Abstract
TWIK-2 (KCNK6) is a member of the two-pore domain (K2P) family of potassium channels which are highly expressed in the vascular system. We tested the hypothesis that TWIK-2 deficiency leads to pulmonary hypertension. TWIK-2 knockout mice and their wildtype littermates at 8 weeks of age had similar mean right ventricular systolic pressures (mRVSP: 24 ± 3 mm Hg& 21 ± 3 mm Hg, respectively.) Significantly, by 20 weeks of age, the mRVSP in TWIK-2 knockout mice increased to 35 ± 3 mm Hg (p ≤ 0.036) while mRVSP in wildtype littermates remained at 22 ± 3 mm Hg. Elevated mRVSP in the TWIK-2 knockout mice was accompanied pulmonary vascular remodeling as determined by a 25% increase in the cross-sectional area of the vessels occupied by the vessel wall. Additionally, secondary branches of the pulmonary artery from 20 week old TWIK-2 knockout mice showed an enhanced contractile response to U46619 (10−6 moles/liter), a thromboxane A2 mimetic, which was completely abolished with the Rho-kinase inhibitor, Y27632 (10−6 and 10−5moles/liter). Treatment of TWIK-2 knockout mice with the Rho-kinase inhibitor, fasudil, in the drinking water for 12 weeks, abolished the development of pulmonary hypertension and attenuated the vessel remodeling.. We conclude that mice deficient the TWIK-2 channel develop pulmonary hypertension between 8 and 20 weeks of age through a mechanism involving Rho-kinase. Our results suggest that down-regulation of TWIK-2 in the pulmonary vasculature may be an underlying mechanism in the development of pulmonary hypertension.
Keywords: Pulmonary Hypertension, Potassium Channel, Two-pore domain potassium channels (K2P), Rho-Kinase, KCNK6, TWIK-2
INTRODUCTION
Pulmonary hypertension (PH) is a pathologic lung condition that occurs due to vascular remodeling. Whereas normal mean pulmonary arterial pressure varies from 10 to 20 mm Hg, PH is defined as mean pulmonary arterial pressure ≥ 25 mmHg (1;2. Although the precise incidence and prevalence of PH is uncertain and underestimated, it is being diagnosed at an increasing rate (3). The World Health Organization (WHO) has stratified PH into five major groups based on the genetic, environmental and pathogenic triggers (4). Although the underlying mechanisms may be different, increased pulmonary vascular resistance resulting from a combination of sustained vasoconstriction, thrombosis, inflammation leading to pulmonary vascular remodeling is common to all groups (5–7). An important consequence of PH is an increase in right ventricular after load which, over time, leads to right ventricular hypertrophy, right heart failure, and ultimately death (1;8).
One of several hypotheses to explain PH involves dysfunction or down regulation of potassium (K+) channels in the pulmonary circulation. (9) K+ channels regulate a number of cellular functions that possibly contribute to the development of PH. (10) In particular, K+ channels function to regulate membrane potential in pulmonary vascular smooth muscle cells (PASMCs). When K+ channels open during normal physiological conditions, K+ moves along its electro-chemical gradient from the cytoplasmic space to the extracellular space. The efflux of positive K+ ions results in a more hyperpolarized membrane potential. When K+ channels close, become dysfunctional, or their expression is down regulated, the cell depolarizes and intracellular Ca2+ increases as a result of voltage-dependent calcium channel activation. Since intracellular free Ca2+ is a second messenger for smooth muscle contraction, K+ channel dysfunction can result in heightened constriction of pulmonary arteries leading to increased pulmonary vascular resistance (10).
Two families of K+ channels, voltage-activated K+ (Kv) and two-pore domain (K2P) have been implicated in PH. Of the Kv channels that are responsible for setting the membrane potential of PAMSCs, Kv1.5 and Kv2.1 are of significance because they inactivate with decreases in O2 tension(9;11;12). During hypoxic episodes, inactivation of these Kv channels produce depolarization to contract pulmonary vascular smooth muscle as described above. Absence of the Kv1.5 channels in mice lead to impaired vasoconstrictive responses to hypoxia. (13) Furthermore, Kv1.5 expression and function are decreased in patients suffering from idiopathic pulmonary arterial hypertension (14)and have been implicated in the mechanism of PH associated with hypoxemia(9;10;15). Although Kv1.5 channel dysfunction is a provocative hypothesis underlying at least some forms of PH, mice lacking Kv1.5 channels demonstrated normal lung perfusion pressures compared to wildtype controls, without evidence for pulmonary hypertension (13). Thus, dysfunctional or down regulation of Kv1.5 may be involved with the pathophysiology of some groups of PH, but cannot completely explain the underlying mechanism.
In addition to the Kv channel family, members of the K2P family, TASK-1 (KCNK3) and TREK-1 (KCNK2), have also been implicated in the regulation of pulmonary vascular resistance and the possible development of PH (16–19). Recently, missense mutations in TASK-1 were identified as possible gene candidates causing idiopathic pulmonary arterial hypertension and familial PH (17). Alternatively, one study presented evidence against the involvement of TASK-1 dysfunction as a cause of PH in mice but left open the possibility that TASK-1 could be involved with PH in other species (20). Regardless, of the evidence for and against TREK-1 and TASK-1 as the underlying mechanism for PH in mice, direct measurements of pulmonary arterial pressure (or its surrogate right ventricular pressure), the standard for diagnosing PH, have not been reported in mice lacking these K2P channels.
TWIK-2 (KCNK6), another member of the K2P family, is also expressed in the vascular system. Of the fifteen known members of the K2P family, TREK-1, TASK-1, and TWIK-2 show the greatest levels of expression for K2P in the vascular system (21–23). Recently, our laboratory reported that mice lacking TWIK-2 are hypertensive at eight weeks of age and have an altered contractile response in isolated aortic ring segments (24). Given the importance of TWIK-2 in the systemic circulation, we questioned if it has a similar role in the pulmonary circulation. We hypothesized that (i) TWIK-2 is involved with regulating pulmonary vascular tone; and (ii) TWIK-2 dysfunction in the pulmonary circulation leads to PH. After our initial results showed that TWIK-2 KO mice develop PH and that branches of the pulmonary artery are hypercontractile to a thromboxane A2 mimetic, we sought to determine the mechanism involved with the onset of PH in TWIK-2 KO mice. Since Rho-kinase promotes contraction of vascular smooth muscle cells by inhibiting the de-phosphorylation of myosin light chain, we tested the hypothesis that (iii) inhibition of Rho-kinase in mice lacking TWIK-2 attenuates hypercontractility of the pulmonary arteries and attenuates the development of PH.
RESULTS
TWIK-2 expression in lung tissue and pulmonary vessels from WT mice
TWIK-2 is expressed in the pulmonary artery and first order branches and lung tissue from 20 week old male KO and WT mice. (Supplementary figure S1A,B) Kv1.5 and TASK-1 were included, since they relate to previous studies involving PH (9;13;16;17). Expression of TWIK-2 (P<0.05; n=7) was absent in the knockout while expression of KV1.5 and TASK-1 was unaltered compared to the WT.
Mice lacking TWIK-2 develop PH and pulmonary vascular remodeling
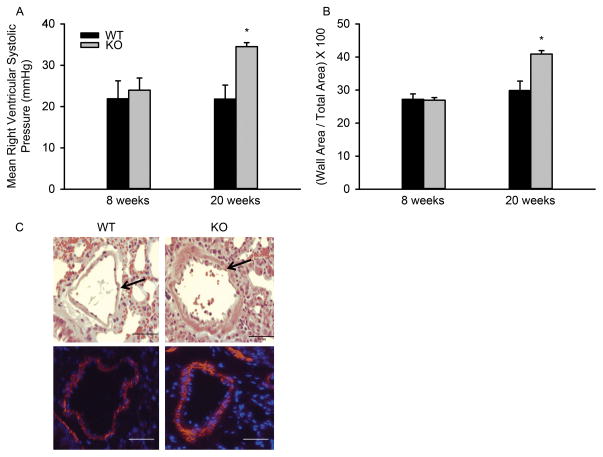
Right ventricular systolic pressure (RVSP), a surrogate measure of pulmonary pressures (25), was measured in TWIK-2 KO male mice and WT male littermates (Figure 1A). At eight weeks of age there was no difference in mean RVSP between genotypes (21 ± 3 and 24 ± 3 mm Hg in WT and TWIK-2 KO mice respectively). However, at 20 weeks, mean RVSP in the TWIK-2 KO increased to 35 ± 3 mm Hg [p≤0.036 compared to WT at 20 weeks (22 ± 3 mm Hg) and TWIK-2 KO at 8 weeks (n=4 for each group)]. The results demonstrate that TWIK-2 KO mice developed PH between 8 and 20 weeks.
Figure 1.
A: Mean right ventricular pressures in 8 and 20 week old TWIK-2 KO and their WT littermates (n=4 per group). *p ≤ 0.036 compared to WT at 20 weeks and TWIK-2 KO at 8 weeks. B: The percent of the cross-sectional area in lung vessels occupied by the vessel wall in TWIK-2 KO mice and their WT littermates at 20 weeks of age. This measure provides an index of wall thickness relative to the vessel size. * p= 0.001 compared to all other groups (n=4 per group). C: H&E stained sections (top panels) and immuno-stained sections of smooth muscle α-actin (bottom panels) from the lung of a 20 week old TWIK-2 KO and its WT littermates. The arrows in the top panels indicate the vessel wall. Red in the bottom panels denotes smooth muscle α-actin and blue represents cell nuclei.
Figure 1C shows H&E staining (top panels) and immunostaining of smooth muscle α-actin (bottom panels) from lung sections of 20 week old TWIK-2 KO mice and their WT littermates. Note the increase in wall thickness (arrows) in lung vessels from TWIK-2 KO mice compared to WT mice (1C, top). Figure 1C (bottom) demonstrates that the vessel wall (arrows in the top two panels) consists of vascular smooth muscle (red in the image). Figure 1B shows the percent of the cross-sectional area in lung vessels occupied by the vessel wall in TWIK-2 KO mice and their WT littermates. This measure provides an index of wall thickness relative to the vessel size. Note that the percent area occupied by the vessel wall is significantly increased in the pulmonary vessels of 20 week old, but not 8 week old TWIK-2 KO mice. Although the results in Figure 1B were derived from the H&E stained images, similar results were obtained when measurements were made using the immunohistochemical images of smooth muscle α-actin (data not shown). The vascular remodeling appeared to correlate with the increase in mean RVSP Figures 1A,B).
Mice lacking TWIK-2 have an increased right ventricular end-diastolic volume, but no alterations in left ventricular size or function
During the development of PH, right ventricular end-diastolic volume is often increased initially as a result of a pressure overload (2;25;26) followed later by a decrease in right ventricle ejection fraction (EF%) as the right ventricle remodels to the pressure overload. Supplementary figure S2A shows that 20 week old TWIK-2 KO mice have an increased right ventricular end-diastolic volume (p=0.02, n=4) when compared to their WT littermate controls. Representative MRI images of the right ventricle at end diastole in a WT and a TWIK-2 KO mouse is shown in supplementary figure S2E. Right ventricular contractility, as measured by the ejection fraction was not altered in TWIK-2 KO mice compared to WT littermates (Supplementary figure S2B).
Given that the TWIK-2 KO mice have increased systemic blood pressures (24), it was important to determine if increased mean RVSP was related to the systemic hypertension. With systemic hypertension, the increase in right ventricular pressures could result indirectly from left ventricular dysfunction or increased left ventricular filling pressures. Supplementary figure S2C and S2D shows left ventricular end-diastolic volume and ejection fraction (EF%) in 20 week old TWIK-2 KO mice. There were no significant differences in either when compared to WT littermates. Furthermore, the left ventricular wall thickness was not significantly different in the WT and TWIK-2 KO mice. At its widest point, the ratio of the left ventricle wall to the total cross sectional area of the left ventricle was 0.50 ± 0.05 and 0.53 ± 0.06 (p=0.66, n=4 each group) in TWIK-2 KO mice and their WT littermates respectively. An additional consideration is that systemic hypertension can lead to elevated left ventricular end diastolic pressures which could be a cause of pulmonary hypertension in the TWIK-2 KO mice. We found no significant increase in the left ventricular end-diastolic pressures (LVEDP) of 18–22 week-old TWIK-2 KO and WT mice. (Data not shown)
TWIK-2 KO mice pulmonary arteries are hypercontractile to a thromboxane A2 mimetic through activation of Rho-kinase
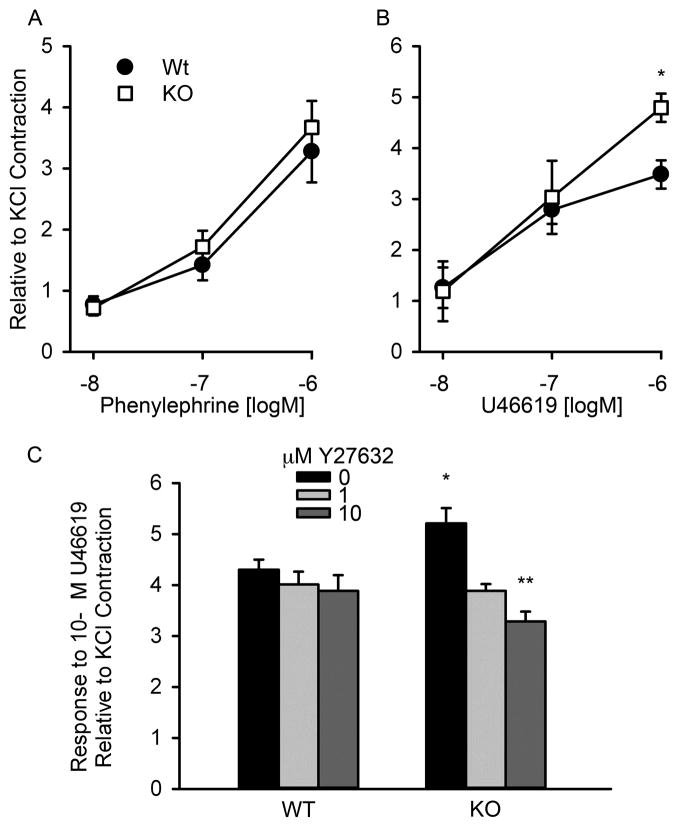
Isometric contractions to phenylephrine, an α-adrenoreceptor agonist, or U46619, a thromboxane A2 (TXA2) mimetic, were measured in rings of first order branches of pulmonary arteries from 20 week old TWIK-2 KO mice and their WT littermates (Figures 2A &2B). While the contractile responses to phenylephrine were similar between genotype, the response to U46619 was increased at a concentration of 10−6 M (p=0.04, n=8 each group) in arterial rings from TWIK-2 KO compared to that observed in arterial rings from WT mice. In addition, contractile responses to 60 mM KCl (n=9) were similar between rings of the first order branches of pulmonary arteries from TWIK-2 KO mice and their WT littermates (data not shown).
Figure 2.
Contractile responses to (A) phenylephrine, an α-adrenoreceptor agonist, (n=4 each group) or (B) U46619, a thromboxane A2 mimetic, in first order branches of the pulmonary artery obtained from 20 week old TWIK-2 KO and WT mice. * p=0.04 compared to 10−6 M U46619 in WT, n=8 for each group. C: Contractile responses to U46619 in first order branches of the pulmonary artery from 20 week old WT and TWIK-2 KO mice in the presence of 1 uM (10−6 moles/liter) and 10 uM (10−5 moles/liter) Y27632, an inhibitor of Rho-kinase. * p≤ 0.002 compared to all other groups, ** p=0.038 compared to KO with 1 uM Y27632.
Since stimulation of the TXA2 receptor can activate downstream rho-kinase signaling, we determined if the enhanced contractile response to U46619 could be blocked by inhibition of Rho-kinase. Figure 2C shows the contractile responses to a single dose of 10−6 M U46619 in arterial rings from 20 week old WT and TWIK-2 KO mice in the absence and presence of the Rho-kinase inhibitor, Y27632 (10−6 and 10−5 moles/liter). Y27632 had no significant effects on the force generated in ring from WT mice; however, it significantly attenuated the contractile response in a dose dependent manner in pulmonary artery rings from TWIK-2 KO mice. With Y27632 pre-treatment, the contractile responses to 10−6 M U46619 were decreased to levels in rings from WT mice. Thus, it appears that Rho-kinase activity was responsible for the hypercontractile response to U46619 in first order branches of the pulmonary artery in TWIK-2 KO mice.
Treatment with a Rho-kinase inhibitor reduced RVSP and pulmonary vascular remodeling in TWIK-2 KO mice
Having demonstrated enhanced contractility with Rho-kinase activation in first order branches of pulmonary arteries and increased Rho-kinase activity in whole lungs from 20 week old TWIK-2 KO mice, we determined if inhibition of Rho-kinase in vivo could be an effective treatment for the hypertension that develops between 8 and 20 weeks in TWIK-2 KO mice. Eight-week old TWIK-2 KO mice and WT littermates were provided with regular drinking water or drinking water with 1 mg/ml fasudil, a Rho-kinase inhibitor. Fasudil was used for these studies since it has been demonstrated as an effective inhibitor of Rho-kinase when administered orally (27). At 20 weeks of age, mean RVSP was measured by right heart catheterization and lungs were harvested for histological analysis. Fasudil treatment abolished the increase in mean right ventricular pressure in TWIK-2 KO mice (Figure 3A) and diminished pulmonary vascular remodeling. Figure 3B shows that fasudil treatment in the TWIK-2 KO mice attenuated vessel remodeling as determined by the percent of the cross-sectional area in lung vessels occupied by the vessel wall. Supplemental Figure S3 shows H&E stained lung sections with the arrow pointing to the arterial wall. Thus, the development of PH in TWIK-2 KO mice appears to be mediated by the Rho-kinase pathway and can be attenuated by Rho-kinase inhibition.
Figure 3.

A: Mean right ventricular systolic pressure in 20 week old TWIK-2 KO mice and their WT littermates. Mice were provided with either 1 mg/ml fasudil in the drinking water or drinking water alone (Control). (n= 3–4 per group) * p=0.007 and 0.02 compared to TWIK-2 KO with fasudil and WT control respectively. B: The percent of the cross-sectional area in lung vessels occupied by the vessel wall in TWIK-2 KO mice and their WT littermates at 20 weeks of age with and without 12 weeks of Fasudil treatment. This measure provides an index of wall thickness relative to the vessel size. * p<0.001 compared to WT Control and TWIK-2 KO mice treated with Fasudil, ** p=0.004 compared to WT Control.
Discussion
The results of our study demonstrate that loss of function of the two-pore domain K+ channel, TWIK-2, produces pulmonary hypertension. Given the importance of the TWIK-2 channel, in the systemic circulation (24), we questioned if it has a similar role in the pulmonary circulation. Consistent with previous studies conducted in multiple species (21;28–30), we report that TWIK-2 is expressed in the lung and pulmonary circulation of mice (Supplementary Figure S1). By using a TWIK-2 knockout mouse in the present study, we conclude the following: (i.) loss of function of TWIK-2 in the pulmonary circulation leads to the development of PH (Figure 1A and 3A) and vascular wall remodeling (Figures 1B, 1C, 3B, and Supplemental Figure S3), (ii.) PH and remodeling occur between 8 and 20 weeks of age in the TWIK-2 KO mice, (iii) the mechanism of this PH phenotype appears possibly mediated by hypercontractility as a result Rho-kinase pathway activation (Figure 2), and (iv.) treatment of TWIK-2 KO mice with a Rho-kinase inhibitor before the onset of PH significantly attenuates pulmonary arterial pressures (Figure 3A) and vascular remodeling (Figure 3B and Supplemental Figure S3). Our studies suggest that TWIK-2 regulates pulmonary vascular tone by inhibiting the activity and/or expression of Rho-kinase.
Although decreased K+ channel activity has been hypothesized to underlie the development of PH, the TWIK-2 KO model is the first K2P+ channel KO mouse model, to our knowledge, known to develop pulmonary hypertension. A recent study reported the important link between the missense mutation of another member of the two-pore domain K+ channel family, TASK-1 or KCNK3, and a familial form of human PH (17). Given that the pulmonary circulation express a number of two-pore domain K+ channels (18;21) and that two members of this family have been implicated with PH to date (present study included), it is reasonable to consider that dysfunction of other members of this family could also result in PH.
One potential confounding factor involved with our model of PH is the systemic hypertension which is present by 8 weeks of age in TWIK-2 KO mice (24). Since the pulmonary and systemic circulations function at independent pressures, PH should be secondary to systemic hypertension only when there is dysfunction in the left side of the heart (classified as WHO Group 2 PAH). We measured left ventricular function and morphology in TWIK-2 KO mice at 20 weeks of age, a time when PH was fully developed. Our studies demonstrate that left ventricular end-diastolic volume and ejection fraction were similar in 20 week old TWIK-2 KO mice and their WT littermates (Supplementary figures 2C and 2D). Furthermore, we found no indication of left ventricular remodeling as determined by wall thickness in the TWIK-2 KO mice at 20 weeks of age (see results) and no changes in left ventricular pressures as measured by left cardiac catheterization (see Methods, Supplementary materials). Thus, we conclude that PH, developing between the ages of 8 and 20 weeks in male TWIK-2 KO mice, is not a result of left-sided heart failure. Instead, our model confers a unique hypertensive phenotype in the pulmonary vasculature resulting from TWIK-2 dysfunction in the pulmonary vessels.
Our studies demonstrate a potentially important role for Rho-kinase in the development of PH in male TWIK-2 KO mice. Rho-kinase, through its inhibition of myosin light chain phosphatase, helps to maintain a contracted statein vascular smooth muscle by preventing the de-phosphorylation of myosin light chain (31). Additionally, Rho-kinase is involved with remodeling in the vessel wall, in part, through enhancing vascular smooth muscle motility and smooth muscle proliferation (31;32). At the arterial level, Rho-kinase activity was enhanced in the TWIK-2 KO mice and appears to be at least partially responsible for PH in the TWIK-2 KO mice by increasing pulmonary vascular resistance through alterations in arterial contractility. A recent publication addressed the complexity of the varying factors involved in pulmonary vasoconstriction and remodeling, including the contributions of hypoxia. (33) While we do not believe Rho-kinase activation is the only mechanism behind TWIK-2 deficiency leading to hypercontractility, the interactions between TWIK-2 and Rho-kinase signaling as well as their combined roles in intracellular calcium homeostasis are potentially revealing and require further study. Enhanced Rho-kinase activity is commonly associated with cardiovascular diseases, in general, including other forms of PH (32;34). For example, long-term inhibition of rho-kinase attenuated chronic hypoxia- and monocrotaline-induced pulmonary hypertension in rodents (34–36). In addition, fasudil acutely decreased pulmonary vascular resistance in humans with PH, although mean pulmonary arterial pressure only slightly decreased (~3 mm Hg) in one study (37) and was not significantly affected in another (38).
It is reasonable to consider that pulmonary artery vascular smooth muscle cells in TWIK-2 KO mice are more depolarized and have an increase in intracellular free Ca2+ compared to that in WT mice (24). Since K+ channels function to regulate membrane potential in pulmonary vascular smooth muscle cells (9;11;12), loss of function of TWIK-2 should depolarize the vascular smooth muscle and increase intracellular Ca2+ through activation of voltage-dependent calcium channels. The depolarization and increased Ca2+ in PASMCs could produce PH through two possible mechanisms. First, intracellular free Ca2+ is a second messenger for smooth muscle cell contraction. With an increase in intracellular Ca2+ resulting from the loss of TWIK-2, a persistent increase in the contractile state of pulmonary arteries and arterioles would serve to increase the pulmonary vascular resistance. Second, both membrane depolarization and increased cytoplasmic Ca2+ are stimuli that activate Rho-kinase (39–41). Activation of Rho-kinase would both increase the sensitivity of the contractile machinery in the vascular smooth muscle to Ca2+ and stimulate vessel remodeling (39–41).
Perspectives
This study is the first to demonstrate a role for the TWIK-2 potassium channel in the regulation of pulmonary vascular tone. The observation that male TWIK-2 KO mice spontaneously develop PH between 8 and 20 weeks of age makes the PH phenotype more physiologic and comparable to human disease compared to other drug-induced models of PH. Of significance the increase in right ventricular end diastolic volume seen in the TWIK-2 KO mice parallel the increase in right ventricular end diastolic volume without right ventricular remodeling that occurs during the early stages of PH in humans (4;25). The presence of right ventricular dilatation is notable since deaths and clinical deterioration in PH are directly related to alterations in right heart pressures and remodeling (1;3). We describe a potential role for enhanced Rho-kinase activity in the development of PH in the TWIK-2 KO mice by increasing pulmonary vascular resistance through alterations in arterial contractility and by vascular remodeling. Given the recently described role of another K2P channel in human PH (17), TWIK-2 dysfunction could be important in the etiology of human PH through either a loss of function mutation in familial forms of PH or by down regulation of TWIK-2 from environmental factors (such as hypoxia) in other forms of PH.
Supplementary Material
Novelty and Significance.
1) What Is New
These studies are the first to characterize the TWIK-2 knockout mouse and describe the development of spontaneous pulmonary hypertension and right ventricular remodeling by 20 weeks of age
These studies describe how TWIK-2 deficiency leads to pulmonary hypertension through rho-kinase mediated hypercontractility
2) What Is Relevant?
Human pulmonary hypertension is a progressive disease resulting in severe right heart failure and death if left untreated. The disease is difficult to recognize and therapies are limited to vasodilators that often have significant side effects and poor tolerance.
These studies present new mechanistic/genetic possibilities in the development of pulmonary hypertension, thus offering new therapeutic targets.
These studies expand the current knowledge of potassium channels and their important contributions to the regulation of pulmonary vascular tone, cellular proliferation and responses to environmental stimuli.
Summary
This study is the first to demonstrate a role for the TWIK-2 potassium channel in the regulation of pulmonary vascular tone. Male TWIK-2 KO mice spontaneously develop PH between 8 and 20 weeks of age, and this PH is attenuated by rho-kinase inhibition. These findings implicate the TWIK-2 channel as having a potential role in the development of pulmonary hypertension.
Acknowledgments
The authors wish to thank Sean Marrelli, Ph.D. for his scientific guidance and appraisal of the manuscript, and Sharon Phillips for her technical assistance.
SOURCES OF FUNDING
The studies were supported by R21HL098921 (RMB), Baylor College of Medicine Seed Funding Award 2011-2012 (LMP) and Veterans Affairs Career Development Award Program (LMP).
Footnotes
CONFLICTS OF INTEREST/DISCLOSURE
None
REFERENCE LIST
- 1.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, Rubin LJ, Tapson VF, Varga J. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119:2250–2294. doi: 10.1161/CIRCULATIONAHA.109.192230. [DOI] [PubMed] [Google Scholar]
- 2.Kuhr FK, Smith KA, Song MY, Levitan I, Yuan JX. New mechanisms of pulmonary arterial hypertension: role of Ca(2)(+) signaling. Am J Physiol Heart Circ Physiol. 2012;302:H1546–H1562. doi: 10.1152/ajpheart.00944.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, Barst RJ, Benza RL, Liou TG, Turner M, Giles S, Feldkircher K, Miller DP, McGoon MD. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest. 2010;137:376–387. doi: 10.1378/chest.09-1140. [DOI] [PubMed] [Google Scholar]
- 4.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna KR, Landzberg M, Machado RF, Olschewski H, Robbins IM, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D34–D41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 5.Stenmark KR, Davie N, Frid M, Gerasimovskaya E, Das M. Role of the adventitia in pulmonary vascular remodeling. Physiology. 2006;21:134–145. doi: 10.1152/physiol.00053.2005. [DOI] [PubMed] [Google Scholar]
- 6.Tuder RM, Marecki JC, Richter A, Fijalkowska I, Flores S. Pathology of pulmonary hypertension. Clin Chest Med. 2007;28:23–24. doi: 10.1016/j.ccm.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrell NW, Adnot S, Archer SL, Dupuis J, Jones PL, MacLean MR, McMurtry IF, Stenmark KR, Thistlethwaite PA, Weissmann N, Yuan JX, Weir EK. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S20–S31. doi: 10.1016/j.jacc.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan JX, Rubin LJ. Pathogenesis of pulmonary arterial hypertension: the need for multiple hits. Circulation. 2005;111:534–538. doi: 10.1161/01.CIR.0000156326.48823.55. [DOI] [PubMed] [Google Scholar]
- 9.Bonnet S, Archer SL. Potassium channel diversity in the pulmonary arteries and pulmonary veins: implications for regulation of the pulmonary vasculature in health and during pulmonary hypertension. Pharmacol Ther. 2007;115:56–69. doi: 10.1016/j.pharmthera.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 10.Moudgil R, Michelakis ED, Archer SL. The role of k+ channels in determining pulmonary vascular tone, oxygen sensing, cell proliferation, and apoptosis: implications in hypoxic pulmonary vasoconstriction and pulmonary arterial hypertension. Microcirculation. 2006;13:615–632. doi: 10.1080/10739680600930222. [DOI] [PubMed] [Google Scholar]
- 11.Archer SL, Souil E, Dinh-Xuan AT, Schremmer B, Mercier JC, El Yaagoubi A, Nguyen-Huu L, Reeve HL, Hampl V. Molecular Identification of the Role of Voltage-gated K+ Channels, Kv1.5 and Kv2.1, in Hypoxic Pulmonary Vasoconstriction and Control of Resting Membrane Potential in Rat Pulmonary Artery Myocytes. Journal of Clinical Investigation. 1998;101:2319–2330. doi: 10.1172/JCI333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Archer SL, Wu XC, Thebaud B, Nsair A, Bonnet S, Tyrrell B, McMurtry MS, Hashimoto K, Harry G, Michelakis ED. Preferential Expression and Function of Voltage-Gated, O2-Sensitive K+ Channels in Resistance Pulmonary Arteries Explains Regional Heterogeneity in Hypoxic Pulmonary Vasoconstriction: Ionic Diversity in Smooth Muscle Cells. Circ Res. 2004;95:308–318. doi: 10.1161/01.RES.0000137173.42723.fb. [DOI] [PubMed] [Google Scholar]
- 13.Archer SL, London B, Hampl V, Wu X, Nsair A, Puttagunta L, Hashimoto K, Waite RE, Michelakis ED. Impairment of hypoxic pulmonary vasoconstriction in mice lacking the voltage-gated potassium channel Kv1.5. FASEB J. 2001;15:1801–1803. doi: 10.1096/fj.00-0649fje. [DOI] [PubMed] [Google Scholar]
- 14.Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest. 2008;118:2372–2379. doi: 10.1172/JCI33452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Juhaszova M, Rubin LJ, Yuan XJ. Hypoxia inhibits gene expression of voltage-gated K+ channel alpha subunits in pulmonary artery smooth muscle cells. J Clin Invest. 1997;100:2347–2353. doi: 10.1172/JCI119774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gurney AM, Osipenko ON, MacMillan D, McFarlane KM, Tate RJ, Kempsill FEJ. Two-Pore Domain K Channel, TASK-1, in Pulmonary Artery Smooth Muscle Cells. Circ Res. 2003;93:957–964. doi: 10.1161/01.RES.0000099883.68414.61. [DOI] [PubMed] [Google Scholar]
- 17.Ma L, Roman-Campos D, Austin ED, Eyries M, Sampson KS, Soubrier F, Germain M, Tregouet DA, Borczuk A, Rosenzweig EB, Girerd B, Montani D, Humbert M, Loyd JE, Kass RS, Chung WK. A novel channelopathy in pulmonary arterial hypertension. N Engl J Med. 2013;369:351–361. doi: 10.1056/NEJMoa1211097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nielsen G, Wandall-Frostholm C, Sadda V, Olivan-Viguera A, Lloyd EE, Bryan RM, Jr, Simonsen U, Kohler R. Alterations of N-3 Polyunsaturated Fatty Acid-Activated K2P Channels in Hypoxia-Induced Pulmonary Hypertension. Basic Clin Pharmacol Toxicol. 2013;113:250–258. doi: 10.1111/bcpt.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olschewski A, Li Y, Tang B, Hanze J, Eul B, Bohle RM, Wilhelm J, Morty RE, Brau ME, Weir EK, Kwapiszewska G, Klepetko W, Seeger W, Olschewski H. Impact of TASK-1 in human pulmonary artery smooth muscle cells. Circ Res. 2006;98:1072–1080. doi: 10.1161/01.RES.0000219677.12988.e9. [DOI] [PubMed] [Google Scholar]
- 20.Manoury B, Lamalle C, Oliveira R, Reid J, Gurney AM. Contractile and electrophysiological properties of pulmonary artery smooth muscle are not altered in TASK-1 knockout mice. J Physiol. 2011;589:3231–3246. doi: 10.1113/jphysiol.2011.206748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gardener MJ, Johnson IT, Burnham MP, Edwards G, Heagerty AM, Weston AH. Functional evidence of a role for two-pore domain potassium channels in rat mesenteric and pulmonary arteries. Br J Pharmacol. 2004;142:192–202. doi: 10.1038/sj.bjp.0705691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gurney A, Manoury B. Two-pore potassium channels in the cardiovascular system. Eur Biophys J. 2009;38:305–318. doi: 10.1007/s00249-008-0326-8. [DOI] [PubMed] [Google Scholar]
- 23.Lloyd EE, Marrelli SP, Bryan RM., Jr cGMP does not activate two-pore domain K+ channels in cerebrovascular smooth muscle. Am J Physiol Heart Circ Physiol. 2009;296:H1774–H1780. doi: 10.1152/ajpheart.00082.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lloyd EE, Crossland RF, Phillips SC, Marrelli SP, Reddy AK, Taffet GE, Hartley CJ, Bryan RM., Jr Disruption of K(2P)6.1 produces vascular dysfunction and hypertension in mice. Hypertension. 2011;58:672–678. doi: 10.1161/HYPERTENSIONAHA.111.175349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tabima DM, Hacker TA, Chesler NC. Measuring right ventricular function in the normal and hypertensive mouse hearts using admittance-derived pressure-volume loops. Am J Physiol Heart Circ Physiol. 2010;299:H2069–75. doi: 10.1152/ajpheart.00805.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vonk NA, Galie N. The role of the right ventricle in pulmonary arterial hypertension. Eur Respir Rev. 2011;20:243–253. doi: 10.1183/09059180.00006511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang BH, Tawara S, Abe K, Takaki A, Fukumoto Y, Shimokawa H. Acute vasodilator effect of fasudil, a Rho-kinase inhibitor, in monocrotaline-induced pulmonary hypertension in rats. J Cardiovasc Pharmacol. 2007;49:85–89. doi: 10.1097/FJC.0b013e31802df112. [DOI] [PubMed] [Google Scholar]
- 28.Medhurst AD, Rennie G, Chapman CG, Meadows H, Duckworth MD, Kelsell RE, Gloger II, Pangalos MN. Distribution analysis of human two pore domain potassium channels in tissues of the central nervous system and periphery. Brain Res Mol Brain Res. 2001;86:101–114. doi: 10.1016/s0169-328x(00)00263-1. [DOI] [PubMed] [Google Scholar]
- 29.Patel AJ, Maingret F, Magnone V, Fosset M, Lazdunski M, Honore E. TWIK-2, an inactivating 2P domain K+ channel. J Biol Chem. 2000;275:28722–28730. doi: 10.1074/jbc.M003755200. [DOI] [PubMed] [Google Scholar]
- 30.Salinas M, Reyes R, Lesage F, Fosset M, Heurteaux C, Romey G, Lazdunski M. Cloning of a new mouse two-P domain channel subunit and a human homologue with a unique pore structure. J Biol Chem. 1999;274:11751–11760. doi: 10.1074/jbc.274.17.11751. [DOI] [PubMed] [Google Scholar]
- 31.de Godoy MA, Rattan S. Role of rho kinase in the functional and dysfunctional tonic smooth muscles. Trends Pharmacol Sci. 2011;32:384–393. doi: 10.1016/j.tips.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Satoh K, Fukumoto Y, Shimokawa H. Rho-kinase: important new therapeutic target in cardiovascular diseases. Am J Physiol Heart Circ Physiol. 2011;301:H287–H296. doi: 10.1152/ajpheart.00327.2011. [DOI] [PubMed] [Google Scholar]
- 33.Cahill E, Rowan SC, Sands M, Banahan M, Ryan D, Howell K, McLoughlin P. The pathophysiological basis of chronic hypoxic pulmonary hypertension in the mouse: vasoconstrictor and structural mechanisms contribute equally. Exp Physiol. 2012;97:796–806. doi: 10.1113/expphysiol.2012.065474. [DOI] [PubMed] [Google Scholar]
- 34.Abe K, Shimokawa H, Morikawa K, Uwatoku T, Oi K, Matsumoto Y, Hattori T, Nakashima Y, Kaibuchi K, Sueishi K, Takeshit A. Long-term treatment with a Rho-kinase inhibitor improves monocrotaline-induced fatal pulmonary hypertension in rats. Circ Res. 2004;94:385–393. doi: 10.1161/01.RES.0000111804.34509.94. [DOI] [PubMed] [Google Scholar]
- 35.Abe K, Tawara S, Oi K, Hizume T, Uwatoku T, Fukumoto Y, Kaibuchi K, Shimokawa H. Long-term inhibition of Rho-kinase ameliorates hypoxia-induced pulmonary hypertension in mice. J Cardiovasc Pharmacol. 2006;48:280–285. doi: 10.1097/01.fjc.0000248244.64430.4a. [DOI] [PubMed] [Google Scholar]
- 36.Fagan KA, Oka M, Bauer NR, Gebb SA, Ivy DD, Morris KG, McMurtry IF. Attenuation of acute hypoxic pulmonary vasoconstriction and hypoxic pulmonary hypertension in mice by inhibition of Rho-kinase. Am J Physiol Lung Cell Mol Physiol. 2004;287:L656–L664. doi: 10.1152/ajplung.00090.2003. [DOI] [PubMed] [Google Scholar]
- 37.Ishikura K, Yamada N, Ito M, Ota S, Nakamura M, Isaka N, Nakano T. Beneficial acute effects of rho-kinase inhibitor in patients with pulmonary arterial hypertension. Circ J. 2006;70:174–178. doi: 10.1253/circj.70.174. [DOI] [PubMed] [Google Scholar]
- 38.Fukumoto Y, Matoba T, Ito A, Tanaka H, Kishi T, Hayashidani S, Abe K, Takeshita A, Shimokawa H. Acute vasodilator effects of a Rho-kinase inhibitor, fasudil, in patients with severe pulmonary hypertension. Heart. 2005;91:391–392. doi: 10.1136/hrt.2003.029470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakurada S, Takuwa N, Sugimoto N, Wang Y, Seto M, Sasaki Y, Takuwa Y. Ca2+-Dependent Activation of Rho and Rho Kinase in Membrane Depolarization-Induced and Receptor Stimulation-Induced Vascular Smooth Muscle Contraction. Circ Res. 2003;93:548–556. doi: 10.1161/01.RES.0000090998.08629.60. [DOI] [PubMed] [Google Scholar]
- 40.Urban NH, Berg KM, Ratz PH. K+ depolarization induces RhoA kinase translocation to caveolae and Ca2+ sensitization of arterial muscle. Am J Physiol Cell Physiol. 2003;285:C1377–C1385. doi: 10.1152/ajpcell.00501.2002. [DOI] [PubMed] [Google Scholar]
- 41.Fernandez-Tenorio M, Porras-Gonzalez C, Castellano A, Del Valle-Rodriguez A, Lopez-Barneo J, Urena J. Metabotropic regulation of RhoA/Rho-associated kinase by L-type Ca2+ channels: new mechanism for depolarization-evoked mammalian arterial contraction. Circ Res. 2011;108:1348–1357. doi: 10.1161/CIRCRESAHA.111.240127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.