Abstract
Co-transmission, the ability of a neuron to release multiple transmitters, has long been recognized in selected circuits. However, the release of multiple primary neurotransmitters from a single neuron is only beginning to be appreciated. Here we consider recent examples of co-transmission as well as co-release – the packaging of multiple neurotransmitters into a single vesicle. The properties associated with each mode of release greatly enhance the possible action of such neurons within circuits. The functional importance of dual- (or multi-) transmitter neurons extends beyond actions on postsynaptic receptors, due in part to differential spatial and temporal profiles of each neurotransmitter. Recent evidence also suggests that the dual-transmitter phenotype can be dynamically regulated during development and following injury or disease.
Keywords: co-release, co-transmission, dual-transmitter neurons, synaptic transmission
Introduction
Neurotransmitter phenotype has long been recognized as a hallmark of neuronal identity. The classical view was that each neuron releases a single neurotransmitter, leading to the “one neuron, one transmitter” hypothesis [1], formalized by John Eccles as Dale’s Principle [2]. However, we now know that many neurons throughout the brain are capable of releasing two or more neurotransmitters [3–5]. For simplicity, in this review we refer to these cells as “dual” transmitter neurons. Some of the first evidence for such dual- (or multi-) transmitter neurons was noted in 1982, when Jan et al. reported a very slow synaptic potential in a subset of sympathetic neurons that accompanied the well-established cholinergic transmission (Figure 1) [6]. This slow potential was mediated by a peptide, LHRH (luteinizing hormone-releasing hormone), indicating that the presynaptic neuron released a neuroactive peptide as well as acetylcholine [6,7]. Such co-transmission, defined broadly as the release of multiple neurotransmitters from a single neuron, has been reported for many neuromodulators including ATP, neuroactive peptides, neurotrophic factors and even ions such as Zn2+ [8–15]. Recent evidence, however, suggests that neurons can co-transmit not only neuromodulators but also multiple primary neurotransmitters including fast-acting neurotransmitters, monoamines and acetylcholine [16–20].
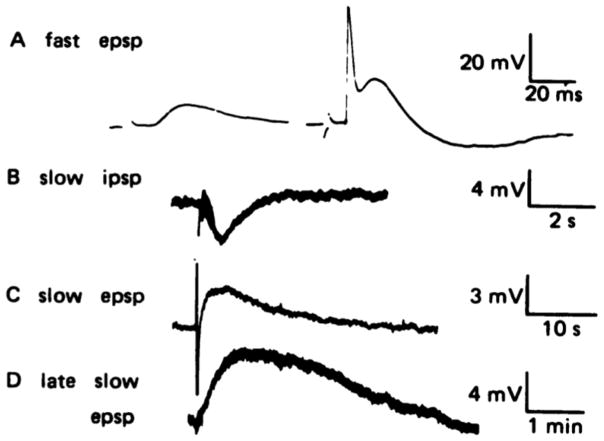
Figure 1. Co-transmission in the sympathetic nervous system.
Stimulation of a sympathetic ganglion neuron revealed (a) a fast, nicotinic EPSP (left) and higher stimulation evoked an action potential (right). (b and c)Such stimuli can also elicit a slow muscarinic IPSP (b) or EPSP (c) depending on the stimulation conditions. (d) Strong stimulation generated a late slow EPSP consistent with co-transmission by the neuromodulator LHRH. Note timescale difference in each panel. Adapted from Figure 1 of Jan et al., 1979.
Although dual-transmitter neurons are found throughout the brain, the functional significance of co-transmission on neuronal circuits has been difficult to dissect. This difficulty arises, in part, because in addition to activating postsynaptic receptors, co-released neurotransmitters can modulate pre- and postsynaptic responses and even modulate the packaging of other neurotransmitters into synaptic vesicles [4]. Additionally, each neurotransmitter may be differentially released in time and space, thereby complicating analysis. A consideration of all these parameters is necessary to understand how dual-transmitter neurons alter the computational capabilities of neuronal circuits. Of note, the functional importance of co-transmission has been better described in select invertebrate systems, where each transmitter can differentially enhance the ability of the circuit to participate in multiple computational tasks [21–24]. Here we focus on recent studies of dual-transmitter neurons, including the mechanisms governing the release of multiple neurotransmitters, and the functional importance of co-transmission and co-release on circuit function in the mammalian CNS.
Co-release vs. Co-transmission
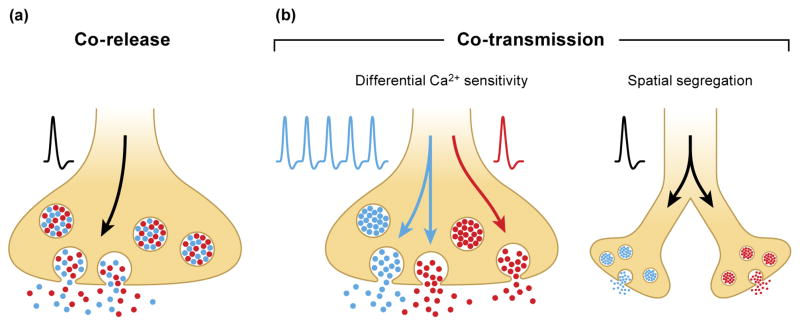
The release of multiple neurotransmitters from a single neuron does not necessarily imply co-release, i.e. that two or more neurotransmitters are packaged into a single population of synaptic vesicles (Figure 2a). Co-transmission can be more broadly defined as the release of multiple neurotransmitters from non-overlapping pools of synaptic vesicles (Figure 2b). The distinction between co-release and co-transmission is important because each mode of release can have different potential impacts on circuit function. For example, release from different sets of vesicles can be differentially regulated by differential Ca2+ sensitivity or the spatial segregation of vesicles (Figure 2b).
Figure 2. Co-release and Co-transmission are distinct modes of release.
(a) With co-release, both neurotransmitters (mixed red and blue) are packaged into the same set of synaptic vesicles. Upon an action potential invading the presynaptic terminal, vesicles containing both neurotransmitters are released into the synaptic cleft. (b) In contrast, co-transmission requires neurotransmitters be sequestered into distinct populations of synaptic vesicles with differential release mediated by differential Ca2+ sensitivities (left panel). For example, a single action potential might release one set of vesicles (red), but multiple action potentials might be required to release both sets of vesicles (red and blue). Alternatively, co-transmission can rely on spatial segregation of vesicle populations to different boutons (right panel) in which case, unique information is transmitted to different postsynaptic targets.
Recent work by Tritsch and colleagues (2012) provides an elegant example of co-release in the ventral tegmental area (VTA) [25••]. Their work indicates that dopaminergic neurons targeting striatal spiny neurons co-release GABA. Surprisingly, the conditional knockout of the vesicular GABA transporter (VGAT) [26–28], failed to eliminate GABA release [25••]. Instead, inhibition or conditional knockout of the vesicular monoamine transporter (VMAT2), which was thought to only package monoamines [29], completely eliminated GABA release [25••]. Although this work did not examine changes at the single vesicle level, the results indicate that GABA is a non-canonical substrate for VMAT2. Although the function of co-released GABA within the VTA circuitry has not yet been well characterized, the ability of VMAT2 to package a non-canonical substrate, such as GABA, into synaptic vesicles suggests that monoaminergic neurons expressing VMAT2 are capable of co-releasing GABA.
Co-transmission, on the other hand, has been demonstrated in a handful of circuits, including the retina where a subpopulation of starburst amacrine cells release acetylcholine (ACh) and GABA [30–32]. Consistent with a co-transmission phenotype, cholinergic synapses are uniformly distributed on the postsynaptic neuron, whereas GABAergic synapses are non-uniformly distributed [32]. Furthermore, the release of GABA and ACh have different Ca2+ sensitivities, suggesting that co-transmission in this circuit involves spatial segregation of vesicles and differential release [32]. Ostensibly, these features allow single starburst amacrine cells to participate in distinct, yet related circuit functions.
Interestingly, the co-transmission phenotype can be confined to a subset of synaptic boutons in an individual neuron. For example, in cultured dopaminergic neurons, immunohistochemical studies suggest that most boutons contain vesicular glutamate and monoamine transporters, but only a single vesicular transporter type could be detected in a significant fraction of terminals [33]. However, these studies could not distinguish whether the transporters were colocalized in the same vesicles, a necessary condition for co-release. Similarly, neurons in the dorsal raphe, the nucleus that provides serotonergic innervation to many brain regions, can also release glutamate [18,34–35•]. In this case, the proportion of terminal varicosities that co-express vesicular glutamate transporter and the vesicular monoamine transporter varies across target regions, and such co-expression is completely lacking in recurrent axon collaterals projecting back to the raphe nucleus [34,35•]. Selectively targeting of the co-transmission phenotype to some boutons, but not others, may allow neurons to convey different information to distinct downstream targets.
Functional implications of co-release in neuronal circuits
Broadly speaking, there are three classes of dual-transmitter neurons: neurons that release two fast-acting neurotransmitters; neurons that release a fast-acting neurotransmitter and a slow-acting monoamine; and neurons that release a fast-acting neurotransmitter and a neuromodulator, defined here as peptides, ions or other small molecules (Figure 3). Each class can have different effects on pre- and postsynaptic neurons, thus altering the functional impact of dual-transmitter neurons within circuits. The effect of dual-transmitter neurons that release two fast acting neurotransmitters (such as glutamate and GABA or GABA and glycine) is primarily determined by postsynaptic factors (Figure 3a). These factors include the kinetics and polarity of postsynaptic currents, the specific receptor types expressed, and the synaptic vs. extrasynaptic distribution of the receptors (Figure 3a). For example, the net effect of neurons that co-release GABA and glycine appears not to depend on the quantity of either neurotransmitter in individual vesicles, but rather by the GABA and/or glycine receptors expressed postsynaptically [36–37••]. Of course, fast acting neurotransmitters can also bind to presynaptic G-protein coupled receptors, such as metabotropic glutamate receptors or GABAB receptors, thereby modulating presynaptic release dynamics. The effect of dual-transmitter neurons releasing a fast-acting neurotransmitter with a slower-acting monoamine is more complex (Figure 3b). This complexity arises from different temporal profiles as well as modulation by presynaptic receptors. Most co-released monoamines act via G-protein coupled receptors, making the response intrinsically slower. Furthermore, activation of G-protein coupled receptors can have multiple postsynaptic effects with different time courses. The list of neuromodulators that can be co-released is extensive, including ions such as Zn2+, neurotrophins, peptides and ATP. Neuromodulators can modulate the presynaptic terminal, elicit postsynaptic currents, provide trophic support to the postsynaptic neuron, directly modulate receptor dynamics, and alter gene expression (Figure 3c). For example, Zn2+can bind to NMDA receptors and prolong the EPSC, thereby altering the computational capabilities afforded by NMDA receptors [38]. Despite the increasing attention to “dual”-transmitter neurons, recent evidence suggests that neurons can release more than two chemical messengers, such as a subset of ventral tegmental neurons that can release dopamine, GABA and glutamate [25••, 39•].
Figure 3. Functional considerations of different classes of co-released transmitters.
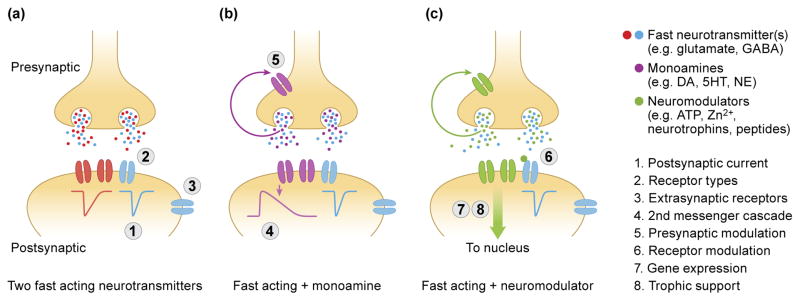
Different classes of dual-transmitter neurons have distinct functional implications for circuit dynamics. (a) The effect of neurons that release two fast-acting neurotransmitters (such as GABA and Glycine) is primarily determined by the postsynaptic receptors; including (1) the kinetics and amplitude of the postsynaptic current, (2) the specific receptor types expressed in the postsynaptic neuron and (3) the synaptic or extrasynaptic location of postsynaptic receptors. (b) For neurons that release a fast-acting neurotransmitter and a monoamine, additional factors that determine the functional output include (4) second messenger cascades and intrinsically slower currents, such as GIRK and (5) modulation of the presynaptic terminal. (c) The action of neurons that release a fast-acting neurotransmitter and a neuromodulator include (6) direct receptor modulation, (7) postsynaptic changes in gene expression and (8) trophic support.
The Spectrum of Co-release and Co-transmission
Co-release of GABA and glycine
Release of GABA and glycine from some inhibitory neurons represents a robust example of co-release [16,37••,40–41]. The first physiological evidence of glycine/GABA co-release was reported in the spinal cord, where miniature IPSCs had both GABA and glycine receptor components [16], suggesting that both neurotransmitters were present in single vesicles. Co-release of GABA and glycine is perhaps not surprising given that the vesicular inhibitory amino acid transporter (VIAAT, also known as VGAT) is expressed in GABAergic and glycinergic neurons [26] and can package both neurotransmitters into synaptic vesicles [27–28,42–43]. Given the low selectivity of VIAAT, it is not immediately clear which factors control the differential release of GABA and glycine in different neuronal populations. However, the availability of neurotransmitter in the cytosol should affect vesicular packaging [37••,41,44]. Consistent with this hypothesis, GABA and glycine compete for vesicular loading, as altering their cytoplasmic concentration changes the GABA/glycine ratio in synaptic vesicles [37••,41,43,45]. In fact, acute changes in cytosolic GABA or glycine concentrations can result in dynamic modulation of IPSC amplitude, independent of vesicle turnover [37••]. These results suggest that the packaging of neurotransmitters into synaptic vesicles, and therefore, the amplitude of IPSCs are dynamically modulated on fast timescales [37••,41].
Co-release of glutamate and dopamine in the VTA
The dopaminergic midbrain has emerged as a surprisingly heterogeneous population of neurons, which is partly reflected in the co-transmission phenotype of individual neurons. The first evidence of co-transmission was noted in cultured dopaminergic neurons, which express the vesicular glutamate transporter (VGLUT2) and upon stimulation elicit fast glutamatergic EPSCs both in autaptic cultures and co-cultures [46–48]. The co-transmitter phenotype was later verified in vivo, as VTA afferents to the nucleus accumbens co-express tyrosine hydroxylase (TH) and VGLUT2 [49–51], however VGLUT2 expression declines with age [52–54]. Although it is difficult to determine the protein composition of a single vesicle, biochemical studies suggest that VMAT2-positive vesicles can co-express VGLUT2 [55••]. However, recent work suggests that while dopamine and glutamate share similar release properties (such as sensitivity to D2 receptor agonists and paired-pulse ratio) they are differentially affected by cocaine, suggesting the two transmitters may be in non-overlapping pools of vesicles [56••].
The transmitter phenotype of neurons within the VTA is heterogeneous, highlighting the ability of these neurons to convey distinct messages to each of their postsynaptic targets. For example, optogenetic stimulation of afferents from the VTA elicits glutamatergic EPSCs in the nucleus accumbens, but not the dorsal striatum [50–51,57]. As mentioned, VTA afferents that target striatal spiny neurons are capable of co-releasing GABA and dopamine through the non-canonical packaging of GABA by VMAT2 [25••]. Interestingly, a presumably separate population of VTA neurons that target cholinergic interneurons release dopamine alone in the dorsal striatum, whereas in the medial shell of the nucleus accumbens this population co-releases dopamine and GABA, and yet in the core they co-release dopamine, GABA and glutamate [39•]. Further evidence suggests that co-released dopamine can modulate the glutamatergic response by actions on pre- and post-synaptic receptors [49]. Taken together, these results indicate that the transmitter phenotype of different populations of “dopaminergic neurons” have widely different transmitter phenotypes depending on the postsynaptic target.
The importance of glutamate co-release from monoaminergic neurons extends beyond the effects of each transmitter on pre- and postsynaptic receptors. If vesicular glutamate transporters and vesicular monoamine transporters are expressed on an individual synaptic vesicle, the amount of monoamine packaged into the vesicle can be enhanced by vesicular synergy, defined as the enhanced packaging of one neurotransmitter as a result of packaging another neurotransmitter into the same vesicle [4,55••]. Mechanistically, expression of the vesicular glutamate transporter is thought to dissipate the vesicle potential while maximizing the pH gradient used to package monoamines, thereby increasing the concentration of monoamine in the vesicle and enhancing monoaminergic transmission [4,55••]. For example, conditional knockout of the vesicular glutamate transporter in dopaminergic neurons caused behavioral deficits associated with dopaminergic transmission, as well as a reduction in the electrochemical detection of dopamine in the VTA [55••,58–60]. Vesicular synergy also occurs in cholinergic neurons, as VGLUT conditional knockouts have a hypocholinergic phenotype [61].
Co-transmission of dopamine and GABA in the olfactory bulb
In the olfactory bulb, a subset of periglomerular interneurons express both tyrosine hydroxylase and GAD70, which suggests they can release both dopamine and GABA [62,63]. Recently, Borisovska and colleagues (2013), using dual patch clamp/amperometric recordings from autaptically cultured dopaminergic periglomerular neurons, reported that stimulation with a single action potential resulted in a fast, GABAergic IPSC but failed to elicit an immediate barrage of amperometric spikes [64••]. Instead, the frequency of amperometric events that detect dopamine release increased slowly over seconds [64••]. The temporal disparity of dopamine and GABA release suggests that these two neurotransmitters are not only packaged into different vesicles, a feature of co-transmission, but also differentially released. In brain slices, optogenetic stimulation of periglomerular neurons produces a temporally biphasic inhibition-excitation, providing further support for of the temporal disparity of dopamine and GABA release [64••,65•].
The neurotransmitter phenotype is plastic
Co-release during development
Emerging evidence suggests that the transmitter phenotype of a neuron is plastic. Even in single transmitter neurons, the neurotransmitter phenotype can be dynamically modulated in response to sensory stimuli. For example, in the hypothalamus, neurons can switch from releasing dopamine to somatostatin with changes in photoperiod [•65]. Similarly in dual-transmitter neurons, there is evidence that the transmitter phenotype can change during normal development. In many circuits co-release is restricted to early periods of development. For example, in the developing auditory brainstem, GABAergic/glycinergic neurons of the medial nucleus of the trapezoid body (MNTB) transiently co-release (or co-transmit) glutamate [67]. Although the role of this co-released glutamate during development is still under examination, the Ca2+ transients elicited by NMDA receptor activation could refine the synaptic map between the MNTB and its target [67–69].
One surprising example of co-release has been reported in the developing hippocampus, in which mossy fibers, long considered to be exclusively glutamatergic, express the GABA synthesis enzyme, GAD70 [70]. In young animals, electrical stimulation of mossy fibers can elicit GABAergic IPSCs in CA3 neurons [17,71••–72]. Although this response could result from the disynaptic activation of inhibitory interneurons, the IPSCs persist in the presence of glutamate receptor antagonists, ostensibly required to activate local interneurons, and persist in conditions of minimal stimulation [17,71••–72]. Furthermore, mossy fiber release of GABA is subject to the same regulation as glutamate release in this pathway [73–75], suggesting that mossy fibers co-release or co-transmit glutamate and GABA [17,71••]. Although GABAergic co-release in this pathway remains contentious [76,77••], stimulation of mechanically dissociated mossy fiber boutons attached to CA3 neurons also elicited GABAergic IPSCs, eliminating possible disynaptic recruitment of GABAergic interneurons [71••]. The developmental pattern of co-release suggests a role in initial circuit formation or synapse stabilization [17,71••–72]. Granule cells, however, undergo continuous adult neurogenesis [78]; therefore it remains possible that co-release occurs from immature granule cells that are generated throughout the life of the animal. Interestingly, the dual-transmitter phenotype can be detected in adult animals following hippocampal seizures [79–81]. Given that seizures dramatically increase granule cell neurogenesis and mossy fiber sprouting [82,83], re-expression of the co-transmission phenotype could result from the increase in immature neurons, or a change in the phenotype of existing, mature granule cells. Interestingly, GAD70 is also unregulated in mossy fibers of human patients with temporal lobe epilepsy [84]. Ostensibly, the increased GABAergic tone within the hippocampus could be neuroprotective.
Dynamic changes in dual-transmitter phenotype with injury and disease
Given the plasticity of the neurotransmitter phenotype, it is perhaps not surprising that co-release may be affected by injury or disease. In the VTA, for example, lesioning with the dopaminergic neurotoxin 6-OHDA causes a rapid increase in the fraction of VTA neurons that co-express vesicular glutamate transporters [52,54]. Furthermore, co-transmission of dopamine and GABA in the olfactory bulb may be modulated in Parkinson’s disease. Prior to the onset of motor symptoms, 73% of sporadic Parkinson’s disease patients have a reduced sense of smell [85,86]. Surprisingly, these olfactory deficits are accompanied by a doubling of tyrosine hydroxylase positive interneurons in the olfactory bulb [87,88]. The increase in TH expression may represent increased neurogenesis of dual-transmitter neurons [89]. However, it is also possible that the existing population of GABA-only periglomerular interneurons upregulate TH expression to become dual-transmitter neurons. Although the contribution of the putative increase in dopamine tone has not been functionally examined, dopamine decreases the release probability of incoming olfactory receptor neurons [63,90], thereby likely decreasing olfactory sensory input. The role of co-transmission in disease has only recently been described and thus further examples are expected in the future.
Highlights.
Co-release and co-transmission are distinct, separate modes of release
There are multiple classes of dual-transmitter neurons
The consequence of co-release extends beyond effects on postsynaptic receptors
The transmitter phenotype of a neuron is plastic, in development and disease
Acknowledgments
We thank all the members of the Westbrook lab for helpful discussion. We also thank Lori Vaskalis for help with illustrations. This work was supported by NIH Grants NS26464 and MH46613 to GLW; as well as a Tartar Trust Fellowship, ARCS Scholarship, and National Science Foundation Graduate Research Fellowship (DGE0925180) to CEV.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Christopher E Vaaga, Email: vaaga@ohsu.edu.
Maria Borisovska, Email: borisovs@ohsu.edu.
Annotated Bibliography
- 1.Dale HP. Pharmacology and Nerve Endings (Walter Ernest Dixon Memorial Lecture): (Section of Therapeutics and Pharmacology) Proc R Soc Med. 1935;28:319–332. doi: 10.1177/003591573502800330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eccles JC, Fatt P, Koketsu K. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to moto-neurons. J Physiol. 1954;126:524–562. doi: 10.1113/jphysiol.1954.sp005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broussard J. Co-transmission of dopamine and glutamate. J Gen Physiol. 2011;139:93–96. doi: 10.1085/jgp.201110659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hnasko TS, Edwards RH. Neurotransmitter corelease: mechanism and physiological role. Annu Rev Physiol. 2012;74:225–243. doi: 10.1146/annurev-physiol-020911-153315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seal RP, Edwards RH. Functional implications of neurotransmitter co-release: Glutamate and GABA share the load. Curr Opin Pharmacol. 2006;6:114–119. doi: 10.1016/j.coph.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Jan YN, Jan LY, Kuffler SW. A peptide as a possible transmitter in sympathetic ganglia of the frog. Proc Natl Acad Sci USA. 1979;76:1501–1505. doi: 10.1073/pnas.76.3.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jan LY, Jan YN. Peptidergic transmission in sympathetic ganglia of the frog. J Physiol. 1982;327:219–246. doi: 10.1113/jphysiol.1982.sp014228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 9.Terman GW, Drake CT, Simmons ML, Milner TA, Chavkin C. Opioid modulation of recurrent excitation in the hippocampal dentate gyrus. J Neurosci. 2000;20:4379–4388. doi: 10.1523/JNEUROSCI.20-12-04379.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Q, Palmiter RD. GABAergic signaling by AgRP neurons prevents anorexia via a melanocortin-independent mechanism. Eur J Pharmacol. 2011;660:21–27. doi: 10.1016/j.ejphar.2010.10.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blomeley C, Bracci E. Substance P depolarizes striatal projection neurons and facilitates their glutamatergic inputs. J Physiol. 2008;586:2143–2144. doi: 10.1113/jphysiol.2007.148965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kay AR, Tóth K. Is zinc a neuromodulator? Sci Signal. 2008;1 doi: 10.1126/stke.119re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sadakata T, Furuichi T. Developmentally regulated Ca2+-dependent activator protein for secretion 2 (CAPS2) is involved in BDNF secretion and is associated with autism susceptibility. Cerebellum. 2009;8:312–322. doi: 10.1007/s12311-009-0097-5. [DOI] [PubMed] [Google Scholar]
- 14.De Biasi S, Rustioni A. Glutamate and substance P coexist in primary afferent terminals in the superficial laminae of spinal cord. Proc Natl Acad Sci USA. 1988;85:7820–7824. doi: 10.1073/pnas.85.20.7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sperk G, Hamilton T, Colmers WF. Neuropeptide Y in the dentate gyrus. Prog Brain Res. 2007;163:285–297. doi: 10.1016/S0079-6123(07)63017-9. [DOI] [PubMed] [Google Scholar]
- 16.Jonas P, Bischofberger J, Sandkühler J. Corelease of two fast neurotransmitters at a central synapse. Science. 1998;281:419–424. doi: 10.1126/science.281.5375.419. [DOI] [PubMed] [Google Scholar]
- 17.Walker MC, Ruiz A, Kullmann DM. Monosynaptic GABAergic signaling from dentate to CA3 with a pharmacological and physiological profile typical of mossy fiber synapses. Neuron. 2001;29:703–715. doi: 10.1016/s0896-6273(01)00245-8. [DOI] [PubMed] [Google Scholar]
- 18.Johnson MD. Synaptic glutamate release by postnatal rat serotonergic neurons in microculture. Neuron. 1994;12:433–442. doi: 10.1016/0896-6273(94)90283-6. [DOI] [PubMed] [Google Scholar]
- 19.Higley MJ, Gittis AH, Oldenburg IA, Balthasar N, Seal RP, Edwards RH, Lowell BB, Kreitzer AC, Sabatini BL. Cholinergic interneurons mediate fast VGluT3-dependent glutamatergic transmission in the striatum. PLoS One. 2011;6 doi: 10.1371/journal/pone.0019155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varga V, Losonczy A, Zemelman BV, Borhegyi Z, Nyriri G, Domonkos A, Hangya B, Holderith N, Magee JC, Freund TF. Fast synaptic subcortical control of hippocampal circuits. Science. 2009;326:449–453. doi: 10.1126/science.1178307. [DOI] [PubMed] [Google Scholar]
- 21.Nusbaum MP, Blitz DM, Swensen AM, Wood D, Marder E. The roles of co-transmission in neural network modulation. Trends Neurosci. 2001;24:146–154. doi: 10.1016/s0166-2236(00)01723-9. [DOI] [PubMed] [Google Scholar]
- 22.DeLong ND, Beenhakker, Nusbaum MP. Presynaptic inhibition selectively weakens peptidergic cotransmission in a small motor system. J Neurophysiol. 2009;102:3492–3504. doi: 10.1152/jn.00833.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chalasani SH, Kato S, Albrecht DR, Nakagawa T, Abbott LF, Bargmann CI. Neuropeptide feedback modifies odor-evoked dynamics in Caenorhabditis elegans olfactory neurons. Nat Neurosci. 2010;13:615–621. doi: 10.1038/nn.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwiatkowski MA, Gabranski ER, Huber KE, Chapline MC, Christie AE, Dickinson PS. Coordination of distinct but interacting rhythmic motor programs by a modulatory projection neuron using different co-transmitters in different ganglia. J Exp Biol. 2013;15:1827–1836. doi: 10.1242/jeb.082503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25••.Tritsch NX, Ding JB, Sabatini BL. Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature. 2012;490:262–266. doi: 10.1038/nature11466. Here the authors demonstrate that VTA dopaminergic neurons co-release GABA. Surprisingly, GABA release did not require the vesicular GABA transporter, but rather relied on the vesicular monoamine transporter (VMAT2), suggesting that GABA is a non-canonical substrate for VMAT2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaudhry FA, Reimer RJ, Bellocchio EE, Danbolt NC, Osen KK, Edwards RH, Storm-Mathisen J. The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. J Neurosci. 1998;18:9733–9750. doi: 10.1523/JNEUROSCI.18-23-09733.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McIntire SL, Reimer SJ, Schuske K, Edwards RH, Jorgensen EM. Identification and characterization of the vesicular GABA transporter. Nature. 1997;389:870–876. doi: 10.1038/39908. [DOI] [PubMed] [Google Scholar]
- 28.Sagné C, El Mestikawy S, Isambert MF, Hamon M, Henry JP, Giros B, Gasnier B. Cloning of functional vesicular GABA and glycine transporter by screening of genome databases. FEBS Lett. 1997;417:177–183. doi: 10.1016/s0014-5793(97)01279-9. [DOI] [PubMed] [Google Scholar]
- 29.Eiden LE, Weihe E. VMAT2: A dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse. Ann NY Acad Sci. 2011;1216:86–98. doi: 10.1111/j.1749-6632.2010.05906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Malley DM, Masland RH. Co-release of acetylcholine and gamma-aminobutyric acid by a retinal neuron. Proc Natl Acad Sci USA. 1989;86:3414–3418. doi: 10.1073/pnas.86.9.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Malley DM, Sandell JH, Masland RH. Co-release of acetylcholine and GABA by the starburst amacrine cells. J Neurosci. 1992;12:1394–1408. doi: 10.1523/JNEUROSCI.12-04-01394.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee S, Kim K, Zhou ZJ. Role of ACh-GABA cotransmission in detecting image motion and motion direction. Neuron. 2010;68:1159–1172. doi: 10.1016/j.neuron.2010.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Onoa B, Li H, Gagnon-Bartsch JA, Elias LA, Edwards RH. Vesicular monoamine and glutamate transporters select distinct synaptic vesicle recycling pathways. J Neurosci. 2010;30:7917–7927. doi: 10.1523/JNEUROSCI.5298-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amilhon B, Lepicard E, Renoir T, Mongeau R, Popa D, Poirel O, Miot S, Gras C, Gardier AM, Gallego J, et al. VGLUT3 (vesicular glutamate transporter type 3) contribution to the regulation of serotonergic transmission and anxiety. J Neurosci. 2010;30:2198–2210. doi: 10.1523/JNEUROSCI.5196-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35•.Gagnon D, Parent M. Distribution of VGLUT3 in highly collateralized axons from the rat dorsal raphe nucleus as revealed by single-neuron reconstructions. PLoS One. 2014;9:e87709. doi: 10.1371/journal.pone.0087709. Individual serotonergic neurons can selectively target a co-transmitter phenotype to a subset of its synaptic boutons. The proportion of boutons participating in co-transmission varies by target region. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dugué GP, Dumoulin A, Triller A, Diedonné S. Target-dependent use of co-released inhibitory transmitters at central synapses. J Neurosci. 2005;25:6490–6498. doi: 10.1523/JNEUROSCI.1500-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37••.Apostolides PF, Trussell LO. Rapid, activity-independent turnover of vesicular transmitter content at a mixed glycine/GABA synapse. J Neurosci. 2013;33:4768–4781. doi: 10.1523/JNEUROSCI.5555-12.2013. Elegant demonstration that acute changes in either GABA or glycine concentration in the cytosol rapidly changes IPSC amplitude, independent of synaptic activity. These data suggests that in these vesicles, neurotransmitters reach equilibrium with cytosolic levels independent of vesicle turnover, allowing for rapid, dynamic modulation of IPSC strength. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tovar KR, Westbrook GL. Amino-terminal ligands prolong NMDA receptor-mediated EPSCs. J Neurosci. 2012;32:8065–8073. doi: 10.1523/JNEUROSCI.0538-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39•.Chuhma N, Mingote S, Moore H, Rayport S. Dopamine neurons control striatal cholinergic neurons via regionally heterogeneous dopamine and glutamate signaling. Neuron. 2014;81:901–912. doi: 10.1016/j.neuron.2013.12.027. Systematic characterization of the co-release phenotype of dopaminergic VTA neurons across multiple afferent targets reveals a rich, region specific heterogeneity of the dual-transmitter phenotype. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rahman J, Latal AT, Besser S, Hirrlinger J, Hülsmann S. Mixed miniature postsynaptic currents resulting from co-release of glycine and GABA recorded from glycinergic neurons in the neonatal respiratory network. Eur J Neurosci. 2013;37:1229–1241. doi: 10.1111/ejn.12136. [DOI] [PubMed] [Google Scholar]
- 41.Ishibashi H, Yamaguchi J, Nakahata Y, Nabekura J. Dynamic regulation of glycine-GABA co-transmission at spinal inhibitory synapses by neuronal glutamate transporter. J Physiol. 2013;591:3821–3832. doi: 10.1113/jphysiol.2012.250647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Christensen H, Fykse EM, Fonnum F. Uptake of glycine into synaptic vesicles isolated from rat spinal cord. J Neurochem. 1990;54:1142–1147. doi: 10.1111/j.1471-4159.1990.tb01941.x. [DOI] [PubMed] [Google Scholar]
- 43.Wojcik SM, Katsurabayashi S, Guillemin I, Friauf E, Rosenmund C, Brose N, Rhee JS. A shared vesicular carrier allows synaptic corelease of GABA and glycine. Neuron. 2006;50:575–587. doi: 10.1016/j.neuron.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 44.Edwards RH. The neurotransmitter cycle and quantal size. Neuron. 2007;55:835–858. doi: 10.1016/j.neuron.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 45.Aubrey KR, Rossi FM, Ruivo R, Alboni S, Bellenchi GC, Le Goff A, Gasnier B, Supplisson S. The transporters GlyT2 and VIAAT cooperate to determine the vesicular glycinergic phenotype. J Neurosci. 2007;27:6273–6281. doi: 10.1523/JNEUROSCI.1024-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sulzer D, Joyce MP, Lin L, Geldwert D, Haber SN, Hattori T, Rayport S. Dopamine neurons make glutamatergic synapses in vitro. J Neurosci. 1998;18:4588–4602. doi: 10.1523/JNEUROSCI.18-12-04588.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joyce MP, Rayport S. Mesoaccumbens dopamine neuron synapses reconstructed in vitro are glutamatergic. Neuroscience. 2000;99:445–456. doi: 10.1016/s0306-4522(00)00219-0. [DOI] [PubMed] [Google Scholar]
- 48.Dal Bo G, St-Gelais F, Danik M, Williams S, Cotton M, Trudeau LE. Dopamine neurons in culture express VGLUT2 explaining their capacity to release glutamate at synapses in addition to dopamine. J Neurochem. 2004;88:1398–1405. doi: 10.1046/j.1471-4159.2003.02277.x. [DOI] [PubMed] [Google Scholar]
- 49.Chuhma N, Choi WY, Mingote S, Rayport S. Dopamine neuron glutamate cotransmission: frequency-dependent modulation in the mesoventromedial projection. Neuroscience. 2009;164:1068–1083. doi: 10.1016/j.neuroscience.2009.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J Neurosci. 2010;30:8229–8233. doi: 10.1523/JNEUROSCI.1754-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tecuapetla F, Patel JC, Xenias H, English D, Tadros I, Shah F, Berlin J, Deisseroth K, Rice ME, Tepper JM, et al. Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens. J Neurosci. 2010;30:7105–7110. doi: 10.1523/JNEUROSCI.0265-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dal Bo G, Bérubé-Carrière N, Mendez JA, Leo D, Riad M, Descarries L, Lévesque D, Trudeau LE. Enhanced glutamatergic phenotype of mesencephalic dopamine neurons after neonatal 6-hydroxydopamine lesion. Neuroscience. 2008;156:59–70. doi: 10.1016/j.neuroscience.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 53.Mendez JA, Bourque MJ, Dal Bo G, Bourdeau ML, Danik M, Williams S, Lacaille JC, Trudeau LE. Developmental and target-dependent regulation of vesicular glutamate transporter expression by dopamine neurons. J Neurosci. 2008;28:6309–6318. doi: 10.1523/JNEUROSCI.1331-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bérubé-Carrière N, Riad M, Dal Bo G, Lévesque D, Trudeau LE, Descarries L. The dual dopamine-glutamate phenotype of growing mesencephalic neurons regresses in mature rat brain. J Comp Neurol. 2009;517:873–891. doi: 10.1002/cne.22194. [DOI] [PubMed] [Google Scholar]
- 55••.Hnasko TS, Chuhma N, Zhang H, Goh GY, Sulzer D, Palmiter RD, Rayport S, Edwards RH. Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron. 2010;65:643–656. doi: 10.1016/j.neuron.2010.02.012. In this paper, the authors examine dopamine/glutamate co-release from the VTA, with particular interest in defining whether expression of VGLUT2 changes the ability of synaptic vesicles to package dopamine. They find that VGLUT2 cKO significantly decreased DA transients suggesting glutamate transporters increase the ability of synaptic vesicles to package dopamine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56••.Adrover MF, Shin JH, Alvarez VA. Glutamate and dopamine transmission from midbrain dopamine neurons share similar release properties but are differentially affected by cocaine. J Neurosci. 2014;34:3183–3192. doi: 10.1523/JNEUROSCI.4958-13.2014. While both optogenetically-elicited dopamine transients and glutamatergic EPSCs share similar release probabilities (sensitivity to D2 agonists and paired pulse ratio) they are differentially affected by cocaine, suggesting dopamine and glutamate may be packaged into different synaptic vesicles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chuhma N, Zhang H, Masson J, Zhuang X, Sulzer D, Hen R, Rayport S. Dopamine neurons mediate a fast excitatory signal via their glutamatergic synapses. J Neurosci. 2004;24:972–981. doi: 10.1523/JNEUROSCI.4317-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Birgner C, Nordenankar K, Lundblad M, Mendez JA, Smith C, le Grevès M, Galter D, Olson L, Fredriksson A, Trudeau LE, et al. VGLUT2 in dopamine neurons is required for psychostimulant-induced behavioral activation. Proc Natl Acad Sci USA. 2010;107:389–394. doi: 10.1073/pnas.0910986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alsiö J, Nordenankar K, Arvidsson E, Birgner C, Mahmoudi S, Halbout B, Smith C, Fortin GM, Olson L, Descarries L, et al. Enhanced sucrose and cocaine self-administration and cue-induced drug seeking after loss of VGLUT2 in midbrain dopamine neurons in mice. J Neurosci. 2011;31:12593–12603. doi: 10.1523/JNEUROSCI.2397-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fortin GM, Bourque MJ, Mendez JA, Leo D, Nordenankar K, Birgner C, Arvidsson E, Rymar VV, Bérubé-Carrière N, Claveau AM, et al. Glutamate corelease promotes growth and survival of midbrain dopamine neurons. J Neurosci. 2012;32:17477–17491. doi: 10.1523/JNEUROSCI.1939-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gras C, Amilhon B, Lepicard EM, Poirel O, Vinatier J, Herbin M, Dumas S, Tzavara ET, Wade MR, Nomikos GG, et al. The vesicular glutamate transporter VGLUT3 synergizes striatal acetylcholine tone. Nat Neurosci. 2008;11:292–300. doi: 10.1038/nn2052. [DOI] [PubMed] [Google Scholar]
- 62.Gall CM, Hendry SH, Seroogy KB, Jones EG, Haycock JW. Evidence for coexistence of GABA and dopamine in neurons of the rat olfactory bulb. J Comp Neurol. 1987;266:307–318. doi: 10.1002/cne.902660302. [DOI] [PubMed] [Google Scholar]
- 63.Maher BJ, Westbrook GL. Co-transmission of dopamine and GABA in periglomerular cells. J Neurophysiol. 2008;99:1559–1564. doi: 10.1152/jn.00636.2007. [DOI] [PubMed] [Google Scholar]
- 64••.Borisovska M, Bensen AL, Chong G, Westbrook GL. Distinct modes of dopamine and GABA release in a dual transmitter neuron. J Neurosci. 2013;33:1790–1796. doi: 10.1523/JNEUROSCI.4342-12.2013. Using dual amperometric and whole cell patch clamp recordings from cultured olfactory bulb dopaminergic interneurons, this study demonstrates that stimulation results in a fast, GABAergic IPSC but a sustained increase in dopamine release over many seconds. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65•.Liu S, Plachez C, Shao Z, Puche A, Shipley MT. Olfactory bulb short axon cell release of GABA and dopamine produces a temporally biphasic inhibition-excitation response in external tufted cells. J Neurosci. 2013;33:2916–2926. doi: 10.1523/JNEUROSCI.3607-12.2013. In olfactory bulb slices, the authors demonstrate that co-release of dopamine and GABA elicits a temporally biphasic inhibition-excitation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66•.Dulcis D, Jamshidi P, Leutgeb S, Spitzer NC. Neurotransmitter switching in the adult brain regulates behavior. Science. 2013;340:449–453. doi: 10.1126/science.1234152. Following a shift in photoperiod, neurons in the hypothalamus can change transmitter phenotype, switching between releasing dopamine and somatostatin. [DOI] [PubMed] [Google Scholar]
- 67.Gillespie DC, Kim G, Kandler K. Inhibitory synapses in the developing auditory system are glutamatergic. Nat Neurosci. 2005;8:332–338. doi: 10.1038/nn1397. [DOI] [PubMed] [Google Scholar]
- 68.Kalmbach A, Kullmann PH, Kandler K. NMDAR-mediated calcium transients elicited by glutamate co-release at developing inhibitory synapses. Front Synaptic Neurosci. 2010;2:27. doi: 10.3389/fnsyn.2010.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Noh J, Seal RP, Garver JA, Edwards RH, Kandler K. Glutamate co-release at GABA/glycinergic synapses is crucial for the refinement of an inhibitory map. Nat Neurosci. 2010;13:232–238. doi: 10.1038/nn.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sloviter RS, Dichter MA, Rachinsky TL, Dean E, Goodman JH, Sollas AL, Martin DL. Basal expression and induction of glutamate decarboxylase and GABA in excitatory granule cells of the rat and monkey hippocampal dentate gyrus. J Comp Neurol. 1996;373:593–618. doi: 10.1002/(SICI)1096-9861(19960930)373:4<593::AID-CNE8>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 71••.Beltrán JQ, Gutiérrez R. Co-release of glutamate and GABA from single, identified mossy fibre giant boutons. J Physiol. 2012;590:4789–4800. doi: 10.1113/jphysiol.2012.236372. Here the authors mechanically dissociate CA3 pyramidal neurons with attached mossy fiber boutons. They show that stimulation of the bouton in this preparation elicits a GABAergic IPSC, suggesting release of GABA and glutamate from mossy fibers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gutiérrez R, Romo-Parra H, Maqueda J, Vivar C, Ramírez M, Morales MA, Lamas M. Plasticity of the GABAergic phenotype of the “glutamatergic” granule cells of the rat dentate gyrus. J Neurosci. 2003;23:5594–5598. doi: 10.1523/JNEUROSCI.23-13-05594.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kamiya H, Shinozaki H, Yamamoto C. Activation of metabotropic glutamate receptor type 2/3 suppresses transmission at rat hippocampal mossy fibre synapses. J Physiol. 1996;493:447–455. doi: 10.1113/jphysiol.1996.sp021395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harris EW, Cotman CW. Long-term potentiation of guinea pig mossy fiber responses is not blocked by N-methyl D-aspartate antagonists. Neurosci Lett. 1986;70:132–137. doi: 10.1016/0304-3940(86)90451-9. [DOI] [PubMed] [Google Scholar]
- 75.Regehr WG, Delaney KR, Tank DW. The role of presynaptic calcium in short-term enhancement at the hippocampal mossy fiber synapse. J Neurosci. 1994;14:523–537. doi: 10.1523/JNEUROSCI.14-02-00523.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Uchigashima M, Fukaya M, Watanabe M, Kamiya H. Evidence against GABA release from glutamatergic mossy fiber terminals in the developing hippocampus. J Neurosci. 2007;27:8088–8100. doi: 10.1523/JNEUROSCI.0702-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77•.Cabezas C, Irinopoulou T, Gauvain G, Poncer JC. Presynaptic but not postsynaptic GABA signaling at unitary mossy fiber synapses. J Neurosci. 2012;32:11835–11840. doi: 10.1523/JNEUROSCI.5543-11.2012. Demonstration that mossy fiber GABAergic transmission can alter presynaptic release probability through activation of presynaptic GABAB receptors, however, this study fails to find any postsynaptic effect of mossy fiber GABA release. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aimone JB, Deng W, Gage FW. Adult neurogenesis: integrating theories and separating functions. Trends Cogn Sci. 2010;14:325–337. doi: 10.1016/j.tics.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gutiérrez R, Heinemann U. Kindling induces transient fast inhibition in the dentate gyrus—CA3 projection. Eur J Neurosci. 2001;13:1371–1379. doi: 10.1046/j.0953-816x.2001.01508.x. [DOI] [PubMed] [Google Scholar]
- 80.Gutiérrez R. Seizures induce simultaneous GABAergic and glutamatergic transmission in the dentate gyrus-CA3 system. J Neurophysiol. 2000;84:3088–3090. doi: 10.1152/jn.2000.84.6.3088. [DOI] [PubMed] [Google Scholar]
- 81.Romo-Parra H, Vivar C, Maqueda J, Morales MA, Guitérrez R. Activity-dependent induction of multitransmitter signaling onto pyramidal cells and interneurons of hippocampal area CA3. J Neurophysiol. 2003;89:3155–3167. doi: 10.1152/jn.00985.2002. [DOI] [PubMed] [Google Scholar]
- 82.Overstreet-Wadiche LS, Bromberg DA, Bensen AL, Westbrook GL. Seizures accelerate functional integration of adult-generated granule cells. J Neurosci. 2006;26:4095–4103. doi: 10.1523/JNEUROSCI.5508-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao CS, Overstreet-Wadiche L. Integration of adult generated neurons during epilepogenesis. Epilepsia. 2008;49:3–12. doi: 10.1111/j.1528-1167.2008.01632.x. [DOI] [PubMed] [Google Scholar]
- 84.Sperk G, Wieselthaler-Hölzl A, Pirker S, Tasan R, Strasser SS, Drexel M, Pifl C, Marschalek J, Ortler M, Trinka E, et al. Glutamate decarboxylase 67 is expressed in hippocampal mossy fibers of temporal lobe epilepsy patients. Hippocampus. 2012;22:590–603. doi: 10.1002/hipo.20923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Doty RL. Olfaction in Parkinson’s disease and related disorders. Neuribol Dis. 2012;46:527–552. doi: 10.1016/j.nbd.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ross GW, Petrovitch H, Abbott RD, Tanner CM, Popper J, Masaki K, Launer L, White LR. Association of olfactory dysfunction with risk for future Parkinson’s disease. Ann Neurol. 2008;63:167–173. doi: 10.1002/ana.21291. [DOI] [PubMed] [Google Scholar]
- 87.Mundiñano IC, Caballero MC, Ordóñez C, Hernandez M, DiCaudo C, Marcilla I, Erro ME, Tuñon MT, Luquin MR. Increased dopaminergic cells and protein aggregates in the olfactory bulb of patients with neurodegenerative disorders. Acta Neuropathol. 2011;122:61–74. doi: 10.1007/s00401-011-0830-2. [DOI] [PubMed] [Google Scholar]
- 88.Huisman E, Uylings HB, Hoogland PV. A 100% increase of dopaminergic cells in the olfactory bulb may explain hyposmia in Parkinson’s disease. Mov Disord. 2004;19:687–692. doi: 10.1002/mds.10713. [DOI] [PubMed] [Google Scholar]
- 89.Gheusi G, Lepousez G, Lledo PM. Adult-born neurons in the olfactory bulb: integration and functional consequences. Curr Top Behav Neurosci. 2013;15:49–72. doi: 10.1007/7854_2012_228. [DOI] [PubMed] [Google Scholar]
- 90.Hsia AY, Vincent JD, Lledo PM. Dopamine depresses synaptic inputs into the olfactory bulb. J Neurophysiol. 1999;82:1082–108. doi: 10.1152/jn.1999.82.2.1082. [DOI] [PubMed] [Google Scholar]