Abstract
Preparation of hydrogels that possess an effective antibiotic release profile and better mechanical properties compared to the traditionally used collagen hydrogels has the potential to minimize post-surgical infections and support wound healing. Towards this goal, we prepared elastin-like polypeptide (ELP)-collagen composite hydrogels that displayed a significantly higher elastic modulus compared to the collagen hydrogels. We then characterized the release behavior of the collagen and ELP-collagen hydrogels loaded with varying dosages (1 – 5% w/w) of a commonly used broad spectrum antibiotic, doxycycline hyclate. Both collagen and ELP-collagen hydrogels showed a gradual time dependent doxycycline release over a period of 5 days. The ELP-collagen hydrogels, in general, showed a slower release of the doxycycline compared to the collagen hydrogels. The released doxycycline was found to be effective against four bacterial strains (Escherichia coli, Pseudomonas aeruginosa, Streptococcus sanguinis, and methicillin-resistant Staphylococcus aureus) in a dose dependent manner. Combined with their improved mechanical properties, the gradual and effective drug release from the biocompatible ELP-collagen hydrogels shown here may be beneficial for drug delivery and tissue engineering applications.
Keywords: Elastin-like polypeptide, collagen, hydrogel, drug delivery
1. INTRODUCTION
Hydrogels have been extensively used for encapsulation of cells and bioactive molecules in a three dimensional environment because of their high water content, biocompatibility, and mechanical and structural properties similar to some tissues and extracellular matrices (ECMs) [1–4]. Special attention has been given to collagen-based hydrogels for local drug delivery applications [5–15]. Unfortunately, native collagen fibers lack the rigidity required for tissue engineering [16] and are rapidly degraded by collagenases [17] to products that elicit inflammation [18]. While crosslinking using chemicals such as gluteraldehyde [19,20] can alleviate some disadvantages [16,17], their leaching from collagen results in cytotoxicity [21]. An alternate approach involving polymer/collagen or ceramic/collagen composites improves mechanical properties and modulates cell response [22–24], but retains other shortfalls (e.g., rapid degradation) associated with collagen being the major component. Minimizing the use of collagen by forming a composite with molecules that mimic elastin, another major component of the ECM, is a specific hallmark of our approach. In this study, we have used a soluble and recombinant form of elastin called elastin-like polypeptide (ELP) for this purpose.
ELPs have been used for tissue engineering and drug delivery applications [25–35]. Researchers have demonstrated enhanced delivery of ELP-bound bioactive agents to solid tumors [28], intraarticular joint space [29], and chronic wounds [30]. On the other hand, delivery of bioactive agents has been attempted from ELP scaffolds. These studies included ELP-poly(ε-caprolactone) composite scaffolds and ELP coatings for delivery of adeno-associated virus [31,32], chemically cross-linked ELP microparticles and films, respectively, for delivery of model drug molecules (bovine serum albumin and prednisone acetate) and antibiotics (vancomycin and cefazolin) [33,34], and ELP nanoparticles for delivery of cancer chemotherapeutics [35].
There have not been many attempts to prepare a composite of collagen and ELP. Garcia et al. reported crosslinking collagen with ELP using microbial transglutaminase and showed selective proliferation of endothelial cells and smooth muscle cells compared to fibroblasts, thus mimicking the behavior of natural elastin [36]. Kinikoglu et al. physically and chemically crosslinked collagen with a specialized ELP containing 6 RGD, 6 aspartic acid, 24 lysine, and 7 histidine residues. These composites showed improved mechanical properties and were favorable substrates for human fibroblasts and epithelial cells [37]. Chaikof and co-workers have demonstrated the preparation of a crosslinked ELP-collagen composite where the collagen fibers were oriented within an ELP matrix [38,39]. These modifications of collagen with ELP may also modulate its drug release behavior and obtain a sustained drug release profile that is essential to ensure complete bacterial eradication from the wounded site and assure good healing [10,13]. However, no drug delivery studies have been reported using crosslinked ELP-collagen composite scaffolds.
Composite formation through crosslinking may lead to unintended drug-matrix interactions (chemical reactions) during the in situ drug loading. Hence, our approach focused on non-crosslinked ELP-collagen composites, versus crosslinked composites reported earlier [36–39]. The non-crosslinked ELP-collagen composites displayed equivalent biocompatibility compared to the collagen hydrogels and allowed MC3T3-E1 pre-osteoblast cell attachment, differentiation, and subsequent mineralization over a 3-week culture period [40,41]. We have also shown that subjecting the non-crosslinked ELP-collagen composites to processing temperatures higher than 37°C resulted in controlled microstructures and tunable drug release properties [42]. The main aim of the present study was to compare mechanical, physical, and drug release characteristics of the collagen and ELP-collagen composite hydrogels and ultimately, evaluate their antibacterial properties. Specifically, the collagen and ELP-collagen (3:1 weight ratio of ELP:collagen) hydrogels were prepared and evaluated by measuring their mechanical and degradation properties. The hydrogels were loaded with varying amounts (1 – 5% w/w) of a commonly used broad spectrum antibiotic in periodontal therapy (doxycycline hyclate) and the release was followed. Our goal was to prepare an ELP-collagen composite that would possess superior mechanical properties, gradual drug release profile, and similar biological properties of collagen. We then tested the efficacy of the released doxycycline against four bacterial strains commonly encountered in clinical settings. These strains included: Escherichia coli (E. coli, Gram-negative, facultative), Pseudomonas aeruginosa (P. aeruginosa, Gram-negative, aerobic), Streptococcus sanguinis (S. sanguinis, Gram-positive, facultative), and methicillin-resistant Staphylococcus aureus (MRSA, Gram-positive, facultative).
2. MATERIALS AND METHODS
2.1. Preparation of collagen and ELP-collagen hydrogels
Expression and purification of ELP has been described in detail elsewhere [40]. In brief, the ELP with a primary sequence of [VPGVG]120, where G = glycine, P = proline, and V = valine and a molecular weight of 51,000 Da was produced using recombinant E. coli BLR(DE3) bacteria. The ELP was purified by an inverse phase transition temperature method by at least three repeated cycles of solubilization in phosphate buffered saline (PBS) at 4°C followed by precipitation and centrifugation at 40°C. The purified ELP was then lyophilized.
Preparation of collagen and ELP-collagen composite hydrogels has been described in detail elsewhere [40]. In brief, 25 mg ELP was dissolved in sterile DI water (118 μL), 10 X PBS (250 μL), and 1 N NaOH (48 μL) and mixed gently with a collagen solution (rat tail, Invitrogen, Carlsbad, CA; 3.84 mg/mL, 2084 μL). The final solution was incubated at 37°C in a humidified environment for 24 h to form hydrogels. Hydrogel specimens for mechanical testing were prepared by incubating 350 μL of the final solution per dog-bone shaped well of a custom-made acrylic mold. Hydrogel specimens for assessing degradation behavior and swelling ratio were prepared by incubating 2 mL of the final solution per well in a 24-well tissue culture polystyrene (TCPS) plate (Costar, Corning Life Sciences, Lowell, MA). Hydrogel specimens for assessing drug release were prepared by incubating 150 μL of the final solution per well in a 96-well TCPS plate (Costar, Corning Life Sciences, Lowell, MA). All hydrogels were washed with PBS to remove un-incorporated ELP and/or collagen.
2.2. Mechanical testing
Uniaxial tensile testing was performed on day 1 and day 7 after formation of the hydrogel specimens using ASTM D882 at an extension rate of 0.5 in/min (12.7 mm/min) on a Sintech 2/G Materials Testing System (MTS, Eden Prairie, MN) [43]. The dimensions (thickness and width) of the dog-bone shaped specimens (n = 6), kept wet by immersion in DI water until testing, were measured using Clemex image analysis microscope and software (Clemex Technologies, Quebec, Canada) and Vernier calipers. Values of tensile strength, Young’s modulus, toughness, and percent elongation at break were calculated using the MTS Testworks 4.0 software.
2.3. Degradation behavior
The degradation behavior of the hydrogels was investigated using two methods. Firstly, the collagen and ELP-collagen hydrogels were incubated in 1 mL of fresh PBS at 37°C under a humidified environment. At each time point, three 2 μL aliquots of the supernatant were removed for each hydrogel and analyzed by the NDS 1000 spectrophotometer (Thermo Scientific, Wilmington, DE). The total supernatant volume was maintained at 1 mL throughout the course of study by replacing the equivalent volume drawn out during each reading. The amount of collagen and/or ELP released into the PBS was determined by measuring the absorbance at 280 nm and comparing to pre-prepared calibration curves for collagen and ELP. The lower detection limit for the NDS 1000 spectrophotometer was 0.1 mg/mL, which allowed reliable measurement of the percent degradation from 0.3% and up. This release represented the degradation of the hydrogel matrix. Secondly, the effect of any degradation of the hydrogel matrix on their mechanical properties was assessed using the uniaxial tensile testing performed on day 7 after formation of the hydrogel specimens.
2.4. Determination of swelling ratio
The collagen and ELP-collagen hydrogels were equilibrated with 1 mL DI water at 37°C for 24 h. After equilibration, the excess water was wicked away by contacting all surfaces of the hydrogels to an absorbent paper for exactly 1 min. The hydrogels were subsequently weighed and the swelling ratio was determined using the following equation: where, Ws and Wd are scaffold weights in swollen and dry states.
2.5. Characterization of drug release
Doxycycline hyclate (1, 2, or 5% w/w; Sigma, St. Louis, MO) were added to ELP-collagen solution and the gelation procedure was followed as described above. Collagen hydrogels loaded with doxycycline were fabricated using the same procedure for comparison. All hydrogels were washed with 100 μL PBS to remove un-incorporated doxycycline. Collagen and ELP-collagen hydrogels without any doxycycline were prepared as controls. Hydrogels were incubated in 100 μL of fresh PBS at 37°C under a humidified environment and the supernatants were analyzed by the NDS 1000 spectrophotometer and the xMark microplate spectrophotometer (Bio-Rad, Hercules, CA). The amount of doxycycline released was determined by measuring the absorbance at 345 nm and comparing to pre-prepared calibration curve.
2.6. Determination of bioactivity of the released doxycycline
In order to test the bioactivity of doxycycline released from the hydrogels, bioassays were performed using a disk diffusion method [44]. E. coli bacteria (BLR-DE3) were multiplied in a Luria Bertani nutrient broth in suspension culture at 275 rpm for 8 h at 37°C and swabbed onto petri dishes containing Luria Bertani agar (MP Biomedicals, Santa Ana, CA). P. aeruginosa strain D2133 was provided by Andrea Swiatlo (G.V. Sonny Montgomery VA Medical Center, Jackson, MS) and was streaked for isolation on tryptic soy agar (EMD Chemicals, Gibbstown, NJ) overnight at 37°C. Pure colonies were grown in liquid suspension in tryptic soy broth (MP Biomedicals, Santa Ana, CA) at 200 rpm for 18 h at 37°C, and subcultured in tryptic soy broth until the bacterial concentration was approximately 108 colony-forming units per mL as determined by the optical density at 600 nm. This logarithmic-phase culture was swabbed onto petri dishes containing tryptic soy agar. S. sanguinis strain 1736 was provided by Dr. Joseph Dajcs (Alcon Laboratories, Fort Worth, TX) and was streaked for isolation on blood agar base No. 2 (OXOID Ltd., Hampshire, England) containing 5% sheep’s blood (Quad Five, Ryegate, MT) overnight at 37°C and 5% CO2. Pure colonies were grown in a static liquid suspension in Todd Hewitt broth (Becton, Dickinson and Company, Sparks, MD) containing 0.5% yeast extract (Fisher Bioagents, Fair Lawn, NJ) for 18 h at 37°C and 5% CO2, and subcultured until the bacterial concentration was approximately 108 colony-forming units per mL. This logarithmic-phase culture was swabbed onto blood agar base No. 2 containing 5% sheep’s blood. MRSA strain VA1312/10 was provided by Andrea Swiatlo (G.V. Sonny Montgomery VA Medical Center) and was cultured under the same conditions as those for P. aeruginosa. The collagen and ELP-collagen hydrogels with or without doxycycline were placed on the surface of the agar plates and incubated at 37°C (plus 5% CO2 for S. sanguinis) for 18 h. The E. coli bacteria were stained with Direct Blue stain (Sigma) for better visualization. The inhibition of bacterial growth was observed by comparing the zones of inhibition created around the hydrogels.
2.7. Statistical analysis
All experiments were performed with n = 6 and reported as mean ± 95% confidence intervals. Statistical evaluation of the results was performed using ANOVA with Games-Howell post hoc test for unequal variances. Values with p ≤ 0.05 were deemed significantly different.
3. RESULTS
Figure 1 shows representative stress-strain curves for collagen and ELP-collagen hydrogels. The specimen dimensions as well as the mechanical properties of the collagen and ELP-collagen hydrogels are summarized in Table 1. On day 1 after formation of the hydrogel specimens, the ELP-collagen hydrogels showed slightly higher tensile strength and toughness values compared to those for the collagen hydrogels (p > 0.05). However, the Young’s modulus was significantly greater for the ELP-collagen hydrogels (22.0 ± 7.5 versus 10.7 ± 2.7 kPa) compared to the collagen hydrogels (p = 0.025). The average percent elongation at break values reduced to 76.0 ± 11.5% from 119.7 ± 31.3 for the ELP-collagen hydrogels compared to the collagen hydrogels (p = 0.034). On day 7 after formation of the hydrogel specimens, the tensile strength, Young’s modulus, toughness, and percent elongation at break values were statistically indistinct for the collagen and ELP-collagen hydrogels (p > 0.05). Interestingly, in case of collagen hydrogels the tensile strength, Young’s modulus, toughness, and percent elongation at break values were generally higher on day 7 compared to those on day 1. ELP-collagen hydrogels showed statistically indistinct mechanical properties for day 1 versus day 7 (p > 0.05).
Figure 1.
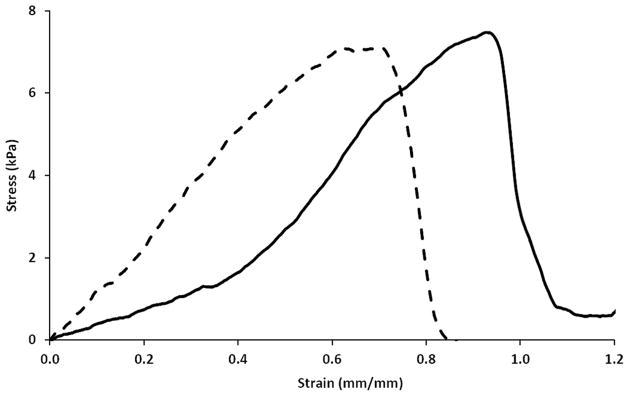
Representative stress-strain curves for collagen hydrogel [solid line] and ELP-collagen hydrogel [dashed line].
Table 1.
Mechanical properties of collagen and ELP-collagen hydrogels. Error bars represent 95% confidence intervals.
| Properties | Hydrogels |
|
|---|---|---|
| Collagen | ELP-collagen | |
| Day 1 | ||
| Thickness (mm) | 1.8 ± 0.2 | 1.5 ± 0.2 |
| Width (mm) | 3.1 ± 0.1 | 3.5 ± 0.3• |
| Tensile Strength (kPa) | 6.6 ± 1.5 | 8.2 ± 2.9 |
| Young’s Modulus (kPa) | 10.7 ± 2.7 | 22.0 ± 7.5• |
| Toughness (kJ/m3) | 2.4 ± 1.0 | 3.3 ± 1.2 |
| Elongation at Break (%) | 119.7 ± 31.3 | 76.0 ± 11.5• |
|
| ||
| Day 7 | ||
| Thickness (mm) | 1.8 ± 0.2 | 2.1 ± 0.3 |
| Width (mm) | 3.6 ± 0.1# | 3.8 ± 0.2 |
| Tensile Strength (kPa) | 10.3 ± 1.5# | 8.2 ± 2.3 |
| Young’s Modulus (kPa) | 14.4 ± 2.2 | 21.4 ± 4.5 |
| Toughness (kJ/m3) | 4.2 ± 0.7# | 3.6 ± 1.4 |
| Elongation at Break (%) | 81.4 ± 11.7 | 68.5 ± 8.5 |
p < 0.05 against the collagen hydrogels at same time point.
p < 0.05 against day 1 for the same type of hydrogels.
The physical properties of the hydrogels were characterized by measuring the swelling ratio and degradation properties. The collagen hydrogel absorbed a significant amount of water and displayed a swelling ratio (Fig. 2) of 38.6 ± 10.7, while the ELP-collagen showed a significantly lower swelling ratio of 10.3 ± 4.2 (p < 0.05). Next, we verified the structural integrity of our hydrogels by studying their degradation, if any, over a 1-week period. For this purpose we analyzed the supernatants collected at the various time points from collagen and ELP-collagen hydrogels to measure any ELP or collagen released from the hydrogels. The UV-Vis spectra of the supernatants collected at 24 h are shown in Figs. 3a(i) and 3b(i) (solid lines) as an example. These spectra did not show an absorbance peak at 280 nm, the characteristic absorbance peak for polypeptide molecules such as ELP and fragments of collagen, and thus, confirmed that a significant amount of collagen or ELP was not released into the supernatant. Figure 4 shows that the percent degradation over the 168 h incubation period was less than 10% for both collagen and ELP-collagen hydrogels, attesting the stability of our hydrogels. We also verified the chemical identity of the doxycycline released from our hydrogels by analyzing the supernatants collected at the various time points from collagen and ELP-collagen hydrogels. The UV-Vis spectrum of freshly prepared pure doxycycline solution (0.25 mg/mL) is shown in Fig. 3a(v) (double line) for reference. This spectrum shows characteristic absorbance peaks for doxycycline at 280 nm and 345 nm. As an example, Figs. 3a and 3b show the UV-Vis spectra of supernatants collected at 24 h from collagen and ELP-collagen hydrogels loaded with 1, 2, or 5% w/w doxycycline, respectively. These spectra also show the same characteristic absorbance peaks at 280 nm and 345 nm and confirm the chemical identity of the doxycycline released.
Figure 2.

Swelling ratio of collagen and ELP-collagen hydrogels. * p ≤ 0.05.
Figure 3.
(a) UV-Vis spectra of supernatant collected at 24 h from collagen hydrogels loaded with (i) 0% w/w, (ii) 1% w/w, (iii) 2% w/w, and (iv) 5% w/w doxycycline. (v) UV-Vis spectrum of the freshly prepared pure doxycycline solution (0.25 mg/mL) is included as reference. (b) UV-Vis spectra of supernatant collected at 24 h from ELP-collagen hydrogels loaded with (i) 0% w/w, (ii) 1% w/w, (iii) 2% w/w, and (iv) 5% w/w doxycycline.
Figure 4.
Degradation behavior of collagen hydrogel (red diamonds) and ELP-collagen hydrogel (purple triangles) confirming the structural integrity of the hydrogels. Error bars indicate 95% confidence intervals.
Figures 5a and 5b show that both the collagen and the ELP-collagen hydrogels rapidly released doxycycline in the initial 48 h followed by a gradual release phase over the next 72 h. The ELP-collagen hydrogel seemed to release a smaller amount of doxycycline compared to the collagen hydrogel. Comparison among the release profiles revealed that a significantly (p < 0.05) lower amount of doxycycline was released from the ELP-collagen hydrogels at all time points for the doxycycline loading of 2 and 5% w/w, but not for 1% w/w. For example, as shown in Fig. 5c, after 72 h, the ELP-collagen hydrogel loaded with 5% w/w doxycycline had released 4.5 ± 0.1 μg doxycycline per mg of scaffold, while corresponding collagen hydrogel had released 5.0 ± 0.1 μg doxycycline per mg of scaffold (p < 0.05).
Figure 5.
Release profiles for (a) collagen and (b) ELP-collagen hydrogels loaded with (blue diamonds) 0, (red squares) 1, (green triangles) 2, and (black circles) 5% w/w doxycycline. The markers represent the actual percent release and the solid lines represent trend lines. (c) Normalized doxycycline release from collagen (blue bars) and ELP-collagen (red bars) hydrogels after 72 h. Error bars represent 95% confidence intervals. * p < 0.05 percent releases from the ELP-collagen hydrogels with same target doxycycline loading measured at same time point against collagen hydrogels.
The bioactivity assays (Figs. 6, 7, 8, and 9) revealed that the doxycycline released from all hydrogels was effective against all the four strains of bacteria tested. The negative controls, which in our case were the collagen and ELP-collagen hydrogels without doxycycline showed no zones of inhibition (Figs. 6a, 6c, 7a, 7c, 8a, 8c, 9a, and 9c). As an example, the zones of inhibition created by doxycycline released from hydrogels loaded with 5% w/w doxycycline are shown in Figs. 6b, 6d, 7b, 7d, 8b, 8d, 9b, and 9d. We observed larger zones of inhibition (p < 0.05) against all four bacterial species tested around the collagen hydrogels than the corresponding ELP-collagen hydrogels (Figs. 6e, 7e, 8e, and 9e). Larger zones of inhibition were also observed for hydrogels loaded with 2% w/w doxycycline against all four bacterial species tested (p < 0.05), but not for hydrogels loaded with 1% w/w doxycycline. More importantly, the zones of inhibition created around the hydrogels were dependent on the doxycycline loading (Figs. 6e, 7e, 8e, and 9e). This was true for both collagen and ELP-collagen hydrogels against all four bacterial species tested. The larger zones of inhibition obtained with collagen hydrogels compared to the ELP-collagen hydrogels as well as the larger zones of inhibition obtained with increasing doxycycline loading could be directly attributed to the higher amount of doxycycline released from the hydrogels by the 24 h time point (Fig. 5). Interestingly, for hydrogels loaded with 1% w/w doxycycline, the ELP-collagen hydrogels performed superior to collagen hydrogels against E. coli and P. aeruginosa (p < 0.05), equivalent to collagen hydrogels against S. sanguinis (p > 0.05), and inferior to collagen hydrogels against MRSA (p < 0.05).
Figure 6.
Bioactivity of (a) collagen hydrogel with no doxycycline, (b) collagen hydrogel loaded with 5% w/w doxycycline, (c) ELP-collagen hydrogel with no doxycycline, and (d) ELP-collagen hydrogel loaded with 5% w/w doxycycline against E. coli. The circles indicate the zones of inhibition. (e) Zones of inhibition measured for the collagen (blue bars) and ELP-collagen (red bars) hydrogels. Error bars represent 95% confidence intervals. * p < 0.05 zones of inhibition for the ELP-collagen hydrogels with same target doxycycline loading measured at same time point against collagen hydrogels. ND = No zone of inhibition detected.
Figure 7.
Bioactivity of (a) collagen hydrogel with no doxycycline, (b) collagen hydrogel loaded with 5% w/w doxycycline, (c) ELP-collagen hydrogel with no doxycycline, and (d) ELP-collagen hydrogel loaded with 5% w/w doxycycline against P. aeruginosa. The circles indicate the zones of inhibition. (e) Zones of inhibition measured for the collagen (blue bars) and ELP-collagen (red bars) hydrogels. Error bars represent 95% confidence intervals. * p < 0.05 zones of inhibition for the ELP-collagen hydrogels with same target doxycycline loading measured at same time point against collagen hydrogels. ND = No zone of inhibition detected.
Figure 8.
Bioactivity of (a) collagen hydrogel with no doxycycline, (b) collagen hydrogel loaded with 5% w/w doxycycline, (c) ELP-collagen hydrogel with no doxycycline, and (d) ELP-collagen hydrogel loaded with 5% w/w doxycycline against S. sanguinis. The circles indicate the zones of inhibition. (e) Zones of inhibition measured for the collagen (blue bars) and ELP-collagen (red bars) hydrogels. Error bars represent 95% confidence intervals. * p < 0.05 zones of inhibition for the ELP-collagen hydrogels with same target doxycycline loading measured at same time point against collagen hydrogels. ND = No zone of inhibition detected.
Figure 9.
Bioactivity of (a) collagen hydrogel with no doxycycline, (b) collagen hydrogel loaded with 5% w/w doxycycline, (c) ELP-collagen hydrogel with no doxycycline, and (d) ELP-collagen hydrogel loaded with 5% w/w doxycycline against MRSA. The circles indicate the zones of inhibition. (e) Zones of inhibition measured for the collagen (blue bars) and ELP-collagen (red bars) hydrogels. Error bars represent 95% confidence intervals. * p < 0.05 zones of inhibition for the ELP-collagen hydrogels with same target doxycycline loading measured at same time point against collagen hydrogels. ND = No zone of inhibition detected.
4. DISCUSSION
Post-surgical infections may lead to impaired healing and eventual second surgery to remove the impacted tissue. Systemic antibiotic treatment methods used to fight these infections are suboptimal due to difficulty in delivering therapeutic antibiotic dosage through the infectious bacterial biofilm. As argued by Ruszczak and Friess, the high antibiotic concentrations needed at the local tissue site to minimize such infections cannot be achieved by systemic injections as well as spreading of drug-loaded powder of a carrier material or polymer beads over the wound site [13]. Sustained release of a broad spectrum antibiotic at the local site from a tissue-engineered scaffold has the potential to minimize post-surgical infections, while supporting new tissue growth. To this end, we have developed a biocompatible composite hydrogel system consisting of constituents that mimic natural ECM components (namely, collagen and elastin) and a broad spectrum antibiotic (namely, doxycycline) commonly used in periodontal therapy.
We explain the various physical and mechanical properties of our composite hydrogels based on the various inter-chain interactions (secondary bonding) occurring between the ELP and collagen components of our composites. Such inter-chain interactions could be the direct or water mediated hydrogen bonding and hydrophobic associations between the ELP and collagen molecules. The strong secondary bonding between ELP and collagen is evidenced by the structural integrity of our non-crosslinked ELP-collagen hydrogels through no ELP release during the degradation studies (Fig. 4) and no deterioration of mechanical properties over a period of 7 days (Table 1). Such strong secondary bonding between the ELP and collagen would also explain the higher elastic modulus of the ELP-collagen hydrogels compared to the collagen hydrogels (Table 1). Additionally, the strong secondary bonding may lead to a smaller swelling ratio for the ELP-collagen hydrogels compared to the collagen hydrogels (Fig. 2). The swelling ratio plays an important role in drug release properties as it determines the drug diffusivity in and out of the hydrogels. A smaller swelling ratio leads to slower drug release [45] and may explain the slower doxycycline release from the ELP-collagen hydrogels compared to the collagen hydrogels (Fig. 5).
All our hydrogels showed a behavior of faster release initially with a slower release rate with time. Such doxycycline release behavior was similar to the one observed by other researchers using a synthetic polymer scaffold (sulfonated styrene-ethylene-butylenes-styrene triblock copolymer) [46], a natural polymer scaffold (chitosan microspheres) [47], and a composite scaffold (gelatin microspheres impregnated in collagen) [48]. Wallace and Rosenblatt have argued that bioactive agents with molecular weights lower than 200 kDa may not experience hindered diffusion in a collagen hydrogel [15]. This may be especially true for the bioactive agents molecules bound in the near surface region of the hydrogel and may explain the faster diffusion of the doxycycline out of our collagen and ELP-collagen hydrogels at initial time points. On the other hand, the subsequent slower release of the doxycycline may be due to a reduced concentration gradient (difference between drug concentrations in the hydrogel versus the surrounding medium) that may result after the initial doxycycline release.
When drugs are incorporated in a polymer matrix, the polymer may interact with the drugs and reduce their bioactivity by changing the molecular configuration [35]. Therefore, it was important to investigate the bioactivity of the released doxycycline. Our doxycycline-loaded collagen and ELP-collagen hydrogels showed varying degree of bioactivity against all four bacterial species tested in the disk diffusion tests (Figs. 6, 7, 8, and 9). As such, the zones of inhibition obtained in our study against P. aeruginosa (~ 7–12 mm) and MRSA (~ 21–30 mm) were equivalent or larger compared to a clinical study which used a commercially available TheraGauze composite wound dressing and showed the zones of inhibition of 8 mm against P. aeruginosa and 25 mm against MRSA [49]. The zones of inhibition obtained by Phaechamud and Charoenteeraboon using chitosan sponge loaded with 50 mg doxycycline against E. coli and S. aureus were approximately 35 mm. It should be noted that our collagen and ELP-collagen hydrogels with 5% w/w doxycycline contained only ~ 33 μg of doxycycline and yet produced 8–12 mm and 25–30 mm zones of inhibition against E. coli and MRSA, respectively [50]. While doxycycline has been shown to be effective against S. sanguinis [51,52], no studies have been reported using release of doxycycline from hydrogels to inhibit S. sanguinis growth. In that sense, to our knowledge, our study represents the first evidence of effective doxycycline release from composite hydrogels against S. sanguinis. It should be noted that our ~ 33 μg doxycycline loading was equivalent to the one used in published standards of antimicrobial susceptibility testing [53]. According to the standards, the zones of inhibition obtained in this study for E. coli and S. sanguinis indicated resistance against doxycycline. Similarly, the zones of inhibition obtained for P. aeruginosa indicated intermediate resistance against doxycycline. MRSA, however, was highly susceptible to doxycycline regardless of hydrogel composition, as its published susceptibility breakpoint is a zone diameter of ≥ 16 mm [53]. Nevertheless, our results clearly demonstrated that collagen and ELP did not interfere with the bioactivity of doxycycline.
5. CONCLUSIONS
We have described the formation, physical characterization, and drug delivery behavior of non-crosslinked ELP-collagen composite hydrogels. Overall, the ELP-collagen composite hydrogels showed enhanced performance in terms of their mechanical properties, showed a more gradual drug release profile, and achieved effective antibacterial performance compared to collagen hydrogels. The addition of ELP resulted in a composite hydrogel with higher elastic modulus that would be beneficial while handling the hydrogels scaffolds for drug delivery and tissue engineering applications. The ELP-collagen composite hydrogels released a smaller amount of doxycycline compared to the collagen hydrogels, likely due to a smaller swelling ratio resulting from the secondary bonding interactions between the components of our composites. The dose-dependent efficacy of the doxycycline released from the ELP-collagen composite hydrogels against bacterial strains commonly encountered in clinical settings will prove valuable in many drug delivery applications. As such, the 3:1 ELP-collagen composite reduced the overall collagen content by 75%, which will likely impact their properties when exposed to a physiological enzyme solution.
Acknowledgments
Sponsored by the School of Dentistry intramural research support program and the National Institute of Dental and Craniofacial Research of the National Institutes of Health under Award Number R03DE024257. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. TRA participated in the Undergraduate and Professional Student Training in Advanced Research Techniques (UPSTART) Program. This work made use of instruments in the Department of Biomedical Materials Science User Facility.
References
- 1.Langer R, Peppas NA. Advances in biomaterials, drug delivery, and bionanotechnology. AIChE J. 2003;49:2990–3006. [Google Scholar]
- 2.Wang X, Kluge JA, Leisk GG, Kaplan DL. Sonication-induced gelation of silk fibroin for cell encapsulation. Biomaterials. 2008;29:1054–1064. doi: 10.1016/j.biomaterials.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q, Wang J, Shahani S, Sun DD, Sharma B, Elisseeff JH, Leong KW. Biodegradable and photocrosslinkable polyphosphoester hydrogel. Biomaterials. 2006;27:1027–1034. doi: 10.1016/j.biomaterials.2005.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Degoricija L, Bansal PN, Sontjens SH, Joshi NS, Takahashi M, Snyder B, Grinstaff MW. Hydrogels for osteochondral repair based on photocrosslinkable carbamate dendrimers. Biomacromolecules. 2008;9:2863–2872. doi: 10.1021/bm800658x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Z, Wang F, Roy S, Sen CK, Guan J. Injectable, highly flexible, and thermosensitive hydrogels capable of delivering superoxide dismutase. Biomacromolecules. 2009;10:3306–3316. doi: 10.1021/bm900900e. [DOI] [PubMed] [Google Scholar]
- 6.Liu W, Griffith M, Li F. Alginate microsphere-collagen composite hydrogel for ocular drug delivery and implantation. J Mater Sci Mater Med. 2008;19:3365–3371. doi: 10.1007/s10856-008-3486-2. [DOI] [PubMed] [Google Scholar]
- 7.Segura T, Chung PH, Shea LD. DNA delivery from hyaluronic acid-collagen hydrogels via a substrate-mediated approach. Biomaterials. 2005;26:1575–1584. doi: 10.1016/j.biomaterials.2004.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergeron E, Leblanc E, Drevelle O, Giguère R, Beauvais S, Grenier G, Faucheux N. The evaluation of ectopic bone formation induced by delivery systems for bone morphogenetic protein-9 or its derived peptide. Tissue Eng Part A. 2012;18:342–352. doi: 10.1089/ten.TEA.2011.0008. [DOI] [PubMed] [Google Scholar]
- 9.Maeda M, Tani S, Sano A, Fujioka K. Microstructure and release characteristics of the minipellet, a collagen-based drug delivery system for controlled release of protein drugs. J Controlled Rel. 1999;62:313–324. doi: 10.1016/s0168-3659(99)00156-x. [DOI] [PubMed] [Google Scholar]
- 10.Fujioka K, Maeda M, Hojo T, Sano A. Protein release from collagen matrices. Adv Drug Del Rev. 1998;31:247–266. doi: 10.1016/s0169-409x(97)00119-1. [DOI] [PubMed] [Google Scholar]
- 11.Lee KY, Yuk SH. Polymeric protein delivery systems. Prog Polym Sci. 2007;32:669–697. [Google Scholar]
- 12.Friess W. Collagen - biomaterial for drug delivery. Eur J Pharma Biopharma. 1998;45:113–136. doi: 10.1016/s0939-6411(98)00017-4. [DOI] [PubMed] [Google Scholar]
- 13.Ruszczak Z, Friess W. Collagen as a carrier for on-site delivery of antibacterial drugs. Adv Drug Del Rev. 2003;55:1679–1698. doi: 10.1016/j.addr.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Malafaya PB, Silva GA, Reis RL. Natural-origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv Drug Del Rev. 2007;59:207–233. doi: 10.1016/j.addr.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Wallace DG, Rosenblatt J. Collagen gel systems for sustained delivery and tissue engineering. Adv Drug Del Rev. 2003;55:1631–1649. doi: 10.1016/j.addr.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Glowacki J, Mizuno S. Collagen scaffolds for tissue engineering. Biopolymers. 2008;89:338–344. doi: 10.1002/bip.20871. [DOI] [PubMed] [Google Scholar]
- 17.Kyle S, Aggeli A, Ingham E, McPherson MJ. Production of self-assembling biomaterials for tissue engineering. Trends Biotechnol. 2009;27:423–433. doi: 10.1016/j.tibtech.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yannas IV, Burke JF. Design of an artificial skin. I. Basic design principles. J Biomed Mater Res. 1980;14:65–81. doi: 10.1002/jbm.820140108. [DOI] [PubMed] [Google Scholar]
- 19.Chvapil M, Owen JA, Clark DS. Effect of collagen crosslinking on the rate of resorption of implanted collagen tubing in rabbits. J Biomed Mater Res. 1977;11:297–314. doi: 10.1002/jbm.820110213. [DOI] [PubMed] [Google Scholar]
- 20.Harriger MD, Supp AP, Warden GD, Boyce ST. Glutaraldehyde crosslinking of collagen substrates inhibits degradation in skin substitutes grafted to athymic mice. J Biomed Mater Res. 1997;35:137–145. doi: 10.1002/(sici)1097-4636(199705)35:2<137::aid-jbm1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 21.Speer DP, Chvapil M, Eskelson CD, Ulreich J. Biological effects of residual glutaraldehyde in glutaraldehyde-tanned collagen biomaterials. J Biomed Mater Res. 1980;14:753–764. doi: 10.1002/jbm.820140607. [DOI] [PubMed] [Google Scholar]
- 22.Parenteau-Bareil R, Gauvin R, Berthod F. Collagen-based biomaterials for tissue engineering applications. Materials. 2010;3:1863–1887. [Google Scholar]
- 23.Huang L, Nagapudi K, Apkarian RP, Chaikof EL. Engineered collagen-PEO nanofibers and fabrics. J Biomater Sci Polym Ed. 2001;12:979–993. doi: 10.1163/156856201753252516. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi K. Effect of new bone substitute materials consisting of collagen and tricalcium phosphate. Bull Tokyo Dent Coll. 2009;50:1–11. doi: 10.2209/tdcpublication.50.1. [DOI] [PubMed] [Google Scholar]
- 25.Urry DW, Parker TM, Reid MC, Gowda DC. Biocompatibility of the bioelastic materials, poly(GVGVP) and its gamma-irradiation cross-linked matrix - summary of generic biological test results. J Bioactive Compat Polym. 1991;6:263–282. [Google Scholar]
- 26.Chilkoti A, Dreher MR, Meyer DE. Design of thermally responsive, recombinant polypeptide carriers for targeted drug delivery. Adv Drug Del Rev. 2002;54:1093–1111. doi: 10.1016/s0169-409x(02)00060-1. [DOI] [PubMed] [Google Scholar]
- 27.MacEwan SR, Chilkoti A. Elastin-like polypeptides: biomedical applications of tunable biopolymers. Biopolymers. 2010;94:60–77. doi: 10.1002/bip.21327. [DOI] [PubMed] [Google Scholar]
- 28.Dreher MR, Liu W, Michelich CR, Dewhirst MW, Chilkoti A. Thermal cycling enhances the accumulation of a temperature-sensitive biopolymer in solid tumors. Cancer Res. 2007;67:4418–4424. doi: 10.1158/0008-5472.CAN-06-4444. [DOI] [PubMed] [Google Scholar]
- 29.Betre H, Liu W, Zalutsky MR, Chilkoti A, Kraus VB, Setton LA. A thermally responsive biopolymer for intra-articular drug delivery. J Controlled Rel. 2006;115:175–182. doi: 10.1016/j.jconrel.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 30.Koria P, Yagi H, Kitagawa Y, Megeed Z, Nahmias Y, Sheridan R, Yarmush ML. Self-assembling elastin-like peptides growth factor chimeric nanoparticles for the treatment of chronic wounds. Proc Nat Acad Sci. 2011;108:1034–1039. doi: 10.1073/pnas.1009881108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee S, Kim JS, Chu HS, Kim GW, Won JI, Jang JH. Electrospun nanofibrous scaffolds for controlled release of adeno-associated viral vectors. Acta Biomater. 2011;7:3868–3876. doi: 10.1016/j.actbio.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 32.Kim JS, Chu HS, Park KI, Won JI, Jang JH. Elastin-like polypeptide matrices for enhancing adeno-associated virus-mediated gene delivery to human neural stem cells. Gene Ther. 2012;19:329–337. doi: 10.1038/gt.2011.84. [DOI] [PubMed] [Google Scholar]
- 33.Na K, Jung J, Lee J, Hyun J. Thermoresponsive pore structure of biopolymer microspheres for a smart drug carrier. Langmuir. 2010;26:11165–11169. doi: 10.1021/la1013285. [DOI] [PubMed] [Google Scholar]
- 34.Adams SB, Jr, Shamji MF, Nettles DL, Hwang P, Setton LA. Sustained release of antibiotics from injectable and thermally responsive polypeptide depots. J Biomed Mater Res B. 2009;90:67–74. doi: 10.1002/jbm.b.31254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu Y, MacKay JA, McDaniel JR, Chilkoti A, Clark RL. Fabrication of elastin-like polypeptide nanoparticles for drug delivery by electrospraying. Biomacromolecules. 2009;10:19–24. doi: 10.1021/bm801033f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia Y, Hemantkumar N, Collighan R, Griffin M, Rodriguez-Cabello JC, Pandit A. In vitro characterization of a collagen scaffold enzymatically cross-linked with a tailored elastin-like polymer. Tissue Eng. 2009;15:887–899. doi: 10.1089/ten.tea.2008.0104. [DOI] [PubMed] [Google Scholar]
- 37.Kinikoglu B, Rodriguez-Cabello JC, Damour O, Hasirci V. A smart bilayer scaffold of elastin-like recombinamer and collagen for soft tissue engineering. J Mat Sci Mater Med. 2011;22:1541–1554. doi: 10.1007/s10856-011-4315-6. [DOI] [PubMed] [Google Scholar]
- 38.Caves JM, Kumar VA, Martinez AW, Kim J, Ripberger CM, Haller CA, Chaikof EL. The use of microfiber composites of elastin-like protein matrix reinforced with synthetic collagen in the design of vascular grafts. Biomaterials. 2010;31:7175–7182. doi: 10.1016/j.biomaterials.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caves JM, Cui W, Wen J, Kumar VA, Haller CA, Chaikof EL. Elastin-like protein matrix reinforced with collagen microfibers for soft tissue repair. Biomaterials. 2011;32:5371–5379. doi: 10.1016/j.biomaterials.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amruthwar SS, Puckett AD, Janorkar AV. Preparation and characterization of novel elastin-like polypeptide-collagen composite scaffolds. J Biomed Mater Res. 2013;101A:2383–2391. doi: 10.1002/jbm.a.34514. [DOI] [PubMed] [Google Scholar]
- 41.Amruthwar SS, Janorkar AV. In vitro evaluation of elastin-like polypeptide - collagen composite scaffold for bone tissue engineering. Dental Materials. 2013;29:211–220. doi: 10.1016/j.dental.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 42.Patel N, Purser CA, Baker RC, Janorkar AV. Effect of processing temperature on the morphology and drug-release characteristics of elastin-like polypeptide - collagen composite coatings. Biomacromolecules. 2013;14:2891–2899. doi: 10.1021/bm4007425. [DOI] [PubMed] [Google Scholar]
- 43.Wheeler TS, Sbravati ND, Janorkar AV. Mechanical & cell culture properties of elastin-like polypeptide, collagen, bioglass, and carbon nanosphere composites. Annals Biomed Eng. 2013;41:2042–2055. doi: 10.1007/s10439-013-0825-3. [DOI] [PubMed] [Google Scholar]
- 44.Barry AL, Coyle MB, Thornsberry C, Gerlach EH, Hawkinson RW. Methods of measuring zones of inhibition with the Bauer-Kirby disk susceptibility test. J Clinical Microbiol. 1979;10:885–889. doi: 10.1128/jcm.10.6.885-889.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y, Zhu W, Wang B, Yu L, Ding J. Postfabrication encapsulation of model protein drugs in a negatively thermosensitive hydrogel. J Pharma Sci. 2005;94:1676–1684. doi: 10.1002/jps.20310. [DOI] [PubMed] [Google Scholar]
- 46.Vachon DJ, Yager DR. Novel sulfonated hydrogel composite with the ability to inhibit proteases and bacterial growth. J Biomed Mater Res. 2006;76A:35–43. doi: 10.1002/jbm.a.30440. [DOI] [PubMed] [Google Scholar]
- 47.Shanmuganathan S, Shanumugasundaram N, Adhirajan N, Ramyaa Lakshmi TSR, Babu M. Preparation and characterization of chitosan microspheres for doxycycline delivery. Carbohydrate Polym. 2008;73:201–211. [Google Scholar]
- 48.Adhirajan N, Shanmugasundaram N, Shanmuganathan S, Babu M. Functionally modified gelatin microspheres impregnated collagen scaffold as novel wound dressing to attenuate the proteases and bacterial growth. Eur J Pharma Sci. 2009;36:235–245. doi: 10.1016/j.ejps.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 49.Echague CG, Hair PS, Cunnion KM. A comparison of antibacterial activity against methicillin-resistant staphylococcus aureus and gram-negative organisms for antimicrobial compounds in a unique composite wound dressing. Adv Skin Wound Care. 2010;23:406–413. doi: 10.1097/01.ASW.0000383213.95911.bc. [DOI] [PubMed] [Google Scholar]
- 50.Phaechamud T, Charoenteeraboon J. Antibacterial activity and drug release of chitosan sponge containing doxycycline hyclate. AAPS Pharm Sci Tech. 2008;9:829–835. doi: 10.1208/s12249-008-9117-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Larsen T, Fiehn NE. Resistance of Streptococcus sanguis biofilms to antimicrobial agents. APMIS. 1996;104:280–284. [PubMed] [Google Scholar]
- 52.Bjorvatn K, Skaug N, Selvig KA. Inhibition of bacterial growth by tetracycline-impregnated enamel and dentin. Scand J Dent Res. 1984;92:508–516. doi: 10.1111/j.1600-0722.1984.tb01290.x. [DOI] [PubMed] [Google Scholar]
- 53.Clinical and Laboratory Standards Institute. Thwenty-third Informational Supplement. Wayne, PA: 2013. Performance Standards for Antimicrobial Susceptibility Testing. CLSI document M100-S23. [Google Scholar]










