Abstract
Background
A cornerstone of neonatal resuscitation teaching suggests that a rapid vagal-mediated bradycardia is one of the first signs of perinatal compromise. As this understanding is based primarily on fetal studies, we investigated whether the heart rate and blood pressure response to total asphyxia is influenced by whether the animal is in utero or ex utero.
Methods
Fetal sheep were instrumented at ∼139 days of gestation and then asphyxiated by umbilical cord occlusion until mean arterial blood pressure decreased to ∼20 mmHg. Lambs were either completely submerged in amniotic fluid (in utero; n = 8) throughout the asphyxia or were delivered and then remained ex utero (ex utero; n = 8) throughout the asphyxia. Heart rate and arterial blood pressure were continuously recorded.
Results
Heart rate was higher in ex utero lambs than in utero lambs. Heart rates in in utero lambs rapidly decreased, while heart rates in ex utero lambs initially increased following cord occlusion (for ∼1.5 min) before they started to decrease. Mean arterial pressure initially increased then decreased in both groups.
Conclusions
Heart rate response to asphyxia was markedly different depending upon whether the lamb was in utero or ex utero. This indicates that the cardiovascular responses to perinatal asphyxia are significantly influenced by the newborn's local environment. As such, based solely on heart rate, the stage and severity of a perinatal asphyxic event may not be as accurate as previously assumed.
Introduction
Our current understanding of the cardiorespiratory responses to birth asphyxia in humans is primarily based on experiments first conducted in the 1960's by Dawes and colleagues [1]. The first sign of compromise is cessation of respiratory efforts, commonly referred to as primary apnea, which is accompanied by a profound bradycardia. This bradycardia is mediated by vagal inputs and is most strongly initiated by both hypoxia and acidosis [2]. During primary apnea, it is taught that the newborn can be stimulated to resume breathing by actions such as drying or slapping of the feet [3]. If cardiorespiratory compromise continues, the newborn may begin irregular gasping before entering “secondary or terminal” apnea when blood pressure also begins to fall. Once in secondary apnea, it is widely considered that a newborn requires intervention, most commonly in the form assisted ventilation [3].
This understanding is based on many different studies, but was first observed in newborn rhesus monkeys that were made asphyxic by placing a water-filled bag over the newborn's head after delivery [4]–[7]. Subsequently, other studies have examined and characterised this response in more detail, mainly using fetal models, with the fetus remaining in utero during asphyxia. In a recent study, we observed that the heart rate response to asphyxia, induced by umbilical cord occlusion, appeared to differ from this characteristic bradycardic response if the lambs were delivered and remained ex utero during the asphyxia period [8]. As the ex utero lambs were intubated and did not have bags of water over their heads, we hypothesised that that the presence of liquid surrounding the face, may influence the physiological response to birth asphyxia. Our aim was to document and report the cardiovascular responses to birth asphyxia in near-term lambs that either remained in utero or were ex utero during the period of asphyxia.
Methods
All experimental procedures were approved by the relevant Monash University Animal Ethics Committee in accordance with the National Health and Medical Research Council (Australia) Australian code of practice for the care and use of animals for scientific purposes (7th Edition, 2004). Pregnant ewes at 139±2 d gestation (term 147 d) were anesthetised and the fetus exposed for instrumentation via a hysterotomy; catheters were implanted into the jugular vein and carotid or femoral artery.
Lambs were then asphyxiated by total occlusion of the umbilical cord. The first group of lambs (n = 8) remained in utero, completely submerged in amniotic fluid, throughout the asphyxia period. The second group of lambs (n = 8) were intubated with a clamped cuffed endotracheal tube (4.5mm) to prevent breathing, delivered and remained ex utero throughout the period of asphyxia. For this study we analysed data on the circulatory response for the first 10 minutes after occlusion of the cord. Heart rate and arterial blood pressure were continuously recorded (DTX Plus Transducer; Becton Dickinson, Singapore) from prior to delivery until the end of the experiment. Arterial blood gases were collected at the end of asphyxia.
Analytical methods
Heart rate (HR) and mean arterial pressure (BP) were averaged over 5 s epochs every 30 s 3 min before and for the 10 min study period after umbilical cord occlusion. The carotid arterial pressure waveform was also analysed for end diastolic pressure (BPED; an indicator of downstream vascular resistance) and pulse amplitude (BPamp; an indicator of stroke volume). Maximum rate of blood pressure increase during systole (max dP/dt; an indicator of cardiac contractility) was calculated by converting arterial pressure into its first derivative with respect to time.
Statistical methods
Data were analysed using 2-way repeated measures ANOVA with group (in utero vs. ex utero) and time as factors. Post hoc comparisons between groups and time points were performed using the Holm-Sidak test. Data are presented as mean ± SEM unless otherwise stated.
Results
Although the duration of asphyxia was similar between groups at ∼11 min (Table 1) and the target BP for terminating the asphyxia was the same, the BP at end asphyxia was higher in the ex utero lambs compared to in utero lambs (22.3±4.7 and 17.8±3.6 mmHg respectively). Similarly, at the end of the asphyxia period, PaCO2 levels were higher and the pH tended to be lower in in utero lambs, compared to ex utero lambs (Table 1).
Table 1. Lamb arterial blood gases and physiological parameters at the end of asphyxia.
| in utero | ex utero | |
| Asphyxia duration (min) | 11.1±1.4 | 11.2±1.3 |
| BP (mmHg) | 17.8±3.6* | 22.3±4.7 |
| pH | 6.2±1.9 | 6.9±0.04 |
| PaCO2 (mmHg) | 161.3±36.7* | 100.8±27.0 |
| PaO2 (mmHg) | 3.4±1.0 | 4.9±5.3 |
| SaO2 (%) | 2.0±0.7* | 9.0±6.8 |
| Lactate (mmol/L) | 10.1±2.2 | 8.3±3.3 |
| BE (mmol/L) | −12.2±4.0 | −17.0±3.0 |
BP, arterial blood pressure; PaCO2, partial pressure of carbon dioxide; PaO2, partial pressure of oxygen; SaO2, oxygen saturation; BE, base excess. Data are mean ± SD. * p<0.05 in utero vs ex utero.
HR in ex utero lambs was significantly higher than in utero lambs for the first 10 min of asphyxia. Almost immediately following umbilical cord occlusion, HR in in utero lambs rapidly decreased, resulting in a ∼50% reduction within 2 min (Figure 1). In contrast, HR in ex utero lambs initially increased following cord occlusion (for ∼1.5 min) by ∼15% before they started to decrease; at 2 min they were not different from before cord occlusion. Mean BP initially increased then decreased in both groups. BP was higher in ex utero lambs from 6.5 to 10 min after asphyxia onset (Figure 2).
Figure 1. Heart rate (HR), expressed as a percentage change from before cord clamping, measured in in in utero (•) or ex utero (○) lambs before and after umbilical cord occlusion (designated as time 0).
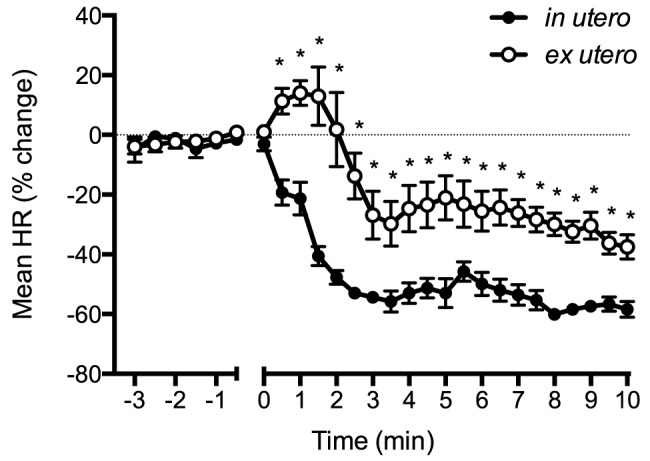
Data are mean ± SEM. * p<0.05 in utero vs ex utero.
Figure 2. Mean blood pressure (BP), expressed as a percentage change from before cord clamping measured in in utero (•) or ex utero (○) lambs before and after umbilical cord occlusion (designated as time 0).
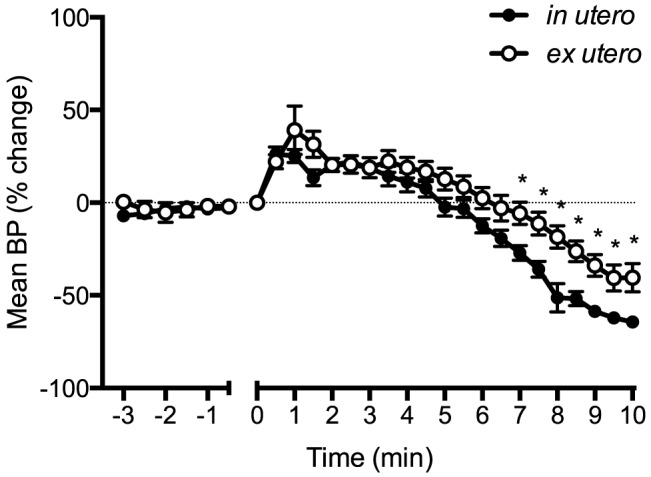
Data are mean ± SEM. * p<0.05 in utero vs ex utero.
BPED initially increased in both groups by ∼40% within 1.5 min after asphyxia onset in both groups before decreasing (Figure 3). After 6 min of asphyxia, BPED in in utero lambs decreased below ex utero lambs. BPamp in in utero lambs increased by ∼70% within 2.5 min following cord occlusion, then decreased to pre-occlusion values by ∼9 min (Figure 4). In contrast, BPamp in ex utero lambs only increased by ∼25% within the first 3 min of asphyxia before decreasing. Max dP/dt was higher in in utero lambs for the first 2 min of asphyxia compared to ex utero lambs (Figure 5), with the max dP/dt immediately increasing by ∼50% within 2.5 min of asphyxia in in utero lambs before decreasing before. Max dP/dt initially decreased in ex utero lambs by ∼25% before increasing.
Figure 3. End diastolic pressure (BPED) expressed as a percentage change from before cord clamping measured in in utero (•) or ex utero (○) lambs before and after umbilical cord occlusion (designated as time 0).

Data are mean ± SEM. * p<0.05 in utero vs ex utero.
Figure 4. Arterial pressure pulse amplitude (BPamp) expressed as a percentage change from before cord clamping measured in in utero (•) or ex utero (○) lambs before and after umbilical cord occlusion (designated as time 0).
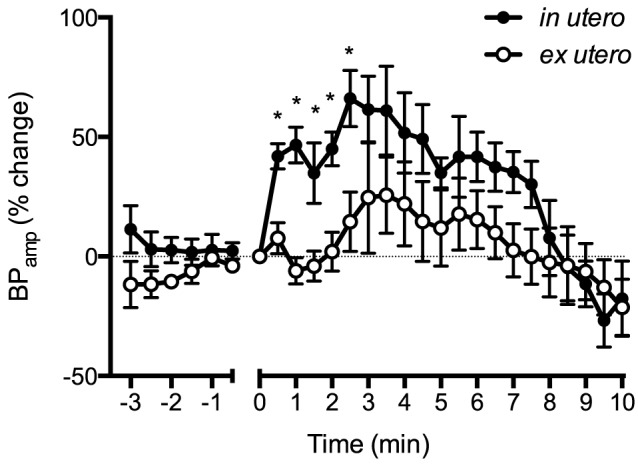
Data are mean ± SEM. * p<0.05 in utero vs ex utero.
Figure 5. Max dP/dt expressed as a percentage change from before cord clamping measured in in utero (•) or ex utero (○) lambs before and after umbilical cord occlusion (designated as time 0).
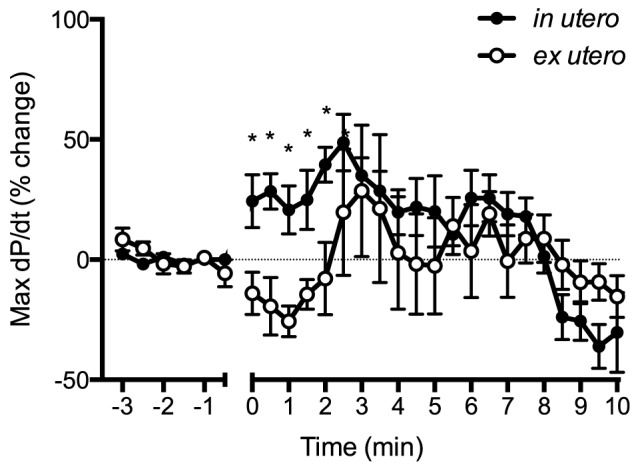
Data are mean ± SEM. * p<0.05 in utero vs ex utero.
Discussion
These data demonstrate that the heart rate and arterial pressure response to asphyxia, induced by umbilical cord occlusion, differs markedly depending upon whether the lambs were in utero and submerged in amniotic fluid or were ex utero during the period of asphyxia. When lambs remained in utero, heart rates fell abruptly in response to umbilical cord occlusion, decreasing by ∼50% within 2 mins (Figure 1). This HR decrease is a well-characterised response to fetal asphyxia, is vagally mediated and is most pronounced when hypoxemia and acidemia are combined [2], [5]. However, if lambs were delivered and asphyxiated ex utero, the heart rate initially increased before slowly decreasing, but only decreased to a maximum of ∼35% of pre-occlusion levels at 10 mins.
We consider that the most likely explanation for these findings is a complicated mix of altered stimuli, leading to a modification of the asphyxia-induced, vagal-mediated, bradycardia in ex utero lambs. This could have resulted from greater physical stimulation during delivery in ex utero lambs (first 30 secs), but this stimulation lasted for <1 min and so is unlikely to account for the continuing differences in heart rate. Indeed, we have recently shown that cord clamping in ex utero lambs that are not physically stimulated fails to elicit this increase in HR [9]. Instead, the HR decreases, albeit at a much slower rate and to a lessor degree than that occurs in response to a vagal-mediated bradycardia, as occurs in utero. As such, we consider that the vagally-mediated bradycardia may have been modified by the absence of fluid surrounding the lambs face. If correct, this suggests that the vagally-mediated bradycardia that occurs in utero in response to asphyxia includes a component of the “diving reflex”. This reflex is a vasovagal response triggered by water contact on the face, resulting in an immediate apnea, bradycardia and peripheral vasoconstriction that is independent of arterial oxygen tension [10], [11]. It is interesting that the original studies described by Dawes and colleagues used fetal monkeys that had water-filled bags placed over their heads to prevent ventilation onset [4]–[7].
It is also interesting that despite major differences in heart rate, the blood pressure was similar between groups, particularly over the first couple of minutes of asphyxia (Figure 2). While this could be partially explained by a greater peripheral vasoconstriction in in utero lambs we found that BPED, an indication of downstream vascular resistance, initially increased similarly in both groups (Figure 3). To compensate for the lower HR, in utero lambs may have increased combined ventricular stroke volume. This is supported by the finding of a greater increase in BPamp in response to asphyxia, compared to ex utero lambs (Figure 4), possibly resulting from increased ventricular contractility. Indeed, max dP/dt, an approximation of heart contractility, was immediately increased in in utero lambs upon the onset of asphyxia (Figure 5). However, as preload is reduced following cord clamping, due to the loss in umbilical venous return, this increase in contractility could only have occurred in response to a large increase in sympathetic drive. Indeed, a reduction in preload would normally result in a reduction in stroke volume and likely explains the reduction in max dP/dt we observed in ex utero lambs immediately following cord clamping. This suggests that the primary difference in cardiac function response between the two groups of lambs is the change in autonomic drive to the heart in response to cord clamping. That is, the change is much greater in in utero lambs, compared to ex utero lambs, but further studies are required to elucidate the exact mechanisms.
The immediate increase in stroke volume after the onset of asphyxia in in utero lambs is curious. It is widely accepted that the neonate's ability to increase stroke volume in response to bradycardia is limited [12] as the fetal heart is usually performing at the top of its cardiac function curve. As such, it is commonly assumed that neonates respond similarly and can only increase cardiac output by increasing heart rate [12]–[14]. This limitation is believed to be due to immaturity of the myocardium and increased myocardial stiffness [15]. Conversely, other studies have found the Frank Starling relationship is effective in the fetal lamb heart and cardiac output also relies on end-diastolic volume and heart contractility [16]–[18]. These mechanisms exist at birth to enhance neonatal ventricular output [17]. However, these studies were conducted in fetuses and newborns days to weeks after birth. As yet, the changes in cardiac function during the transition at birth are not well understood.
Whatever the mechanism, it is clear that the heart rate response to perinatal asphyxia is complex and possibly includes a component of the “diving reflex” when it occurs in utero. As a result, heart rates are not solely determined by oxygenation levels and may not be a good indicator of the extent of the asphyxia or of the underlying cardiovascular function. Indeed, in ex-utero lambs, the heart rate initially increased which may have been in response to the physical stimuli. Alternatively it may reflect a sympathetic mediated “stress” response to the hypoxia, as previously described in newborns, which is masked by an over-riding vagal response in utero [19]–[21].
It is interesting that, at the end of the asphyxia period, PaCO2 levels were higher and SaO2 levels were lower in in utero lambs compared to ex utero lambs. As the recorded values are well outside normal ranges, it is possible that the differences are not real and reflect measurement errors, particularly the saturation levels. However, as the arterial pressure decrease was significantly lower in in utero lambs, it is possible that they were more compromised, particularly as arterial pressure was lower than in ex utero lambs at the end of the asphyxia period.
These data suggest that our current understanding of the cardiovascular responses to a perinatal asphyxic event do not indicate the stage and severity as accurately as previously assumed. In view of our findings, further studies are urgently required to fully characterise the cardiovascular responses to perinatal asphyxia and to understand how vagal reflexes, such as the diving reflex, may impact on these responses.
Supporting Information
Heart rate (% change from fetal) of individual in utero and ex utero asphyxia animals from start of asphyxia.
(PDF)
Mean blood pressure (% change from fetal) of individual in utero and ex utero asphyxia animals from start of asphyxia.
(PDF)
End diastolic pressure (% change from fetal) of individual in utero and ex utero asphyxia animals from start of asphyxia.
(PDF)
Pulse amplitude (% change from fetal) of individual in utero and ex utero asphyxia animals from start of asphyxia.
(PDF)
Max dP/dt (% change from fetal) of individual in utero and ex utero asphyxia animals from start of asphyxia.
(PDF)
Acknowledgments
We thank Ms. Karyn Rodgers for their assistance with these experiments.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the Supporting Information files.
Funding Statement
Funding was provided by the National Health and Medical Research Council (NHMRC) of Australia program grant (No. 384100), fellowship (GRP: 1026890, TJM: APP1043294, SBH: 545921), a Rebecca L. Cooper Medical Research Foundation Fellowship (GRP) and the Victorian Government's Operational Infrastructure Support Program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Dawes GS (1968) Foetal and neonatal physiology: a comparative study of the changes at birth. Year Book Medical Publishers; Chicago, USA.
- 2. Giussani DA, Spencer JA, Moore PJ, Bennet L, Hanson MA (1993) Afferent and efferent components of the cardiovascular reflex responses to acute hypoxia in term fetal sheep. J Physiol 461: 431–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kattwinkel J (2011) Neonatal Resuscitation (NRP) Textbook, 6th edition. American Academy of Pediatrics and American Heart Association.
- 4. Daniel SS, Dawes GS, James LS, Ross BB, Windle WF (1966) Hypothermia and the resuscitation of asphyxiated fetal rhesus monkeys. J Pediatr 68: 45–53. [DOI] [PubMed] [Google Scholar]
- 5. Adamsons K Jr, Behrman R, Dawes GS, Dawkins MJ, James LS, et al. (1963) The Treatment of Acidosis with Alkali and Glucose during Asphyxia in Foetal Rhesus Monkeys. J Physiol 169: 679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Adamsons K Jr, Behrman R, Dawes GS, James LS, Koford C (1964) Resuscitation by Positive Pressure Ventilation and Tris-Hydroxymethylaminomethane of Rhesus Monkeys Asphyxiated at Birth. J Pediatr 65: 807–818. [DOI] [PubMed] [Google Scholar]
- 7. Dawes GS, Jacobson HN, Mott JC, Shelley HJ, Stafford A (1963) The Treatment of Asphyxiated, Mature Foetal Lambs and Rhesus Monkeys with Intravenous Glucose and Sodium Carbonate. J Physiol 169: 167–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klingenberg C, Sobotka KS, Ong T, Allison BJ, Schmolzer GM, et al. (2013) Effect of sustained inflation duration; resuscitation of near-term asphyxiated lambs. Arch Dis Child Fetal Neonatal Ed 98: F222–227. [DOI] [PubMed] [Google Scholar]
- 9. Bhatt S, Alison BJ, Wallace EM, Crossley KJ, Gill AW, et al. (2013) Delaying cord clamping until ventilation onset improves cardiovascular function at birth in preterm lambs. J Physiol 591: 2113–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tchobroutsky C, Merlet C, Rey P (1969) The diving reflex in rabbit, sheep and newborn lamb and its afferent pathways. Respir Physiol 8: 108–117. [DOI] [PubMed] [Google Scholar]
- 11. Gooden BA (1994) Mechanism of the human diving response. Integr Physiol Behav Sci 29: 6–16. [DOI] [PubMed] [Google Scholar]
- 12. Shaddy RE, Tyndall MR, Teitel DF, Li C, Rudolph AM (1988) Regulation of cardiac output with controlled heart rate in newborn lambs. Pediatr Res 24: 577–582. [DOI] [PubMed] [Google Scholar]
- 13. Gilbert RD (1980) Control of fetal cardiac output during changes in blood volume. Am J Physiol 238: H80–86. [DOI] [PubMed] [Google Scholar]
- 14. Rudolph AM, Heymann MA (1976) Cardiac output in the fetal lamb: the effects of spontaneous and induced changes of heart rate on right and left ventricular output. Am J Obstet Gynecol 124: 183–192. [DOI] [PubMed] [Google Scholar]
- 15. Romero T, Covell J, Friedman WF (1972) A comparison of pressure-volume relations of the fetal, newborn, and adult heart. Am J Physiol 222: 1285–1290. [DOI] [PubMed] [Google Scholar]
- 16. Kirkpatrick SE, Pitlick PT, Naliboff J, Friedman WF (1976) Frank-Starling relationship as an important determinant of fetal cardiac output. Am J Physiol 231: 495–500. [DOI] [PubMed] [Google Scholar]
- 17. Anderson PA, Glick KL, Killam AP, Mainwaring RD (1986) The effect of heart rate on in utero left ventricular output in the fetal sheep. J Physiol 372: 557–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Anderson PA, Killam AP, Mainwaring RD, Oakeley AE (1987) In utero right ventricular output in the fetal lamb: the effect of heart rate. J Physiol 387: 297–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stahlman M, Gray J, Young WC, Shepard FM (1967) Cardiovascular response of the neonatal lamb to hypoxia and hypercapnia. Am J Physiol 213: 899–904. [DOI] [PubMed] [Google Scholar]
- 20. Brady JP, Ceruti E (1966) Chemoreceptor reflexes in the new-born infant: effects of varying degrees of hypoxia on heart rate and ventilation in a warm environment. J Physiol 184: 631–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Purves MJ (1966) The effects of hypoxia in the new-born lamb before and after denervation of the carotid chemoreceptors. J Physiol 185: 60–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Heart rate (% change from fetal) of individual in utero and ex utero asphyxia animals from start of asphyxia.
(PDF)
Mean blood pressure (% change from fetal) of individual in utero and ex utero asphyxia animals from start of asphyxia.
(PDF)
End diastolic pressure (% change from fetal) of individual in utero and ex utero asphyxia animals from start of asphyxia.
(PDF)
Pulse amplitude (% change from fetal) of individual in utero and ex utero asphyxia animals from start of asphyxia.
(PDF)
Max dP/dt (% change from fetal) of individual in utero and ex utero asphyxia animals from start of asphyxia.
(PDF)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the Supporting Information files.


