Abstract
The age-associated increase in arterial stiffness has long been considered to parallel or to cause the age-associated increase in blood pressure (BP). Yet, the rates at which pulse wave velocity (PWV), a measure of arterial stiffness, and BP trajectories change over time within individuals who differ by age and sex have not been assessed and compared. This study determined the evolution of BP and aortic PWV trajectories over a 9.4-year follow-up in over 4,000 community dwelling men and women of 20–100 years of age at entry into the SardiNIA Study. Linear mixed effects model analyses revealed that PWV accelerates with time over the observation period, at about the same rate over the entire age range in both men and women. In men, the longitudinal rate at which BP changed over time, however, did not generally parallel that of PWV acceleration: at ages above 40 years the rates of change in SBP and PP increase plateaued and then declined so that SBP, itself, also declined at older ages while PP plateaued. In women, SBP, DBP and MBP increased at constant rates across all ages, producing an increasing rate of increase in PP. Therefore, increased aortic stiffness is implicated in the age-associated increase in SBP and PP. These findings indicate that PWV is not a surrogate for BP and that arterial properties other than arterial wall stiffness that vary by age and sex also modulate the BP trajectories during aging and lead to the dissociation of PWV, PP and SBP trajectories in men.
Keywords: Aging, arteries, blood pressure, longitudinal studies, pulse wave velocity, gender
INTRODUCTION
Interactions among arterial stiffness, blood pressure (BP), and aging over time present a complex conundrum1–7. Stiffening of central arteries after age 40 is a characteristic feature of life and is accompanied by an age-associated change in BP.1 Epidemiological studies demonstrate that increased aortic stiffness, indexed as an increased pulse wave velocity (PWV),8,9 is an independent risk factor for cardiovascular (CV) events, even when the impact of age, BP, and other known risk factors are taken into account.10–12 These findings suggest that prevention or reduction of PWV may carry substantial health benefits.13 Cross sectional studies show that BP is strongly associated with PWV. BP is transmitted into the arterial wall, where its increase progressively stimulates the less distensible collagen fibers, thus resulting in a progressively stiffer artery14. Therefore, the age associated increase in arterial stiffness has long been considered to parallel the age-associated increase in BP1. Yet, the rates at which PWV and BP accelerate within individuals who differ in age and sex is largely unknown, but their definition is required to unravel the conundrum of interactions of arterial stiffness and BP as age increases, and is also required for correct power analyses and the age/sex composition of clinical trials aiming to intervene on PWV. Therefore an understanding of the age-BP-arterial stiffness conundrum is a major public health priority.
The present study determined the evolution of BP and PWV trajectories in persons of different ages by measuring both PWV and BP (systolic, diastolic, mean, and pulse pressure) trajectories over 9.4 years of follow-up in over 4,000 community dwelling men and women of 20–100 years of age at entry into the study. We discovered that the association of BP and aortic PWV is much more complicated than appreciated previously, possibly due to concurrent age-, gender-, and pressure-dependent aortic remodeling.
SUBJECTS AND METHODS
Study population
The SardiNIA Study investigates the genetics and epidemiology of complex traits/phenotypes, including CV risk factors and arterial properties, in a community-dwelling Sardinian founder population.15,16 The study population for the present analysis consisted of 4358 volunteers (1810 men and 2548 women) of a wide age range (20 to 101 years) followed for two or three visits (mean = 2.4 visits) with a mean follow-up of 5.4 years and up to 9.4 years resulting in 11968 observations. The Supplement provides details about participant recruitment and missing visits. Supplement Figure S1 and Supplement Table S1 shows that the age-sex distribution at initial visit for subjects in the present analyses is similar for men and women.
Blood Pressure
Blood pressure was measured in both arms with a mercury sphygmomanometer using an appropriately sized cuff. The blood pressure values used in this study are the average of the second and third measurements during each visit on both the right and left arm. For further details, see the online supplement.
Arterial stiffness
Carotid-femoral PWV was measured as previously described,17 using nondirectional transcutaneous Doppler probes (Model 810A, 9 to 10-Mhz probes, Parks Medical Electronics, Inc, Aloha, OR). For further details, see the supplement.
Statistical analyses
Participants enter the SardiNIA study at different ages and were followed for two or three visits. Thus both cross-sectional and longitudinal perceptions can be gleaned from the measured variables. All analyses were performed using SAS for Windows (Version 9.2, Cary, NC). Data are presented as mean ± SD unless otherwise specified. Both cross-sectional and longitudinal changes were modeled with a single linear mixed-effects regression model,18, 19 which easily accommodates unbalanced, unequally spaced observations and, consequently, is an ideal tool for analyzing both cross-sectional and longitudinal changes in data from this observational study. Additional details including a glossary of model terms and interpretations are in the supplement. The fit of the mixed-effects models to the data are addressed by plots of the residuals and plots of observed vs. predicted values. These plots are presented in the supplement and show that the models provide adequate fits to the data.
Each model fit represents a specific hypothesis being tested and each model contains a number of model effects. In conducting the backward elimination variable selection procedure it is prudent to guard against false positive results. To do so, for each model fit, a Bonferroni adjusted significance level is used: for the basic models that contain 5 model effects, the adjusted significance level is 0.05/5 = 0.01 and for the models with additional covariates, the full model contains 15 model effects so the adjusted significance level is 0.05/15 = 0.00333.
RESULTS
Description of study population
Average age, PWV, PP, SBP, DBP, mean BP, heart rate (HR), body mass index (BMI), and percent of subjects in each sex are listed in Table 1. While there is no difference in the mean follow-up time between men and women participants (p = 0.3338), there is a significant difference in mean follow-up time by age decade (p < 0.0001).
Table 1.
Description of study population
| Variable | Mean ± SD |
|---|---|
| Age (years) | 43.7 ± 17·6 |
| Follow-up Time (years) | 5.4 ± 2.0 |
| Women (%) | 58·5 |
| PWV (m/sec) | 6.71 ± 1.89 |
| SBP (mmHg) | 125.5 ± 17.8 |
| DBP (mmHg) | 77.7 ± 10.6 |
| PP (mmHg) | 47.9 ± 12.2 |
| MBP (mmHg) | 93.6 ± 12.1 |
| HR | 66.7 ± 11.0 |
| BMI (kg/m2) | 25.6 ± 4.4 |
| Hypertension (%) | 29.1 |
| Diabetes (%) | 4.8 |
| Prevalent MI (%) | 0.9 |
| Prevalent Stroke (%) | 0.8 |
Combined PWV Trajectories over Time in Men and Women
To determine if there are significant sex effects on PWV, we first fit mixed-effects models18 to ln(PWV) (see Supplemental Statistical Analysis section for motivation of modelling the transformed PWV) that included data on both men and women. There were significant sex differences in PWV measured both cross-sectionally and longitudinally (see Supplement). Consequently, we subsequently constructed separate models for men and women for ln(PWV) and for each of the blood pressure variables.
Sex-Specific Longitudinal PWV Trajectories by Entry Age
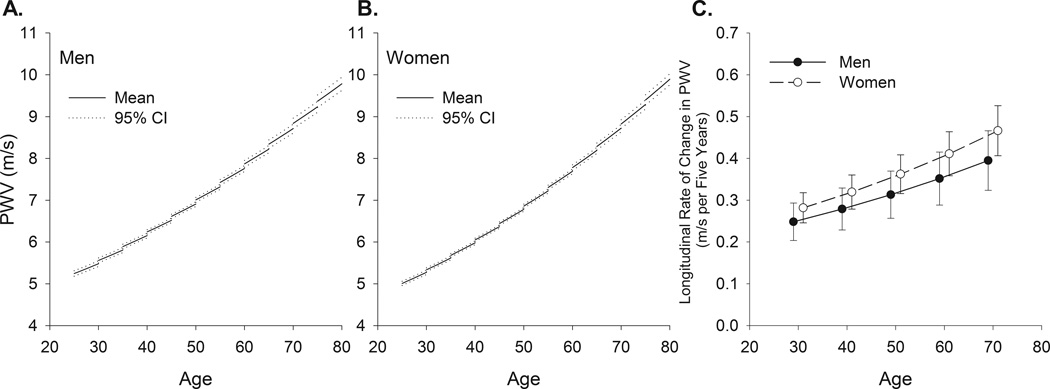
Table 2A lists the estimates for the fitted models for PWV in men and women. The graphs in Figures 1A and B are generated from the equations provided by the mixed-effects models in Table 2A. The PWV model formula for men is provided in the legend of Figure 1. The cross-sectional association of PWV with age at entry is represented by the left, or beginning, point of each line segment. The average longitudinal rates of change of PWV for subjects of different entry ages are illustrated by the slopes of the five-year line segments in the plot. Also included on the plots are 95% confidence bands for each line segment. Thus, the plots in Figure 1A and B show how PWV changes with entry age among different subjects, and the average rate of change of PWV during the observations period within the same individuals.
Table 2.
| A: Final Mixed-Effects Model Results for Basic Models for ln(PWV) | ||||
|---|---|---|---|---|
| ln(PWV) | ||||
| Variable | MEN | WOMEN | ||
| Estimate | p-value | Estimate | p-value | |
| Intercept | 1.3671 | <.0001 | 1.296 | <0·0001 |
| Entry Age | 0.0116 | <.0001 | 0.0126 | <0·0001 |
| (Entry Age)2 | ||||
| Time | 0.008744 | <.0001 | 0.0103 | <0·0001 |
| Entry Age ×Time | ||||
| (Entry Age)2 ×Time | ||||
| Corr(Obs, Pred) | 0.890 | 0·892 | ||
| B: Final Mixed-Effects Model Results for Basic Models for BP in Men | ||||||
|---|---|---|---|---|---|---|
| Variable | PP | DBP | SBP | |||
| Estimate | p-value | Estimate | p-value | Estimate | p-value | |
| Intercept | 67·066 | <0·0001 | 44.147 | <0.0001 | 112·760 | <0·0001 |
| Entry Age | −1·0881 | <0·0001 | 1.3856 | <0.0001 | 0·2253 | 0·0880 |
| (Entry Age)2 | 0·01390 | <0·0001 | −0.01155 | <0.0001 | 0·00311 | 0·0235 |
| Time | −2·0182 | <0·0001 | 1.2943 | <0.0001 | −1·3342 | 0·0263 |
| Entry Age ×Time | 0·08066 | 0·0001 | −0.02652 | <0.0001 | 0·08243 | 0·0021 |
| (Entry Age)2 ×Time | −0·00074 | 0·0006 | −0·00104 | 0·0002 | ||
| Corr(Obs, Pred) | 0·823 | 0.794 | 0·860 | |||
| C: Final Mixed-Effects Model Results for Basic Models for BP in Women | ||||||
|---|---|---|---|---|---|---|
| Variable | PP | DBP | SBP | |||
| Estimate | p-value | Estimate | p-value | Estimate | p-value | |
| Intercept | 46·596 | <0·0001 | 48.819 | <.0001 | 91.7116 | <.0001 |
| Entry Age | −0·3946 | <0·0001 | 0.9306 | <.0001 | 0.6701 | <.0001 |
| (Entry Age)2 | 0·00756 | <0·0001 | −0.00652 | <.0001 | ||
| Time | −0·5207 | <0·0001 | 0.1540 | <.0001 | 0.2114 | <.0001 |
| Entry Age ×Time | 0·01302 | <0·0001 | ||||
| (Entry Age)2 ×Time | ||||||
| Corr(Obs, Pred) | 0·824 | 0.813 | 0·889 | |||
Figure 1.
Longitudinal trajectories and 95% confidence bands in PWV in men (panel A) and women (panel B) and five year longitudinal rates of change in PWV with 95% confidence intervals (panel C) from mixed-effects models in men and women of different ages at entry into the study. Note that rates of change at all ages are positive. Since the data becomes more sparse with advancing age (Supplement Figure S1 and Table S1), the longitudinal plots of the modelled data are only constructed for entry ages up to 75 years. Note: The equation to predict ln(PWV) for a man is: ln(PWV) = 1.3671 + 0.0116 × (Entry Age) + 0.008744 × Time. The predicted value of PWV is obtained as eln(PWV). Predicted values for PWV in women or for other variables can be obtained in a similar fashion by inserting appropriate values of the explanatory variables into the relevant equations.
The pattern and the magnitude of the increase in the rate of change of PWV over time varied by entry age and sex. In both men and women, the average rate of change in PWV increased by about 60% from entry age 30 to entry age 70 (Figure 1C).
Effects of Antihypertensive Medications on PWV Trajectories
As expected, the use of antihypertensive medications increased with advancing age, with no sex differences (p= 0.22) (Supplement Table S5). To disentangle the possible effect of antihypertensive medications on the longitudinal changes in PWV, we constructed additional models. It was found that antihypertensive medications did not significantly affect the cross-sectional or longitudinal conclusions for PWV. Thus trends described in PWV (Table 2, and Figures 1) are independent of use of antihypertensive medications that, as expected, is greater at older ages. Additional specific details are discussed in the supplement (Effects of Antihypertensive Medications on PWV Trajectories).
Blood Pressure Trajectories over Time by Sex and Age at Study Entry
SBP
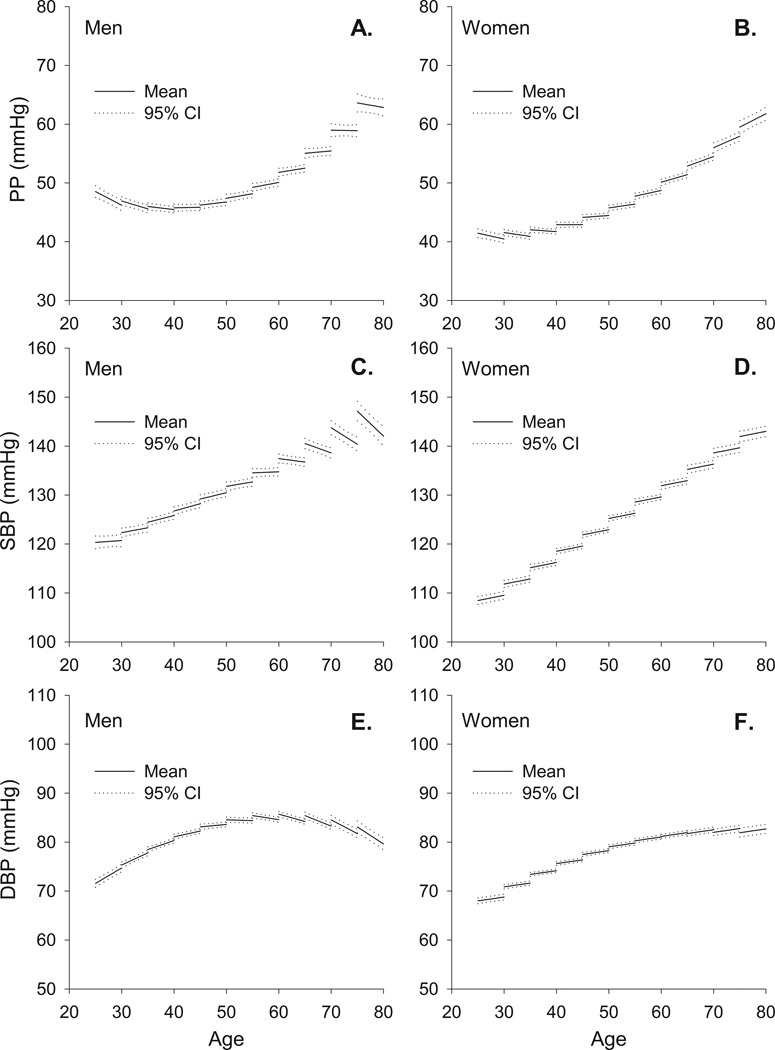
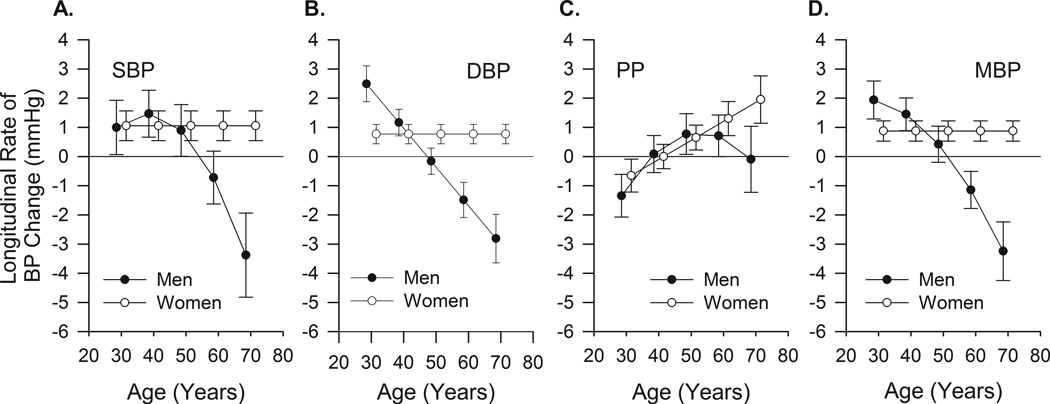
Table 2B and C lists the model effects for SBP and Figure 2C and D illustrate the models. Cross-sectional BP (i.e. at entry age) differences are similar to those described in the literature. The average longitudinal rates of change in SBP vary in a non-linear fashion. In men the average longitudinal rate of change of SBP increases with age at entry until 40 years, beyond which the rate of increase declines (Figures 3 and 4). Note that beyond entry age 50 years of age, the average longitudinal rate of change in SBP becomes negative (the rate of change in SBP crosses the zero axis), and SBP itself begins to decline. Thus, although when measured cross-sectionally in different men of different ages SBP increases with age, longitudinal analyses in the same men over time indicate that the maximum average rate at which SBP increases is achieved by entry age 40, and that beyond entry age 50, the average rate of change becomes negative, and, on average, SBP declines. In women, the rate of increase in SBP remains constant and positive at all ages studied (Figures 3 and 4).
Figure 2.
Longitudinal trajectories and 95% confidence bands in PP, SBP, and DBP from mixed-effects models in men (Panels A, C, and E) and women (Panel B, D, and F) of different ages at entry into the study.
Figure 3.
Five year longitudinal rates of change in SBP (panel A), DBP (panel B), PP (panel C) and MBP (panel D) with 95% confidence intervals from mixed-effects models in men and women of different ages at entry into the study. Note that beyond 50 years the rates of change in the BPs in men become negative, indicating a fall in the absolute pressure. Note that rates above zero imply increases in the parameter.
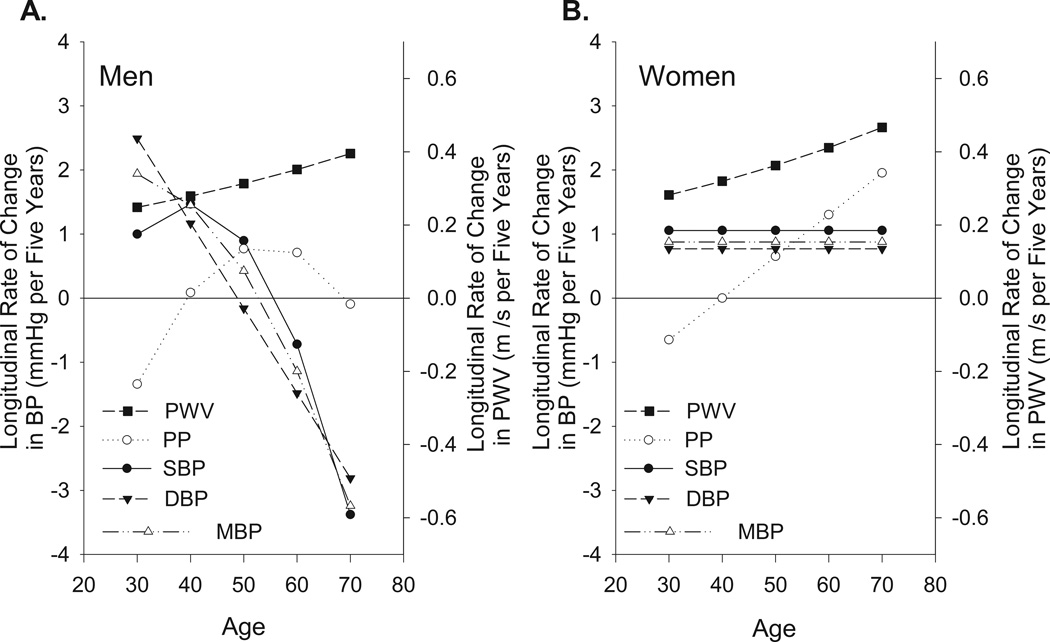
Figure 4.
Combined data from Figures 1C and 3 to compare five year longitudinal rates of change in PWV, PP, SBP, DBP, and MBP from mixed-effects models in men (panel A) and women (panel B) of different ages at entry into the study. Note, the general dissociation between the rate at which the BPs and PWV change in men.
DBP
Table 2B and C provides the parameter estimates for the various terms in the model for DBP for men and women. The models show that the average rate of DBP change, in men, exhibits a monotonic decline between entry ages 30 and 70 years, becoming negative at age 50. In contrast to men, in women the average longitudinal change in DBP is positive and constant at all entry ages (Figures 3 and 4).
Pulse Pressure
Table 2B and C provides the parameter estimates for PP and Figure 2A and B illustrate the fitted models. Figures 2A, 3, and 4 show that in men the average longitudinal rate of PP increase plateaus at entry age 55, and the rate of PP increase declines thereafter until entry age 70, when there is little change in PP. In women, in contrast to men, the average longitudinal rate of change of PP (dictated by rates of change in SBP and DBP) increases monotonically with every decade after the entry age of 30 years (Figure 3 and 4B).
MBP
Supplement Table S7 and Figure S5 present the mean blood pressure (MBP) analyses. The average longitudinal rates of change in MBP are illustrated in Figures 3 and 4. In men, MBP increases up through about age 50 changing to declines at older ages. In women, the average rate of MBP increases at a constant rate at all entry ages (Figures 3 and 4B).
Comparison of Longitudinal Trajectories of PWV and BP by Entry Age
In order to directly visualize and compare the longitudinal rates of change of PWV and BP trajectories as a function of entry age, the PWV changes in Figure 1C are superimposed on the BP changes in Figure 4. Note that in women (Figure 4B) the average rates of change in both PWV and PP over time increase monotonically at each successive entry age. In men, however, the average rates of change of PP and SBP and those of PWV over time exhibit substantial dissociation (Figure 4A). While the rates of change in PWV increase steadily over the age span as in women, the rates of change in PP in men initially increase but decline after age 50 and the rates of change in SBP decline after age 40.
Effects of BP and PWV on each other’s Trajectories
To control for BP on rates of change in PWV, and for PWV on rates of change in the BPs, mixed effects models for PWV with each BP as an additional covariate, and for each BP with PWV as a covariate were fit to the data (these models also control for HR and BMI). Supplement Tables S8 and S9 provide the parameter estimates of the final mixed-effects models. Figures S6, S7, and S8 display graphs of some of the fitted models.
Associations of PWV with BPs at entry age and over time
In men, when PWV is included as a covariate in the BP models it has only a main model effect on SBP, DBP, and PP, but no association with the BP’s over time (Table S9A). Higher PWV, however, is associated with lower longitudinal trajectories in MBP (due to the significantly negative Time×PWV interaction, Table S9A). In women, PWV is associated with cross-sectional differences in all BP’s (Table S9B). As entry age increases, the association of PWV on DBP and MBP decreases, whilst that of PWV on PP increases (Table S9B). In women, PWV is associated with longitudinal changes in SBP and DBP.
Associations of BP with PWV at entry age and over time
No BP’s are associated with longitudinal changes in PWV over time (Tables S8A – D). However, higher BP is associated with higher PWV at older entry ages for PP in men and women and for SBP in women (due to the significantly positive (Entry Age)×BP interaction terms in Tables S8A and D). In DBP and MBP in men and women and SBP in men, BP is associated with higher PWV levels (due to the positive parameter estimate of the main effect of the BP terms in the models).
The perspectives given above are for average trajectories and rates of change. Figure S11 in the Supplement provides graphs that show the associations among individual rates of changes and initial values of the covariates.
DISCUSSION
This is the first study to simultaneously examine both cross-sectional (measured at entry age) and longitudinal rates of change of PWV and BP over time at each entry age in a large, general population of men and women of broad age range at study entry. Our cross-sectional measurements of PWV and BP in different subjects who differ in entry age are similar to those reported previously in cross-sectional studies14,20–24. In addition to potential cohort effects, age and other factors present in different individuals prior to entry into the study, e.g. profile of intensity of risk factors (intensity and duration) that are associated with PWV or BP, may impact the interpretation of cross-sectional data. However, our longitudinal findings revealed a dissociation between trajectories of PWV and BP in men, challenging the conventional wisdom of a direct simple relationship between arterial stiffness and the rise in systolic and pulse pressure with aging.
Longitudinal PWV Trajectories
The longitudinal trajectories of PWV that occur over time do not depend upon entry age and their rates of change do not differ by sex (Figures 1 – 4). A few prior longitudinal studies of arterial stiffness indexed by both PWV and BP4, 24–27 have been conducted in small samples. A recent report from the Baltimore Longitudinal Study of Aging (BLSA)27, however, describes longitudinal changes in PWV in 1200 subjects that are similar to those in the present study. Interestingly, the average rate of PWV increase in the present study (about 0.05 m/s per year for a 50 year old male) is strikingly similar to that reported recently in a smaller study population in which the rate of increase from the mid 50s to the mid 60s depended upon an angiotensin II type 1 receptor genotype.24
Interactions of PWV, BP, and Age: The Conundrum
Divergence of PWV and BP Trajectories Over Time in men but not in women
A most important and novel observation of the present study is that the longitudinal rate of change or absolute direction of longitudinal BP change in men over the span of entry ages does not track with that of PWV. Both SBP and PWV increase across the younger entry ages in men but at older entry ages while SBP increases, the rate of BP increase in each subsequent age decade decreases, while the rate at which PWV increases accelerates. In contrast to men, in women the rate of increase in both PWV and the BP’s remain positive across the age range studied.
While PWV and BP are clearly intertwined, our linear mixed-effects models permitted us to statistically verify associations among BP and PWV (Tables S8 and S9). As expected from the existing literature, both PWV and BP are each related to cross-sectional differences of the other. However, in some cases there are differences in the associations of each on the other’s longitudinal trajectories.
Interestingly, PWV was not associated with the longitudinal change in PP over time in either sex (Tables S9A and B). In addition, in men PWV was not associated with the longitudinal change in SBP and in women, there is an association of PWV on longitudinal changes in SBP but this association declines as Entry Age increases. As might be expected, PWV was inversely associated with the longitudinal change in DBP over time (Table S9B) in women, but not in men. This result confirms prior cross-sectional perspectives that DBP falls as central arteries stiffen. However, in men, PWV was associated with longitudinal changes in only MBP;but in women PWV was associated with longitudinal changes in SBP and DBP, but the association PWV on longitudinal changes in SBP declines as entry age increases.
While PP and entry age are associated with PWV in men and women (Table S8A) and SBP and entry age are associated with PWV only in women (Table S8D), none of the BPs is associated with differences in longitudinal PWV trajectories (Tables S8A–D). In Framingham, BP evaluated at the previous visit is not independently associated with PWV increases that occur over time after accounting for PWV at the previous visit4, an approach that differs from that of the present study, in which BP at the same visit as PWV is used as a covariate in the linear mixed models to describe longitudinal changes in PWV while accounting for various covariates. In contrast to the present study, in the BLSA, a higher SBP is associated with an accelerated rate of PWV increase in both men and women.27
The cross sectional perspective of associations of SBP with age gleaned from the present results is that SBP continues to increase with each successive entry age decade (Figure 2) while the longitudinal perspective, however, indicates that, at older ages in men, in fact, SBP decreases over time (Figures 2, 3, and 4). This suggests that the cross sectional increase with Entry Age would have been even steeper if SBP prior to entry into the study in older men had not already begun to plateau or decline. Clearly, the waning of the rate at which SBP increases at older ages in men is the major factor that drives the divergence of the rates of change in PWV, SBP, and PP. While central BP was not measured in the present study, brachial and central SBP converge with increasing age. The reduction in the rate at which SBP increases and later declines in men, but not in women, explains the long recognized convergence or cross-over of cross-sectional measures of PP in women vs. men with advancing age.28
One explanation for the divergence of the rates of change in PWV and SBP at older ages in men is that the reduction in the rate of increase and eventual decline in SBP reflects a concomitant deterioration in cardiodynamics and heart-arterial coupling or change in wave reflection. A reduction in stroke volume, however, is not likely to be a cause because stroke volume does not decline with age in either men or women29.
Given that characteristic impedance, a major determinant of SBP, is a function of both PWV and aortic diameter squared, per the water hammer equation,30 another explanation for the dissociation between SBP and PWV longitudinal trajectories in men is an increase in aortic diameter. Indeed, a greater rate of aortic root dilatation with increasing age in men than in women has been reported.31,32 This study employed echo and did not assess thoracic or abdominal aortic diameter or aortic tortuosity. Nonetheless, it is tempting to speculate, therefore, that an increase in the rate of aortic remodeling (dilatation) in men in the context of a continual increase in PWV, in the absence of a change in stroke volume, is a factor implicated in the SBP reduction in older men. An increased rate at which the aorta remodels (dilates) in men vs. women, could also be an explanation for the decline in the rate of increase followed by a reduction in the rate of change DBP in men (Figures 2 and 4). In women, neither PP nor SBP decline, on average, at older ages, and in fact, the rate at which PP increases remains positive and PP continues to increase into advanced age (Figures 3, 4B) because the rate at which SBP increases is greater than that of DBP (Figures 3 and 4). A sex specific combination of aortic stiffening and aortic dilatation in women may account for the monotonic rates at which systolic and diastolic BP change and PP increases in women but not in men. Inferences regarding sex differences in blood pressure and aortic diameter have been previously drawn from cross-sectional data30, 33–35.
Finally, an age-associated increase in blood pressure, per se, may have a causal association with the age-associated aortic dilatation. Indeed, that the reduction in SBP is greater in those individuals with a higher SBP at entry age (Figure S12) might offer a clue to an association of BP on aortic remodeling. A chronic increase in BP, itself, via an effect to promote aortic dilatation, reduces aortic impedance, thus limiting a further BP increase, or even resulting in a reduction in BP in the context of a progressive increase in arterial wall stiffness. Hence, the conundrum of aging, arterial stiffness, and BP likely results from both feed-forward and negative feedback interactions of BP, aortic wall stiffness, and aortic diameter over time as age increases. Thus, one plausible scenario is that, as the aortic wall stiffens with age, SBP increases;this chronic increase in SBP leads to greater aortic dilatation in men than in women which reduces the rate at which SBP continues to increase in men. The dissociation of the continuous rate at which PWV accelerates over time from the rate at which BP changes as age increases in men, clearly indicates that while interrelated, changes in PWV and BP are intrinsically regulated by independent mechanisms.
Potential Limitations
This study only used two or three repeated observations on each participant. This allows us to draw valid conclusions about longitudinal changes for only up to about five years. The participants in this study enter at ages from 20 to over 80 years old. While this broad range of subject age at the study onset allows for the characterization of longitudinal PWV and BP trajectories over a wide range of entry ages, persons of older entry age have survived to that age and consequently some survivor bias, in addition to other cohort effects, may impact Entry Age effects. But longitudinal changes during the observational period do not suffer directly from this bias but are affected by age at entry. Secular trends that occur during the observation period, e.g. changes in lifestyle and diet, might be reflected in the data. Secular trends, that develop over long periods of time and require long duration, are unlikely to be a major issue in this study, however, as the data on each of the three waves were collected within a relatively short time period.
PERSPECTIVES
The age-associated increase in arterial stiffness has long been considered to parallel or to cause the age-associated increase in blood pressure. Yet, the rates at which PWV, a measure of arterial stiffness, and BP trajectories change over time within individuals who differ by age and sex have not been assessed and compared. This study determined the evolution of BP and aortic PWV trajectories over a 9.4-year follow-up in over 4,000 community dwelling men and women of 20–100 years of age at entry into the SardiNIA Study. Analyses revealed that PWV accelerates with time over the observation period, at about the same rate over the entire age range in both men and women. In men, the longitudinal rate at which BP changed over time, however, did not generally parallel that of PWV acceleration. These findings indicate that PWV is not a surrogate for BP and that arterial properties other than arterial wall stiffness that vary by age and sex also modulate the BP trajectories during aging and lead to the dissociation of PWV, PP and SBP trajectories in men. Thus, knowledge of the longitudinal trajectories of PWV and BP over time in the same individuals and whether the rates of change are constant or vary with age helps to elucidate the conundrum of the interrelationship among aortic stiffness, age, and BP. These rates also provide crucial information for the design of future proof of concept studies for interventions on arterial stiffening.
Supplementary Material
Novelty and Significance.
a) What Is New?
The age-associated increase in arterial stiffness has long been considered to parallel or to cause the age-associated increase in blood pressure (BP).
We assessed PWV and BP with two or three repeated measurements over a 9.4 year follow-up in a 4000+ community dwelling cohort of men and women, 20–101 years of age at entry into the SardiNIA study.
In individuals beyond entry age 40 years, the longitudinal rate at which PWV increases becomes dissociated from the rate at which SBP and PP change in men.
b) What Is Relevant?
Age-associated central arterial stiffening, measured by PWV, and its associated increase in SBP and PP are independent risk factors for cardiovascular events.
Knowledge of the longitudinal trajectories of PWV and BP over time in the same individuals and whether the rates of change are constant or vary with age helps to elucidate the conundrum of the interrelationship among aortic stiffness, age, and BP.
The definition of the rates of change in PWV and BP over time in individuals who entered the present study at different ages provides crucial information for the design of future proof of concept studies for interventions on arterial stiffening.
c) Summary
The association of BP and aortic PWV is much more complicated than appreciated previously, possibly due to concurrent age-, gender-, and pressure-dependent aortic remodeling.
PWV increases with time in men and women but, in men, this is not paralleled by an increase in SBP and PP. The dissociation of the longitudinal changes in aortic PWV, SBP, and PP in older men in a large, general population observed in our study indicates that PWV is not a surrogate for BP and that therapies to reduce PWV aimed primarily at blood pressure reduction in older men may not be optimally effective.
In order to delay age-associated arterial stiffening in humans and its attendant exponential increase in risk for arterial diseases, future interventional clinical trials need to begin to explore novel therapeutic strategies aimed at arterial wall mechanisms that are implicated in arterial stiffness.
ACKNOWLEDGMENTS
We thank numerous Sardinians involved with this study. See the supplement for details. We thank Paul Pullen for assistance with processing the PWV data and Ruth Sadler for editorial assistance. This research was supported (in part) by the Intramural Research Program of the NIH, National Institute on Aging.
Funding:
The SardiNIA team was supported by Contract NO1-AG-1-2109 from the NIA. This research was supported in part by the Intramural Research Program of the NIH, NIA (USA).
Footnotes
Disclosures:
None
Contributor Information
Angelo Scuteri, Hospital San Raffaele Pisana – Istituto Ricovero e Cura a Carattere Sceintifico (IRCCS), Rome, Italy.
Christopher H. Morrell, Laboratory of Cardiovascular Science, National Institute of Aging, Baltimore, MD, USA; Loyola University Maryland, Baltimore, MD, USA.
Marco Orru, Istituto di Ricerca Genetica e Biomedica (IRGB), Consiglio Nazionale delle Ricerche, c/o Cittadella Universitaria di Monserrato, Monserrato, Cagliari, Italy.
James B. Strait, Laboratory of Cardiovascular Science, National Institute of Aging, Baltimore, MD, USA.
Kirill V. Tarasov, Laboratory of Cardiovascular Science, National Institute of Aging, Baltimore, MD, USA.
Majd AlGhatrif, Johns Hopkins School of Medicine, Baltimore, MD, USA.
Liana Anna Pina Ferreli, Istituto di Ricerca Genetica e Biomedica (IRGB), Consiglio Nazionale delle Ricerche, c/o Cittadella Universitaria di Monserrato, Monserrato, Cagliari, Italy.
Francesco Loi, Istituto di Ricerca Genetica e Biomedica (IRGB), Consiglio Nazionale delle Ricerche, c/o Cittadella Universitaria di Monserrato, Monserrato, Cagliari, Italy.
Maria Grazia Pilia, Istituto di Ricerca Genetica e Biomedica (IRGB), Consiglio Nazionale delle Ricerche, c/o Cittadella Universitaria di Monserrato, Monserrato, Cagliari, Italy.
Alessandro Delitala, Istituto di Ricerca Genetica e Biomedica (IRGB), Consiglio Nazionale delle Ricerche, c/o Cittadella Universitaria di Monserrato, Monserrato, Cagliari, Italy.
Harold Spurgeon, Laboratory of Cardiovascular Science, National Institute of Aging, Baltimore, MD, USA.
Samer S. Najjar, MedStar Heart Institute, Washington DC, USA.
Edward G. Lakatta, Laboratory of Cardiovascular Science, National Institute of Aging, Baltimore, MD, USA.
REFERENCES
- 1.Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension. 2005;46:454–462. doi: 10.1161/01.HYP.0000177474.06749.98. [DOI] [PubMed] [Google Scholar]
- 2.Izzo JL., Jr Arterial stiffness and the systolic hypertension syndrome. Curr Opin Cardiol. 2004;19:341–352. doi: 10.1097/01.hco.0000126581.89648.10. [DOI] [PubMed] [Google Scholar]
- 3.Najjar SS, Scuteri A, Shetty V, Wright JG, Muller DC, Fleg JL, Spurgeon HP, Ferrucci L, Lakatta EG. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore longitudinal study of aging. J Am Coll Cardiol. 2008;51:1377–1383. doi: 10.1016/j.jacc.2007.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaess B, Rong J, Larson M, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic Stiffness, Blood Pressure Progression, and Incident Hypertension. Journal of the American Medical Association. 2012;308:875–881. doi: 10.1001/2012.jama.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liao D, Arnett DK, Tyroler HA, Riley WA, Chambless LE, Szklo M, Heiss G. Aterial Stiffness and the development of hypertension. The ARIC study. Hypertension. 1999;34:201–206. doi: 10.1161/01.hyp.34.2.201. [DOI] [PubMed] [Google Scholar]
- 6.Dernellis J, Panaretou M. Aortic Stiffness is an independent predictor of progression to hypertension in nonhypertensive subjects. Hypertension. 2005;45:426–431. doi: 10.1161/01.HYP.0000157818.58878.93. [DOI] [PubMed] [Google Scholar]
- 7.O’Rourke MF, Nichols WW. Aortic diameter, aortic stiffness and wave reflection increase with age and isolated systolic hypertension. Hypertension. 2005;45:652–658. doi: 10.1161/01.HYP.0000153793.84859.b8. [DOI] [PubMed] [Google Scholar]
- 8.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. European Network for Non-invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Shlomo Y, Spears M, Boustred C, et al. Aortic Pulse Wave Velocity Improves Cardiovascular Event Prediction: An Individual Participant Meta-Analysis of Prospective Observational Data From 17,635 Subjects. J Am Coll Cardiol. 2014;63:636–646. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 11.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A. Health ABC Study. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scuteri A, Lakatta EG. Bringing prevention in geriatric medicine: evidences supporting the new challenge. Exp Gerontol. 2013;48:64–68. doi: 10.1016/j.exger.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nichols W, O'Rourke M, Vlachopoulos C. McDonald's Blood Flow in Arteries, Sixth Edition: Theoretical, Experimental and Clinical Principles. CRC Press; 2011. [Google Scholar]
- 15.Pilia G, Chen WM, Scuteri A, et al. Heritability of cardiovascular and personality traits in 6,148 Sardinians. PLoS Genet. 2006;2:e132. doi: 10.1371/journal.pgen.0020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scuteri A, Najjar SS, Orru' M, Albai G, Strait J, Tarasov KV, Piras MG, Cao A, Schlessinger D, Uda M, Lakatta EG. Age- and gender-specific awareness, treatment, and control of cardiovascular risk factors and subclinical vascular lesions in a founder population: The SardiNIA Study. Nutr Metab Cardiovasc Dis. 2009;19:532–541. doi: 10.1016/j.numecd.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scuteri A, Najjar SS, Orru' M, Usala G, Piras MG, Ferrucci L, Cao A, Schlessinger D, Uda M, Lakatta EG. The central arterial burden of the metabolic syndrome is similar in men and women: the SardiNIA Study. Eur Heart J. 2010;31:602–613. doi: 10.1093/eurheartj/ehp491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York: Springer; 2000. [Google Scholar]
- 19.Morrell CH, Brant LJ, Ferrucci L. Model choice can obscure results in longitudinal studies. Journal of Gerontology: Medical Sciences. 2009;64A:215–222. doi: 10.1093/gerona/gln024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scuteri A, Orru' M, Morrell CH, Tarasov K, Schlessinger D, Uda M, Lakatta EG. Associations of large artery structure and function with adiposity: effect of age, gender, and hypertension. The SardiNIA Study. Atherosclerosis. 2012;221:189–197. doi: 10.1016/j.atherosclerosis.2011.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McEniery CM, Yasmin, Maki-Petaja KM, McDonnell BJ, Munnery M, Hickson SS, Franklin SS, Cockcroft JR, Wilkinson IB. Anglo-Cardiff Collaboration Trial Investigators. The impact of cardiovascular risk factors on aortic stiffness and wave reflections depends on age: the Anglo-Cardiff Collaborative Trial (ACCT III) Hypertension. 2010;56:591–597. doi: 10.1161/HYPERTENSIONAHA.110.156950. [DOI] [PubMed] [Google Scholar]
- 22.Scuteri A, Chen CH, Yin FCP, Chih TT, Spurgeon HA, Lakatta EG. Functional correlates of central arterial geometric phenotypes. Hypertension. 2001;38:1471–1475. doi: 10.1161/hy1201.099291. [DOI] [PubMed] [Google Scholar]
- 23.Scuteri A, Manolio TA, Marino EK, Arnold AM, Lakatta EG. Prevalence of specific variant carotid geometric patterns and incidence of cardiovascular events in older persons. The Cardiovascular Health Study. JACC. 2004;43:187–193. doi: 10.1016/j.jacc.2003.08.035. [DOI] [PubMed] [Google Scholar]
- 24.Benetos A, Giron A, Joly L, Temmar M, Nzietchueng R, Pannier B, Bean K, Thomas F, Labat C, Lacolley P. Influence of the AGTR1 A1166C genotype on the progression of arterial stiffness: A 16-year longitudinal study. Am J Hypertens. 2013;26:1421–1427. doi: 10.1093/ajh/hpt141. [DOI] [PubMed] [Google Scholar]
- 25.Wildman RP, Farhat GN, Patel AS, Mackey RH, Brockwell S, Thompson T, Sutton-Tyrrell K. Weight change is associated with change in arterial stiffness among healthy young adults. Hypertension. 2005;45:187–192. doi: 10.1161/01.HYP.0000152200.10578.5d. [DOI] [PubMed] [Google Scholar]
- 26.El Khoudary SR, Barinas-Mitchell E, White J, Sutton-Tyrrell K, Kuller LH, Curb JD, Shin C, Ueshima H, Masaki K, Evans RW, Miura K, Edmundowicz D, Sekikawa A ERA JUMP Study Group. Adiponectin, systolic blood pressure, and alcohol consumption are associated with more aortic stiffness progression among apparently healthy men. Atherosclerosis. 2012;225:475–480. doi: 10.1016/j.atherosclerosis.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.AlGhatrif M, Strait JB, Morrell CH, Canepa M, Wright J, Elango P, Scuteri A, Najjar SS, Ferrucci L, Lakatta EG. Longitudinal Trajectories of Arterial Stiffness and the Role of Blood Pressure: The Baltimore Longitudinal Study of Aging. Hypertension. 2013;62:934–941. doi: 10.1161/HYPERTENSIONAHA.113.01445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McEniery CM, Wilkinson IB. The pressures of aging. Hypertension. 2013;62:823–824. doi: 10.1161/HYPERTENSIONAHA.113.01998. [DOI] [PubMed] [Google Scholar]
- 29.Fleg JL, O'Connor F, Gerstenblith G, Becker LC, Clulow J, Schulman SP, Lakatta EG. Impact of age on the cardiovascular response to dynamic upright exercise in healthy men and women. J Appl Physiol (1985) 1995;78:890–900. doi: 10.1152/jappl.1995.78.3.890. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell GF, Lacourcière Y, Ouellet JP, Izzo JL, Jr, Neutel J, Kerwin LJ, Block AJ, Pfeffer MA. Determinants of elevated pulse pressure in middle-aged and older subjects with uncomplicated systolic hypertension. Circulation. 2003;108:1592–1598. doi: 10.1161/01.CIR.0000093435.04334.1F. [DOI] [PubMed] [Google Scholar]
- 31.Lam CS, Xanthakis V, Sullivan LM, Lieb W, Aragam J, Redfield MM, Mitchell GF, Benjamin EJ, Vasan RS. Aortic Root Remodeling Over the Adult Life Course: Longitudinal Data From the Framingham Heart Study. Circulation. 2010;122:884–890. doi: 10.1161/CIRCULATIONAHA.110.937839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.AlGhatrif M, Strait JB, Morrell CH, Canepa M, Wright J, Elango P, Scuteri A, Najjar SS, Ferrucci L, Lakatta EG. Attenuated Aortic Dilatation, Not Increased Wall Stiffness Best Explains the Rise in Pulse Pressure in Women With Aging: Results From the Baltimore Longitudinal Study of Aging. Circulation. 2013;128(22 Supplement) S, Meeting Abstract: 18061, Published: NOV 26 2013. [Google Scholar]
- 33.Mitchell GF, Gudnason V, Launer LJ, Aspelund T, Harris TB. Hemodynamics of increased pulse pressure in older women in the community-based Age, Gene/Environment Susceptibility – Reykjavik Study. Hypertension. 2008;51:1123–1128. doi: 10.1161/HYPERTENSIONAHA.107.108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farasat SM, Morrell CH, Scuteri A, Ting CT, Yin FC, Spurgeon HA, Chen CH, Lakatta EG, Najjar SS. Pulse Pressure Is Inversely Related to Aortic Root Diameter Implications for the Pathogenesis of Systolic Hypertension. Hypertension. 2008;51:196–202. doi: 10.1161/HYPERTENSIONAHA.107.099515. [DOI] [PubMed] [Google Scholar]
- 35.Farasat SM, Morrell CH, Scuteri A, Ting CT, C P Yin F, Spurgeon HA, Chen CH, G Lakatta E, Najjar SS. Do Hypertensive Individuals Have Enlarged Aortic Root Diameters? Insights from Studying the Various Subtypes of Hypertension. American Journal of Hypertension. 2008;21:558–563. doi: 10.1038/ajh.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.