Summary
The mouse model has greatly contributed to understanding molecular mechanisms involved in the regulation of progesterone (P4) plus estrogen (E) dependent blastocyst implantation process. However, little is known about contributory molecular mechanisms of the P4-only dependent blastocyst implantation process that occurs in species like hamsters, guineapigs, rabbits, pigs, rhesus monkeys and perhaps humans. We utilized the hamster as a model of P4-only dependent blastocyst implantation and performed cross-species microarray (CSM) analyses to reveal differentially expressed genes at the blastocyst implantation site (BIS), in order to advance the understanding of molecular mechanisms of implantation. Upregulation of 112 genes and downregulation of 77 genes at the BIS were identified using a mouse microarray platform, while use of the human microarray revealed 62 up- and 38 down-regulated genes at the BIS. Excitingly, a sizable number of genes (30 up- and 11 down-regulated genes) were identified as a shared pool by both CSMs. Real-time RT-PCR and in situ hybridization validated expression patterns of several up- and down-regulated genes identified by both CSMs at the hamster and mouse BIS to demonstrate the merit of CSM findings across species, in addition to revealing genes specific to hamsters. Functional annotation analysis found that genes involved in the spliceosome, proteasome and ubiquination pathways are enriched at the hamster BIS, while genes associated with tight junction, SAPK/JNK signaling, PPARalpha/RXRalpha signalings are repressed at the BIS. Overall, this study provides a pool of genes and evidence of their participation in up- and down-regulated cellular functions/pathways at the hamster BIS.
Introduction
The blastocyst implantation process in mammals is considered a remarkable evolutionary strategy for pregnancy success. A crucial component of this process includes the preparation of a receptive uterus under the control of maternal ovarian steroids (Paria et al. 1993). Studies have revealed that ovarian progesterone (P4) is indispensable for the preparation of the uterus, while ovarian estrogen (E) utilization strategies vary, even within the rodent family members. For example, the uterus of mice, rats and gerbils require exposure to both P4 and E, whereas only ovarian P4 ensures preparation of the receptive uterus and blastocyst implantation in hamsters and guinea pigs (Reese et al. 2008). Other species like rabbits, pigs, horses, rhesus monkeys, and possibly humans utilize a similar P4-dependent blastocyst implantation as found in hamsters and guinea pigs (Reese et al. 2008). Interestingly, studies have suggested that in some of these P4-dependent species such as rabbits, pigs, horses, hamsters and humans the blastocyst represents a potential alternative source of estrogen for implantation (reviewed in Reese et al. 2008). Despite the striking commonality of maternal P4-dependent blastocyst implantation in hamsters and guinea pigs with the majority of other species, the use of hamsters or guinea pigs in identifying the molecules and/or molecular pathways that are enriched or suppressed at the blastocyst implantation site (BIS) compared with the interimplantation site (IIS) has been limited.
The uterus of mice, hamsters and humans consists of two cellular compartments, the myometrium and endometrium. The endometrium is divided into two layers and consists of a simple columnar epithelium and an underlying stroma that contains glands made of epithelial cells, blood vessels, and bone marrow-derived immune cells, i.e. macrophages and lymphocytes. Bone-marrow-derived immune cells are also found in the myometrial compartment. Histological and ultrastructural studies of the early stages of implantation in the mouse, hamster and human have demonstrated that trophoblast cells of the blastocyst make contact with uterine epithelial cells to initiate the process of implantation. While hamsters and mice exhibit an eccentric type of implantation, where the blastocyst lies within a uterine crypt, blastocyst implantation in humans is interstitial, where the blastocyst is completely embedded within the stromal tissue of the uterus. However, unlike the human in which polar trophoblast cells of the blastocyst initiate initial contact with endometrial uterine luminal cells, mural trophoblastic cells of blastocysts initiate contact with endometrial luminal epithelial cells in mice and hamsters (Reese et al. 2008; Lee & DeMayo 2004). Luminal epithelial cells that are in contact with the blastocyst are larger than luminal epithelial cells of the interimplantation site (WARD 1948). Ultrastructural studies have demonstrated that in contrast to mice and humans where the epithelial cell surface becomes flattened and microvilli are replaced with pinopods (Aplin 1991), epithelial microvilli persist during implantation in hamsters (Parkening 1976a). Finally, while loss of mucin1 (a component of epithelial apical glycocalyx) occurs from the uterine epithelium at the time of implantation in mice (DeSouza et al. 1999), no evidence of reduction of mucin1 from apical surface of the uterine epithelium was observed in hamsters and humans (Reese et al. 2008; Hey et al. 1995). The uterine glands are few in number in the vicinity of the BIS as compared with IIS where the glands are more numerous. Contact between the trophoblast and luminal epithelial cells at the BIS, initiate transformation of stromal fibroblasts into decidual cells at the antimesometrial area. In hamsters and mice, decidualization is evident in the early phase of implantation. In contrast, the process of decidualization at the implantation site of humans is not evident until about 5 days after the embryo initiates contact with the stroma (Parkening 1976b; Parkening 1976a; Aplin 1991). A dramatic redistribution of uterine macrophages takes place at the BIS of mice, such that macrophages egress from the implantation site. In this regard, macrophage distribution at the day 5 BIS of hamsters is unknown due to lack of such studies in this species. Currently, the molecules involved in initiating the blastocyst implantation process have largely been derived from studies in mice using various approaches such as expression of individual genes, phenotypic characterization of genetically altered mice and transcriptomics. Despite these in-depth studies in mice, our understanding of the molecular regulation of the implantation process in humans is incomplete. Since several species from the rodent to primate showed ovarian P4-dependent blastocyst implantation, and the hamster and human showed certain striking similarities in early implantation events, the hamster could serve as an excellent rodent model for identifying common gene networks at the blastocyst implantation site (BIS) of P4-dependent species or explaining differences in the molecular mechanisms between the P4- and E-dependent blastocyst implantation processes and that perhaps might be more relevant in understanding the blastocyst implantation mechanisms in the human. However, this becomes difficult due to the unavailability of Syrian hamster microarray platforms and lack of transgenic technologies. The transcriptome of the Chinese hamster ovary (CHO) cell line has been unveiled by next generation sequencing (Becker et al. 2011; Rupp et al. 2014). However, Schmucki and coworkers (Schmucki et al. 2013) have demonstrated that the CHO transcriptome has limited value in identifying transcripts of lipid metabolism in the Syrian golden hamster liver, due to: 1) different CHO cell lines containing different chromosome numbers owing to the effects of passaging and toxicant exposures; 2) extensive use of these cell lines in industries perhaps leading to genomic instability by chemical and radiation exposures (Hammond et al. 2012; Xu et al. 2011) and 3) Syrian hamster chromosome numbers (44) are twice the number of chromosomes (22) found in Chinese hamsters. Thus, our study explored the utility of using gene arrays of closely related mouse species and distantly related human species in identifying differential gene expression patterns at the hamster BIS keeping in mind that the information obtained from these CSM hybridizations not identify important and/or rare transcripts due to insufficient gene sequence homology.
We hypothesize that blastocyst implantation in the hamster results from the simultaneous change in expression of a pool of genes that has functions pertinent to P4-dependent implantation. Results of our microarray analysis identified a set of upregulated and downregulated genes at the BIS as compared with their expression pattern at the IIS of hamsters. This pool of differentially expressed genes at the BIS of hamsters contains some previously identified genes involved in mouse implantation, as well as newly revealed additional genes, allowing us to discern relevant biological processes or pathways specific for the P4-dependent implantation site.
Materials and Methods
Reagents
All reagents used in this study were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise specified.
Animals
Sexually-mature CD1 mice and Syrian Golden hamsters were purchased from Charles River Laboratories (Wilmington, MA). Animals were maintained under controlled environmental conditions [room temp. 23±2°C; relative humidity (50–60%); lighting conditions (12 h light/12 h dark, switched on/off at 0700/1900 h)] in our Institutional Animal Facility with unlimited access to food and water ad libitum. All experimental protocols using these animals were approved by the Vanderbilt University Animal Care and Use Committee.
Preparation of animals for experiments and tissue collections
Female hamsters that showed at least three consecutive 4-day estrous cycles were mated with fertile males overnight on the evening of proestrus. The presence of sperm in the vaginal smear the next morning (estrus) indicated the first day of pregnancy. For mouse pregnancies, female and male mice were bred overnight and the presence of vaginal plug the following morning indicated day 1 of pregnancy (Zhang & Paria 2006).
Uterine blastocyst implantation sites (BISs) and inter-implantation sites (IISs) from hamsters and mice on day 5 of pregnancy were identified after an intravenous injection of Chicago Blue B dye solution (Zhang & Paria 2006). For microarray and real-time RT-PCR analysis, BISs were dissected from IISs and subjected to total RNA extraction in TRIzol reagent according to the manufacturer’s instruction (Life technologies, Gaithersburg, MD) (Reese et al. 2001; Lei et al. 2013). Three sets of RNA were isolated from three different animals.
Microarray analysis
Total RNA was collected as previously described (Reese et al. 2001) then analyzed for purity and quantified. Three separate pair-wise labeling, hybridization, and scanning analyses were performed using GeneChip® Mouse Expression 430A or GeneChip® Human Genome U133A containing over 22,000 probe sets, representing 14,500 well-characterized mouse and human genes. All microarray data have been deposited at Gene Expression Omnibus (GEO) database (number: GSE59474). Raw.CEL files were uploaded into Partek Genomics Suite version 6.6 (Partek Incorporated, St. Louis, MO) and processed using Robust Multi-chip Average (RMA) normalization (Bolstad et al. 2003; Irizarry et al. 2003). Following RMA normalization, Partek was used to perform pairwise comparisons of average group values and one-way ANOVA for analysis of BIS versus IIS. Principal component analysis and hierarchal cluster analyses were conducted using Partek default settings. Only probes that resulted in a fold-change of at least 1.5 and p<0.05 without multiple hypothesis correction, were considered as significantly altered genes. In order to compare differentially expressed gene lists obtained from mouse and human microarray platforms, human and mouse orthologs were searched using the bioDBnet website (http://biodbnet.abcc.ncifcrf.gov). Functional analyses of canonical pathways, and molecular and cellular functions that were altered by differentially expressed genes, was performed using Ingenuity Pathway Analysis (IPA) software. In addition, identification of overrepresented gene ontologies and KYOTO Encyclopedia of Genes and Genome (KEGG) pathways were performed using DAVID (http://david.abcc.ncifcrf.gov). Statistical analyses [including Benjamini & Hochberg (B-H) correction for multiple hypothesis testing] were performed to identify relevant gene sets.
Quantitative real-time reverse transcription polymerase chain reaction
To confirm the microarray results, representative genes were chosen at random for analysis with quantitative real-time reverse transcription polymerase chain reaction (RT-PCR). DNase treated total RNAs (1 μg) were reversed transcribed using oligo(dT) primers according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA). One microliter of the first strand was amplified in 25 μl of total volume in an iCycler (Bio-Rad laboratories, Inc., Hercules, CA) using iQ™ SYBER Green Supermix; Bio-Rad). The following PCR protocol was used: 95°C for 3 minutes followed by 40 cycles of 95°C for 10 seconds and 60°C for 30 seconds. All reactions were run in triplicates. The quantification was performed by the iQ™ 5 Standard Edition Optical System Version 2.0 (Lei et al. 2013). Data from real-time PCR analysis was normalized to hypoxanthine phosphoribosyltransferase (Hprt), a commonly used reference gene (Wang et al. 2011). Primer sequences for each gene are listed in Table 1.
Table 1.
Primers used for cloning and real-time RT-PCR
| Gene name | Sequence (5′ to 3′) S, Sense; AS, Antisense | Accession # | Size (bp) | Application |
|---|---|---|---|---|
| Actg2 | S: CGCCCTAGACATCAGGGT AS: TTCTGGTGCTACTCGAAGC |
NM_009610 | 189 | Real-time RT-PCR |
| Acvr2a | S: CGGCATTGTTTTGCTACCTG AS: TGTGTGACTTCCATCTCCGG |
XM_005068951 | 200 | Real-Time RT-PCR |
| Atp1b1 | S: ATGGGTTGTGTTGTGCTCC AS: ACATGATGCCTCCAGAGA |
XM_005071373 | 179 | Real-time RT-PCR |
| Cd24a | S: ACCCACGCAGATTTACTGCA AS: CGTTTCCTGGCCTGAGTCTC |
NM_009846 | 191 | Real-time RT-PCR |
| E2f8 | S: GAGAAATCCCAGCCGAGTC AS: CATAAATCCGCCGACGTT |
NM_001013368 | 157 | Real-time RT-PCR |
| E2f8 | S: CAGTCAAGTGAACCCAGGAA AS: CGGGTTGTAAGTAGATGGCA |
XM_005083212 | 234 | Cloning |
| Fst | S: GCCTGTGGGATTTCAAGGTT AS: CAGCTTCCTTCATGGCACAC |
XM_005082770 | 135 | Real-time RT-PCR |
| Fst | S: TCTGCCAGTTCATGGAGGAC AS: CACATTCGTTGCGGTAGGTT |
XM_005082770 | 378 | Cloning |
| Gadd45a | S: CGTGCTAGTGACGAACCCAC AS: ATTGAGATGCCATCACCGTT |
XM_005076070 | 139 | Real-time RT-PCR |
| Hnrnpd | S: ATCCTATCACAGGGCGATCA AS: GGCCCGTTTAGGATCAATGA |
XM_005068122 | 125 | Real-time RT-PCR |
| Hoxb6 | S: AACAGTTCCTCTTTTGGGCC AS: TCCTTTTTCCACTTCATGCG |
XM_005075884 | 200 | Real-time RT-PCR |
| Hprt | S: CTTGCTGGTGAAAAGGACCTC TCGAAG AS: TGAAGTACTCATTATAGTCA AGGGCAT |
NM_013556 | 115 | Real-time RT-PCR |
| Itm2b | S: AGCTGCTCGCTACCAGACAA AS: GATGGACGTGTTCAGAGGA |
XM_005070971 | 197 | Real-time RT-PCR |
| Klf9 | S: CAGTCTGGAGAGTCCCGATG AS: CCAGAGTGGAGGAGGGAGA |
XM_005063225 | 139 | Real-time RT-PCR |
| Lmna | S: GCTGCACTGAGCACTGCTCT AS: GGGTCTGCAGCCTGTTCTC |
XM_005080125 | 163 | Real-time RT-PCR |
| Nppc | S: AGCGGTCTGGGATGTTAGTG AS: CCTCCCCTCCCCAAATAATA |
NM_010933 | 202 | Real-time RT-PCR & Cloning |
| Prdx4 | S: CGGATCACTCCCTGCATCTA AS: TGAGCTCCTTGAATTCTCCG |
NM_016764 | 96 | Real-time RT-PCR |
| Psmb3 | S: GTGACCACGGACTTCCAGAA AS: TACAGAAGGTTGGCCACCAT |
XM_005139336 | 185 | Real-Time RT-PCR |
| Ran | S: CTTGGAGTTTGTTGCCATGC AS: TCCAGCTTCGCTTTCTCACA |
XM_005079847 | 148 | Real-Time RT-PCR |
| Ran | S: TTTGTTGCCATGCCTGCTC AS: GCCCATTCATCTCCTTCAGC |
XM_005079847 | 265 | Cloning |
| Ube2c | S: CCCCAGTGGCTACCCTTACA AS: TGCGGACCACTTATCCTTGA |
XR_219744 | 118 | Real-time RT_PCR |
| Ube2c | S: CCTGAATCAGACAACCTGTTC AS: GCGGACCACTTATCCTTGAG |
XR_219744 | 212 | Cloning |
| Ypel3 | S: ACAGGTCTTCATGCTGTCGC AS: CCAGCCGTTGTCTTTGATCA |
XM_005064410 | 150 | Real-Time RT-PCR |
In situ hybridization using radioactive probes
Plasmids bearing hamster or mouse cDNAs for Nppc and E2f8 were linearized and transcribed using appropriate RNA polymerases and labeled with 35S for in situ hybridizations. All labeled sense and antisense cRNA probes used for hybridizations had specific activities of approximately ≅ 2 × 109 dpm/μg. The protocol was followed as previously described by our group. Briefly, frozen uterine sections were mounted onto poly-L-lysine-coated slides and fixed in cold 4% paraformaldehyde solution in phosphate buffered saline for 15 min on ice. After prehybridization, sections were hybridized to 35S-labeled antisense probes at 45°C for 4 h in 50% formamide hybridization buffer. Sections were also hybridized with 35S-labeled sense probes as negative control. After hybridization and washing, sections were incubated with RNase A at 37°C for 20 min. RNase A resistant hybrids were detected by autoradiography using Kodak NTB-2 liquid emulsion (Eastman Kodak Company, Rochester, NY). The slides were then stained with hematoxylin and eosin (Lei et al. 2013; Wang et al. 2004).
In situ hybridization with digoxigenin-labeled probe
After linearization of plasmids bearing hamster Fst, Ran and Ube2c cDNAs, digoxigenin-labeled antisense and sense cRNA probes were transcribed in vitro using the DIG RNA Labeling Kit (Roche Applied Science, Mannheim, Germany). In situ hybridization with digoxigenin-labeled probe was performed as described previously (Lei et al. 2012). After fixing with 4% paraformaldehyde solution in PBS, frozen sections were hybridized with antisense or sense cRNA probes at 55°C for 16 h, respectively. Sections were then washed and incubated in sheep anti-Digoxigenin-AP (1:5,000; Roche Applied Science). The signal was visualized with diluted NBT/BCIP Stock Solution (Roche Applied Science). All of the sections were counterstained with 1% methyl green.
Statistical analysis
All experiments were repeated at least three times using different specimens. Statistically significant (p<0.05) differences in gene expression between each pair of experiments and the corresponding control samples were analyzed using Student’s t-test.
Results
1) Overview of differentially expressed genes identified by the mouse and human gene chip microarray analyses
Exploratory analysis of the microarray data was performed, anticipating a clear and distinct pattern of gene expression at the hamster BIS due to the presence of the implanted blastocyst at this site compared with the IIS. Principal component analysis (PCA), on the entire probe sets within the mouse and human genome microarray platforms, provided a 3-dimensional view of global gene expression changes between independent samples. We found BIS were easily distinguishable from IIS for each of the three individual chip sets (Fig. 1A: blue dots vs. red dots, respectively) for both of the microarray platforms. Despite variation in probe expression patterns among individual IIS observed in both microarrays, there was a clear separation from the BIS, which were very similar to one another across the genome.
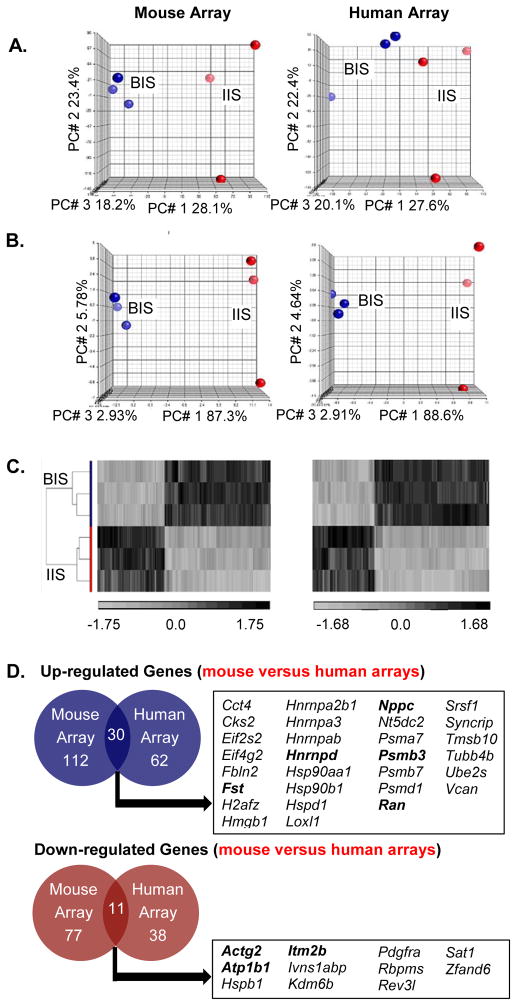
Figure 1. Transcriptome of the hamster uterine blastocyst implantation site (BIS) is distinct from interimplantation site (IIS).
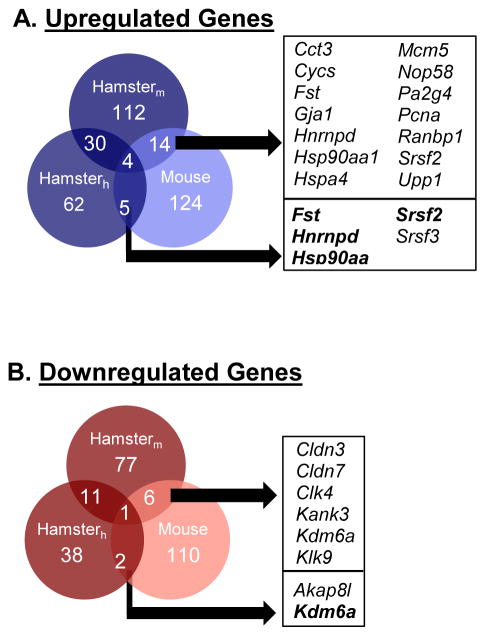
A. Principal Component Analysis (PCA) on the entire probe sets using mouse and human microarray platforms showed two distinct transcriptomes with clear separation of BIS samples (red) from IIS samples (blue). The effect of blastocyst implantation is visible in all three repeats. B. PCA of differentiated genes [at least 1.5-fold (p<0.05)] demonstrated a clearer separation of the BIS (red) from the IIS (blue). C. Hierarchical clustering (heatmap) showed complete separation of differentially expressed 227 (mouse array: left panel) and 123 (human array: right panel) probes (at least 1.5-fold, p value < 0.05) between the BIS and IIS. Values shown are log base 2, and black, light grey, and medium gray indicate the highest, lowest, and median normalized signal values, respectively. Vertical dendrograms represent the three individual samples of hamster BIS and IIS. D. Venn diagrams illustrate the overlap of gene transcripts that were at least 1.5-fold (p<0.05) different between hamster embryo implantation and interimplantation sites in both human and mouse Affymetrix cDNA microarrays. Official symbols of the shared genes are presented in the box and bolded gene transcripts represent those whose mRNA levels were validated using real-time RT-PCR analysis.
Microarray comparison of transcriptomal profiles between BIS and IIS tissues showed that, among 2581 significantly expressed probes, 227 probes were differentially expressed with at least 1.5-fold changes and confidence interval of 95% using the mouse microarray platform. Furthermore, use of the human array platform revealed a total of 1948 oligonucleotide probes that were significantly expressed, with 123 probes expressed at least 1.5-fold difference (p<0.05) between BIS and IIS. PCA analysis performed using only the differentially expressed probes between BIS and IIS, more successfully distinguished BIS and IIS samples (Fig. 1B) compared to PCA using the entire probe set (Fig. 1A).
In order to visually illustrate the pattern of the differentially expressed genes at the BIS and IIS, we constructed a heat map (representing the highest and lowest normalized signal values, black and grey respectively) using unsupervised hierarchical clustering (HC; Fig. 1C). HC showed that the 227 and 123 differentially expressed probes obtained by using mouse and human microarray platforms, respectively, were distinctly separated into BIS and IIS, as observed with the PCA analysis (Fig. 1B), suggesting that these transcripts may have biological relevance to the blastocyst implantation process in hamsters.
In Affymetrix gene expression arrays, several genes are represented by more than one oligonucleotide probe from different regions of a same gene, in order to measure internal consistency of the data set. We found 189 genes were represented by the 227 differentially expressed (≥1.5-fold, p<0.05) mouse probes, and 100 genes were represented by the 123 differentially expressed human probes. Genes identified by multiple probe sets are listed in Table 2.
Table 2.
Differentially expressed genes identified by more than one probe.
| Gene Symbol/#Probes | ||||
|---|---|---|---|---|
|
| ||||
| Mouse | Human | |||
| Upregulated | Cct3 | 4 | Eif4a1 | 2 |
| Chpt1 | 2 | Fst | 2 | |
| Eif4a1 | 2 | Gspt1 | 2 | |
| Eif5a | 2 | H2afz | 2 | |
| Gapdh | 4 | Hnrnpd | 3 | |
| Gja1 | 3 | Psme3 | 2 | |
| Hnrnpab | 4 | Srsf1 | 3 | |
| Hsp90aa1 | 2 | Syncrip | 2 | |
| Hyou1 | 2 | Tuba1b | 5 | |
| Ran | 3 | Tuba1c | 2 | |
| Sdc1 | 2 | Tubb3 | 2 | |
| Set | 2 | |||
| Srsf1 | 4 | |||
| Syncrip | 3 | |||
| Tmsb10 | 2 | |||
| Ywhag | 2 | |||
|
| ||||
| Downregulated | App | 2 | Atp1b1 | 2 |
| Atp1b1 | 2 | Fhl1 | 3 | |
| Ccnl2 | 2 | |||
| Cd24a | 2 | |||
| Hspb1 | 2 | |||
| Igkc | 2 | |||
| Ogt | 2 | |||
| Sgms1 | 2 | |||
| Zbtb16 | 2 | |||
| Zfand6 | 2 | |||
Among the 189 differentially expressed genes identified by the mouse array, 112 (59%) showed upregulation and 77 (41%) exhibited downregulation at the BIS compared with the IIS (Fig. 1D). Of the 100 genes identified using the human array, 62 (62%) were enriched in the BIS, whereas 38 (38%) showed increased expression in the IIS (Fig. 1D). Remarkably, use of two different microarray platforms identified a sizable number of shared genes that were differentially expressed between BIS and IIS (Fig. 1D, official gene symbols provided); 30 of which were upregulated, while 11 were downregulated at the BIS. A complete list of all significantly differentially expressed genes obtained by both mouse and human microarray platforms is presented in supplemental Tables S1&S2, while the top ten upregulated and downregulated genes in the BIS identified by the mouse and human array platforms can be found in Tables 3&4, respectively.
Table 3.
Top 10 differentially expressed genes identified by mouse microarray platforms.
| Variation | Gene Symbol | Gene Name | Fold-change | p-value |
|---|---|---|---|---|
| Up | Fst | Follistatin | 3.275 | 1.14E-03 |
| Cycs | Cytochrome c, somatic | 3.022 | 2.14E-04 | |
| Hnrnpab | Heterogeneous nuclear ribonucleoprotein A/B | 2.482 | 3.37E-06 | |
| Hyou1 | Hypoxia up-regulated 1 | 2.466 | 4.78E-03 | |
| Manf | Mesencephalic astrocyte-derived neurotrophic factor | 2.233 | 1.13E-02 | |
| Tmsb10 | Thymosin, beta 10 | 2.231 | 4.14E-03 | |
| Nppc | Natriuretic peptide type C | 2.212 | 2.93E-04 | |
| Nhp2 | NHP2 ribonucleoprotein | 2.200 | 3.00E-02 | |
| Ada | Adenosine deaminase | 2.159 | 1.04E-02 | |
| Lrrc59 | Leucine rich repeat containing 59 | 2.190 | 7.80E-05 | |
| Down | Hspb1 | Heat shock protein 1 | −2.150 | 3.21E-03 |
| Ivns1abp | Influenza virus NS1A binding protein | −2.150 | 6.81E-04 | |
| Igkc | immunoglobulin kappa constant | −2.184 | 1.46E-02 | |
| Tagln | Transgelin | −2.400 | 1.13E-02 | |
| Sat1 | Spermidine/spermine N1-acetyl transferase 1 | −2.425 | 8.03E-03 | |
| Itm2b | Integral membrane protein 2B | −2.556 | 2.58E-03 | |
| Actg2 | Actin, gamma 2, smooth muscle, enteric | −3.337 | 2.01E-02 | |
| Ogt | O-linked N-acetylglucosamine (GlcNAc) transferase (UDP-N-acetylglucosamine:polypeptide- | −3.393 | 3.08E-05 | |
| Cd24a | CD24a antigen | −3.960 | 8.89E-06 | |
| Atp1b1 | ATPase, Na+/K+ transporting, beta 1 polypeptide | −4.002 | 8.31E-04 |
Genes bolded were confirmed to be differentially expressed using real-time RT-PCR analysis.
Table 4.
Top 10 differentially expressed genes identified by human microarray platform.
| Variation | Gene Symbol | Gene Name | Fold-change | p-value |
|---|---|---|---|---|
| Up | Fst | Follistatin | 3.891 | 4.74E-04 |
| Vcan | Versican | 2.762 | 1.26E-03 | |
| Cks2 | CDC28 protein kinase regulatory subunit 2 | 2.551 | 6.12E-03 | |
| Eif5a | Eukaryotic translation initiation factor 5A | 2.532 | 3.99E-04 | |
| Hsp90aa1 | Heat shock protein 90kDa alpha (cytosolic), class A member 1 | 2.397 | 1.36E-03 | |
| Ran | RAN, member RAS oncogene family | 2.359 | 3.05E-04 | |
| H2afz | H2A histone family, member Z | 2.120 | 2.40E-03 | |
| Loxl1 | Lysyl oxidase-like 1 | 2.119 | 5.74E-03 | |
| Tuba1b | Tubulin, alpha 1b | 2.077 | 1.83E-04 | |
| Ndc1 | NDC1 transmembrane nucleoporin | 1.943 | 3.85E-03 | |
| Down | Ncoa2 | Nuclear receptor coactivator 2 | −1.787 | 2.72E-02 |
| Glul | Glutamate-ammonia ligase | −1.791 | 9.03E-03 | |
| Bcl6 | B-cell CLL/lymphoma 6 | −1.896 | 5.51E-03 | |
| Srsf7 | Serine/arginine-rich splicing factor 7 | −1.900 | 1.10E-03 | |
| H2afy | H2A histone family, member Y | −1.904 | 2.46E-02 | |
| Sat1 | Spermidine/spermine N1-acetyltransferase 1 | −1.985 | 1.32E-02 | |
| Hspb1 | Heat shock 27kDa protein 1 | −2.056 | 8.58E-03 | |
| Hoxb6 | Homeobox B6 | −2.077 | 7.22E-03 | |
| Fhl1 | Four and a half LIM domains 1 | −2.327 | 5.53E-03 | |
| Atp1b1 | ATPase, Na+/K+ transporting, beta 1 polypeptide | −4.074 | 1.85E-04 |
Genes bolded were confirmed to be differentially expressed using real-time RT-PCR analysis.
2) Real time RT-PCR and in situ hybridization validation of differentially expressed genes obtained from the mouse and human arrays
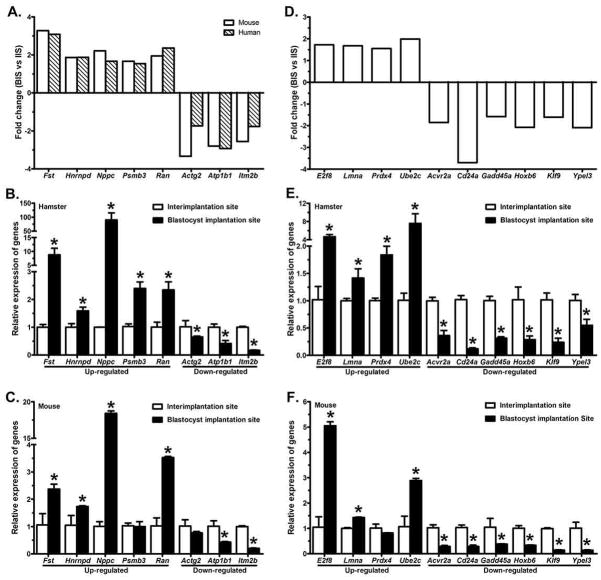
A total of 18 protein coding genes, identified by either or both array platforms, were randomly selected for validation studies using real-time RT-PCR analysis (Fig. 2). Fold-change in expression obtained from the microarray analysis of these genes is presented in Figs. 2A&D. In order to determine whether the differential expression pattern of these genes is specific to the hamster BIS, we chose to also examine their relative mRNA abundance at the BIS and IIS of the mouse. Of the 18 genes examined, 18 had mRNA levels that were significantly different between hamster BIS and IIS (Figs. 2B&E), while only 15 transcripts were differentially expressed between mouse BIS and IIS (Figs. 2C&F).
Figure 2. Real-time RT-PCR validated the expression of 18 altered genes relative to the house keeping gene, Hprt.
A–C: gene transcripts that were identified by both mouse and human gene array platforms; D-F: gene transcripts that were identified by either mouse or human gene array platform; A&D: fold-change from microarrays; B&E: real-time RT-PCR analysis of differentially expressed genes in hamsters; C&F: real-time RT-PCR analysis of differentially expressed genes in mice. Real-time RT-PCR data is shown as mRNA levels in the blastocyst implantation site (black bars) relative to the interimplantation site (white bars). Changes in gene expression were determined in triplicate by real-time PCR. Error bars represent ± SD (n=3). *p<0.05 (student’s t test).
Of the shared gene transcripts examined, all 5 up-regulated (Fst, Hnrnpd, Nppc, Psmb3 and Ran) and 3 down-regulated (Actg2, Atp1b2 and Itm2b) showed significant (p<0.05) differential expression at the BIS compared with the IIS of the hamsters (Fig. 2B). Among these 8 genes, one up-regulated (Psmb3) and one down-regulated (Actg2) gene showed no significant (p>0.05) change between the BIS and IIS of the mouse (Fig. 2C). As previously stated, we randomly selected a total of 11 differentially expressed genes that were either identified only by the mouse or the human arrays (i.e. unshared; Fig. 2D) for validation by RT-PCR. All 3 upregulated genes (E2f8, Prdx4 and Ube2c) that were identified by the mouse array showed significant (p<0.05) upregulation at the BIS compared with the IIS of hamsters (Fig. 2E). Among these 3 genes, E2f8 and Ube2c showed significant (p<0.05) upregulation at the BIS of the mouse, while Prdx4 showed no significant (p>0.05) differences between the BIS and IIS of mice (Fig. 2F). The protein coding Lmna gene that was identified by the human array showed significantly (p<0.05) higher expression at the BIS compared to the IIS of both the hamster (Fig. 2E) and mouse (Fig. 2F). In agreement with the microarray results, all 6 downregulated protein coding genes (Acvr2a, Cd24a, Klf9 and Ypel3 identified by the mouse array; Gadd45a and Hoxb6 identified by the human array) at the BIS showed significantly (p<0.05) decreased expression at the BIS compared with their expression patterns at the IIS of both species (Figs. 2E&2F).
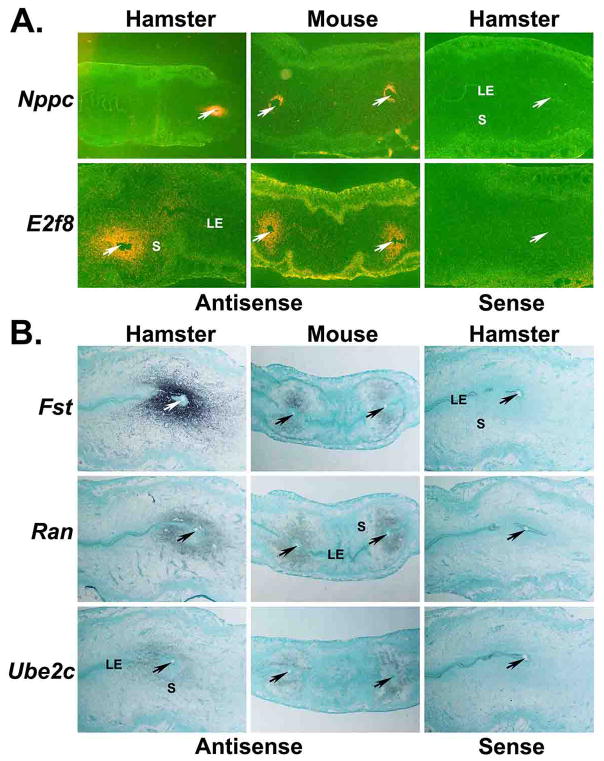
Next, we randomly selected five genes (Nppc, E2f8, Fst, Ran and Ube2C), whose upregulation at the BIS was confirmed by real-time PCR analysis, for further analysis using in situ hybridization in order to examine their uterine site of localization and cell-type specific expressions using longitudinal uterine sections containing BIS and ISS from hamsters and mice on day 5 of pregnancy. Nppc and E2f8 signals were localized in stromal cells, but not in the luminal epithelium, at the BISs of both the hamster and mouse (Fig. 3A). There was no expression of these genes in cells of the implanted blastocyst. Nppc and E2f8 signals were not noted in any uterine cells away from the BIS. At the BIS of the hamster and mouse, Fst, Ran and Ube2c mRNA expressions were found in the stromal cells away from the implanted blastocyst, but not in the subluminal stromal cells (Fig. 3B). Expression of mRNAs of Ran and Ube2c, but not Fst, was observed in the implanted blastocyst. Hybridization signals of these genes were not eminent in any uterine cell types of the IISs of either hamsters or mice (Fig. 3B). Together, results of real-time PCR and in situ hybridization showed correlative mRNA expression patterns of Nppc, E2f8, Fst, Ran and Ube2C at the BIS.
Figure 3. Longitudinal sections of the day 5 blastocyst implantation sites from hamsters and mice were processed for in situ hybridization to demonstrate site and cell-specific expression of differentially expressed genes.
A: representative dark-field images (20×) of three experiments using 35S-labled probes. B: representative bright-field images (20×) of three experiments using digoxigenin-labeled probes. Arrows indicate location of the implanted blastocyst. LE, luminal epithelium; S; stroma
3) Functional classification of significantly upregulated and downregulated genes at the BIS
During the microarray data analysis, we identified differential expression of multiple subtypes of the same gene, as well as multiple members of the same family of genes at the BIS, providing potential insight into enriched biological molecular & cellular functions or pathways involved in the blastocyst implantation process. In particular, several proteasomes (Psma7, Psmb3, Psmb7, Psmc4, Psmd1 and Psme3), tubulins (Tuba1b, Tuba1c, Tuba3a, Tubb3, Tubb4b and Tubb5), eukaryotic translation initiation factors (Eif2s2, Eif4a1, Eif4g2, Eif5a and Eif6), heat shock proteins (Hspd1, Hspa4, Hspa5, Hsp90aa1 and Hsp90b1), heterogeneous nuclear ribonucleoproteins (Hnrnpab, Hnrnpa2b1, Hnrnpa3, Hnrnpd and Hnrnpu) and ubiquitins (Ube2c, Ube2k, Ube2m and Ube2s) were identified as upregulated by either or both microarray platforms at the hamster BIS. Similarly, we also noticed that downregulated genes at the BIS, identified by using the mouse and human microarray platforms, also contained two families of genes that had more than 3 members: homeobox (Hoxa9, Dlx5, Hoxb6, Hoxb8 and Hox11as) and zinc-finger proteins/domains (Zbtb16, Zfp36l2, Zfand6 and Zc3h11a). Biological function was assessed by analyzing enriched molecular & cellular functions and canonical pathways using IPA software, as well as cluster analysis of gene ontology (biological processes) and KEGG pathways using the DAVID online tool.
Molecular and Cellular Functions
Ingenuity pathway analysis revealed that the enriched molecular and cellular functions at BIS, identified by both microarray platforms, included: cellular growth & proliferation, RNA post-transcriptional modification, cellular development, protein synthesis and cell death (Table 5). Similar molecular and cellular functions were enriched in IIS (Table 5), as the BIS, including: cell death & survival, cellular growth & proliferation, cellular development, RNA post-transcriptional modification (mouse array only) and protein synthesis (human array only). A unique molecular and cellular function was found to be enriched only in IIS compared to BIS: cell cycle (mouse array only), cellular assembly and organization (human array only).
Table 5.
Enriched or repressed molecular and cellular functions identified by the Ingenuity Pathway Analysis at the hamster blastocyst implantation site (BIS)
| Order/Platform | Molecular and Cellular Function | B-H p-value | |
|---|---|---|---|
| Upregulated at BIS | |||
| 1 | Mouse | Cellular growth and proliferation | 5.72E-13 |
| 1 | Human | 1.11E-07 | |
| 2 | Mouse | RNA Post-Transcriptional Modification | 1.85E-08 |
| 2 | Human | 7.48E-07 | |
| 3 | Mouse | Cell death and survival | 6.02E-08 |
| 5 | Human | 1.96E-05 | |
| 4 | Mouse | Cellular development | 1.30E-06 |
| 3 | Human | 2.19E-06 | |
| 5 | Mouse | Protein Synthesis | 3.78E-06 |
| 4 | Human | 1.48E-05 | |
| Downregulated at BIS | |||
| 1 | Mouse | Cell death and survival | 1.22E-07 |
| Human | 1.23E-05 | ||
| 2 | Mouse | Cellular Growth and Proliferation | 6.75E-05 |
| Human | 2.27E-04 | ||
| 3 | Mouse | Cell Development | 5.05E-03 |
| Human | 1.65E-03 | ||
| 4 | Mouse | RNA post-transcriptional modification | 6.07E-03 |
| 4 | Human | Cell assembly and organization | 1.80E-03 |
| 5 | Mouse | Cell cycle | 6.07E-03 |
| 5 | Human | Protein Synthesis | 3.69E-03 |
Biological Pathways and Processes
Canonical pathway analysis of both arrays by IPA revealed the most enriched molecular pathways (-log(B-H p-value ≥ 1.00) at the BIS including protein ubiquitination, hypoxia signaling in the cardiovascular system, aldosterone signaling in epithelial cells, glucocorticoid receptor signaling, aryl hydrocarbon receptor signaling, epithelial adherens junction and gap junction signaling and RAN signaling pathways (Table 6). Often, individual genes were found in more than one category. As for example, genes associated with the protein ubiquitin pathway were also associated with hypoxia signaling in the cardiovascular system, aldosterone signaling in epithelial cells and glucocorticoid receptor signaling. Cluster analysis of GO biological processes and KEGG pathway analysis of upregulated genes identified at the BIS by both arrays showed dominance of processes related to splicesome, proteasome, cell cycle, macromolecular and protein complex biogenesis/assembly, gap junction, pathogenic E. coli infection, cytoskeletal organization, protein localization/transport’ RNA transport/localization and mesenchymal development (Table 7). The pathways that were diminished at the BIS included: tight junction signaling, SAPK/JNK signaling, PPARalpha/RXRalpha activation & function and epithelial adherens junction signaling. However, these pathways were only significantly [(-log (B-H p-value) greater than or equal to 1.0)] altered using genes provided by the mouse array platform (Table 8). Cluster analysis of KEGG pathways and GO biological processes (Table 9) identified regulation of steroid and lipid metabolic processes as the most enriched annotation cluster, which included genes identified as the SAP/JNK pathway using IPA.
Table 6.
Overrepresented canonical pathways at the blastocyst implantation site identified by the Ingenuity Pathway Analysis
| Canonical pathway | −log(B-H p-value) | Representative genes (M: mouse array; H: Human array; S: Shared between mouse and human arrays | |
|---|---|---|---|
| Protein Ubiquitination Pathway | 5.49 | M: Hspa4, Hspa5, Psmc4, Ube2c | S: Hsp90aa1, Hsp90b1, Hspd1, Psma7, Psmb3, Psmb7, Psmd1, Ube2s |
| 5.02 | H: Psme3, Ube2m | ||
| Hypoxia Signaling in the Cardiovascular System | 1.61 | M: Ube2c | S: Hsp90aa1, Hsp90b1, Ube2s |
| 2.89 | H: Ube2m | ||
| Aldosterone Signaling in Epithelial Cells | 1.21 | M: Hspa4, Hspa5 | S: Hsp90aa1, Hsp90b1, Hspd1 |
| 1.30 | |||
| Glucocorticoid Receptor Signaling | 1.00 | M: Fkbp4, Hspa4, Hspa5 | S: Hmgb1, Hspa4, Hspa5 |
| 1.35 | H: Polr2e | ||
| Aryl Hydrocarbon Receptor Signaling | 0.93 | M: Aldh1a2, Mcm7 | S: Hsp90aa1, Hsp90b1 |
| 1.35 | H: Ccna2 | ||
| Epithelial Adherens Junction Signaling and Gap Junction Signaling | 0.55 | M: Tuba3a/Tuba3b (Tuba3e), Tubb5 (Tubb) | S: Tubb4b |
| 2.76 | H: Tubb3, Tuba1a, Tuba1b, Tuba1c | ||
| RAN Signaling | 2.11 | M: Kpnb1, Ranbp1 | S: Ran |
| 1.93 | H: Xpo1 | ||
Table 7.
Enriched biological processes at the blastocyst implantation site identified by DAVID.
| AC # | DAVID: GO Biological Process/KEGG Cluster | ES | Benjamini p-value | Representative genes (M: mouse array; H: Human array; S: Shared between mouse and human arrays | |
|---|---|---|---|---|---|
| 1 | RNA processing/splicing, Splicesome | 3.97 | 1.70E-02 | M: Hnrnpu, Nhp2, Pabpn2, Pa2g4, Ppih, Snrpa, Snrpa1, Sfpq, Snrpd2, Tra2b, Wdr12 | S: Hnrnpa2b1, Hnrnpa3, Hnrnpd, Syncrip |
| 4 | 2.37 | 1.50E-02 | H: Gspt1, Polr2e, Prmt5, Tardbp1, Wt1 | ||
| 1 | Cell cycle, proteosome, ubiquitin-mediated proteolysis | 3.09 | 2.30E-04 | H: Ccna2, Eif4g2, Eif5a, Fst, Gspt1, Hmgb1, Hnrnpab, Hnrnpd, Hspd1, Nppc, Psme3, Ran, Sssca1, Tardbp, Tubb3, Ube2m, Wt1, Ywhae | S: Hsp90b1, Psma7, Psmb3, Psmb7, Psmd1, Ube2s |
| 7 | Proteosome/Ubiquitin-mediated proteolysis | 1.29 | 1.20E-02 | M: Pcolce, Psmc4, Psmd1, Ube2c, Ube2k, Vcp | |
| 2 | Cell Cycle | 2.99 | 8.00E-05 | H:Ccna2, Eif4g2, Fzd2, Gspt1, Nasp, Ppm1g, Psmd1, Psme3, Sssca1, Tardbp, Tubb3, Ywhag | S: Cks2, Ran |
| 6 | 1.29 | 3.10E-01 | M: Anp32b, E2f8, Khdrbs1, Mcm5, Mcm7, Nudc, Ranbp1, Spag5, Tubb5, Ube2c | ||
| 3 | Macromolecular and Protein Complex Biogenesis/Assembly, Gap Junction, Pathogenic E. Coli Infection | 2.55 | 7.30E-03 | H: Cks2, Hsp90aa1, Hspd1, Mif, Nasp, Polr2e, Prmt5, Ran, Tuba1b, Tuba1c, Tubb3, Xpo1 | S: H2afz |
| 2 | Macromolecular and Protein Complex Biogenesis/Assembly | 2.29 | 1.20E-01 | M: Calr, Eif6, Fkbp4, Kpnb1, Rrm1, Set, Tuba3a, Tubb5 | |
| 6 | Cytoskeletal organization | 1.15 | H: Cks2, Ran, Tmsb10, Tuba1b, Tuba1c, Tubb3 | ||
| 3 | Protein localization/transport | 1.98 | 2.30E-01 | M: Kpnb1, Nop58, Nutf2, Pcna, Ranbp1, Rrbp1, Tomm40, Vcp | S: Eif5a, Ran, Tmsb10, Ywhag |
| 5 | 1.42 | 4.60E-02 | H: Atp2a2, Hnrnpa2b1, Hsp90aa1, Hsp90b1, Nasp, Xpo1 | ||
| 4 | RNA transport/localization | 1.78 | 3.30E-01 | M: Eif5a, Eny2, G3bp2, Hnrnpa2b1 | |
| 5 | Mesenchyme Development | 1.33 | 6.00E-01 | M: Aldh1a2, Hnrnpab, Trim28 | |
AC = annotation cluster, ES = enrichment score (≥1, p<0.05) provided by DAVID bioinformatics database.
Table 8.
Underrepresented canonical pathways at the blastocyst implantation site identified by the Ingenuity Pathway Analysis
| Canonical pathway | −Log (B-H p-value) | Representative genes (M: mouse array; H: Human array; S: Shared between mouse and human arrays | |
|---|---|---|---|
| Tight Junction Signaling | 1.58 | M: Cldn3, Cldn7, Jun, Myh11 | S: Actg2 |
| 0.46 | |||
| SAPK/JNK Signaling | 1.58 | M: Jun, Irs1, Map3k1, Map3k3 | |
| 0.46 | H: Gadd45a | ||
| RXR Activation and Function | |||
| PPARα/RXRα Activation | 1.58 | M: Abca1, Acaa1, Acvr2a, Jun, Irs1 | |
| LPS/IL-1 Mediated Inhibition of RXR Function | 1.05 | M: Abca1, Gstp1, Jun, Map3k1 | |
| 0.43 | H: Apoe | ||
| Epithelial Adherens Junction Signaling | 1.21 | M: Acvr2a, Myh11, Wasl | S: Acgt2 |
| 0.46 | |||
Table 9.
Diminished biological processes at the blastocyst implantation site identified by DAVID.
| AC # | DAVID: GO Biological Process/KEGG Cluster | ES | FDR | Representative genes (M: mouse array; H: Human array; S: Shared between mouse and human arrays | |
|---|---|---|---|---|---|
| 1 | Regulation of steroid and lipid metabolic processes | 1.95 | 4.40E-01 | H: Apoe, Glul,, Igfbp7, Kcnma1 | S: Hspb1, Pdgfra |
| 6 | Response to peptide hormone stimulus and organic substance | 1.11 | 7.00E-01 | M: App, Btg2, Irs1, Jun, Kdm3a, Map3k1, Mcl, Ube2b | |
| 8 | Response to steroid hormone stimulus and organic substance | 1.06 | 6.40E-01 | H: Apoe, Glul, Igfbp7, Kcnma1 | |
| 2 | Regulation of cell proliferation and growth | 1.93 | 5.20E-01 | H: Apoe, Bcl6, Btg1, Igfbp7, Jag1, NfIB, Pdgfra | |
| 6 | Regulation of cell proliferation/migration, cell surface receptor linked signal transduction | 1.35 | 5.50E-01 | H: Apoe, Bcl6, Btg1, Igfbp7, Jag1, NfIB, Pdgfra, Sptbn1 | |
| 1 | Phosphorylation | 1.78 | 3.60E-01 | M: Acvr2a, App, Atp6v0b, Clk1, Clk4, Enpp2, Map3k1, Map3k3, Pdgfra | |
| 3 | Posttranscriptional regulation of gene expression | 1.44 | 6.80E-01 | M: Acvr2a, App, Eif4a2, Hspb1, Jun, Map3k1, Ube2b, Zfp36l2 | |
| 3 | Regulation of apoptosis/cell death | 1.85 | 3.70E-01 | H: Apoe, Bcl6, Btg1, Cadm1, Hoxb6, Kcnma1, Son | S: Hspb1, Itm2b |
| 7 | 1.06 | 4.30E-01 | M: Abca1, Acvr2a, App, Atp1a2, Btg2, Ccnl2, Dlx5, Eif4a2, Enpp2, Gstp1, Hoxb8, Irs1, Jun, Kdm3a, Klf9, Map3k1, Map3k3, Mcl1, Pdgfra, Rbm39, S100a6Serinc3, Sgms1, Suv420h1, Ube2b, Wasl, Zbtb16, Zfp36l2 | ||
| 4 | Circulatory/vascular system process | 1.79 | 5.80E-01 | H: Actg2, Apoe, Kcnma1 | |
| 9 | Blood vessel/vasculature development | 1.02 | 7.20E+01 | H: Apoe, Jag1, Sox17 | |
| 4 | Response to abiotic stimulus Ear development | 1.37 | 5.40E-01 | M: App, Atp1a2, Btg2, Hspb1, Jun, Map3k1, Ube2b | |
| 5 | 1.55 | 6.30E-01 | H: Jag1, Kcnma1, Pdgfra | ||
| 2 | Anterior/posterior pattern formation, embryonic morphogenesis | 1.46 | 4.40E-01 | M: Acvr2a, Btg2, Dlx5, Hoxb8, Pdgfra, Zbtb16 | |
AC = annotation cluster, ES = enrichment score (≥1, p<0.05) provided by DAVID bioinformatics database
Top Ranked Gene Networks
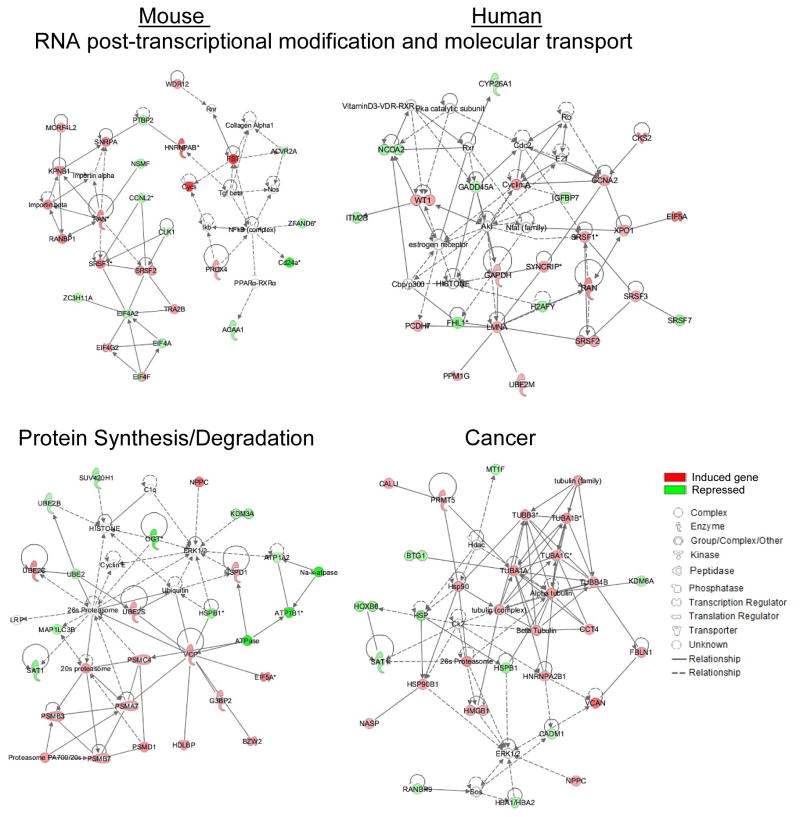
We next investigated possible gene networks of up- and down-regulated genes identified by both arrays. The top ranked networks included several genes involved in the most enriched molecular and cellular function and canonical pathways identified by IPA. The top networks constructed by IPA based on the functional and biological connectivity of the 190 genes identified as differentially expressed using the mouse array platform included: RNA post-transcriptional modification & molecular transport with a score of 45 and protein synthesis/degradation with a score of 40 (Fig. 4, left panel). Similarly, IPA identified the top networks using the 100 differentially expressed genes at hamster BIS using the human array platform, including: cancer and RNA post-transcriptional modification & molecular transport, with scores of 51 and 43, respectively (Fig. 4 right panel). Importantly, the gene network related to RNA post-transcriptional modification and molecular transport was identified by both arrays (Fig. 4).
Figure 4. Most significant gene networks of differentially expressed genes at the blastocyst implantation site of the hamster.
Ingenuity Pathway Analysis constructed the top biological networks enriched and repressed at BIS based on the functional and biological connectivity of the 190 and 100 genes identified using mouse and human CSH arrays, respectively. The top two networks are shown, and are graphically represented as nodes (genes) and edges (biological relationship between genes). Red and green colors represent induced or repressed genes, respectively, while clear nodes are those that IPA included based on the relationship. Color gradations are based upon gene regulation at the fold-change level.
4) Cross-species microarray data identified common altered genes at the blastocyst implantation site of mouse and hamsters
Genes that are differentially expressed between whole BIS and IIS of mice have been identified using the techniques of RNA differential display (Nie et al. 2000), serial analysis of gene expression (Ma et al. 2006) and cDNA microarray (Reese et al. 2001). Temporal gene expression profile in the luminal epithelial cells of the mouse implantation site was also studied using cDNA microarray (Chen et al. 2006). The global gene expression profile of human (Kao et al. 2002) and mouse (Xiao et al. 2014) endometrium during the window of implantation have also been investigated. However, to determine whether our cross-species hybridization microarray analyses identified novel transcripts enriched in BIS from hamsters compared to mice, we compared our differentially expressed genes list obtained from each microarray platforms to our previously reported mouse microarray study (Fig. 5A), which is the only study to date examining trancriptomal differences between whole BIS and IIS on day 5 of pregnancy using microarray analysis. We should note that a different Affymetrix GeneChip (U74A) was used for the array study using mouse BIS, compared to the GeneChip U430A used in the current hamster study. Nevertheless, using the same 1.5-fold, p<0.05 cutoff threshold, a total of 124 enhanced transcripts from mouse BIS were compared to our 174 enhanced transcripts from hamster BIS. There were a total of 19 genes that were similarly upregulated in BIS from mice and hamsters on day 5 of pregnancy, listed in Fig. 5A. Of these 19 genes, 4 were similarly identified as upregulated in hamster BIS using both microarray platforms: Fst (follistatin), Hsp90aa1 (heat shock protein 90, alpha (cytosolic), class A member 1), Hnrnpd (heterogeneous nuclear riboprotein D) and Srsf2 (serine/arginine-rich splicing factor 2). When compared to the 115 genes found to be downregulated in hamster BIS using our cross-hybridization arrays, we found only 8 genes that were similarly downregulated at BIS of mice, out of 110 from our previously mouse study (Fig. 5B). Of these 8 genes, remarkably only 1 gene was identified as downregulated in hamster BIS by both array platforms: Kdm6a (lysine (K)-specific demethylase 6A).
Figure 5. Comparison of the hamster cross-species microarray data to the mouse microarray data revealed a subset of conserved genes at the blastocyst implantation sites of the mouse and hamster.
Hamster cross-species hybridization microarray data from this study [mouse (Hamsterm) and human (Hamsterh)] was compared to our previously reported mouse microarray experiment comparing BIS to IIS on day 5 of pregnancy. Our differential gene set was compared to the previously microarray using similar parameters (1.5-fold, p<0.05). The number of shared genes between these two microarray studies is stated in the Venn diagram, for both the upregulated and downregulated data sets. Symbols for the shared genes are provided in the boxes.
Discussion
Blastocyst implantation is a complex uterine site-specific process that is a crucial event of mammalian pregnancy. It is possible that this event is regulated by an evolutionarily common genetic pool with conserved functions across species. However, given the variation in the mode and ovarian steroid hormonal regulation of blastocyst implantation among species (Reese et al. 2008), genetic profiling of the BISs of various species may become useful for the identification of a separate gene pool that differs between the P4-dependent compared to the P4 plus E–dependent implantation process. This study unveils for the first time a differentially expressed gene profile at the BIS of hamsters using CSM techniques, and demonstrates putative biological processes/pathways/gene networks that may contribute to the process of implantation in general and/or ovarian P4-dependent implantation process in hamsters.
Both array platforms yielded a relatively small number of differentially expressed genes at the hamster BIS. This finding is somewhat unexpected, but is quite explicable by the fact that most cross-species microarray probes identify genes that show high sequence conservation. In spite of this limitation, PCA and hierarchial heat-map clustering of differentially expressed probes and genes revealed two distinctive transcriptomes in uterine sites with or without the implanted blastocyst, indicating that the gene signature at the BIS is distinct from the IIS. Furthermore, correlation between the microarray data and the real-time PCR results verified the trends (up- or down-regulation) of 18 differentially expressed genes (unshared or shared) at various levels (higher- or smaller-fold difference) at the hamster BIS, clearly confirming that genes identified by both arrays are not an artifact and may represent the uterine functional state. We noted some quantitative differences between array results and real-time RT-PCR based data. We assume that this variation is likely based on the greater technological sensitivity of the latter. This interpretation is also supported by our in situ hybridization method (a secondary procedure used to validate our microarray as well as real-time PCR results) that demonstrated strong accumulation of mRNAs of 5 up-regulated genes at the BIS compared with the IIS. Taken together, our findings indicate the qualitative accuracy of our cross-species array results and reliably identified transcripts having higher or smaller differences in expression between RNA samples from BIS and IIS. To our knowledge, there is no published manuscript comparing the uterine gene expression profile of the hamster BIS and IIS using microarray analysis. Therefore, we believe that we have identified a group of genes that perhaps can be used to define up- or down-regulated molecular signaling at the hamster BIS. Among these genes, those that failed to show differential expression at the mouse BIS such as Psmb3, Actg2 and Prdx4, perhaps, represent hamster BIS specificity. Future studies focusing on these genes may provide insights into the cellular basis of P4-dependent implantation.
Uterine stromal proliferation, differentiation and cell death must be coordinately executed at the BIS to establish the physiological state of pregnancy following blastocyst implantation. The present study successfully identified a considerable number of differentially expressed genes that are involved in regulating the processes and/or pathways of RNA splicing and transport, protein synthesis and degradation, cell proliferation, differentiation, growth and death. Interestingly, our microarray results also revealed that RNA-post transcriptional modification, protein synthesis and degradation, and cancer are the top biological networks at the BIS. This is not surprising given the enhanced cellular growth and constant remodeling observed at the BIS (Alexander et al. 1996). Moreover, the process of decidualization at BISs is also considered an inflammatory event (Mor et al. 2011), which is a widely accepted component of tumor development and progression (Coussens & Werb 2002). In general, a cell’s adaptation to new physiological conditions in all tissues depends on degradation of specific proteins and RNA synthesis, processing and transport for new protein synthesis. Thus, analysis of the spliceosome, proteasome and cancer gene networks at the BIS may be useful for better understanding of how the blastocyst influences uterine growth and remodeling at the site of implantation.
Collectively, this study identified a pool of differentially expressed genes and their possible relationship with cellular and molecular processes or pathways at the hamster BIS. After validation of expression of several of these genes and functional annotation of differentially expressed genes, we speculate that determining the role of these genes at the implantation site will greatly aid in improving our understanding of the molecular mechanisms of blastocyst implantation in general as well as P4-dependent blastocyst implantation. Future RNA-Seq studies comparing the hamster BIS and IIS will be useful for validation of our CSM results, as well as for uncovering any unidentified differentially expressed transcripts, which may differ in nucleotide sequences compared to their respective mouse and/or human. Recently, researchers from the Broad Institute of MIT and Harvard have succeeded in sequencing the genome of the Syrian hamster, and deposited the data online (GenBank: APMT 00000000.1) for public assess. This genetic data will be precious for transcript identification in our future RNA-Seq approach towards a better understanding of the molecular pathways associated with blastocyst implantation in hamsters.
Supplementary Material
Acknowledgments
Funding
This work was supported by National Institutes of Health grant HD044741.
We acknowledge the technical support provided by Heidi Nguyen.
Footnotes
Declaration of interest
The authors declare that there are no conflicts of interest
Reference List
- Alexander CM, Hansell EJ, Behrendtsen O, Flannery ML, Kishnani NS, Hawkes SP, Werb Z. Expression and function of matrix metalloproteinases and their inhibitors at the maternal-embryonic boundary during mouse embryo implantation. Development. 1996;122:1723–1736. doi: 10.1242/dev.122.6.1723. [DOI] [PubMed] [Google Scholar]
- Aplin JD. Implantation, trophoblast differentiation and haemochorial placentation: mechanistic evidence in vivo and in vitro. J Cell Sci. 1991;99:681–692. doi: 10.1242/jcs.99.4.681. [DOI] [PubMed] [Google Scholar]
- Becker J, Hackl M, Rupp O, Jakobi T, Schneider J, Szczepanowski R, Bekel T, Borth N, Goesmann A, Grillari J, Kaltschmidt C, Noll T, Puhler A, Tauch A, Brinkrolf K. Unraveling the Chinese hamster ovary cell line transcriptome by next-generation sequencing. J Biotechnol. 2011;156:227–235. doi: 10.1016/j.jbiotec.2011.09.014. [DOI] [PubMed] [Google Scholar]
- Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- Chen Y, Ni H, Ma XH, Hu SJ, Luan LM, Ren G, Zhao YC, Li SJ, Diao HL, Xu X, Zhao ZA, Yang ZM. Global analysis of differential luminal epithelial gene expression at mouse implantation sites. J Mol Endocrinol. 2006;37:147–161. doi: 10.1677/jme.1.02009. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSouza MM, Surveyor GA, Price RE, Julian J, Kardon R, Zhou X, Gendler S, Hilkens J, Carson DD. MUC1/episialin: a critical barrier in the female reproductive tract. J Reprod Immunol. 1999;45:127–158. doi: 10.1016/s0165-0378(99)00046-7. [DOI] [PubMed] [Google Scholar]
- Hammond S, Kaplarevic M, Borth N, Betenbaugh MJ, Lee KH. Chinese hamster genome database: an online resource for the CHO community at www.CHOgenome.org. Biotechnol Bioeng. 2012;109:1353–1356. doi: 10.1002/bit.24374. [DOI] [PubMed] [Google Scholar]
- Hey NA, Li TC, Devine PL, Graham RA, Saravelos H, Aplin JD. MUC1 in secretory phase endometrium: expression in precisely dated biopsies and flushings from normal and recurrent miscarriage patients. Hum Reprod. 1995;10:2655–2662. doi: 10.1093/oxfordjournals.humrep.a135762. [DOI] [PubMed] [Google Scholar]
- Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao LC, Tulac S, Lobo S, Imani B, Yang JP, Germeyer A, Osteen K, Taylor RN, Lessey BA, Giudice LC. Global gene profiling in human endometrium during the window of implantation. Endocrinology. 2002;143:2119–2138. doi: 10.1210/endo.143.6.8885. [DOI] [PubMed] [Google Scholar]
- Lee KY, DeMayo FJ. Animal models of implantation. Reproduction. 2004;128:679–695. doi: 10.1530/rep.1.00340. [DOI] [PubMed] [Google Scholar]
- Lei W, Feng XH, Deng WB, Ni H, Zhang ZR, Jia B, Yang XL, Wang TS, Liu JL, Su RW, Liang XH, Qi QR, Yang ZM. Progesterone and DNA damage encourage uterine cell proliferation and decidualization through up-regulating ribonucleotide reductase 2 expression during early pregnancy in mice. J Biol Chem. 2012;287:15174–15192. doi: 10.1074/jbc.M111.308023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei W, Nguyen H, Brown N, Ni H, Kiffer-Moreira T, Reese J, Millan JL, Paria BC. Alkaline phosphatases contribute to uterine receptivity, implantation, decidualization, and defense against bacterial endotoxin in hamsters. Reproduction. 2013;146:419–432. doi: 10.1530/REP-13-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XH, Hu SJ, Ni H, Zhao YC, Tian Z, Liu JL, Ren G, Liang XH, Yu H, Wan P, Yang ZM. Serial analysis of gene expression in mouse uterus at the implantation site. J Biol Chem. 2006;281:9351–9360. doi: 10.1074/jbc.M511512200. [DOI] [PubMed] [Google Scholar]
- Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci. 2011;1221:80–87. doi: 10.1111/j.1749-6632.2010.05938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie GY, Li Y, Batten L, Griffiths B, Wang J, Findlay JK, Salamonsen LA. Uterine expression of alternatively spliced mRNAs of mouse splicing factor SC35 during early pregnancy. Mol Hum Reprod. 2000;6:1131–1139. doi: 10.1093/molehr/6.12.1131. [DOI] [PubMed] [Google Scholar]
- Paria BC, Huet-Hudson YM, Dey SK. Blastocyst’s state of activity determines the “window” of implantation in the receptive mouse uterus. Proc Natl Acad Sci USA. 1993;90:10159–10162. doi: 10.1073/pnas.90.21.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkening TA. An ultrastructural study of implantation in the golden hamster. II. Trophoblastic invasion and removal of the uterine epithelium. J Anat. 1976a;122:211–230. [PMC free article] [PubMed] [Google Scholar]
- Parkening TA. An ultrastructural study of implantation in the golden hamster. III. Initial formation and differentiation of decidual cells. J Anat. 1976b;122:485–498. [PMC free article] [PubMed] [Google Scholar]
- Reese J, Das SK, Paria BC, Lim H, Song H, Matsumoto H, Knudtson KL, DuBois RN, Dey SK. Global gene expression analysis to identify molecular markers of uterine receptivity and embryo implantation. J Biol Chem. 2001;276:44137–44145. doi: 10.1074/jbc.M107563200. [DOI] [PubMed] [Google Scholar]
- Reese J, Wang H, Ding T, Paria BC. The hamster as a model for embryo implantation: insights into a multifaceted process. Semin Cell Dev Biol. 2008;19:194–203. doi: 10.1016/j.semcdb.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp O, Becker J, Brinkrolf K, Timmermann C, Borth N, Puhler A, Noll T, Goesmann A. Construction of a public CHO cell line transcript database using versatile bioinformatics analysis pipelines. PLoS One. 2014;9:e85568. doi: 10.1371/journal.pone.0085568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmucki R, Berrera M, Kung E, Lee S, Thasler WE, Gruner S, Ebeling M, Certa U. High throughput transcriptome analysis of lipid metabolism in Syrian hamster liver in absence of an annotated genome. BMC Genomics. 2013;14:237. doi: 10.1186/1471-2164-14-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Luan L, Ding T, Brown N, Reese J, Paria BC. Dynamics of zonula occludens-2 expression during preimplantation embryonic development in the hamster. Theriogenology. 2011;76:678–686. doi: 10.1016/j.theriogenology.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su Y, Deb K, Raposo M, Morrow JD, Reese J, Paria BC. Prostaglandin E2 is a product of induced prostaglandin-endoperoxide synthase 2 and microsomal-type prostaglandin E synthase at the implantation site of the hamster. J Biol Chem. 2004;279:30579–30587. doi: 10.1074/jbc.M400573200. [DOI] [PubMed] [Google Scholar]
- WARD MC. The early development and implantation of the golden hamster, Cricetus auratus, and the associated endometrial changes. Am J Anat. 1948;82:231–275. doi: 10.1002/aja.1000820204. [DOI] [PubMed] [Google Scholar]
- Xiao S, Diao H, Zhao F, Li R, He N, Ye X. Differential gene expression profiling of mouse uterine luminal epithelium during periimplantation. Reprod Sci. 2014;21:351–362. doi: 10.1177/1933719113497287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Nagarajan H, Lewis NE, Pan S, Cai Z, Liu X, Chen W, Xie M, Wang W, Hammond S, Andersen MR, Neff N, Passarelli B, Koh W, Fan HC, Wang J, Gui Y, Lee KH, Betenbaugh MJ, Quake SR, Famili I, Palsson BO, Wang J. The genomic sequence of the Chinese hamster ovary (CHO)-K1 cell line. Nat Biotechnol. 2011;29:735–741. doi: 10.1038/nbt.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Paria BC. Importance of uterine cell death, renewal, and their hormonal regulation in hamsters that show progesterone-dependent implantation. Endocrinology. 2006;147:2215–2227. doi: 10.1210/en.2005-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.