Abstract
The DASH-Sodium trial demonstrated beneficial effects on blood pressure (BP) of the DASH diet with lower sodium intake compared to typical American diet. The subsequent OMNIHEART trial reported additional BP benefits from replacing carbohydrate in the DASH diet with either protein or monounsaturated fats. The primary aim of this study is to assess possible BP benefits of an OMNIHEART-like diet in free-living Americans using cross-sectional U.S. population data of the INTERMAP Study. INTERMAP data include four 24-h dietary recalls, two timed 24-h urine collections, eight BP readings for 2,195 individuals ages 40-59 from eight US INTERMAP population samples. Analyses are conducted using two approaches: 1. Regression of BP on a linear OMNIHEART nutrient score calculated for each individual, and 2. A Bayesian approach comparing estimated BP levels of an OMNIHEART-like nutrient profile with a typical American nutrient profile. After adjustment for potential confounders, an OMNIHEART score higher by one point was associated with systolic/diastolic BP differences of −1.0/−0.5 mmHg (both P<0.001). Mean systolic/diastolic BPs were 111.3/68.4 and 115.2/70.6 mmHg for Bayesian OMNIHEART and Control profiles respectively, after controlling for possible confounders, with BP differences of −3.9/−2.2 mmHg, Pr(difference ≤ 0) ≈ 0.98/0.96. Findings were comparable for men and women, for non-hypertensive participants, and with adjustment for antihypertensive treatment. Our findings from data on US population samples indicate broad generalizability of OMNIHEART results beyond the trial setting, and support recommendations for an OMNIHEART-style diet for prevention/control of population-wide adverse BP levels.
Keywords: blood pressure, population studies, nutrition, statistics
Introduction
Adverse blood pressure (BP) – prevalent worldwide – is an independent major risk factor for cardiovascular diseases 1. Public health measures with an emphasis on primary and primordial prevention are needed to address this problem 2. The Dietary Approaches to Stop Hypertension-Sodium (DASH-Sodium) feeding trial examined the effects on BP of the DASH diet (rich in fruit, vegetables, grains, legumes, nuts/seeds, and low-fat/fat-free dairy products, with reduced total and saturated fats, cholesterol, sugars) 3 at higher, intermediate, and lower levels of sodium for 30 days, compared with a “typical American” control diet 4. Both DASH vs. control and lower (target 50 mmol/24-h or 1.2 g/24-h) vs. higher (target 150 mmol/24-h or 3.5g/24-h) sodium resulted in significant reductions in BP; the greatest difference was seen for the DASH diet with lower sodium level compared to control diet with higher sodium (−8.9 mm Hg systolic, P<0.001). The DASH diet appears to be achievable and palatable for sustained periods in free-living populations 5,6. DASH plus reduced sodium is a recommended lifestyle modification for the prevention and management of prehypertension and hypertension 7. The Optimal Macronutrient Intake Trial for Heart Health (OMNIHEART) study compared the effects of three heart-healthy diets on BP: a carbohydrate-rich diet, a diet rich in protein (predominantly from nonmeat sources), and a diet rich in unsaturated fat (predominantly monounsaturated. Compared with the carbohydrate diet, both the protein and unsaturated fat diets significantly lowered systolic and diastolic BP in all participants and in those with hypertension 8.
A key unresolved question is the extent that BP lowering achieved by the DASH-Sodium and OMNIHEART diets over the short-term in trial settings translate into BP differences in free-living in populations. The International Study of Macronutrients, Micronutrients and Blood Pressure (INTERMAP) has reported several favorable single nutrient-BP associations 9-15. Here we use INTERMAP data to assess BP differences associated with the three OMNIHEART-like nutrient profiles compared to a typical American nutrient profile.
Methods
Population Samples and Field Methods (1996-1999)
INTERMAP surveyed 4,680 men and women ages 40-59 from Japan (4 population samples), People's Republic of China (3 samples), UK (2 samples), and US (8 samples). Participants were randomly recruited from general and occupational population samples 16. Each person attended the clinic on four occasions: visits 1 and 2 on consecutive days, visits 3 and 4 on consecutive days on average 3 weeks later. For BP measurement, each participant – having emptied his/her bladder – was seated for 5 minutes, feet flat on the floor, in a quiet room, with no physical activity, eating, drinking, or smoking in the preceding half hour. Blood pressure was measured twice at each visit with a random-zero sphygmomanometer; Korotkoff sounds I and V were criteria for systolic BP and diastolic BP. Measurements of height and weight, and questionnaire data on daily alcohol consumption over the previous seven days were obtained at two visits. Dietary data were collected at each visit by a trained interviewer with use of the multi-pass 24-hour recall method 17. Questionnaire data were obtained on possible confounders. Each participant provided two 24-hour urine collections, start and end timed at the research center; measurements included urinary volume, sodium, potassium, calcium, magnesium, creatinine 16, as well as amino acids and multiple urinary metabolites 18. We focus here on data for the 2,195 US INTERMAP participants. Dietary data for the US participants were converted to nutrient intakes (83 nutrients, including six individual sugars and the sum of glucose, sucrose and fructose from sugar-sweetened beverages) with use of the Nutrition Data System (version 2.91) maintained by the Nutrition Coordinating Center, University of Minnesota17, 19. Measurements/person were averaged for BP and nutrient variables across the four visits; for 24-hour urinary excretions, across the two collections.
The study received institutional ethics committee approval for each site; all participants gave written informed consent.
Statistical Methods
To investigate associations of OMNIHEART-like nutrient profiles with BP, two statistical approaches were used, 1) more commonly used nutrient score analyses and 2) Bayesian profile regression. The score analyses utilized a measure of “closeness” based on a score derived from a linear combination of individual nutrients. This score weighted each nutrient equally and did not take into account possible interactions between nutrients. The Bayesian profile approach, on the other hand, analyzed dietary patterns holistically, and thus encapsulated the complex manner in which individual nutrients combine to affect BP. These two statistical approaches were implemented as follows:
OMNIHEART Score Statistical Methods
OMNIHEART-protein (OMNI-PRO), OMNIHEART-monounsaturated fatty acids (OMNI-MFA), and OMNIHEART-carbohydrate (OMNI-CHO) scores (adapted from Mellen et al. 20) were calculated for each participant and included as explanatory variables in separate regressions with BP. One point was given for each OMNIHEART nutrient target that was met, and half a point was given for an intermediate intake. The target nutrients and their values are given in Tables S1 to S3. Associations of the three OMNIHEART scores with systolic and diastolic BP were assessed by multiple regression, with and without adjustment for weight and height. Two models were used, the first unadjusted for possible confounders; the second adjusted for age, gender, sample, dietary supplement use, cardiovascular disease or diabetes diagnosis, physical activity, family history of high BP. Analyses were done for men and women combined and separately; excluding hypertensive individuals (see Table S6 footnotes); excluding individuals with cardiovascular disease or diabetes; and adding 10/5 mm Hg to the systolic/diastolic BP of individuals taking antihypertensive medication, to account for possible antihypertensive treatment bias 21.
Bayesian Profile Regression Statistical Methods
An iterative Bayesian dimension-reduction and clustering technique was used 22, 23. At every iteration of the clustering technique the 2,195 study participants plus the three hypothetical OMNIHEART profiles and the hypothetical Control profile were assigned probabilistically to clusters based on the similarity of their nutrient profiles (comprising 61 nutrients) as determined by the mixture-model setup described in the Online Supplemental Material.
Bayesian profile regression analysis 22, 23 matched three OMNIHEART nutrient profiles and a typical American nutrient profile (per the DASH-Sodium trial4) to US INTERMAP participants, and then compared BP levels estimated for each OMNIHEART profile, with those estimated for the typical American profile. To perform Bayesian clustering and profile regression the following data were computed: (i) for cluster assignments, a nutrient profile for each participant, based on 59 of the 83 available dietary nutrients (expressed as energy densities), plus urinary sodium and urinary potassium excretion (expressed as mmol/24-hour, 1 mmol sodium = 23 mg, 1 mmol potassium = 39 mg) (Table S4); (ii) mean systolic and diastolic BP levels for each participant; (iii) data on possible confounders (see below). The profile regression approach allowed expected BP levels to be compared between individuals with hypothetical pre-specified nutrient profiles which may or may not have existed in the data. Thus nutrient profiles were constructed for (i) a hypothetical individual specified to match OMNI-PRO criteria; (ii) a nutrient profile to match OMNI-MFA criteria; (iii) a nutrient profile to match OMNI-CHO criteria; and (iv) a nutrient profile to match the typical American criteria (denoted “Control”) (Tables S1 to S3).
The hypothetical OMNIHEART and Control profiles were defined from published OMNIHEART and DASH-Sodium nutrient targets and were based on eight or more variables in the nutrient profiles (Tables S1 to S3) 3, 4, 24. For each of the putatively favorable nutrients the OMNIHEART target was set as the median value among INTERMAP participants with intake (or urinary excretion) greater than or equal to the published OMNIHEART target (Tables S1 to S3). For each of the putatively unfavorable nutrients the OMNIHEART targets were set as the median value among INTERMAP participants with intake (or urinary excretion) less than the published OMNIHEART target. Control targets were set by the opposite: i.e., the median of putatively favorable nutrient intake below the published Control target; the median of putatively unfavorable nutrient intakes above the published control target (Table S1-S3).
Statistical analyses were performed by J.M. and I.J.B. using the freely available package PreMiuM 25, 26 for R statistical software 27 and SAS version 9.3 (SAS Institute, Cary, NC, USA).
Results
Mean systolic/diastolic BP was 120.4/75.7 mm Hg in men, 116.8/71.1 mm Hg in women (Table S5). Mean vegetable protein, starch, and fiber intakes were 5.2% of energy, 22.8% of energy, and 2.1 g/1,000 kJ respectively. Mean dietary magnesium, dietary calcium, and urinary potassium were 35.4 mg/1,000 kJ, 86.8 mg/1,000 kJ, and 57.7 mmol/24-h (2.3 g/24-h). Mean saturated fatty acids intake, dietary cholesterol, and urinary sodium were 10.7 % kcal, 31.4 mg/1,000 kJ, and 162.6 mmol/24-h (3.7 g/24-h). Mean OMNI-PRO scores were 1.5 for men and 1.9 for women – marginally lower than the mean OMNI-MFA and OMNI-CHO scores.
One-hundred and thirty-three participants (6%) had an OMNI-PRO score of 4.5 or above, i.e., met at least half of the nutrient criteria (Figure S1). Eight participants (0.4%) met more than half the nutrient targets set out in Table S1 for the OMNI-PRO Bayesian profile; 447 (20%) met more than half of the targets for the Control Bayesian profile (Figure S2).
OMNIHEART-Protein score analysis
In multiple regression analysis adjusted for confounders, OMNI-PRO score higher by one point was associated with systolic/diastolic BP lower by 1.6/0.9 mm Hg (P <0.001), 1.0/0.5 mm Hg (P <0.001) with additional control for weight and height (Table 1). Associations were of similar magnitude for men and women, in analyses adjusted for antihypertensive treatment, and with exclusion of individuals with a cardiovascular disease or diabetes diagnosis. BP differences were smaller in analyses excluding hypertensive individuals (Table 1).
Table 1.
Estimated mean difference in blood pressure (mm Hg) and 95% confidence intervals for OMNIHEART score (protein arm) higher by 1 point, multiple regression models, US INTERMAP Participants (N=2,195)
| Model | Systolic blood pressure | Diastolic blood pressure | |||
|---|---|---|---|---|---|
| Without adjustment for weight, height | With adjustment for weight, height | Without adjustment for weight, height | With adjustment for weight, height | ||
| Difference (95% CI) mm Hg | Difference (95% CI) mm Hg | Difference (95% CI) mm Hg | Difference (95% CI) mm Hg | ||
| Overall (N=2,195) | * | −1.74 (−2.16, −1.33)‡ | −0.98 (−1.38, −0.58)‡ | −0.96 (−1.25, −0.67)‡ | −0.50 (−0.78, −0.22)‡ |
| † | −1.55 (−1.96, −1.14)‡ | −0.98 (−1.38, −0.58)‡ | −0.87 (−1.15, −0.59)‡ | −0.54 (−0.82, −0.26)‡ | |
| Men (N=1,103) | * | −1.52 (−2.08, −0.95)‡ | −0.99 (−1.54, −0.44)‡ | −0.71 (−1.14, −0.27)‡ | −0.35 (−0.78, 0.07) |
| † | −1.34 (−1.91, −0.78)‡ | −0.86 (−1.41, −0.31)‡ | −0.68 (−1.10, −0.26)‡ | −0.33 (−0.75, 0.08) | |
| Women (N=1,092) | * | −1.68 (−2.28, −1.08)‡ | −0.83 (−1.41, −0.24)‡ | −0.82 (−1.19, −0.44)‡ | −0.48 (−0.86, −0.10‡ |
| † | −1.74 (−2.33, −1.15)‡ | −1.06 (−1.63, −0.48)‡ | −1.03 (−1.40, −0.65)‡ | −0.71 (−1.08, −0.33)‡ | |
| Excluding hypertensive individuals (N=1,600) | * | −1.31 (−1.69, −0.94)‡ | −0.62 (−0.98, −0.26)‡ | −0.71 (−1.00, −0.43)‡ | −0.26 (−0.54, 0.03) |
| † | −1.08 (−1.45, −0.71)‡ | −0.57 (−0.93, −0.21)‡ | −0.60 (−0.87, −0.32)‡ | −0.28 (−0.56, −0.01‡ | |
| Excluding individuals with a cardiovascular disease or diabetes diagnosis (N=1,852) | * | −1.59 (−2.02, −1.16)‡ | −0.87 (−1.29, −0.45)‡ | −0.99 (−1.30, −0.68)‡ | −0.49 (−0.79, −0.19)‡ |
| † | −1.43 (−1.86, −1.00)‡ | −0.86 (−1.28, −0.44)‡ | −0.86 (−1.15, −0.56)‡ | −0.51 (−0.80, −0.22)‡ | |
| Adjusted for antihypertensive treatment (N=2,195) | * | −1.90 (−2.36, −1.43)‡ | −0.94 (−1.39, −0.50)‡ | −1.04 (−1.35, −0.73)‡ | −0.48 (−0.78, −0.18)‡ |
| † | −1.75 (−2.20, −1.29)‡ | −1.02 (−1.45, −0.58)‡ | −0.97 (−1.26, −0.67)‡ | −0.56 (−0.85, −0.27)‡ | |
Unadjusted model
Adjusted for age, gender, sample, cardiovascular disease or diabetes diagnosis, family history of high blood pressure, dietary supplement use, physical activity
Statistically significant C.I.
Similar patterns of findings were observed for OMNI-MFA and OMNI-CHO scores, although the BP differences for the latter were generally smaller (Tables S6 and S7).
Bayesian profile regression analysis
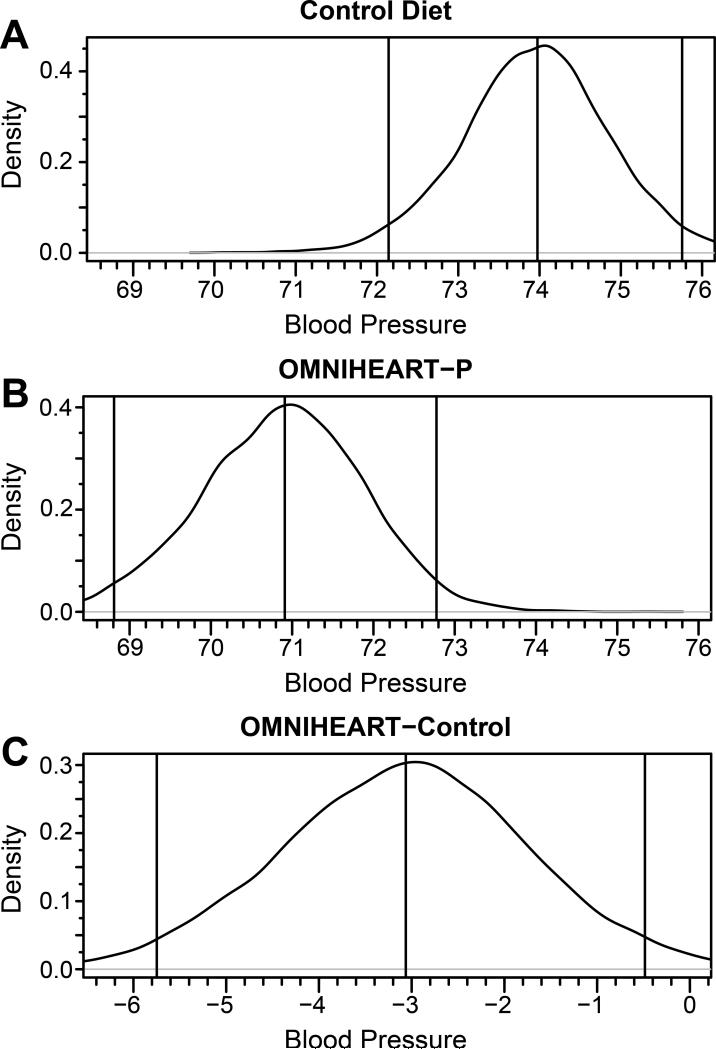
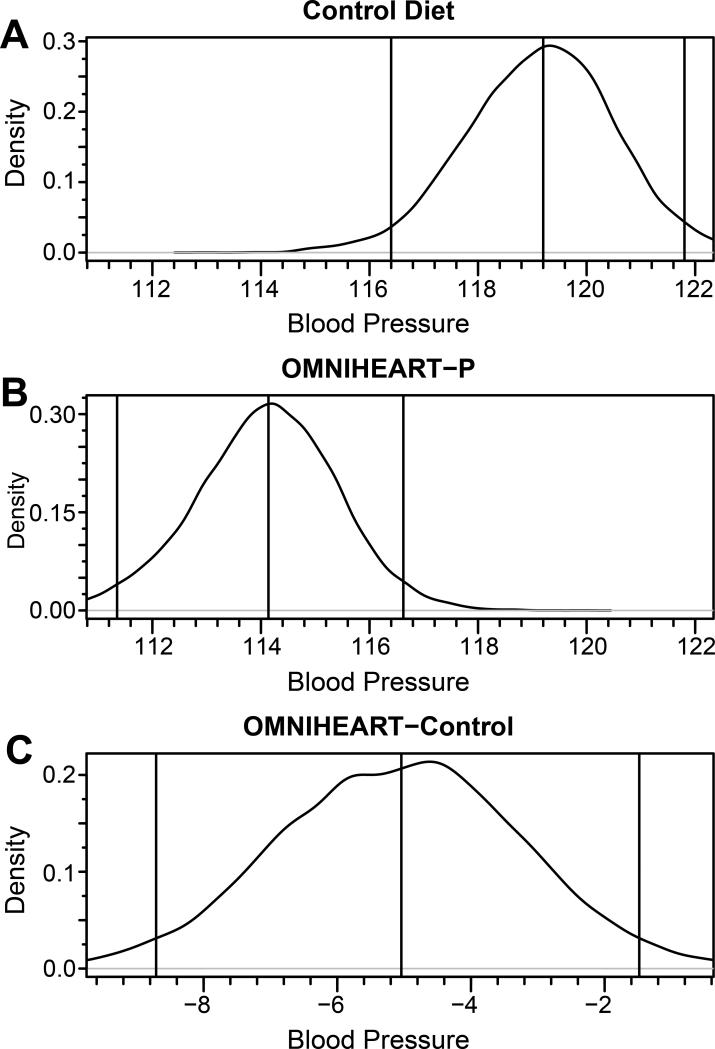
Mean posterior systolic and diastolic BPs were lower for the OMNI-PRO profile compared to Control profile in all models tested (Tables 2 and 3). In analysis of men and women combined, mean posterior systolic/diastolic BPs for OMNI-PRO profile were 114.1/70.9 mm Hg compared with 119.2/74.0 for Control profile, i.e., OMNI-PRO minus Control BPs were −5.0/−3.1 mm Hg, Pr(diff≤0) ≈ 1.00/0.99. With adjustment for multiple possible confounders including gender, weight, height, and medical history of cardiovascular disease or diabetes, the differences were −3.9/−2.2 mm Hg, Pr(diff≤0) ≈ 0.98/0.96. The unadjusted posterior distributions for systolic and diastolic BP for OMNIPRO and Control nutrient profiles are illustrated in Figures 1 and 2. The distributions were approximately normal; there was a clear “shift to the left” (toward lower BP values) for the OMNI-PRO profile compared to Control for both systolic and diastolic BP.
Table 2.
Posterior means and 95% credibility intervals for systolic blood pressure for OMNIHEART (protein arm) and Control nutrient profiles, US INTERMAP participants, Bayesian analyses
| Model | OMNIHEART | Control | Difference (OMNIHEART-Control) | ||
|---|---|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Pr(diff≤0) | ||
| Overall (N=2,195) | * | 114.1 (111.4, 116.6) | 119.2 (116.4, 121.8) | −5.04 (−8.71, −1.48) | 1.00 |
| † | 111.3 (107.6, 114.6) | 115.2 (111.9, 118.4) | −3.91 (−7.69, −0.27) | 0.98 | |
| Men (N=1,103) | * | 116.6 (113.0, 120.6) | 121.4 (118.9, 123.9) | −4.83 (−9.20, −0.13) | 0.98 |
| † | 113.9 (110.0, 117.8) | 117.7 (114.3, 120.9) | −3.74 (−7.76, 0.27) | 0.97 | |
| Women (N=1,092) | * | 113.0 (109.3, 116.6) | 117.9 (114.6, 121.1) | −4.90 (−9.79, −0.03) | 0.98 |
| † | 107.7 (102.7, 112.4) | 112.3 (107.8, 116.6) | −4.59 (−9.23, −0.07) | 0.98 | |
| Excluding hypertensive individuals (N=1,600) | * | 111.9 (109.4, 114.0) | 115.6 (113.3, 118.1) | −3.81 (−7.06, −0.66) | 0.99 |
| † | 112.3 (109.5, 115.1) | 114.4 (111.6, 117.1) | −2.03 (−5.02, 0.87) | 0.92 | |
| Excluding individuals with cardiovascular disease or diabetes diagnosis (N=1,852) | * | 113.6 (110.7, 116.3) | 118.8 (116.2, 121.4) | −5.25 (−9.18, −1.44) | 1.00 |
| † | 111.8 (108.2, 115.3) | 115.8 (112.4, 119.1) | −4.00 (−7.74, −0.32) | 0.98 | |
| Adjusted for antihypertensive treatment (N=2,195) | * | 115.7 (112.3, 118.8) | 120.5 (117.2, 123.5) | −4.78 (−9.34, −0.36) | 0.98 |
| † | 111.7 (107.6, 115.3) | 115.3 (111.5 119.0) | −3.70 (−7.58, 0.18) | 0.97 |
Unadjusted model
Adjusted for age, gender, sample, cardiovascular disease or diabetes diagnosis, family history of high blood pressure, special diet, dietary supplement use, physical activity, weight, height
Table 3.
Posterior means and 95% credibility intervals for systolic blood pressure for OMNIHEART (protein arm) and Control nutrient profiles, US INTERMAP participants, Bayesian analyses
| Model | OMNIHEART | Control | Difference (OMNIHEART-Control) | ||
|---|---|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Pr(diff≤0) | ||
| Overall (N=2,195) | * | 70.9 (68.8, 72.8) | 74.0 (72.1, 75.6) | −3.06 (−5.75, −0.48) | 0.99 |
| † | 68.4 (65.9, 70.8) | 70.6 (68.3, 72.8) | −2.23 (−4.69, −0.31) | 0.96 | |
| Men (N=1,103) | * | 74.6 (71.9, 77.2) | 75.1 (72.6, 77.9) | −0.54 (−4.40, −3.09) | 0.62 |
| † | 70.1 (66.9, 73.1) | 70.6 (67.6, 74.1) | −0.55 (−4.34, 2.80) | 0.62 | |
| Women (N=1,092) | * | 69.6 (67.2, 71.8) | 71.6 (69.5, 73.6) | −2.00 (−5.10, −0.97) | 0.90 |
| † | 64.6 (61.2, 67.7) | 66.9 (64.0, 69.7) | −2.33 (−5.47, −0.55) | 0.95 | |
| Excluding hypertensive individuals (N=1,600) | * | 69.5 (67.1, 71.8) | 71.83 (70.1, 73.7) | −2.34 (−5.35, −0.46) | 0.95 |
| † | 68.8 (66.4, 71.3) | 70.6 (68.4, 72.6) | −1.80 (−4.16, 0.77) | 0.92 | |
| Excluding individuals with cardiovascular disease or diabetes diagnosis (N=1,852) | * | 71.1 (69.1, 73.1) | 74.2 (72.5, 76.0) | −3.09 (−5.66, −0.45) | 0.99 |
| † | 68.6 (66.1, 71.1) | 71.1 (68.7, 73.3) | −2.41 (−5.00, −0.25) | 0.96 | |
| Adjusted for antihypertensive treatment (N=2,195) | * | 72.1 (69.6, 74.0) | 74.7 (72.7, 76.5) | −2.67 (−5.78, −0.08) | 0.97 |
| † | 68.6 (65.9, 71.2) | 70.6 (68.1, 72.9) | −2.00 (−4.64, 0.61) | 0.93 |
Unadjusted model
Adjusted for age, gender, sample, cardiovascular disease or diabetes diagnosis, family history of high blood pressure, special diet, dietary supplement use, physical activity, weight, height
Figure 1.
Unadjusted posterior systolic blood pressure distribution obtained from Bayesian profile regression for Control nutrient profiles (A), OMNIHEART-P (protein arm) (B), and from OMNIHEARTP minus Control (C), US INTERMAP participants. Vertical lines represent (from left to right) lower bound of the 95% credible interval, mean, and upper bound of the 95% credible interval. The distribution from the Control nutrient profile (A) had a median of 119.2 (116.4, 121.8). The distribution from the OMNIHEART-P profile (B) had a median of 114.1 (111.3, 116.6). The distribution of the difference between the two profiles (C) had a median of −5.0 (−8.7, −1.5) and the probability that the difference was less than or equal to zero was approximately 1.
Figure 2.
Unadjusted posterior diastolic blood pressure distribution obtained from Bayesian profile regression for Control nutrient profiles (A), OMNIHEART (protein arm) (B), and from OMNIHEART (protein arm) minus Control (C), US INTERMAP participants. Vertical lines represent (from left to right) lower bound of the 95% credible interval, mean, and upper bound of the 95% credible interval. The distribution from the Control nutrient profile (A) had a median of 74.0 (72.1, 75.8). The distribution from the OMNIHEART-P profile (B) had a median of 70.9 (68.8, 72.8). The distribution of the difference between the two profiles (C) had a median of −3.1 (--5.7, −0.5) and the probability that the difference was less than or equal to zero was approximately 1.
Findings from sensitivity analyses, comprising gender-specific models, exclusion of hypertensive participants, or adding +10/+5 mm Hg for those using antihypertensive medication, were qualitatively similar to the foregoing (Tables 2 and 3).
Equivalent analyses based on OMNI-MFA and OMNI-CHO profiles yielded similar findings (Tables S8 to S11).
Discussion
We found that compared to a typical American dietary pattern, an OMNIHEART-like dietary pattern was associated with lower BP in cross-sectional U.S. population data of the INTERMAP Study. Our findings, on 2,195 free-living Americans surveyed prior to the publication of the DASH and OMNIHEART trials, are consistent with the results of the trials, and with prior INTERMAP work on relations of multiple dietary factors to BP 3, 4, 8, 28.
Gao et al. 29 assessed DASH diet adherence and associations with hypertension at baseline among 5,972 US adults of the Multi-Ethnic Study of Atherosclerosis (MESA), using an additive DASH score based on 9 nutrient targets (not including sodium) assessed by food frequency questionnaire. Less than 30% of MESA participants met a single DASH nutrient target. Compared with normotensive participants, those with uncontrolled hypertension were less likely to meet DASH targets for saturated fat, calcium, and magnesium intakes. Compared to individuals with uncontrolled hypertension, controlled hypertensive participants were more likely to meet all DASH nutrient targets, indicating that hypertension awareness led to modified dietary behavior. In the INTERMAP Study, restricting the analysis to non-hypertensive participants reduced but did not eliminate the BP difference, suggesting that dietary modification in response to hypertension awareness did not account for the beneficial BP difference observed for the DASH nutrient profile.
Strengths of our study include four standardized multi-pass in-depth 24-h dietary recalls, two timed 24-h urine collections, repeated blood pressure measurements with extensive quality control; random selection of free-living Americans from defined populations, also extensive high quality data on dietary nutrients and availability of data on multiple confounders. We did not adjust analyses for smoking or socioeconomic status as smoking has not been found to be consistently associated with clinic-measured blood pressure levels30, and previous INTERMAP analysis had demonstrated that the inverse association between years of education (a proxy for socioeconomic status) and blood pressure was explained almost entirely by dietary differences31. We included height and weight in regression models rather than body mass index because previous work had shown weight adjusted for height to be more strongly associated with blood pressure levels than body mass index32.
Both statistical methods gave similar results despite differences in modeling assumptions. The frequentist OMNIHEART score approach measured OMNIHEART adherence using a relatively crude metric that did not take into account subtleties related to which combination of nutrients were in compliance, while the Bayesian approach was more sophisticated in this regard. The fact that both approaches gave similar results suggests that associations with BP of OMNIHEART diet adherence are robust with regard to variations in nutrient intakes meeting the OMNIHEART targets for any particular individual.
Limitations include: the cross-sectional nature of the findings – temporality cannot be assessed, and the possibility of reverse causation cannot be completely excluded (although sensitivity analysis data in non-hypertensive participants indicate that bias of this type was unlikely); effect-size underestimation due to limited reliability in nutrient measurement (regression-dilution bias) despite multiple standardized measurements33-35; results limited to adults ages 40-59 years.
The beneficial BP differences estimated here for OMNIHEART-like nutrient profiles compared with a typical American nutrient profile in free-living Americans are compatible with the findings of the OMNIHEART trial 8. Our results from observational population data thus lend further support for recommendations for a reduced sodium OMNIHEART-like diet for prevention and control of population-wide adverse BP levels 36.
Perspectives
The DASH-Sodium trial demonstrated beneficial effects on BP of the DASH diet with lower sodium intake, and the subsequent OMNIHEART trial reported additional benefits for BP by replacing carbohydrate in the DASH diet with either protein or monounsaturated fats. Linear nutrient score and Bayesian approaches were applied to cross-sectional INTERMAP data to assess whether short-term BP lowering achieved by the DASH-Sodium and OMNIHEART diets in a trial settings were translated into BP differences observed in population data. Both linear nutrient score and Bayesian approaches indicated favorable BP differences associated OMNIHEART-like diets compared to a typical American diet, supporting recommendations for a reduced sodium OMNIHEART-style diet for prevention and control of population-wide adverse BP levels.
Supplementary Material
What Is New?
To the best of our knowledge, this is the first study examining the association between an OMNIHEART-like lower sodium diet with lower BP in free-living Americans in cross-sectional U.S. population data.
What is Relevant?
This study addresses a key unresolved question regarding the extent to which BP lowering results achieved in the DASH-Na and OMNIHEART diets trials translate to free-living populations.
Summary.
The beneficial BP differences estimated here for OMNIHEART-like lower sodium compared with a typical American higher sodium nutrient profiles in free-living Americans are compatible with the findings of the OMNIHEART trial, thus lending further support for recommendations for a reduced sodium OMNIHEART-like diet for prevention and control of population-wide adverse BP levels.
Acknowledgements
The INTERMAP Study was accomplished through the fine work of staff at local, national, and international centers – a partial listing of colleagues is published in 12.
The authors’ responsibilities were as follows – JM: developed the Bayesian profile regression method, devised the concept for the analysis, performed statistical analyses, interpreted the data, and drafted the manuscript; IJB: devised the concept for the analysis, performed statistical analyses, interpreted the data, and drafted the manuscript; MP and SL: assisted with development of the Bayesian profile regression method, contributed to the writing of the manuscript; NTM: assisted with data management, contributed to the writing of the manuscript; QC: assisted with data management, contributed to the writing of the manuscript; SR: assisted with development of the Bayesian profile regression method, contributed to the writing of the manuscript; LVH: contributed to the writing of the manuscript; MLD: contributed to the writing of the manuscript; JS: designed and obtained funds for the INTERMAP Study, interpreted the data, contributed to the writing of the manuscript; PE: designed and obtained funds for the INTERMAP Study, interpreted the data, contributed to the writing of the manuscript.
Sources of Funding
Sources of support: The INTERMAP Study is supported by grant R01-HL050490 from the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Maryland, US; and by national agencies in China, Japan (the Ministry of Education, Science, Sports, and Culture, Grant-in-Aid for Scientific Research [A], No. 090357003), and the UK (a project grant from the West Midlands National Health Service Research and Development, and grant R2019EPH from the Chest, Heart and Stroke Association, Northern Ireland). P.E.'s research is supported in part by the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre at Imperial College Healthcare NHS Trust. He is an NIHR senior investigator. J.M. is supported by Medical Research Council grant G0901841. S.L. is supported by the Leverhulme Trust (ECF-2011-576).
Footnotes
Disclosures
None.
References
- 1.Lawes CM, Vander Hoorn S, Law MR, Elliott P, MacMahon S, Rodgers A. Blood pressure and the global burden of disease 2000. Part 1: estimates of blood pressure levels. Journal of hypertension. 2006;24:413–422. doi: 10.1097/01.hjh.0000199801.72563.6f. [DOI] [PubMed] [Google Scholar]
- 2.Lenfant C. Task force on Research in Epidemiology and Prevention of Cardiovascular Diseases. Circulation. 1994;90:2609–2617. doi: 10.1161/01.cir.90.6.2609. [DOI] [PubMed] [Google Scholar]
- 3.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. The New England journal of medicine. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 4.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, 3rd, Simons-Morton DG, Karanja N, Lin PH, Group DA-SCR . The New England journal of medicine. Vol. 344. DASH-Sodium Collaborative Research Group; 2001. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. pp. 3–10. [DOI] [PubMed] [Google Scholar]
- 5.Lin PH, Appel LJ, Funk K, Craddick S, Chen C, Elmer P, McBurnie MA, Champagne C. The PREMIER intervention helps participants follow the Dietary Approaches to Stop Hypertension dietary pattern and the current Dietary Reference Intakes recommendations. Journal of the American Dietetic Association. 2007;107:1541–1551. doi: 10.1016/j.jada.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 6.Karanja N, Lancaster KJ, Vollmer WM, Lin PH, Most MM, Ard JD, Swain JF, Sacks FM, Obarzanek E. Acceptability of sodium-reduced research diets, including the Dietary Approaches To Stop Hypertension diet, among adults with prehypertension and stage 1 hypertension. Journal of the American Dietetic Association. 2007;107:1530–1538. doi: 10.1016/j.jada.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr., Jones DW, Materson BJ, Oparil S, Wright JT, Jr., Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA : the journal of the American Medical Association. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 8.Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER, 3rd, Conlin PR, Erlinger TP, Rosner BA, Laranjo NM, Charleston J, McCarron P, Bishop LM. OmniHeart Collaborative Research G. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA : the journal of the American Medical Association. 2005;294:2455–2464. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- 9.Brown IJ, Elliott P, Robertson CE, Chan Q, Daviglus ML, Dyer AR, Huang CC, Rodriguez BL, Sakata K, Ueshima H, Van Horn L, Zhao L, Stamler J, Group IR. Dietary starch intake of individuals and their blood pressure: the International Study of Macronutrients and Micronutrients and Blood Pressure. Journal of hypertension. 2009;27:231–236. doi: 10.1097/HJH.0b013e32831a7294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elliott P, Kesteloot H, Appel LJ, Dyer AR, Ueshima H, Chan Q, Brown IJ, Zhao L, Stamler J, Group ICR. Dietary phosphorus and blood pressure: international study of macro- and micro-nutrients and blood pressure. Hypertension. 2008;51:669–675. doi: 10.1161/HYPERTENSIONAHA.107.103747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elliott P, Stamler J, Dyer AR, Appel L, Dennis B, Kesteloot H, Ueshima H, Okayama A, Chan Q, Garside DB, Zhou B. Association between protein intake and blood pressure: the INTERMAP Study. Archives of internal medicine. 2006;166:79–87. doi: 10.1001/archinte.166.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miura K, Stamler J, Nakagawa H, Elliott P, Ueshima H, Chan Q, Brown IJ, Tzoulaki I, Saitoh S, Dyer AR, Daviglus ML, Kesteloot H, Okayama A, Curb JD, Rodriguez BL, Elmer PJ, Steffen LM, Robertson C, Zhao L. International Study of M-M, Blood Pressure Research G. Relationship of dietary linoleic acid to blood pressure. The International Study of Macro-Micronutrients and Blood Pressure Study [corrected]. Hypertension. 2008;52:408–414. doi: 10.1161/HYPERTENSIONAHA.108.112383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stamler J, Brown IJ, Daviglus ML, Chan Q, Kesteloot H, Ueshima H, Zhao L, Elliott P, Group IR. Glutamic acid, the main dietary amino acid, and blood pressure: the INTERMAP Study (International Collaborative Study of Macronutrients, Micronutrients and Blood Pressure). Circulation. 2009;120:221–228. doi: 10.1161/CIRCULATIONAHA.108.839241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tzoulaki I, Brown IJ, Chan Q, Van Horn L, Ueshima H, Zhao L, Stamler J, Elliott P. International Collaborative Research Group on M-M, Blood P. Relation of iron and red meat intake to blood pressure: cross sectional epidemiological study. Bmj. 2008;337:a258. doi: 10.1136/bmj.a258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ueshima H, Stamler J, Elliott P, Chan Q, Brown IJ, Carnethon MR, Daviglus ML, He K, Moag-Stahlberg A, Rodriguez BL, Steffen LM, Van Horn L, Yarnell J, Zhou B, Group IR. Food omega-3 fatty acid intake of individuals (total, linolenic acid, long-chain) and their blood pressure: INTERMAP study. Hypertension. 2007;50:313–319. doi: 10.1161/HYPERTENSIONAHA.107.090720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stamler J, Elliott P, Dennis B, Dyer AR, Kesteloot H, Liu K, Ueshima H, Zhou BF, Group IR. INTERMAP: background, aims, design, methods, and descriptive statistics (nondietary). Journal of human hypertension. 2003;17:591–608. doi: 10.1038/sj.jhh.1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dennis B, Stamler J, Buzzard M, Conway R, Elliott P, Moag-Stahlberg A, Okayama A, Okuda N, Robertson C, Robinson F, Schakel S, Stevens M, Van Heel N, Zhao L, Zhou BF, Group IR. INTERMAP: the dietary data--process and quality control. Journal of human hypertension. 2003;17:609–622. doi: 10.1038/sj.jhh.1001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holmes E, Loo RL, Stamler J, Bictash M, Yap IK, Chan Q, Ebbels T, De Iorio M, Brown IJ, Veselkov KA, Daviglus ML, Kesteloot H, Ueshima H, Zhao L, Nicholson JK, Elliott P. Human metabolic phenotype diversity and its association with diet and blood pressure. Nature. 2008;453:396–400. doi: 10.1038/nature06882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schakel SFDBH, Wold AC, Conway R, Zhao LC, Okuda N, Okayama A, Moag-Stahlberg A, Robertson C, Van Heel N, Buzzard IM, Stamler J. Enhancing data on nutrient composition of foods eaten by participants in the INTERMAP study in China, Japan, the United Kingdom, and the United States. J Food Comp Anal. 2003;16:395–408. doi: 10.1016/S0889-1575(03)00043-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mellen PB, Gao SK, Vitolins MZ, Goff DC., Jr Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988-1994 and 1999-2004. Archives of internal medicine. 2008;168:308–314. doi: 10.1001/archinternmed.2007.119. [DOI] [PubMed] [Google Scholar]
- 21.Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Statistics in medicine. 2005;24:2911–2935. doi: 10.1002/sim.2165. [DOI] [PubMed] [Google Scholar]
- 22.Molitor J, Papathomas M, Jerrett M, Richardson S. Bayesian profile regression with an application to the National Survey of Children's Health. Biostatistics. 2010;11:484–498. doi: 10.1093/biostatistics/kxq013. [DOI] [PubMed] [Google Scholar]
- 23.Papathomas M, Molitor J, Richardson S, Riboli E, Vineis P. Examining the joint effect of multiple risk factors using exposure risk profiles: lung cancer in nonsmokers. Environ Health Perspect. 2011;119:84–91. doi: 10.1289/ehp.1002118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swain JF, McCarron PB, Hamilton EF, Sacks FM, Appel LJ. Characteristics of the diet patterns tested in the optimal macronutrient intake trial to prevent heart disease (OmniHeart): options for a heart-healthy diet. Journal of the American Dietetic Association. 2008;108:257–265. doi: 10.1016/j.jada.2007.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hastie DI, Liverani S, Richardson S. PReMiuM: Dirichlet process Bayesian clustering, profile regression. R package version 3.0.20. 2013 [Google Scholar]
- 26.Liverani S, Hastie DI, Papathomas M, Richardson S. PReMiuM: An R package for profile regression mixture models using Dirichlet processes. R package version 3.0.20. 2013 doi: 10.18637/jss.v064.i07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Team RC. R: A language and environment for statistical computing. Foundation for Statistical Computing; Vienna, Austria: 2013. [Google Scholar]
- 28.Stamler J. Nutritional and Metabolic Bases of Cardiovascular Disease. Wiley-Blackwell; 2011. Improved Nutrition: Key to Solving the Populationwide Blood Pressure Problem. pp. 303–320. [Google Scholar]
- 29.Gao SK, Fitzpatrick AL, Psaty B, Jiang R, Post W, Cutler J, Maciejewski ML. Suboptimal nutritional intake for hypertension control in 4 ethnic groups. Archives of internal medicine. 2009;169:702–707. doi: 10.1001/archinternmed.2009.17. [DOI] [PubMed] [Google Scholar]
- 30.Mann SJ, James GD, Wang RS, Pickering TG. Elevation of ambulatory systolic blood pressure in hypertensive smokers. A case-control study. JAMA : the journal of the American Medical Association. 1991;265:2226–2228. [PubMed] [Google Scholar]
- 31.Stamler J, Elliott P, Appel L, Chan Q, Buzzard M, Dennis B, Dyer AR, Elmer P, Greenland P, Jones D, Kesteloot H, Kuller L, Labarthe D, Liu K, Moag-Stahlberg A, Nichaman M, Okayama A, Okuda N, Robertson C, Rodriguez B, Stevens M, Ueshima H, Horn LV, Zhou B. Higher blood pressure in middle-aged American adults with less education-role of multiple dietary factors: the INTERMAP study. Journal of human hypertension. 2003;17:655–775. doi: 10.1038/sj.jhh.1001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dyer AR, Elliott P, Shipley M. Body mass index versus height and weight in relation to blood pressure. Findings for the 10,079 persons in the INTERSALT Study. Am J Epidemiol. 1990;131:589–596. doi: 10.1093/oxfordjournals.aje.a115543. [DOI] [PubMed] [Google Scholar]
- 33.Dyer AR, Liu K, Sempos CT. Nutrient data analysis techniques and strategies. In: Berdanier CD, Dwyer JT, Feldman EB, editors. Handbook of Nutrition and Food. CRC Press; Boca Raton, FL: 2007. pp. 93–103. [Google Scholar]
- 34.Grandits GA, Bartsch GE, Stamler J. Method issues in dietary data analyses in the Multiple Risk Factor Intervention Trial. The American journal of clinical nutrition. 1997;65:211S–227S. doi: 10.1093/ajcn/65.1.211S. [DOI] [PubMed] [Google Scholar]
- 35.Liu K. Measurement error and its impact on partial correlation and multiple linear regression analyses. Am J Epidemiol. 1988;127:864–874. doi: 10.1093/oxfordjournals.aje.a114870. [DOI] [PubMed] [Google Scholar]
- 36.Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM, American Heart A. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension. 2006;47:296–308. doi: 10.1161/01.HYP.0000202568.01167.B6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.