial hyper-responsiveness when challenged with bronchodilators.61 However, physicians frequently diagnose asthma based on symptoms without confirmation by pulmonary function tests or spirometry.63
Confirmation of asthma may be more difficult in patients who are taking regular asthma-controlling medications. Patients on inhaled corticosteroids (ICS), even for <3 months, can experience not only an improvement in symptoms but also a decrease in demonstrable airway responsiveness, even returning to the normal range on bronchial challenge testing. Objective testing, which may include negative bronchial challenge testing results or absence of change in postbronchodilator forced expiratory volume in 1 s (FEV1) can indicate either a well-controlled asthmatic or a non-asthmatic. Even among subjects not started on ICSs, confirming a physician diagnosis of asthma can be difficult if there are minimal symptoms, and hence probably minimal airway inflammation, at the time of testing.63 Because of the overlap in respiratory symptoms and compromised function, obesity creates a unique challenge in the use of symptoms for the diagnosis of asthma and places particular importance on the use of lung function test/spirometry. Indeed, obese individuals presenting for evaluation of acute respiratory symptoms are also more likely to receive a misdiagnosis of asthma.62,64 Overdiagnosis of asthma can lead to inappropriate treatment, with increased risk of side effects and increased costs.60,65–67 Multiple studies report that asthma could be excluded after extensive testing in a third of physician-diagnosed asthma.63,67,68 Underdiagnosis is also an important issue.69 Impaired dyspnea perception and poor perception of airflow obstruction may lead to undertreatment of asthma in the obese. In addition to confirming previous reports on overdiagnosis of asthma, a substantial proportion of morbidly obese asthma patients were underdiagnosed. There were few differences between the underdiagnosed and the overdiagnosed subjects.
Treatment of asthma in the obese patient
Asthma treatment involves environmental trigger avoidance, treatment of co-morbid conditions, and use of pharmacotherapy with a goal of reducing both impairment and risk for exacerbations. The National Heart, Lung, and Blood Institute (NHLBI) Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma provides clinicians with comprehensive treatment recommendations.64 The pharmacotherapy recommendations within this guideline follow a stepwise approach that is tailored to a patient’s level of impairment and risk. The treatments are directed at the underlying physiologic mechanisms of asthma and include short-acting beta agonists for symptom relief with a combination of ICSs, long-acting beta agonists (LABA), and leukotriene receptor antagonists to achieve long-term control. Currently, the NHLBI guidelines do not differentiate pharmacotherapy medication choices or dosing for asthma patients with co-morbid obesity. However, preliminary evidence suggests that the obese asthma phenotype may adversely affect standard treatment regimens, and a one-size fits all approach may not be best.65 A recent review summarizes the growing number of studies that demonstrate obese asthmatics have less response as measured by spirometry and/or symptoms to ICS with comparable decreases seen in efficacy for treatment with combined ICS/LABA.32,63,66,67 Overweight and obese children have been shown to require increased amounts of B-agonists and higher oral steroid use.69 Similarly, inhaled budesonide was less effective at improving measures of lung function and reducing ED visits/hospitalization for overweight/obese children when compared to their normal weight counterparts.70 It is perhaps in part due to these differences in therapeutic effects that lead to an increase in disease severity and exacerbations for obese patients with a nearly fivefold risk of asthma-related hospitalizations.32 Further research is needed to explore if pharmacotherapy can be more specifically tailored to the different physiologic mechanisms of disease activation in the obese asthma phenotypes.
In terms of obesity management in asthmatic patients, the NHLBI guidelines encourage clinicians to evaluate and treat co-morbid obesity, suggesting that despite limited evidence, such treatment may lead to better asthma control. In a 2012 Cochrane review, only four acceptable randomized controlled trials looked at the effect of weight loss strategies on asthma outcomes.71 The trials had methodological limitations, but one trial did show a benefit of weight reduction on asthma control. Another systematic review of asthma and weight loss found reversibility of at least one asthma outcome irrespective of whether weight loss was a result of surgical or medical intervention.72 Potential positive effects of weight loss have been demonstrated albeit at the extremes of both BMI and actual weight lost. In a cohort of 12 severely obese asthmatics, bariatric surgery and the resultant BMI decrease from 51.2 to 34.4 led to improved lung function, improved performance on methacholine challenge, and a decrease in self-reported asthma symptoms.73 Also in a study of 500 morbidly obese patients who underwent laparoscopic adjustable gastric banding surgery, greater than 80% of the patients who had asthma symptoms before surgery reported resolution or improvement in their symptoms.74 While existing studies do not provide definitive guidance on how to integrate weight loss into asthma treatment strategies, the constellation of evidence seems to suggest that weight loss would lead to improved asthma symptoms and decreased exacerbations.
Changing the paradigm of asthma treatment to include weight management and perhaps different pharmacotherapeutic regimens will require special attention to the unique barriers that underserved populations face in the treatment of asthma. These barriers include limited access to care and medications, cultural differences in understanding and accepting treatment options, and decreased health literacy.16,64,75–78 To address these complexities, innovative approaches to treatment are needed.
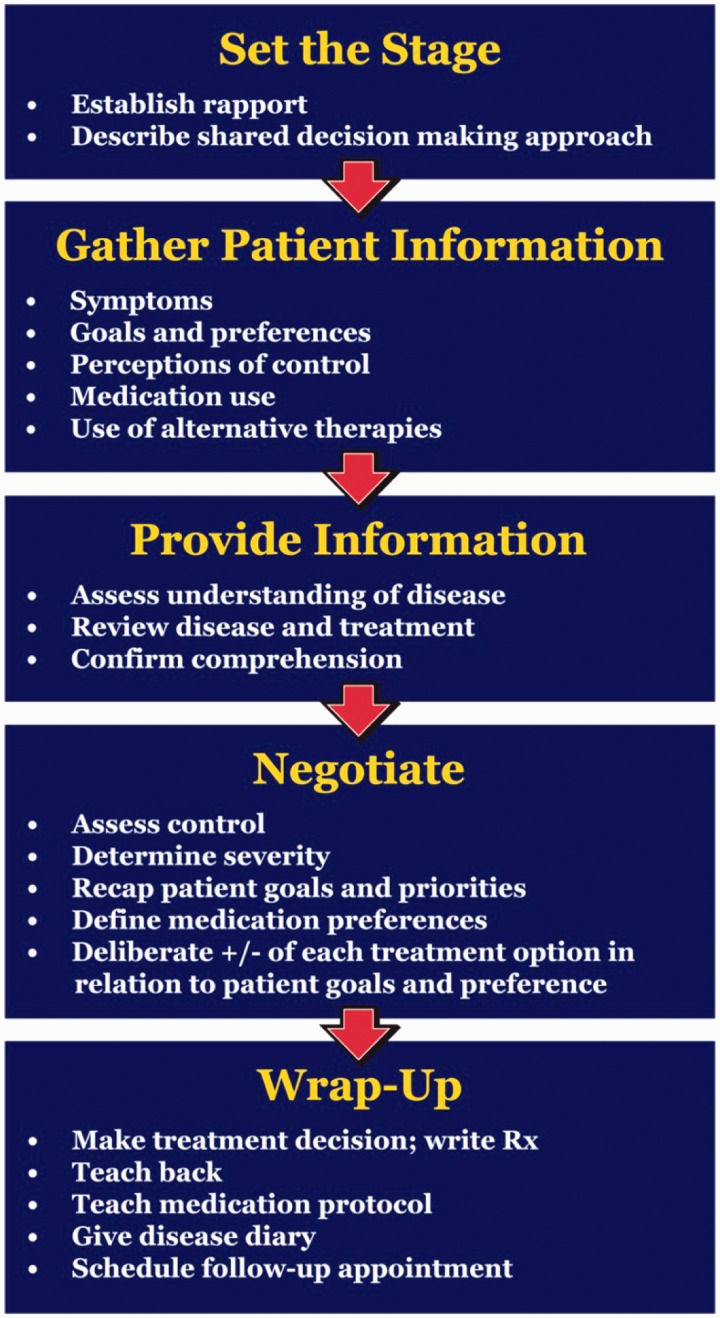
One example of a novel approach in asthma management is shared decision making (SDM) (Figure 2). SDM is a promising modality that it incorporates cultural beliefs, change theory, and health literacy by partnering with patients in understanding their asthma and developing management plans.
Figure 2.
Schematic representation of the elements of shared decision making (A color version of this figure is available in the online journal)
Patient-centered management of asthma in primary care – can SDM improve outcomes for obese patients with asthma?
Identifying new mechanisms that improve the delivery of asthma care is an important step toward advancing patient outcomes, avoiding preventable ED visits and hospitalizations, while simultaneously reducing overall healthcare costs.33
Unfortunately, many patients with asthma lack adequate control of their symptoms, thus negatively impacting their overall quality of life. Indeed, almost 50% of adults with asthma have symptoms more than once weekly and 17% have daily symptoms.79,80 Consequently, over 30% of adults with asthma go to the ED for their asthma at least once per year, resulting in one or more days of missed work.79,80
The current approach to asthma management involves monitoring symptoms and lung function, encouraging use of controller meds and trigger avoidance, patient education, and collaborative patient–provider relationships that include written asthma action plans.81 Adhering to NHLBI guidelines has been shown to be efficacious in a variety of pediatric populations, including high-risk populations such as inner city, poor, and/or African American populations.82–84 However, when patients see their primary care provider for preventative care, the majority are unlikely to be adequately evaluated and treated based on current guidelines.85,86 Clinical practice guidelines have had limited effect on changing provider behavior. Little is known about the process and factors involved in changing physician behaviors to improve guideline adherence.87,33 However, SDM may offer a solution to improve adherence to guidelines and improve patient-oriented outcomes. Only one study, by Dr Sandra Wilson and colleagues, has been published in this area.88
Building off the work of Wilson, our group led the Asthma Comparative Effectiveness (ACE) Study funded by the AHRQ to create a toolkit to assist providers with implementation of a SDM approach to asthma care that would be effective in everyday practice.89,90 Initial results show that use of the asthma SDM toolkit is associated with improved quality of life, medication adherence, reduction in utilization of acute care services, and hence cost savings. SDM interventions may not be as effective in obese patients. Using data from a randomized controlled trial of 612 adults with poorly controlled asthma,91 Ayala et al. hypothesized that obese patients would have benefitted less from the SDM intervention than did overweight or normal weight patients. Standard BMI categories were defined. Overweight SDM patients negotiated a higher daily controller dose than normal weight control. BMI negatively modified the SDM intervention effect on controller fill/refill adherence. Obese SDM patients also received a smaller intervention benefit (but still significantly better than usual care) than SDM patients in other weight groups.
The observed beneficial effects of SDM in our study and others did not change in relation to BMI for any of the clinical outcomes. Like normal weight and overweight SDM patients, obese SDM patients demonstrated significantly better clinical outcomes compared with obese patients in usual care for asthma-related health care utilization, use of rescue medication, the FEV1 and FEV1/FEV6 ratio, and the odds of reporting no asthma control problems. These results demonstrate that a SDM approach to treatment choice can clinically benefit adult patients with poorly controlled asthma, regardless of BMI.91 We performed a retrospective review of data from our ACE study. In this unpublished analysis, we hypothesized that obese asthmatics undergoing SDM have a higher baseline asthma severity and greater reduction in exacerbations following SDM. BMI was used as a determinant of obesity for adults and children. Asthma severity/control was determined by the number of asthma-related ED visits, asthma-related inpatient events, prednisone orders as a marker of exacerbations, and quality of life surveys. Baseline evaluation before the SDM intervention showed obese adults with asthma have approximately twice the rate of prednisone orders, 3× the ED visit rate, and almost double the hospitalization rate compared with non-obese patients. By gender both obese men and women had more ED visits and hospitalizations than non-obese asthmatics, while obese women had more prednisone orders than non-obese women, a trend not seen in men.
For both children and adults in the obese and non-obese, there was a trend of decreased asthma severity for the 12 months following the SDM toolkit intervention compared to the 12 months before. Obese children saw a 67% decrease in asthma severity after the SDM compared to 38% for non-obese children, though the result was not statistically significant. Findings confirmed that obesity was associated with more severe asthma symptoms at baseline in adults, particularly in women. Additionally, SDM was associated with a drop in severity for all groups. SDM appears to be effective in improving asthma severity in obese patients and may be a mechanism for explicitly incorporating weight loss strategies. Possible SDM solutions include: incorporating education about the effects of obesity on asthma control, asking patients about goals around weight loss, and providing them with education and strategies for weight loss.
Increasingly, evidence suggests that obesity and asthma are physiologically linked through a chronic inflammatory response. Increased cost through more frequent hospitalizations and reduced quality of life lead to a need to address obesity during asthma management. This would make including weight loss strategies in primary care a compelling component of asthma management. Future care involving patient-centered approaches such as SDM may offer new strategies for management of the obese asthmatic patient.
Author Contributions
This work was conceived by SM, AW, MD, and HT. Literature search was conducted by SM, HT, and AW. SM, HT, and AW contributed to the writing of the manuscript and production of the figures.
Acknowledgments
We would like to gratefully acknowledge Jake Emmerson, Peter Salathe, Catherine Courtland, and Dr Alisahah Cole for their contributions to this manuscript. This work was supported by the Agency for Healthcare Research and Quality (AHRQ) Grant Number R18HS19946-G1. The authors declare that there is no conflict of interest.
REFERENCES
- 1.Kudo M, Ishigatsubo Y, Aoki I. Pathology of asthma. Front Microbiol 2013; 4: 263–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Initiative for Asthma (GINA). The global strategy for asthma management and prevention,http://www.Ginasthma.org (2011, accessed 22 October 2013).
- 3.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980–2007. Pediatrics 2009; 123: S131–45. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control. Vital signs: asthma prevalence, disease characteristics, and self-management education: United States, 2001–2009. MMWR Morb Mortal Wkly Rep 2011; 60: 547–52. [PubMed] [Google Scholar]
- 5.Flores G. Technical report—racial and ethnic disparities in the health and health care of children. Pediatrics 2010; 125: e979–1020. [DOI] [PubMed] [Google Scholar]
- 6.Velsor-Friedrich B, Militello LK, Kouba J, Harrison PR, Manion A, Doumit R. Pediatric obesity and asthma quality of life. Nurs Clin North Am 2013; 48: 259–70. [DOI] [PubMed] [Google Scholar]
- 7.Manion AB. Asthma and obesity: The dose effect. Nurs Clin North Am 2013; 48: 151–8. [DOI] [PubMed] [Google Scholar]
- 8.Bloom B, Cohen RA, Freeman G. Summary health statistics for U.S. children: National Health Interview Survey, 2009. Vital Health Stat 10 2010; 247: 1–82. [PubMed] [Google Scholar]
- 9.American Lung Association. Trends in morbidity and mortality, 2011.
- 10.Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, Liu X. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief 2012; 94: 1–8. [PubMed] [Google Scholar]
- 11.McDaniel M, Paxson C, Waldfogel J. Racial disparities in childhood asthma in the United States: evidence from the National Health Interview Survey, 1997 to 2003. Pediatrics 2006; 117: e868–77. [DOI] [PubMed] [Google Scholar]
- 12. AHRQ U.S. Department of Health and Human Services, Agency for Healthcare Research and Quality, National Healthcare Quality and Disparities Reports. www.ahrq.gov/qual/qrdr11/6maternalchildhealth/T641411.htm (2011, accessed 31 October 2013)
- 13.Carroll CL, Uygungil B, Zucker AR, Schramm CM. Identifying an at-risk population of children with recurrent near-fatal asthma exacerbations. J Asthma 2010; 47: 460–4. [DOI] [PubMed] [Google Scholar]
- 14. CDC. Asthma prevalence, health care use and mortality: United States, 2003–2005, http://www.cdc.gov/nchs (2006, accessed 31 October 2013)
- 15.Akinbami L. The state of childhood asthma, United States, 1980–2005. Advance data from vital health statistics, Hyattsville, MD: National Center for Health Statistics, 2006. [PubMed] [Google Scholar]
- 16.Mansour ME, Lanphear BP, DeWitt TG. Barriers to asthma care in urban children: parent perspectives. Pediatrics 2000; 106: 512–9. [DOI] [PubMed] [Google Scholar]
- 17.Mansour ME, Rose B, Toole K, Luzader CP, Atherton HD. Pursuing perfection: An asthma quality improvement initiative in school-based health centers with community partners. Public Health Rep 2008; 123: 717–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMullen A, Yoos HL, Anson E, Kitzmann H, Halterman JS, Arcoleo KS. Asthma care of children in clinical practice: do parents report receiving appropriate education? Pediatr Nurs 2007; 33: 37–44. [PubMed] [Google Scholar]
- 19.Bahadori K, Doyle-Waters MM, Marra C, Lynd L, Alasaly K, Swiston J, FitzGerald JM. Economic burden of asthma: a systematic review. BMC Pulm Med 2009; 9: 24–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeiger RS, Hay JW, Contreras R, Chen W, Quinn VP, Seal B, Schatz M. Asthma costs and utilization in a managed care organization. J Allergy Clin Immunol 2008; 121: 885–92.e5. [DOI] [PubMed] [Google Scholar]
- 21.Ivanova JI, Bergman R, Birnbaum HG, Colice GL, Silverman RA, McLaurin K. Effect of asthma exacerbations on health care costs among asthmatic patients with moderate and severe persistent asthma. J Allergy Clin Immunol 2012; 129: 1229–35. [DOI] [PubMed] [Google Scholar]
- 22.Akinbami LJ, Sullivan SD, Campbell JD, Grundmeier RW, Hartert TV, Lee TA, Smith RA. Asthma outcomes: healthcare utilization and costs. J Allergy Clin Immunol 2012; 129: S49–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuhlbrigge A, Adams R, Guilbert T, Grant E, Lozano P, Janson S, Martinez F, Weiss K, Weiss S. The burden of asthma in the United States: Level and distribution are dependent on interpretation of the National Asthma Education and Prevention Program guidelines. Am J Resp Crit Care Med 2002; 166: 1044–9. [DOI] [PubMed] [Google Scholar]
- 24.Anderko L, Bartz C, Lundeen S. Practice-based research networks: Nursing centers and communities working collaboratively to reduce health disparities. Nurs Clin North Am 2005; 40: 747–58 xi–xii. [DOI] [PubMed] [Google Scholar]
- 25.Iglehart JK. Prioritizing comparative-effectiveness research—IOM recommendations. N Engl J Med 2009; 361: 325–8. [DOI] [PubMed] [Google Scholar]
- 26. CDC. Overweight and obesity, http://www.cdc.gov/obesity/adult/defining.html (2012, accessed 7 September 2013)
- 27.Maffeis C, Tato L. Long-term effects of childhood obesity on morbidity and mortality. Horm Res 2001; 55: 42–5. [DOI] [PubMed] [Google Scholar]
- 28.CDC. Differences in prevalence of obesity among black, white, and hispanic adults–United States. MMWR Morb Mortal Wkly Rep 2009; 58: 740–4. [PubMed] [Google Scholar]
- 29.Wang Y, Beydoun MA. The obesity epidemic in the United States—gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev 2007; 29: 6–28. [DOI] [PubMed] [Google Scholar]
- 30.Ford ES, Mannino DM. Time trends in obesity among adults with asthma in the United States: findings from three national surveys. J Asthma Off J Assoc Care Asthma 2005; 42: 91–5. [PubMed] [Google Scholar]
- 31.Akerman MJ, Calacanis CM, Madsen MK. Relationship between asthma severity and obesity. J Asthma Off J Assoc Care Asthma 2004; 41: 521–6. [DOI] [PubMed] [Google Scholar]
- 32.Pradeepan S, Garrison G, Dixon AE. Obesity in asthma: approaches to treatment. Curr Allergy Asthma Rep 2013; 13: 434–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tapp H, Hebert L, Dulin M. Comparative effectiveness of asthma interventions within a practice based research network. BMC Health Serv Res 2011; 11: 188–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McWilliams A, Tapp H, Barker J, Dulin M. Cost analysis of the use of emergency departments for primary care services in Charlotte, North Carolina. North Carolina Med J 2011; 72: 265–71. [PubMed] [Google Scholar]
- 35.Rodriguez MA, Winkleby MA, Ahn D, Sundquist J, Kraemer HC. Identification of population subgroups of children and adolescents with high asthma prevalence: Findings from the Third National Health and Nutrition Examination Survey. Arch Pediatr Adolesc Med 2002; 156: 269–75. [DOI] [PubMed] [Google Scholar]
- 36.Figueroa-Munoz JI, Chinn S, Rona RJ. Association between obesity and asthma in 4–11 year old children in the UK. Thorax 2001; 56: 133–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mannino DM, Mott J, Ferdinands JM, Camargo CA, Friedman M, Greves HM, Redd SC. Boys with high body masses have an increased risk of developing asthma: Findings from the National Longitudinal Survey of Youth (NLSY). Int J Obes (Lond) 2006; 30: 6–13. [DOI] [PubMed] [Google Scholar]
- 38.Dixon AE, Holguin F, Sood A, Salome CM, Pratley RE, Beuther DA, Celedon JC, Shore SA. An official American Thoracic Society Workshop report: Obesity and asthma. Proc Am Thorac Soc 2010; 7: 325–35. [DOI] [PubMed] [Google Scholar]
- 39.Jay M, Wijetunga NA, Stepney C, Dorsey K, Chua DM, Bruzzese JM. The relationship between asthma and obesity in urban early adolescents. Pediatr Allergy Immunol Pulmonol 2012; 25: 159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mosen DM, Schatz M, Magid DJ, Camargo CA., Jr The relationship between obesity and asthma severity and control in adults. J Allergy Clin Immunol 2008; 122: 507–11.e6. [DOI] [PubMed] [Google Scholar]
- 41.Kudo M, Ishigatsubo Y, Aoki I. Pathology of asthma. Front Microbiol 2013; 4: 263–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lloyd CM, Hessel EM. Functions of T cells in asthma: more than just T(H)2 cells. Nat Rev Immunol 2010; 10: 838–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wenzel SE. Asthma phenotypes: The evolution from clinical to molecular approaches. Nat Med 2012; 18: 716–25. [DOI] [PubMed] [Google Scholar]
- 44.Farzan S. The asthma phenotype in the obese: distinct or otherwise? J Allergy (Cairo) 2013; 2013: 602908–602908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sideleva O, Dixon A. The many faces of asthma in obesity. J Cell Biochem 2014;115:421–6. [DOI] [PubMed]
- 46.Sideleva O, Suratt BT, Black KE, Tharp WG, Pratley RE, Forgione P, Dienz O, Irvin CG, Dixon AE. Obesity and asthma: an inflammatory disease of adipose tissue not the airway. Am J Respir Crit Care Med 2012; 186: 598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sideleva O, Black K, Dixon AE. Effects of obesity and weight loss on airway physiology and inflammation in asthma. Pulm Pharmacol Ther 2013; 26: 455–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med 2007; 175: 661–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jensen ME, Wood LG, Gibson PG. Obesity and childhood asthma—mechanisms and manifestations. Curr Opin Allergy Clin Immunol 2012; 12: 186–92. [DOI] [PubMed] [Google Scholar]
- 50.Skloot G, Permutt S, Togias A. Airway hyperresponsiveness in asthma: A problem of limited smooth muscle relaxation with inspiration. J Clin Invest 1995; 96: 2393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fredberg JJ. Airway smooth muscle in asthma. Perturbed equilibria of myosin binding. Am J Respir Crit Care Med 2000; 161: S158–60. [DOI] [PubMed] [Google Scholar]
- 52.Busk M, Busk N, Puntenney P, Hutchins J, Yu Z, Gunst SJ, Tepper RS. Use of continuous positive airway pressure reduces airway reactivity in adults with asthma. Eur Respir J 2013; 41: 317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mahadev S, Farah CS, King GG, Salome CM. Obesity, expiratory flow limitation and asthma symptoms. Pulm Pharmacol Ther 2013; 26: 438–43. [DOI] [PubMed] [Google Scholar]
- 54.Sood A, Qualls C, Schuyler M. Leptin, adiponectin, and asthma: findings from a population-based cohort study. Ann Allergy Asthma Immunol 2010; 104: 355–355. [DOI] [PubMed] [Google Scholar]
- 55.Huang K, Rabold R, Abston E, Schofield B, Misra V, Galdzicka E, Lee H, Biswal S, Mitzner W, Tankersley CG. Effects of leptin deficiency on postnatal lung development in mice. J Appl Physiol 2008; 105: 249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsuchiya T, Shimizu H, Horie T, Mori M. Expression of leptin receptor in lung: Leptin as a growth factor. Eur J Pharmacol 1999; 365: 273–9. [DOI] [PubMed] [Google Scholar]
- 57.Davis A, Lipsett M, Milet M, Etherton M, Kreutzer R. An association between asthma and BMI in adolescents: results from the California Healthy Kids Survey. J Asthma 2007; 44: 873–9. [DOI] [PubMed] [Google Scholar]
- 58.Chinn S, Rona RJ. Can the increase in body mass index explain the rising trend in asthma in children? Thorax 2001; 56: 845–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guerra S, Sherrill DL, Bobadilla A, Martinez FD, Barbee RA. The relation of body mass index to asthma, chronic bronchitis, and emphysema. Chest 2002; 122: 1256–63. [DOI] [PubMed] [Google Scholar]
- 60.Hjellvik V, Tverdal A, Furu K. Body mass index as predictor for asthma: A cohort study of 118,723 males and females. Eur Respir J 2010; 35: 1235–42. [DOI] [PubMed] [Google Scholar]
- 61.Nystad W, Meyer HE, Nafstad P, Tverdal A, Engeland A. Body mass index in relation to adult asthma among 135,000 Norwegian men and women. Am J Epidemiol 2004; 160: 969–76. [DOI] [PubMed] [Google Scholar]
- 62.Myers TR, Tomasio L. Asthma: 2015 and beyond. Respir Care 2011; 56: 1389–407 discussion 407–10. [DOI] [PubMed] [Google Scholar]
- 63.Boulet LP, Franssen E. Influence of obesity on response to fluticasone with or without salmeterol in moderate asthma. Respir Med 2007; 101: 2240–7. [DOI] [PubMed] [Google Scholar]
- 64.Expert Panel Report 3 (EPR-3). Guidelines for the diagnosis and management of asthma—summary report 2007. J Allergy Clin Immunol 2007; 120: S94–138. [DOI] [PubMed] [Google Scholar]
- 65.Boulet LP. Asthma and obesity. Clin Exp Allergy J Br Soc Allergy Clin Immunol 2013; 43: 8–21. [DOI] [PubMed] [Google Scholar]
- 66.Telenga ED, Tideman SW, Kerstjens HA, Hacken NH, Timens W, Postma DS, van den Berge M. Obesity in asthma: More neutrophilic inflammation as a possible explanation for a reduced treatment response. Allergy 2012; 67: 1060–8. [DOI] [PubMed] [Google Scholar]
- 67.Peters-Golden M, Swern A, Bird SS, Hustad CM, Grant E, Edelman JM. Influence of body mass index on the response to asthma controller agents. Eur Respir J 2006; 27: 495–503. [DOI] [PubMed] [Google Scholar]
- 68.Sutherland ER, Lehman EB, Teodorescu M, Wechsler ME. Body mass index and phenotype in subjects with mild-to-moderate persistent asthma. J Allergy Clin Immunol 2009; 123: 1328–34.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Quinto KB, Zuraw BL, Poon KY, Chen W, Schatz M, Christiansen SC. The association of obesity and asthma severity and control in children. J Allergy Clin Immunol 2011; 128: 964–9. [DOI] [PubMed] [Google Scholar]
- 70.Forno E, Celedon JC. Predicting asthma exacerbations in children. Curr Opin Pulm Med 2012; 18: 63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Adeniyi FB, Young T. Weight loss interventions for chronic asthma. Cochrane Database Syst Rev 2012; 7: CD009339–CD009339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eneli IU, Skybo T, Camargo CA., Jr Weight loss and asthma: A systematic review. Thorax 2008; 63: 671–6. [DOI] [PubMed] [Google Scholar]
- 73.Boulet LP, Turcotte H, Martin J, Poirier P. Effect of bariatric surgery on airway response and lung function in obese subjects with asthma. Respir Med 2012; 106: 651–60. [DOI] [PubMed] [Google Scholar]
- 74.Spivak H, Hewitt MF, Onn A, Half EE. Weight loss and improvement of obesity-related illness in 500 U.S. patients following laparoscopic adjustable gastric banding procedure. Am J Surg 2005; 189: 27–32. [DOI] [PubMed] [Google Scholar]
- 75.McLean DE, Bowen S, Drezner K, Rowe A, Sherman P, Schroeder S, Redlener K, Redlener I. Asthma among homeless children: undercounting and undertreating the underserved. Arch Pediatr Adolesc Med 2004; 158: 244–9. [DOI] [PubMed] [Google Scholar]
- 76.Northridge ME, Meyer IH, Dunn L. Overlooked and underserved in Harlem: a population-based survey of adults with asthma. Environ Health Perspect 2002; 110: 217–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moudgil H, Marshall T, Honeybourne D. Asthma education and quality of life in the community: A randomised controlled study to evaluate the impact on white European and Indian subcontinent ethnic groups from socioeconomically deprived areas in Birmingham, UK. Thorax 2000; 55: 177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pachter LM, Weller SC, Baer RD, de Alba Garcia JE, Trotter RT, 2nd, Glazer M, Klein R. Variation in asthma beliefs and practices among mainland Puerto Ricans, Mexican-Americans, Mexicans, and Guatemalans. J Asthma 2002; 39: 119–34. [DOI] [PubMed] [Google Scholar]
- 79.NC State Center for Health Statistics, http://www.schs.state.nc.us/schs/brfss/2008/nc/all/asymptom.html (2008, accessed 19 March 2013).
- 80.Anderko L, Bartz C, Lundeen S. Wellness for a lifetime: Improving lifestyle behaviors of low-income, ethnically diverse populations. Ann Fam Med 2005; 3: S35–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Denlinger LC, Sorkness CA, Chinchilli VM, Lemanske RF., Jr Guideline-defining asthma clinical trials of the National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network and Childhood Asthma Research and Education Network. J Allergy Clin Immunol 2007; 119: 3–11 quiz 2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kattan M, Mitchell H, Eggleston P, Gergen P, Crain E, Redline S, Weiss K, Evans R, 3rd, Kaslow R, Kercsmar C, Leickly F, Malveaux F, Wedner HJ. Characteristics of inner-city children with asthma: The National Cooperative Inner-City Asthma Study. Pediatr Pulmonol 1997; 24: 253–62. [DOI] [PubMed] [Google Scholar]
- 83.Kattan M, Crain EF, Steinbach S, Visness CM, Walter M, Stout JW, Evans R, 3rd, Smartt E, Gruchalla RS, Morgan WJ, O'Connor GT, Mitchell HE. A randomized clinical trial of clinician feedback to improve quality of care for inner-city children with asthma. Pediatrics 2006; 117: e1095–103. [DOI] [PubMed] [Google Scholar]
- 84.Szefler SJ, Mitchell H, Sorkness CA, Gergen PJ, O’Connor GT, Morgan WJ, Kattan M, Pongracic JA, Teach SJ, Bloomberg GR, Eggleston PA, Gruchalla RS, Kercsmar CM, Liu AH, Wildfire JJ, Curry MD, Busse WW. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: A randomised controlled trial. Lancet 2008; 372: 1065–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wisnivesky JP, Lorenzo J, Lyn-Cook R, Newman T, Aponte A, Kiefer E, Halm EA. Barriers to adherence to asthma management guidelines among inner-city primary care providers. Ann Allergy Asthma Immunol 2008; 101: 264–70. [DOI] [PubMed] [Google Scholar]
- 86.Adeniyi FB, Young T. Weight loss interventions for chronic asthma. Cochrane Database Syst Rev 2012; 7: CD009339–CD009339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, Rubin HR. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA 1999; 282: 1458–65. [DOI] [PubMed] [Google Scholar]
- 88.Wilson SR, Strub P, Buist AS, Knowles SB, Lavori PW, Lapidus J, Vollmer WM, Better Outcomes of Asthma Treatment Study G. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. Am J Respir Crit Care Med 2010; 181: 566–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tapp H, Kuhn L, Alkhazraji T, Steuerwald M, Ludden T, Wilson S, Mowrer L, Mohanan S, Dulin M. Adapting community based participatory research (CBPR) methods to the implementation of an asthma shared decision making intervention in ambulatory practices. J Asthma 2014. [DOI] [PMC free article] [PubMed]
- 90.Wilson SR, Strub P, Buist AS, Knowles SB, Lavori PW, Lapidus J, Vollmer WM. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. Am J Respir Crit Care Med 2010; 181: 566–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ayala E, Wilson SR, Ma J, Knowles SB, Buist AS, Strub P, Lavori PW. Influence of body mass index on effects of a shared asthma treatment decision-making intervention. Am J Respir Crit Care Med 2012; 185: 591–3. [DOI] [PMC free article] [PubMed] [Google Scholar]