The term “endothelial dysfunction” generally refers to a maladapted endothelial phenotype characterized by reduced nitric oxide (NO) bioavailability, increased oxidative stress, elevated expression of pro-inflammatory and pro-thrombotic factors, and reduced endothelial derived vasodilation.1 Hyperglycemia, insulin resistance, dyslipidemia, hyperuricemia, increased dietary fructose and fat are conditions that predispose endothelial dysfunction, an early precursor to increased vascular and cardiac stiffness and atherosclerosis all risk factors for hypertension, myocardial infarction, stroke, limb ischemia, and heart failure. Thus, endothelial dysfunction is an important risk factor for cardiovascular disease (CVD) related morbidity and mortality.2
Recently, cross-sectional studies suggested that endothelial dysfunction also independently predicts the incidence of type 2 diabetes (T2D). For example, a prospective study of the children and spouses of children from the original Framingham Heart Study cohort found high levels of endothelial cell-derived Willebrand factor (vWF) increased the risk of developing T2D independent of other risk factors for diabetes including obesity, abnormal glucose metabolism, and inflammation.3 Similarly, in a large, prospective, nested case-control study from an ethnically diverse cohort of U.S. postmenopausal women (Women's Health Initiative Observational Study), higher levels of circulating E-selectin and intercellular adhesion molecule-1 were consistently associated with increased risk of developing T2D.4 Both studies support a potential causal role for endothelial dysfunction in insulin resistance.
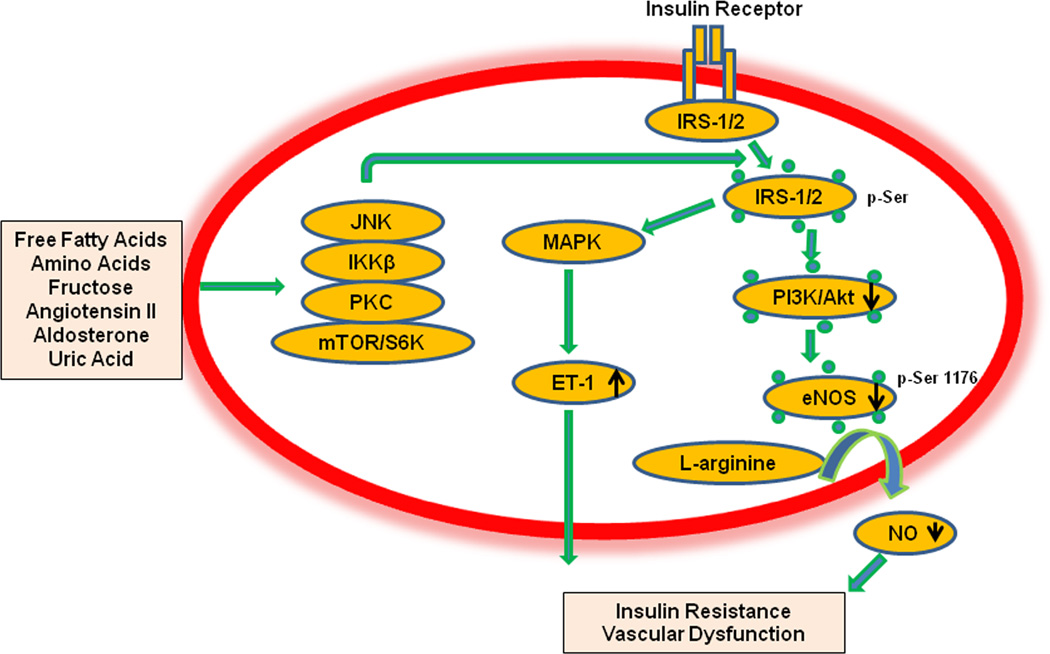
Animal studies have demonstrated impaired vascular insulin metabolic signaling due to activation of serine/threonine kinases in the condition of endothelial dysfunction. Activation of various serine kinases such S6 kinase, increases serine phosphorylation of insulin receptor substrate (IRS) proteins and subsequently results in vascular insulin resistance and associated endothelial dysfunction (Fig. 1).2 Increased serine phosphorylation of IRS protein leads to decreased activity of insulin downstream signaling pathways, including phosphatidylinositide 3-kinases (PI3K) and protein kinase B (Akt), which culminates in reduced endothelial nitric oxide synthase (eNOS) activation, increased vascular smooth muscle calcium sensitization, and reduced vasodilation.5 Further, the hyperinsulinemia associated with systemic insulin resistance stimulates production of the vasoconstrictor endothelin-1 (ET-1) via a mitogen-activated protein kinase (MAPK)-dependent signaling pathway.5 Thus, endothelial insulin resistance is accompanied by reduced PI3K-NO pathway and heightened MAPK-ET-1 pathway (Fig.1).5 Indeed, vascular homeostasis is tightly controlled by endothelial cells (EC) secreting the vasodilatory substances, such as NO, endothelium-derived hyperpolarizing factor (EDHF), prostacyclin, and vasoconstrictory substances such as ET-1, angiotensin II, aldosterone and thromboxane A2.6 The net balance of these EC vasoactive substances have been proposed to mediate the link between insulin resistance and/or hyperinsulinemia and CVD. Thus, endothelial dysfunction has been suggested as a common underlying mechanism that links systemic and vascular insulin resistance and development of T2D. It is noted that systemic insulin resistance and T2D can then accelerate EC functional impairment and this sets up a bi-directionality between endothelial dysfunction and T2D, wherein endothelial dysfunction and systemic metabolic abnormalities interact in a vicious cycle to accelerate CVD.6
Figure 1.
Proposed molecular mechanism for insulin resistance in endothelial dysfunction. Abbreviation: JNK, c-Jun N-terminal kinase; IKKβ, IκB kinase; PKC, protein kinase C; mTOR, mammalian target of rapamycin; S6K, ribosomal S6 kinases; MAPK, mitogen-activated protein kinase; PI3-K/Akt, phosphatidylinositide 3-kinases/protein kinase B; ET-1, endothelin-1; NO, nitric oxide; eNOS, endothelial NO synthase. IRS-1/2, insulin receptor substrate 1/2;
The Hoorn Study was designed to determine the prevalence of T2D and associated risk factors in a population-based cohort study of 2484 patients from 1989.7 In this issue,8 investigators reported an interaction between endothelial dysfunction and impaired glucose metabolism (IGM), insulin resistance and T2D respectively with regard to risk of CVD events. Impaired glucose metabolism and insulin resistance was ascertained via oral glucose tolerance test and HOMA-IR testing, respectively. Endothelial function was evaluated by flow-mediated dilatation (FMD) of the brachial artery. The results of this study confirmed and extended previous reports on the joint interactive effects of endothelial dysfunction, impaired glucose metabolism and insulin resistance on incident cardiovascular events. 7 The present study provides strong evidence that endothelial dysfunction, T2D and insulin resistance synergistically increase CVD, and therefore identifies endothelial dysfunction as a key therapeutic target in persons with underlying metabolic abnormalities.
However, there are some caveats of these studies which need consideration. Although FMD is a non-invasive approach and has become the most widely used technique to measure endothelial function, FMD measures the endothelial vasomotor response during reactive hyperemia, and does not identify abnormalities related to the EC production of vasoactive substances in basal and other states under which endothelial function is evaluated.9 A new technique, low-flow-mediated constriction (L-FMC), may provide complementary information to FMD since L-FMC response is not solely based on NO availability, but also mediated by other EC derived substances including ET-1, EDHF, and cyclooxygenase.10 Secondly, this study provides information on other medications used by study participants that could potentially affect systemic and vascular insulin sensitivity and CVD events. Several therapeutic interventions that may impact insulin sensitivity and CVD have been reported, such as metformin, statin therapy, renin-angiotensin-aldosterone antagonists, and arginine supplementation. It is noted that DREAM trial in the 5,269 patients followed them for 3 years, ramipril increased regression to normoglycemia but did not significantly reduce the primary end point of new-onset T2D.1 The reason is that ACEI may need a relative long time to restore the islet β cell function or increase β cell number which is function of T2D.
Nevertheless, this study provides further evidence that endothelial dysfunction is inextricably related to metabolic abnormalities and that this combination of hemodynamic and metabolic abnormalities synergistically increases CVD event risk. It is conceivable that therapeutic strategies directed at improving vascular insulin metabolic signaling and endothelial dysfunction may lead to an improvement of systemic metabolic abnormalities and combinatorially reduce CVD. Future studies should further confirm that endothelial function protection is indeed associated with a concomitant reduction in T2D and CVD events.
Acknowledgments
Source of Funding
JRS is funded by NIH (R01 HL73101-01A and R01 HL107910-01) and the Veterans Affairs Merit System (0018). The authors would like to thank Brenda Hunter for her editorial assistance.
Footnotes
Disclosure
No potential conflicts of interest relevant to this article were report.
References
- 1.DREAM Trial Investigators. Bosch J, Yusuf S, Gerstein HC, Pogue J, Sheridan P, Dagenais G, Diaz R, Avezum A, Lanas F, Probstfield J, Fodor G, Holman RR. Effect of ramipril on the incidence of diabetes. N Engl J Med. 2006;355:1551–1562. doi: 10.1056/NEJMoa065061. [DOI] [PubMed] [Google Scholar]
- 2.Sowers JR. Diabetes mellitus and vascular disease. Hypertension. 2013;61:943–947. doi: 10.1161/HYPERTENSIONAHA.111.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frankel DS, Meigs JB, Massaro JM, Wilson PW, O'Donnell CJ, D'Agostino RB, Tofler GH. Von Willebrand factor, type 2 diabetes mellitus, and risk of cardiovascular disease: the framingham offspring study. Circulation. 2008;118:2533–2539. doi: 10.1161/CIRCULATIONAHA.108.792986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song Y, Manson JE, Tinker L, Rifai N, Cook NR, Hu FB, Hotamisligil GS, Ridker PM, Rodriguez BL, Margolis KL, Oberman A, Liu S. Circulating levels of endothelial adhesion molecules and risk of diabetes in an ethnically diverse cohort of women. Diabetes. 2007;56:1898–1904. doi: 10.2337/db07-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim JA, Jang HJ, Martinez-Lemus LA, Sowers JR. Activation of mTOR/p70S6 kinase by ANG II inhibits insulin-stimulated endothelial nitric oxide synthase and vasodilation. Am J Physiol Endocrinol Metab. 2012;15(302):E201–E208. doi: 10.1152/ajpendo.00497.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jia G, Aroor AR, Whaley-Connell AT, Sowers JR. Fructose and uric acid: is there a role in endothelial function? Curr Hypertens Rep. 2014;16:434. doi: 10.1007/s11906-014-0434-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Jager J, Dekker JM, Kooy A, Kostense PJ, Nijpels G, Heine RJ, Bouter LM, Stehouwer CD. Endothelial dysfunction and low-grade inflammation explain much of the excess cardiovascular mortality in individuals with type 2 diabetes: the Hoorn Study. Arterioscler Thromb Vasc Biol. 2006;26:1086–1093. doi: 10.1161/01.ATV.0000215951.36219.a4. [DOI] [PubMed] [Google Scholar]
- 8.Thomas T van Sloten, Ronald MA Henry, Jacqueline M Dekker, Giel Nijpels, Thomas Unger, Miranda T Schram, Coen DA Stehouwer. Endothelial dysfunction plays a key role in increasing cardiovascular risk in type 2 diabetes: the Hoorn Study. Hypertension. 2014 doi: 10.1161/HYPERTENSIONAHA.114.04221. In Press. [DOI] [PubMed] [Google Scholar]
- 9.Poredos P, Jezovnik MK. Testing endothelial function and its clinical relevance. J Atheroscler Thromb. 2013;20:1–8. doi: 10.5551/jat.14340. [DOI] [PubMed] [Google Scholar]
- 10.Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Lüscher TF, Shechter M, Taddei S, Vita JA, Lerman A. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126:753–767. doi: 10.1161/CIRCULATIONAHA.112.093245. [DOI] [PMC free article] [PubMed] [Google Scholar]