Abstract
Obesity is a global epidemic and a common risk factor for endothelial dysfunction, and the subsequent development of diabetes and vascular diseases such as hypertension. Epoxyeicosatrienoic acids (EETs) are CYP450-derived metabolites of arachidonic acid (AA) that contribute to vascular protection by stimulating vasodilation and inhibiting inflammation. Heme oxygenase-1 (HO-1) is a stress response protein that plays an important cytoprotective role against oxidative insult in diabetes and cardiovascular disease. We recently demonstrated interplay between EETs and HO-1 in the attenuation of adipogenesis. We examined whether adipocyte dysfunction in mice fed a high fat (HF) diet could be prevented by endothelial-specific targeting of the human CYP epoxygenase, CYP2J2. Tie2-CYP2J2 transgenic mice, fed a HF diet, had a reduction in body weight gain, blood glucose, insulin levels and inflammatory markers. Tie2-CYP2J2 gene targeting restored HF-mediated decreases in vascular HO-1, Cyp2C44, sEH, peNOS, pAKT, and pAMPK protein expression, thus improving vascular function. These changes translated into decreased inflammation and oxidative stress within adipose tissue and decreased PPARγ, C/EBPα, Mest, and aP2 expression and increased UCP1 and UCP2 expression, reflecting the effect of vascular EET overproduction on adipogenesis. The current study documents a direct link between endothelial-specific EET production and adipogenesis, further implicating the EET-HO-1 crosstalk as an important cytoprotective mechanism in the amelioration of vascular and adipocyte dysfunction resulting from diet-induced obesity.
Keywords: EET, HO-1, Mest, aP2, eNOS, AMPK
INTRODUCTION
Obesity has become a major and ever increasing epidemic worldwide and is a major risk factor in the development of vascular diseases such as diabetes and hypertension, and other associated complications 1–3, including vascular dysfunction and insulin resistance. Fat acts not only as a reservoir for triglyceride storage, but also as a dynamic organ that secretes bioactive factors to influence adjacent vasculature 4,5. Adipose tissue is highly vascularized and, vice versa, most vasculature is surrounded by adipose tissue. Thus, it is essential to investigate the role of each in the regulation of adipogenesis and vascular homeostasis. Additionally, emerging studies implicate a mechanistic link between vascular and adipocyte dysfunction as it relates to obesity-derived cardiovascular complications 6,7. This may be due to an increase of reactive oxygen species (ROS) 8.
Epoxyeicosatrienoic acids (EETs) are metabolites of arachidonic acid that are produced by a family of Cytochrome P450 (CYP) monooxygenases/epoxygenases 9,10. Upon formation, EETs are subjected to hydrolysis by soluble epoxide hydrolase (sEH) to their respective dihydroxyepoxytrienoic acids (DHETs) as well as to esterification primarily to glycerophospholipids. Vasodilatory, anti-inflammatory and anti-apoptotic actions of EETs are well established and it is well documented that sEH inhibition significantly increases cellular and circulating EET levels11,12. EET agonists prevent both vascular dysfunction 13 and adiposity in vitro 14 and in mice fed high-fat (HF) diets 7. EET mediated increases in heme oxygenase (HO)-1 provide vascular protection and regulate adipogenesis 15. HO, an essential stress response gene, has two isoforms: HO-1 (inducible) and HO-2 (constitutive) 16. Each catalyzes the degradation of heme to equimolar quantities of biliverdin, carbon monoxide and iron 16. As part of a cell’s antioxidant defense system, increased expression of HO-1/-2 promotes resistance to injury from heme, a pro-oxidant, and damaging ROS 16. Induction of HO-1 decreases adipogenesis via suppression of transcription factors, including peroxisome proliferators-activated receptor-gamma (PPARγ), aP2, and MEST proteins, while concomitantly increasing adiponectin and improving insulin sensitivity 17–19. PPARγ is a master regulator of adipogenesis and activates the expression of genes, such as aP2, to trigger the synthesis of fatty acids and triglycerides 20,21. Paternally expressed 1 (Peg 1)/ Mesoderm specific transcript (Mest), when upregulated, results in adipocyte enlargement during adipose tissue expansion 22,23 that is associated with increased release of inflammatory adipokines and enhanced insulin resistance 17,24. Although obesity is associated with oxidative stress and increased levels of ROS, we have demonstrated that the expression and activity of both CYP-epoxygenases and HO-1 are downregulated in obesity 17,19,25,26.
EETs and HO-1 are interdependent; 11,12-EET stimulates HO-1 expression and its vasodilatory action is dependent on HO activity 27,28. However, the role of EETs on adipocyte HO-1 levels and insulin resistance is largely unknown. HO-1 levels are decreased in models of diabetes 29, atherosclerosis 30, and metabolic syndrome 31. Because excess fat is a major component of all of these diseases, it is plausible that diminished HO activity contributes to increased oxidative stress, inflammation and subsequent adipocyte dysfunction 16. In rodents fed HF diets, the decrease in CYP epoxygenase expression and EET biosynthesis was associated with increased ROS production and reduced levels of HO-1 and adiponectin 25,32–35. Specifically secreted by adipocytes, adiponectin has antiartherogenic, antihypertensive, and insulin-sensitizing properties 36,37. Furthermore, adipocytes derived from obese mice have decreased EET levels, and the administration of an EET-agonist decreased adiposity and increased HO-1 and adiponectin levels 7,15.
The protective effects of both EETs and HO-1 are supported by improved vascular function and reduced adipogenesis via targeted expression of key enzymes in specific cell populations. Lentiviral-targeted HO-1 expression in rat endothelium improved vascular function in accordance with decreased oxidative stress and inflammation 38. Furthermore, selective HO-1 expression in adipocytes attenuated adiposity and vascular dysfunction in mice fed a HF diet 6. Therefore, studying the effects of HO-1 and EET on adipocyte function is critical to better understanding how adipocyte dysfunction leads to increased inflammation, a known risk factor for vascular dysfunction and, subsequently, the development of hypertension in mammals that consume a HF diet.
MATERIALS AND METHODS
A detailed description of experimental protocols, methods and materials is included in a supplemental material (please see http://hyper.ahajournals.org)
RESULTS
Effect of endothelial CYP2J2 gene targeting on body weight, visceral fat and subcutaneous fat accumulation
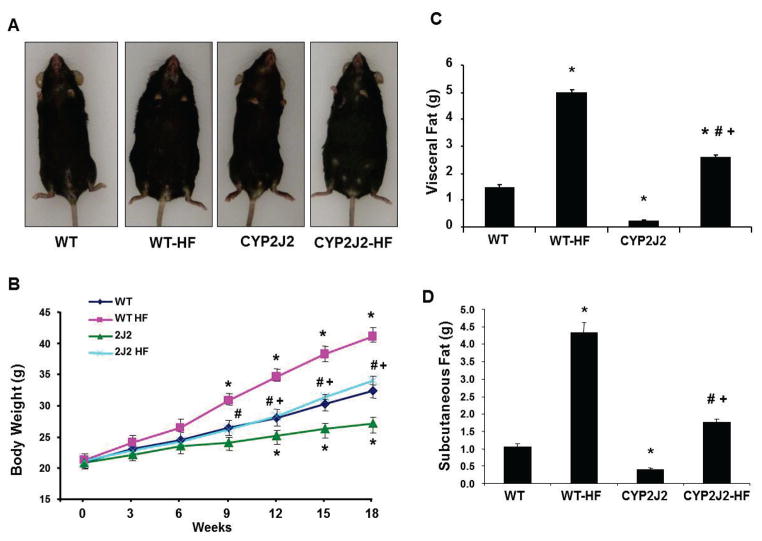
We utilized a B6 transgenic mouse strain (Tie2-CYP2J2-Tr) that, in endothelial cells, selectively expresses a primary CYP epoxygenase (CYP2J2) responsible for EET biosynthesis 39, which were fed either a normal or a HF diet over a period of 18 weeks. There was no significant difference in food intake between the WT and CYP2J2 mice. Body weight of WT mice fed a HF diet (WT-HF) was increased by 53% over that of WT mice fed a normal diet (Figure 1A, 1B). This phenomenon was paralleled in HF versus normal diet Tie2-CYP2J2 mice; however, body weight was significantly lower in Tie2-CYP2J2-Tr-HF mice in comparison to WT-HF mice. Measurements of visceral fat paralleled that of body weight with more pronounced differences between WT and transgenic mice; Tie2-CYP2J2-Tr were leaner than WT mice and a HF diet increased visceral fat in WT by about 4-fold. While the fold increase in visceral fat in HF-fed Tie2-CYP2J2-Tr was greater, it remained significantly lower than in HF-fed WT mice (Figure 1C). A similar pattern was seen with regard to subcutaneous fat content, which was more than 2-fold higher in WT-HF mice than Tie2-CYP2J2-Tr-HF mice (Figure 1D).
Figure 1.
A,B) Appearance and body weight over a period of 18 weeks; C) visceral fat; and D) subcutaneous fat content in mice fed a normal diet (WT and CYP2J2) and mice fed a HF diet (WT-HF and CYP2J2-HF) (n=10, *p<0.05 vs. WT; †p<0.05 vs. CYP2J2; #p<0.05 vs. WT-HF).
Effect of endothelial CYP2J2 gene targeting on blood glucose, insulin, adiponectin and IL-6 levels
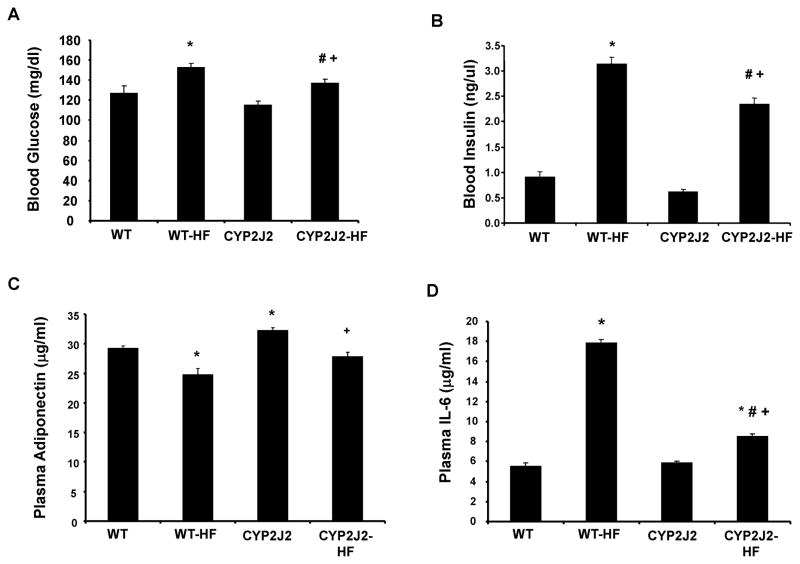
Fasting blood glucose and insulin levels were measured as indicators of systemic complications stemming from excessive body weight gain (Figure 2A–B). Blood glucose and insulin levels increased in WT-HF mice in comparison to WT mice. Tie2-CYP2J2-Tr-HF mice displayed lower blood glucose and insulin levels compared to WT-HF mice (Figure 2A and B).
Figure 2.
A) Fasting blood glucose; B) insulin; C) Adiponectin; and D) IL-6 levels in mice fed a normal diet (WT and CYP2J2) and mice fed a HF diet (WT-HF and CYP2J2-HF) (n=10,*p<0.05 vs. WT; †p<0.05 vs. CYP2J2; #p<0.05 vs. WT-HF).
Circulating adiponectin was lower in WT-HF mice versus WT controls (Figure 2C). Tie2-CYP2J2-Tr mice exhibited increased adiponectin levels compared to corresponding WT and these levels decreased on a high fat diet. Nonetheless, adiponectin levels in Tie2-CYP2J2-Tr-HF mice were significantly higher relative to WT-HF mice. In contrast to the attenuation of serum adiponectin levels, a HF diet increased circulating levels of IL-6 (Figure 2D). IL-6 levels increased by 3-fold in WT-HF mice as compared to WT controls. IL-6 levels in Tie2-CYP2J2-Tr mice fed a normal diet were comparable to the corresponding WT mice, yet were strikingly reduced in Tie2-CYP2J2-Tr-HF mice when compared to WT-HF mice.
Effect of endothelial CYP2J2 gene targeting on vascular parameters
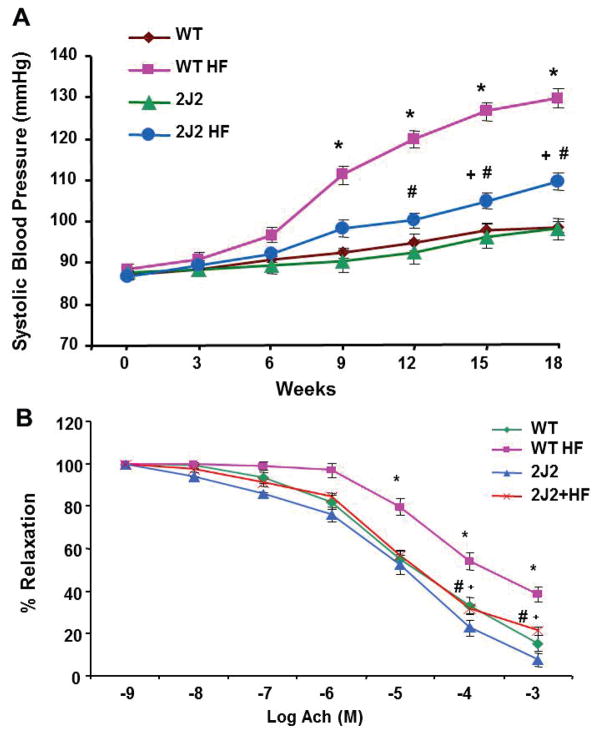
Systolic blood pressure (BP), measured by the tail cuff plethysmography method (please see http://hyper.ahajournals.org), significantly increased over the 18-week period of HF diet in WT and Tie-CYP2J2-Tr mice (Figure 3A). Systolic BP of Tie2-CYP2J2-Tr mice fed a normal diet was comparable to WT control mice. However, the HF-mediated BP increase in Tie-CYP2J2-Tr mice was significantly attenuated as compared to WT mice (109±2 vs 129±5 mmHg, n=10). Vascular function, measured as relaxations to acetylcholine in renal interlobar arteries, was impaired in WT mice fed a HF diet. As seen in Figure 3B, at 10−3 M acetylcholine, the percent of relaxation in WT mice fed a normal diet was 92±8 vs. 59±6 in WT-HF mice (p<0.05). Tie2-driven endothelial expression of CYP2J2 restored maximal relaxations in HF mice to levels no different from those of WT mice fed a normal diet.
Figure 3.
A) Systolic blood pressure in mice fed a normal diet (WT and CYP2J2) and mice fed a HF diet (WT-HF and CYP2J2-HF) for 18 weeks. (n=10, *p<0.05 vs. WT; +p<0.05 vs. CYP2J2; #p<0.05 vs. WT-HF). B) Relaxation-response curves to acetylcholine in interlobar arteries. (n=5; *p<0.05 vs. WT; #p<0.05 vs. WT HF; +p<0.05 vs. CYP2J2)
Effect of endothelial CYP2J2 gene targeting on vascular expression of epoxygenase pathway and key cytoprotective molecules
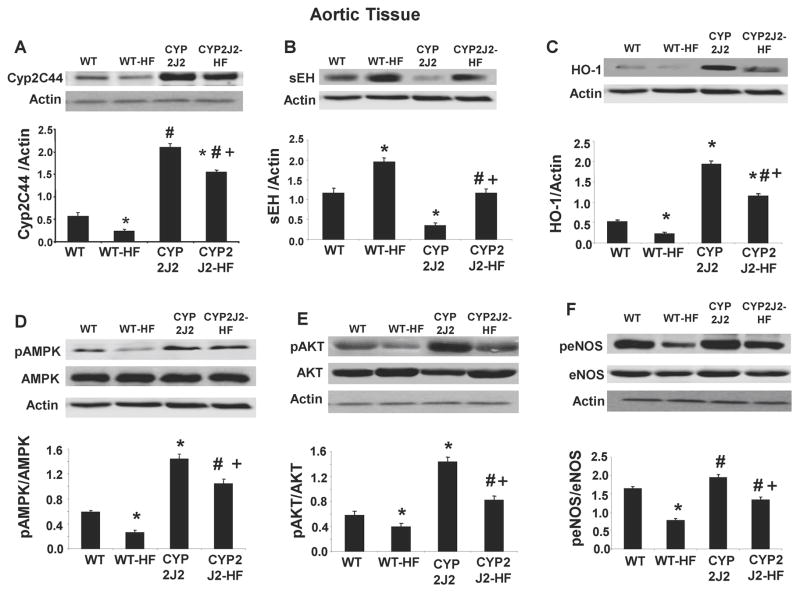
Western blot analysis demonstrated a 70% increase in Cyp2C44 expression (a major AA epoxygenase in mice) in Tie2-CYP2J2-Tr mice on control diet as compared to control WT mice (Figure 4A, B). Cyp2C44 expression was significantly decreased in response to a HF diet in both WT and Tie2-CYP2J2. Expression of sEH in Tie2-CYP2J2-Tr mice was significantly lower compared to WT mice (Figure 4B). However, Tie2-CYP2J2-Tr-HF mice exhibited increased sEH expression although the increase was tempered compared to that observed in HF-WT mice.
Figure 4.
Western blots and densitometry analyses of vascular tissue A) Cyp2C44, B) sEH, C) HO-1 D) pAMPK, E) pAKT, F) peNOS proteins in mice fed a normal diet (WT and CYP2J2) and mice fed a HF diet (WT-HF and CYP2J2-HF). ( n=4; *p<0.05 vs. WT; #p<0.05 vs. WT HF; +p<0.05 vs. CYP2J2).
We further evaluated the effect of endothelial-specific CYP2J2 expression on the levels of key cytoprotective molecules in the vasculature. HO-1 expression was 4-fold higher in Tie2-CYP2J2-Tr mice compared to WT mice (Figure 4C) and while HF diet decreased HO-1 levels in both strains, it remained significantly higher in the Tie2-CYP2J2-Tr-HF mice. Phosphorylation of AMPK showed a similar pattern; it was 2.5-fold higher in Tie2-CYP2J2-Tr than in WT and was decreased by HF diet by only 40% remaining significantly higher than in WT-HF mice (Figure 4D). The effect of Tie2-CYP2J2 transduction and HF diet on pAKT signaling and eNOS phosphorylation at ser1177 mirrored that of pAMPK (Figure 4E–F). Tie2-CYP2J2-Tr mice displayed higher levels of pAKT signaling (2-fold) and peNOS (25%) relative to WT mice. In WT mice, a HF diet decreased pAKT and peNOS levels by 35 and 50%, respectively. Although a HF diet in Tie2-CYP2J2-Tr mice also resulted in a decrease in pAKT and peNOS, these levels remained significantly higher than those in HF-WT mice (Figure 4E–F).
Effect of endothelial CYP2J2 gene targeting on adipocyte HO-1 expression and macrophage infiltration
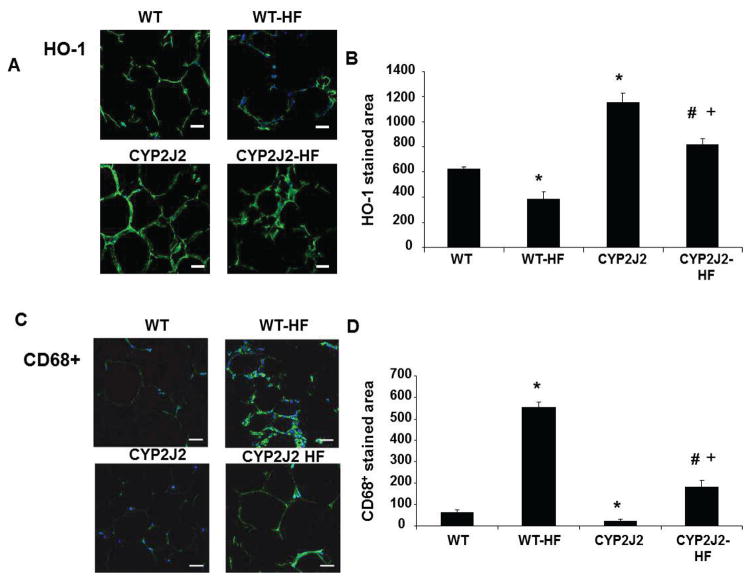
Immunohistochemical staining of adipose tissue (Figure 5A) and quantification (Figure 5B) of HO-1 expression indicated a reduction in HO-1 protein in WT mice fed a HF diet as compared to WT fed a normal diet (p<0.05). A parallel relationship was observed in Tie2-CYP2J2-Tr-HF mice, which exhibited lower HO-1 levels than Tie2-CYP2J2 mice fed a normal diet (p<0.05). Nonetheless, WT-HF mice expressed less HO-1 than Tie2-CYP2J2-Tr-HF mice (p<0.05).
Figure 5.
HO-1 and CD68+ expression in visceral fat from mice fed a normal diet (WT and CYP2J2) and mice fed a HF diet (WT-HF and CYP2J2-HF). (A and B) HO-1 immunohistochemistry and quantitative analysis. (C and D) CD68+ Immunohistochemistry and quantitative analysis. (Bar=20μm; n=5; *p<0.05 vs. WT; #p<0.05 vs. WT-HF; +p<0.05 vs. CYP2J2).
Because obesity and excessive adipogenesis are positively correlated with increased inflammation 40,41, we assessed infiltration of macrophages (CD68+ cells) to adipose tissue. Immunohistochemical staining (Figure 5C) illustrates a 8-fold increase in the CD68+ stained area in adipose tissue of WT-HF mice compared to WT mice fed a normal diet. Tie2-CYP2J2-Tr mice had decreased CD68+ regions as compared to WT mice (32±3 vs 69±8, p<0.05). Additionally, in comparison to WT-HF mice, CD68+ regions in Tie2-CYP2J2-Tr-HF mice were attenuated (199±14) (Figure 5D).
Effect of endothelial CYP2J2 gene targeting on key signaling pathways for adipogenesis and energy metabolism
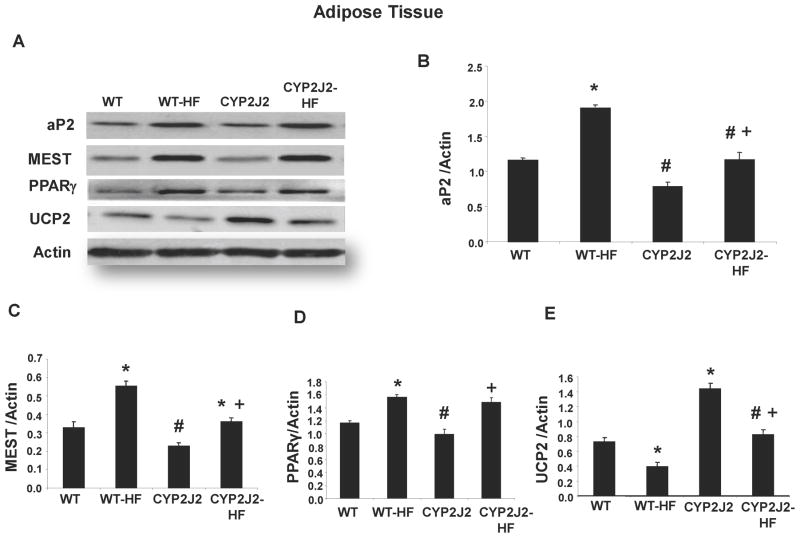
We examined the levels of aP2, Mest and PPARγ to see if they could account for the decrease in fat content in adipose tissues from mice with endothelial expression of CYP2J2 fed a HF diet. Western blot analysis demonstrated in WT-HF mice an upregulation of Mest (2 fold), aP2 (1.5 fold), and PPARγ (1.3 fold) vs. WT mice fed a normal diet (Figure 6). In contrast, Tie2-CYP2J2-Tr mice displayed reduced expression of each of these adipogenic markers in comparison to WT mice. HF diet increased expression of Mest, aP2 and PPARγ in Tie2-CYP2J2-Tr mice (Figure 6B–D). However, the levels of these markers remained significant low (except for PPARγ) in comparison to WT-HF mice (Figure 6D).
Figure 6.
A) Representative Western blots and (B–E) densitometry analyses of adipose tissue B) mest, C) ap2, D) PPARγ E) UCP2 proteins in mice fed a normal diet (WT and CYP2J2) and mice fed a HF diet (WT-HF and CYP2J2-HF). (n=4; *p<0.05 vs. WT; #p<0.05 vs. WT-HF; +p<0.05 vs. CYP2J2).
Uncoupling protein 2 (UCP2) is a marker of energy metabolism and its uncoupled energy is used for heat production in fat tissue. WT mice fed a HF diet exhibited a decreased expression of UCP2 in adipose tissue in comparison to WT mice fed a normal diet (Figure 6A,E). Adipocyte UCP2 levels in Tie2-CYP2J2-Tr mice were 2-fold higher than WT mice and although a HF diet decreased its level, it remained higher than in WT-HF adipose tissue. Measurements of UCP1 protein levels in adipose tissues (Figure S1; Data Supplement at http://hyper.ahajournals.org) followed the same pattern as that of UCP2. In addition, measurements of gp91phox, a subunit of NADPH oxidase and a marker of oxidative stress, in aortic and adipose tissues indicated that increased endothelial expression of CYP2J2 resulted in reduced oxidative stress following a HF diet (Figure S2; Data Supplement at http://hyper.ahajournals.org)
DISCUSSION
This is the first study to demonstrate that targeting endothelial cells in mice fed a HF diet with human CYP2J2 decreased adiposity and vascular dysfunction, improved metabolic parameters, and attenuated serum levels of inflammatory cytokines. These improvements were associated with increased HO-1 expression in both the vasculature and in adipose tissue. Moreover, the beneficial effects of endothelial targeting of CYP2J2, a major EET producing enzyme in humans 42, are reflected by the attenuation of adipocyte expression of the adipogenic markers Mest, aP2 and PPARγ. In addition, increased HO-1 expression correlated with an increase in circulating adiponectin levels, which play a protective role in the development of insulin resistance and inflammation 43. Altogether, these data indicate the existence of a crosstalk between vascular EETs and HO-1 to modulate metabolic function and, thus, may provide a therapeutic target for patients with metabolic syndrome.
The current results support and substantiate our previous findings that indicated the protective effect of EETs on adipose and vascular tissues. Tie2-CYP2J2-Tr mice display increased levels of plasma and tissue EETs 39,44. We recently reported a significant reduction of EETs in mice fed a HF diet, which was accompanied by elevated levels of markers of inflammation and oxidative stress; administration of an EET agonist inhibited the expression of these markers of vascular and adipocyte dysfunction 7. In agreement with that report, our present results showed a significant decrease in gp91phox levels in CYP2J2 Tr mice in vascular and adipose tissues as compared to WT mice and, additionally, demonstrated the specific ability of endothelial cell-generated EETs to exhibit cytoprotective effects by attenuating oxidative stress in adipose tissue. EET regioisomers are potent arachidonic acid-derived vasodilators and anti-inflammatory molecules 11,45 produced by enzymes of the CYP2C and CYP2J gene families 9. Studies have shown vascular protection against angiotensin II-mediated renal injury by CYP2C23 induction 46. Our previous findings in human mesenchymal stem cells implicated CYP2J2 as the primary source of EET generation 18 and provided evidence that EETs are anti-adipogenic 21. In vivo, systemic administration of an EET agonist improves insulin sensitivity and lowers adiposity 7. In addition to this study, we have provided several lines of evidence that the EET-mediated improvement of adipocyte function is a result of increased HO-1 levels 14,15,18,47. We have also shown that EETs stimulate HO-1 expression via the bach1-GSK3β pathway 7. The targeting of CYP2J2 to the vascular endothelium results in increased production of EETs in vascular beds 39. This in situ vascular endothelial-specific CYP2J2 expression and amplification of EET production led to decreased body weight gain and improved insulin sensitivity and vascular function. It is likely that the beneficial effect of EET is primarily due to local EET production in the vasculature of the fat tissue. The mechanisms by which vascular endothelial EETs affect adipocyte function are yet to be fully explored. We have previously shown that EETs induce HO-1 in vascular endothelium as well as in adipocytes 14,18,28. Hence, based on our studies and a recent report suggesting the existence of an EET receptor 48, we postulate that vascular endothelial EETs affect adipocyte function via a receptor-mediated mechanism in an autocrine and/or paracrine fashion to increase vascular and adipose HO-1 and consequently reduce vascular dysfunction, inflammation, hypertrophic adipogenesis and insulin resistance.
Our previous studies demonstrated that HO-1 induction attenuates adipogenesis and improves insulin sensitivity in obesity-induced diabetic rats 19 and in obese mice 26 while promoting healthy adipocyte function via adiponectin synthesis and release 14. A similar improvement in vascular function via targeting HO-1 expression specifically to adipocytes (aP2-HO-1) has recently been described 6. In addition, aP2-HO-1 transduced mice, fed a HF diet, had a decreased inflammation, lower blood glucose, and increased adiponectin vs. WT-HF mice. Increased vascular and adipocyte HO-1 expression in Tie2-CYP2J2-Tr mice in the current study supports these findings and is manifest by an improved vascular response and increased adiponectin levels. Furthermore, recent analysis in diabetic patients shows an impaired HO-1 system, chronic inflammation and oxidative stress, which was accompanied by diminished adiponectin synthesis and release 49.
Obesity downregulates both CYP epoxygenases and HO-1; yet with the Tie2-CYP2J2-Tr mouse model, we demonstrate restoration of these two key cytoprotective enzyme systems through an increase in EET production specifically in the vascular endothelial cell population. Our data suggest a paracrine effect of endothelial EET on adipocyte function that is reflected by improved vascular function and decreased adipogenesis. Moreover, whereas a HF diet in WT mice led to decreased activation of MAP kinase signaling (i.e. pAMPK, pAKT), Tie2-CYP2J2-Tr mice exhibited restored activation to levels well beyond that of WT controls. AMPK activation is controlled by the energy state of the cell (i.e. glucose availability) via a decrease in ATP and a rise in cellular AMP. Therefore, high circulating glucose levels, such as those obtained with a HF diet, result in elevated ATP and a subsequent decrease in AMPK activation. The ability of Tie2-driven endothelial CYP2J2 expression to restore AMPK activation in the presence of a HF diet suggests that endothelial EET production improves adipocyte function despite the presence of high levels of glucose.
The relationship between EETs and AMPK has yet to be clarified. Others have shown in diabetic mice and hypertensive rats that CYP2J3 gene delivery increased EET generation and adiponectin levels, reduced blood pressure and improved insulin sensitivity through AMPK activation 50,51. We showed that administration of an EET agonist to animals on a HF diet restored the decreased adipocyte AMPK activation 7. The increase in adiponectin levels with Tie2-driven endothelial CYP2J2 expression supports a mechanistic link between EETs and AMPK, as other studies demonstrate that adiponectin provides vascular and renal protection. These data illustrate potential autocrine regulation of adipocyte AMPK activity, via adiponectin synthesis. Additionally, increased HO-1 protein levels are associated with increases in the AMPK and AKT signaling pathway 16, supporting the role of HO-1 in mediating EET-AMPK signaling.
We further demonstrate that changes in vascular signaling are associated with changes in adipogenic proteins. Increased expression of CYP2J2 resulted in decreased levels of Mest, aP2 and PPARγ in accordance with previous studies demonstrating the EET-mediated abrogation of adipogenesis 7. Furthermore, the role of HO-1 in improving adipocyte function in the current model is substantiated by previous work showing reduced adipogenesis via targeting HO-1 to adipocytes. Uncoupling proteins (UCP1 and 2) are expressed in adipose tissue and play an important role in the control of energy expenditure by uncoupling respiration from ATP synthesis, thereby dissipating energy as heat and affecting energy metabolism efficiency 52,53. In agreement with these reports, our results show that CYP2J2 Tr mice have increased adipose UCP1 and 2 levels compared to WT mice and this could be one of the mechanisms involved in decreased adiposity in CYP2J2 Tr mice. Taken together and summarized in Figure 7, we provide evidence to support the paracrine effect of endothelial EET production to improve both vascular and adipocyte function through increased levels of HO-1. Thus, EET and HO-1 appear to form a module that serves as a molecular “switch” to genetically reprogram the adipocyte phenotype to express lower levels of Mest and prevent hypoadiponectinemia.
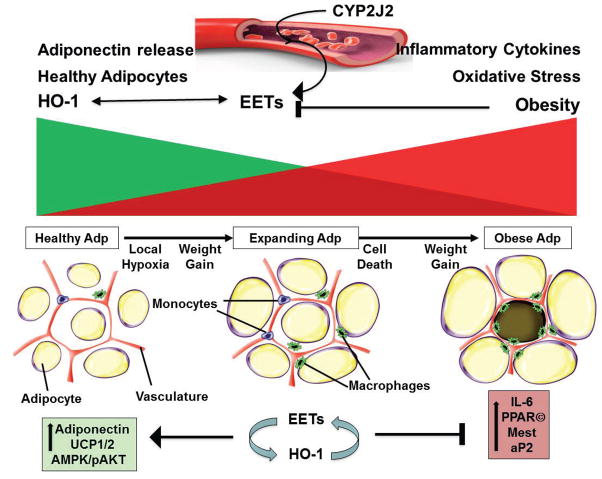
Figure 7.
Proposed scheme demonstrating crosstalk between vascular endothelial EETs and vascular/adipose HO-1 to attenuate vascular dysfunction and adipogenesis. High fat diets, obesity and the metabolic syndrome are all associated with suppression of HO-1, a major anti-inflammatory and cytoprotective circuit, in vascular and adipose tissues 18,20,24,26. The targeting of CYP2J2 to the vascular endothelium increases EET production in vascular beds. This in situ vascular endothelial-specific CYP2J2 expression and amplification of EET production led to decreased body weight gain and improved insulin sensitivity and vascular function through mechanisms that include induction of vascular and adipose HO-1 and activation of anti-inflammatory and anti-adipogenic pathways (e.g., adiponectin) to reduce inflammation, hypertrophic adipogenesis and insulin resistance.
One of the consequences of obesity-mediated adipocyte dysfunction is vascular dysfunction, which is a prelude to vascular disease and hypertension 54. With the growing prevalence of obesity and impaired glycemic control, the correlation between these conditions, and an elevated predisposition for the development of vascular disease, research emphasis is increasingly being targeted to the mechanistic basis and functional outcomes of these relationships. The current study is the first to show a direct link between endothelial-specific EET production and HO-1 induction to inhibit adipogenesis. Thus, our studies offer a portal to a role of HO-1-EET interplay in adipocyte-derived regulation of inflammation that is directly related to vascular dysfunction in obesity.
PERSPECTIVES
The global epidemic of obesity continues unabated with sequelae of type 2 diabetes and metabolic syndrome, and its consequent and severe vascular disease that ranges from early endothelial dysfunction and hypertension to end stage cardiac, cerebral and renal disease. Since levels of vascular EET are decreased in obesity and EET analogues attenuate adiposity, we hypothesized that the vascular protective properties of EETs are an integral component of the adipocyte HO-1-adiponectin axis that controls adipocyte-vascular interactions in obesity-mediated metabolic and vascular complications. Indeed, specific targeting of CYP2J2, a major EET producing enzyme, to the vascular endothelium impacted not only the vasculature, attenuating HF-induced endothelial dysfunction, but also adipose tissue, changing the phenotype of adipocytes and ameliorating the HF diet-induced metabolic conditions including insulin resistance and adiposity by a mechanism that increases the HO-1-adiponectin signaling pathway. EET and HO-1 appear to form a module that serves as a molecular “switch” to genetically reprogram the adipocyte phenotype to express lower levels of Mest and prevent hypoadiponectinemia. Therefore, the CYP2J2 gene targeting mediated sustained increase of EET levels merits further investigation to elucidate whether this new and different approach can have clinical value in treating obesity related metabolic diseases including diabetes, atherosclerosis and vascular disease. A deeper understanding of the mechanisms involved will provide new approaches for the treatment of obesity, diabetes and atherosclerosis and improve the effectiveness of cell based treatment of vascular diseases.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
1) What is New
We provide evidence supporting the paracrine effect of endothelial EET production to improve both vascular and adipocyte function through increased levels of HO-1.
Endothelial-targeted EET overexpression reprograms adipocyte phenotype; it restores AMPK activation and adiponectin production in the presence of a HF diet.
The targeting of CYP2J2 to the vascular endothelium results in increased production of EETs in vascular beds. This in situ vascular endothelial-specific CYP2J2 expression and amplification of EET production improved adipocyte function and led to decreased body weight gain and enhanced insulin sensitivity.
This report uncovers a mechanistic link between the vascular system and adipose tissue and provides an experimental and conceptual basis for both devising new strategies targeting the EET-HO-1-adiponectin module and therapeutic models to ameliorate adipocyte dysfunction associated with hypertension, insulin resistance and vascular disease.
2) What is relevant?
The consequences of obesity-mediated adipocyte dysfunction may lead to vascular dysfunction, which is a prelude to vascular disease and hypertension.
The existence of a close relationship between the vascular system and adipocytes whereby improvement of vascular function by the EET-HO-1 axis positively impacts adipocyte function.
An experimental and conceptual basis for the development of new therapeutic strategies that target the EET-HO-1 module to ameliorate adipocyte and vascular dysfunction associated with the metabolic syndrome.
3) Summary
This is the first study to demonstrate that targeting the vascular endothelium with human CYP2J2 in mice fed a HF diet decreased adiposity and vascular dysfunction, improved metabolic parameters and increased levels of HO-1 expression in both the vasculature and in adipose tissue. Furthermore, this study clarifies the relationship between EET-HO-1 and their ability to increase adiponectin and decrease Mest during adipogenesis and demonstrates how they influence the cellular/vascular homeostasis responses in an obese animal model. Finally, this report provides a portal into the mechanisms involved in obesity related to the development of vascular dysfunction and hypertension as well as potential therapeutic targets for the treatment of the metabolic syndrome and cardiovascular disease.
Acknowledgments
All authors had full access to the data and take responsibility for its integrity. All authors have read and agree with the manuscript as written.
SOURCES OF FUNDING
This work was supported by National Institutes of Health grants HL55601 (NGA) and HL34300 (MLS). This work was also supported, in part, by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01 ES025034 to DCZ)
Footnotes
CONFLICT OF INTEREST AND DISCLOSURE
None.
References
- 1.Hall JE. The kidney, hypertension, and obesity. Hypertension. 2003;41:625–633. doi: 10.1161/01.HYP.0000052314.95497.78. [DOI] [PubMed] [Google Scholar]
- 2.Brook RD, Bard RL, Rubenfire M, Ridker PM, Rajagopalan S. Usefulness of visceral obesity (waist/hip ratio) in predicting vascular endothelial function in healthy overweight adults. Am J Cardiol. 2001;88:1264–1269. doi: 10.1016/s0002-9149(01)02088-4. [DOI] [PubMed] [Google Scholar]
- 3.de Vigili KS, Kiwanuka E, Tiengo A, Avogaro A. Visceral obesity is characterized by impaired nitric oxide-independent vasodilation. Eur Heart J. 2003;24:1210–1215. doi: 10.1016/s0195-668x(03)00206-9. [DOI] [PubMed] [Google Scholar]
- 4.Gu P, Xu A. Interplay between adipose tissue and blood vessels in obesity and vascular dysfunction. Rev Endocr Metab Disord. 2013;14:49–58. doi: 10.1007/s11154-012-9230-8. [DOI] [PubMed] [Google Scholar]
- 5.Nishimura S, Manabe I, Nagasaki M, Hosoya Y, Yamashita H, Fujita H, Ohsugi M, Tobe K, Kadowaki T, Nagai R, Sugiura S. Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes. 2007;56:1517–1526. doi: 10.2337/db06-1749. [DOI] [PubMed] [Google Scholar]
- 6.Cao J, Peterson SJ, Sodhi K, Vanella L, Barbagallo I, Rodella LF, Schwartzman ML, Abraham NG, Kappas A. Heme oxygenase gene targeting to adipocytes attenuates adiposity and vascular dysfunction in mice fed a high-fat diet. Hypertension. 2012;60:467–475. doi: 10.1161/HYPERTENSIONAHA.112.193805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sodhi K, Puri N, Inoue K, Falck JR, Schwartzman ML, Abraham NG. EET agonist prevents adiposity and vascular dysfunction in rats fed a high fat diet via a decrease in Bach 1 and an increase in HO-1 levels. Prost Other Lipid Mediat. 2012;98:133–142. doi: 10.1016/j.prostaglandins.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin Y, Berg AH, Iyengar P, Lam TK, Giacca A, Combs TP, Rajala MW, Du X, Rollman B, Li W, Hawkins M, Barzilai N, Rhodes CJ, Fantus IG, Brownlee M, Scherer PE. The hyperglycemia-induced inflammatory response in adipocytes: the role of reactive oxygen species. J Biol Chem. 2005;280:4617–4626. doi: 10.1074/jbc.M411863200. [DOI] [PubMed] [Google Scholar]
- 9.Zeldin DC. Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem. 2001;276:36059–36062. doi: 10.1074/jbc.R100030200. [DOI] [PubMed] [Google Scholar]
- 10.Capdevila JH, Falck JR, Harris RC. Cytochrome P450 and arachidonic acid bioactivation. Molecular and functional properties of the arachidonate monooxygenase. J Lipid Res. 2000;41:163–181. [PubMed] [Google Scholar]
- 11.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spiecker M, Liao JK. Vascular protective effects of cytochrome p450 epoxygenase-derived eicosanoids. Arch Biochem Biophys. 2005;433:413–420. doi: 10.1016/j.abb.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Imig JD. Eicosanoids and renal damage in cardiometabolic syndrome. Expert Opin Drug Metab Toxicol. 2008;4:165–174. doi: 10.1517/17425255.4.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim DH, Vanella L, Inoue K, Burgess A, Gotlinger K, Manthati VL, Koduru SR, Zeldin DC, Falck JR, Schwartzman ML, Abraham NG. Epoxyeicosatrienoic acid agonist regulates human mesenchymal stem cell-derived adipocytes through activation of HO-1-pAKT signaling and a decrease in PPARgamma. Stem Cells Dev. 2010;19:1863–1873. doi: 10.1089/scd.2010.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sodhi K, Inoue K, Gotlinger K, Canestraro M, Vanella L, Kim DH, Manthati VL, Koduru SR, Falck JR, Schwartzman ML, Abraham NG. Epoxyeicosatrienoic acid agonist rescues the metabolic syndrome phenotype of HO-2-null mice. J Pharmacol Exp Ther. 2009;331:906–916. doi: 10.1124/jpet.109.157545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60:79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- 17.Li M, Kim DH, Tsenovoy PL, Peterson SJ, Rezzani R, Rodella LF, Aronow WS, Ikehara S, Abraham NG. Treatment of obese diabetic mice with a heme oxygenase inducer reduces visceral and subcutaneous adiposity, increases adiponectin levels, and improves insulin sensitivity and glucose tolerance. Diabetes. 2008;57:1526–1535. doi: 10.2337/db07-1764. [DOI] [PubMed] [Google Scholar]
- 18.Vanella L, Kim DH, Sodhi K, Barbagallo I, Burgess AP, Falck JR, Schwartzman ML, Abraham NG. Crosstalk between EET and HO-1 downregulates Bach1 and adipogenic marker expression in mesenchymal stem cell derived adipocytes. Prostaglandins Other Lipid Mediat. 2011;96:54–62. doi: 10.1016/j.prostaglandins.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicolai A, Li M, Kim DH, Peterson SJ, Vanella L, Positano V, Gastaldelli A, Rezzani R, Rodella LF, Drummond G, Kusmic C, L’Abbate A, Kappas A, Abraham NG. Heme Oxygenase-1 Induction Remodels Adipose Tissue and Improves Insulin Sensitivity in Obesity-Induced Diabetic Rats. Hypertension. 2009;53:508–515. doi: 10.1161/HYPERTENSIONAHA.108.124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanella L, Sodhi K, Kim DH, Puri N, Maheshwari M, Hinds TD, Jr, Bellner L, Goldstein D, Peterson SJ, Shapiro JI, Abraham NG. Increased heme-oxygenase 1 expression decreases adipocyte differentiation and lipid accumulation in mesenchymal stem cells via upregulation of the canonical Wnt signaling cascade. Stem Cell Res Ther. 2013;4:28. doi: 10.1186/scrt176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim DH, Vanella L, Inoue K, Burgess A, Gotlinger K, Manthati VL, Koduru SR, Zeldin DC, Falck JR, Schwartzman ML, Abraham NG. EET-Agonist Regulates Human Mesenchymal Stem Cells-Derived Adipocytes Through Activation of HO-1-pAKT Signaling and a decrease in PPARgamma. Stem Cells Dev. 2010 doi: 10.1089/scd.2010.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamei Y, Suganami T, Kohda T, Ishino F, Yasuda K, Miura S, Ezaki O, Ogawa Y. Peg1/Mest in obese adipose tissue is expressed from the paternal allele in an isoform-specific manner. FEBS Lett. 2007;581:91–96. doi: 10.1016/j.febslet.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi M, Kamei Y, Ezaki O. Mest/Peg1 imprinted gene enlarges adipocytes and is a marker of adipocyte size. Am J Physiol Endocrinol Metab. 2005;288:E117–E124. doi: 10.1152/ajpendo.00244.2004. [DOI] [PubMed] [Google Scholar]
- 24.Peterson SJ, Drummond G, Kim DH, Li M, Kruger AL, Ikehara S, Abraham NG. L-4F treatment reduces adiposity, increases adiponectin levels and improves insulin sensitivity in obese mice. J Lipid Res. 2008;49:1658–1669. doi: 10.1194/jlr.M800046-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peterson SJ, Kim DH, Li M, Positano V, Vanella L, Rodella LF, Piccolomini F, Puri N, Gastaldelli A, Kusmic C, L’Abbate A, Abraham NG. The L-4F mimetic peptide prevents insulin resistance through increased levels of HO-1, pAMPK, and pAKT in obese mice. J Lipid Res. 2009;50:1293–1304. doi: 10.1194/jlr.M800610-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burgess A, Li M, Vanella L, Kim DH, Rezzani R, Rodella L, Sodhi K, Canestraro M, Martasek P, Peterson SJ, Kappas A, Abraham NG. Adipocyte heme oxygenase-1 induction attenuates metabolic syndrome in both male and female obese mice. Hypertension. 2010;56:1124–1130. doi: 10.1161/HYPERTENSIONAHA.110.151423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sacerdoti D, Bolognesi M, Di PM, Gatta A, McGiff JC, Schwartzman ML, Abraham NG. Rat mesenteric arterial dilator response to 11,12-epoxyeicosatrienoic acid is mediated by activating heme oxygenase. Am J Physiol Heart Circ Physiol. 2006;291:H1999–H2002. doi: 10.1152/ajpheart.00082.2006. [DOI] [PubMed] [Google Scholar]
- 28.Sacerdoti D, Colombrita C, Di PM, Schwartzman ML, Bolognesi M, Falck JR, Gatta A, Abraham NG. 11,12-epoxyeicosatrienoic acid stimulates heme-oxygenase-1 in endothelial cells. Prostaglandins Other Lipid Mediat. 2007;82:155–161. doi: 10.1016/j.prostaglandins.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Goodman AI, Chander PN, Rezzani R, Schwartzman ML, Regan RF, Rodella L, Turkseven S, Lianos EA, Dennery PA, Abraham NG. Heme oxygenase-2 deficiency contributes to diabetes-mediated increase in superoxide anion and renal dysfunction. J Am Soc Nephrol. 2006;17:1073–1081. doi: 10.1681/ASN.2004121082. [DOI] [PubMed] [Google Scholar]
- 30.Brydun A, Watari Y, Yamamoto Y, Okuhara K, Teragawa H, Kono F, Chayama K, Oshima T, Ozono R. Reduced expression of heme oxygenase-1 in patients with coronary atherosclerosis. Hypertens Res. 2007;30:341–348. doi: 10.1291/hypres.30.341. [DOI] [PubMed] [Google Scholar]
- 31.Deshane J, Wright M, Agarwal A. Heme oxygenase-1 expression in disease states. Acta Biochim Pol. 2005;52:273–284. [PubMed] [Google Scholar]
- 32.Imig JD. Epoxide hydrolase and epoxygenase metabolites as therapeutic targets for renal diseases. Am J Physiol Renal Physiol. 2005;289:F496–F503. doi: 10.1152/ajprenal.00350.2004. [DOI] [PubMed] [Google Scholar]
- 33.Wang MH, Smith A, Zhou Y, Chang HH, Lin S, Zhao X, Imig JD, Dorrance AM. Downregulation of renal CYP-derived eicosanoid synthesis in rats with diet-induced hypertension. Hypertension. 2003;42:594–599. doi: 10.1161/01.HYP.0000090123.55365.BA. [DOI] [PubMed] [Google Scholar]
- 34.Huang H, Morisseau C, Wang J, Yang T, Falck JR, Hammock BD, Wang MH. Increasing or stabilizing renal epoxyeicosatrienoic acid production attenuates abnormal renal function and hypertension in obese rats. Am J Physiol Renal Physiol. 2007;293:F342–F349. doi: 10.1152/ajprenal.00004.2007. [DOI] [PubMed] [Google Scholar]
- 35.Zhao X, Quigley JE, Yuan J, Wang MH, Zhou Y, Imig JD. PPAR-alpha activator fenofibrate increases renal CYP-derived eicosanoid synthesis and improves endothelial dilator function in obese Zucker rats. Am J Physiol Heart Circ Physiol. 2006;290:H2187–H2195. doi: 10.1152/ajpheart.00937.2005. [DOI] [PubMed] [Google Scholar]
- 36.Huang KC, Chen CL, Chuang LM, Ho SR, Tai TY, Yang WS. Plasma adiponectin levels and blood pressures in nondiabetic adolescent females. J Clin Endocrinol Metab. 2003;88:4130–4134. doi: 10.1210/jc.2003-030158. [DOI] [PubMed] [Google Scholar]
- 37.Iwashima Y, Katsuya T, Ishikawa K, Ouchi N, Ohishi M, Sugimoto K, Fu Y, Motone M, Yamamoto K, Matsuo A, Ohashi K, Kihara S, Funahashi T, Rakugi H, Matsuzawa Y, Ogihara T. Hypoadiponectinemia is an independent risk factor for hypertension. Hypertension. 2004;43:1318–1323. doi: 10.1161/01.HYP.0000129281.03801.4b. [DOI] [PubMed] [Google Scholar]
- 38.Cao J, Sodhi K, Inoue K, Quilley J, Rezzani R, Rodella L, Vanella L, Germinario L, Stec DE, Abraham NG, Kappas A. Lentiviral-human heme oxygenase targeting endothelium improved vascular function in angiotensin II animal model of hypertension. Hum Gene Ther. 2011;22:271–282. doi: 10.1089/hum.2010.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee CR, Imig JD, Edin ML, Foley J, Degraff LM, Bradbury JA, Graves JP, Lih FB, Clark J, Myers P, Perrow AL, Lepp AN, Kannon MA, Ronnekleiv OK, Alkayed NJ, Falck JR, Tomer KB, Zeldin DC. Endothelial expression of human cytochrome P450 epoxygenases lowers blood pressure and attenuates hypertension-induced renal injury in mice. FASEB J. 2010;24:3770–3781. doi: 10.1096/fj.10-160119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tabet F, Rye KA. High-density lipoproteins, inflammation and oxidative stress. Clin Sci (Lond) 2009;116:87–98. doi: 10.1042/CS20080106. [DOI] [PubMed] [Google Scholar]
- 41.Tsuchida A, Yamauchi T, Takekawa S, Hada Y, Ito Y, Maki T, Kadowaki T. Peroxisome proliferator-activated receptor (PPAR)alpha activation increases adiponectin receptors and reduces obesity-related inflammation in adipose tissue: comparison of activation of PPARalpha, PPARgamma, and their combination. Diabetes. 2005;54:3358–3370. doi: 10.2337/diabetes.54.12.3358. [DOI] [PubMed] [Google Scholar]
- 42.Askari A, Thomson SJ, Edin ML, Zeldin DC, Bishop-Bailey D. Roles of the epoxygenase CYP2J2 in the endothelium. Prostaglandins Other Lipid Mediat. 2013;107:56–63. doi: 10.1016/j.prostaglandins.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iwaki M, Matsuda M, Maeda N, Funahashi T, Matsuzawa Y, Makishima M, Shimomura I. Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes. 2003;52:1655–1663. doi: 10.2337/diabetes.52.7.1655. [DOI] [PubMed] [Google Scholar]
- 44.Deng Y, Edin ML, Theken KN, Schuck RN, Flake GP, Kannon MA, Degraff LM, Lih FB, Foley J, Bradbury JA, Graves JP, Tomer KB, Falck JR, Zeldin DC, Lee CR. Endothelial CYP epoxygenase overexpression and soluble epoxide hydrolase disruption attenuate acute vascular inflammatory responses in mice. FASEB J. 2011;25:703–713. doi: 10.1096/fj.10-171488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Campbell WB. New role for epoxyeicosatrienoic acids as anti-inflammatory mediators. Trends Pharmacol Sci. 2000;21:125–127. doi: 10.1016/s0165-6147(00)01472-3. [DOI] [PubMed] [Google Scholar]
- 46.Muller DN, Theuer J, Shagdarsuren E, Kaergel E, Honeck H, Park JK, Markovic M, Barbosa-Sicard E, Dechend R, Wellner M, Kirsch T, Fiebeler A, Rothe M, Haller H, Luft FC, Schunck WH. A peroxisome proliferator-activated receptor-alpha activator induces renal CYP2C23 activity and protects from angiotensin II-induced renal injury. Am J Pathol. 2004;164:521–532. doi: 10.1016/s0002-9440(10)63142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burgess A, Vanella L, Bellner L, Schwartzman ML, Abraham NG. Epoxyeicosatrienoic acids and heme oxygenase-1 interaction attenuates diabetes and metabolic syndrome complications. Prostaglandins Other Lipid Mediat. 2012;97:1–16. doi: 10.1016/j.prostaglandins.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Y, Falck JR, Manthati VL, Jat JL, Campbell WB. 20-Iodo-14,15-epoxyeicosa-8(Z)-enoyl-3-azidophenylsulfonamide: photoaffinity labeling of a 14,15-epoxyeicosatrienoic acid receptor. Biochemistry (Mosc) 2011;50:3840–3848. doi: 10.1021/bi102070w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Issan Y, Hochhauser E, Kornowski R, Leshem-Lev D, Lev E, Sharoni R, Vanella L, Puri N, Laniado-Schwartzman M, Abraham NG, Porat E. Endothelial progenitor cell function inversely correlates with long-term glucose control in diabetic patients: association with the attenuation of the heme oxygenase-adiponectin axis. Can J Cardiol. 2012;28:728–736. doi: 10.1016/j.cjca.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu X, Zhao CX, Wang L, Tu L, Fang X, Zheng C, Edin ML, Zeldin DC, Wang DW. Increased CYP2J3 expression reduces insulin resistance in fructose-treated rats and db/db mice. Diabetes. 2010;59:997–1005. doi: 10.2337/db09-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu X, Tu L, Feng W, Ma B, Li R, Zheng C, Li G, Wang DW. CYP2J3 gene delivery up-regulated adiponectin expression via reduced endoplasmic reticulum stress in adipocytes. Endocrinology. 2013;154:1743–1753. doi: 10.1210/en.2012-2012. [DOI] [PubMed] [Google Scholar]
- 52.Fedorenko A, Lishko PV, Kirichok Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell. 2012;151:400–413. doi: 10.1016/j.cell.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Langin D, Larrouy D, Barbe P, Millet L, Viguerie-Bascands N, Andreelli F, Laville M, Vidal H. Uncoupling protein-2 (UCP2) and uncoupling protein-3 (UCP3) expression in adipose tissue and skeletal muscle in humans. Int J Obes Relat Metab Disord. 1999;23 (Suppl 6):S64–S67. doi: 10.1038/sj.ijo.0800950. [DOI] [PubMed] [Google Scholar]
- 54.Dorresteijn JA, Visseren FL, Spiering W. Mechanisms linking obesity to hypertension. Obes Rev. 2012;13:17–26. doi: 10.1111/j.1467-789X.2011.00914.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.