1. Introduction
Research in the last two decades has revealed powerful statistical learning abilities in infants and adults, including the extraction of statistical regularities from a variety of inputs including artificial and natural speech (Pelucchi, Hay, & Saffran, 2009; Saffran, Aslin, & Newport, 1996), non-linguistic auditory stimuli (Saffran, Johnson, Aslin, & Newport, 1999), and visual arrays and sequences of shapes (Bulf, Johnson, & Valenza, 2011; Fiser & Aslin, 2001, 2002a, 2002b; Kirkham, Slemmer, Richardson, & Johnson, 2007). Statistical learning is characterized by detection of regularities in ones environment without an explicit awareness or intention to learn (Perruchet & Pacton, 2006), and it may play a critical role in language acquisition and social behavior (Romberg & Saffran, 2010; Roseberry, Richie, Hirsh-Pasek, Golinkoff, & Shipley, 2011; Wu, Gopnik, Richardson, & Kirkham, 2011). Autism spectrum disorder (ASD) is a neurodevelopmental disorder defined by a dyad of impairments in social communication function and the presence of restricted interests or repetitive behaviors (American Psychiatric Association, 2013). Given the potential relevance of statistical learning to social communication, surprisingly few studies have investigated this domain in children with ASD, perhaps in part because of the reliable behavioral output required in traditional statistical learning tasks.
In the present study, we designed an event related electrophysiological (EEG) shape learning paradigm, adapted from a task developed by Kirkham, Slemmer and Johnson (2002), and we examined the EEG correlates of visual statistical learning in young children with ASD. This is not only the first study to identify electrophysiological markers of visual statistical learning in young children, but it is also the first to investigate this cognitive domain in children with ASD. Additionally, in an effort to capture the cognitive heterogeneity in the autism spectrum, we moved beyond the examination of whole group differences to an analysis linking clinical features with our EEG measures of interest, with focus on the relation between non-verbal cognition and visual statistical learning.
1.1 Implicit learning in ASD
Statistical learning represents one approach to studying the broader cognitive construct of implicit learning, with the latter defined as learning without the intention to learn or without the conscious awareness of the knowledge that has been acquired (Cohen & Squire, 1980; Reber & Squire, 1994; Travers, Klinger, Mussey, & Klinger, 2010). As a whole, implicit learning represents a core cognitive domain that emerges early in development and, unlike explicit memory, remains relatively independent of overall intellectual ability (Komatsu, Naito, & Fuke, 1996; Mitchell, 1993; Perrig, 1995; Wyatt & Conners, 1998). Implicit learning has been identified as a mediator of language acquisition, social development, and motor skills (Cleeremans, 2008; Perruchet & Pacton, 2006), and it could serve as a possible precursor to or a correlate of deficits in cognitive and social skills that define ASD. Implicit learning paradigms studied in ASD include measures of contextual cueing (Brown, Aczel, Jimenez, Kaufman, & Grant, 2010), serial reaction time (SRT) (Barnes et al., 2008; Brown et al., 2010; Gordon & Stark, 2007; Mostofsky, Goldberg, Landa, & Denckla, 2000), probabilistic classification (Brown et al., 2010), and motor sequence learning (Gidley-Larson & Mostofsky, 2008). It is known that these different tasks do not rely on the same cognitive abilities (Howard et al., 2004), and results from studies using these paradigms in ASD have been mixed, with several reporting deficits in learning (Gidley-Larson & Mostofsky, 2008; Gordon & Stark, 2007; Klinger & Dawson, 2001; Mostofsky et al., 2000) and others documenting intact learning, as defined by performance that does not significantly differ from typically developing, age-matched individuals (Barnes et al., 2008; Brown et al., 2010; Molesworth, Bowler, & Hampton, 2005; Travers et al., 2010). To our knowledge, visual statistical learning in ASD remains unexamined. Given its early availability (Bulf et al., 2011) and high degree of continuity across development (Fiser & Aslin, 2002a, 2002b) in typical populations, it may be an ideal task for understanding the nature and limits of implicit learning in children with ASD.
With the exception of the Gordon et al. study (2007), the studies of implicit learning described previously have focused exclusively on high-functioning children with ASD, defined as having above average intelligence on standardized measures of IQ. Examination of this somewhat narrow population facilitates behavioral studies, as high-functioning children with ASD can follow directions more easily and engage in paradigms requiring sustained attention. Such studies have laid a critical foundation for our understanding of the broader domain of implicit learning in ASD. However, younger and lower functioning children with ASD have been neglected when, in fact, one could argue they represent the population least understood and most in need of characterization to inform interventions. Additionally, the focus on higher-functioning children limits our ability to capture subtle differences in cognitive and behavioral domains that may inform the heterogeneity within the ASD population. To address this concern, in our study we focused on young children with ASD with a broad range of cognitive abilities, and we designed a task (described subsequently) whose primary outcome measure is defined by an electrophysiological response rather than overt behavior.
Additionally, all of the studies described above rely on behavioral output as the measure of learning. In this context, Brown et al. (2010) raised an important question about whether performance in a behavioral task truly represents implicit learning or, instead, the recruitment of more explicit cognitive processes. Were the latter the case, the focus on high functioning individuals might inherently bias the results towards “intact” learning, not because implicit learning is truly intact but because the participants are using other pathways to learn the task. This proposition begs the question: Does similar behavior equate to common mechanisms of cognitive processing? Studies of word segmentation in ASD, described in the following section, provide evidence that the two may not be synonymous.
1.2 Statistical language learning in ASD
Recent research has characterized both behavioral and neural correlates of statistical language learning in children with ASD. Word segmentation, or the identification of word boundaries within a continuous stream of speech, is one of the first steps of language learning (Romberg & Saffran, 2010). Mayo and Eigsti (2012) investigated statistical language learning in school age high functioning children with ASD using a similar set of stimuli to that employed by Saffran et al. (1996). Participants were exposed passively to streams of tri-syllabic combinations while engaged in a drawing activity. In the test phase, participants were presented with words and non-words and asked to choose the item that sounded more like the language to which they were exposed. There were no group differences in accuracy between TD and ASD groups, suggesting intact statistical learning in this group. However, the authors were quick to note that “in the absence of behavioral differences, there may nonetheless be neural differences in how individuals with HFA approach the task…it is possible that the HFA group was able to compensate for a distinctive implicit learning process by employing additional or alternative strategies” (Mayo & Eigsti, 2012).
In an investigation of the neural markers of statistical learning,Scott-Van Zealand and colleagues (2010) performed an fMRI study with high functioning children with ASD, ages 9-16, using a word segmentation paradigm adapted from methods described by Saffran et al. (1996). Children listened to two artificial languages containing statistical or statistical + prosodic cues to word boundaries and a random speech stream. The study found that children with ASD did not show the learning-related changes in basal ganglia and left temporo-parietal cortex seen in typical controls. Additionally, the level of language impairment in the ASD group inversely correlated with signal increases in the same regions during exposure to the artificial languages. These two studies highlight the fact that behavioral output may not truly reflect neural processing, with this disconnect possibly more pronounced in young or lower functioning children with ASD (Scott-Van Zeeland et al., 2010), providing additional motivation for our use of a learning task that does not require an overt behavioral response.
1.3 Visual statistical learning in typical development
While not characterized in ASD, statistical learning in the visual domain has been studied in typically developing infants through habituation paradigms. Kirkham et al. (2002) investigated infants' detection of statistical regularities from sequentially presented visual information. Two-, five-, and eight-month-old infants were exposed to a continuous stream of six colored, looming shapes (e.g., turquoise square, blue cross, yellow circle, pink diamond, green triangle, and red octagon). The shapes were organized into three pairs. In the exposure phase, the shape pairs were presented in random order until the pairs were presumably “learned” as indicated by habituation of looking times. In the test phase, infants were presented with the familiar pairs and a novel (random) sequence presented on alternating trials. The authors found that infants demonstrated a significant novelty preference based on looking time, suggesting that they were sensitive to statistical regularities defining the sequence of shapes. No statistically reliable differences in novelty preference were found between the three age groups, suggesting that visual statistical learning in typical development occurs early (by 2 months of age) and remains robust across early development. To determine just how early visual statistical learning occurs in infancy, Bulf et al. (2011) tested newborn infants in a similar shape-sequence paradigm and found that newborns could detect the statistical structure of four (but not six) shape sequences.
To our knowledge, no published studies have investigated the association between visual statistical learning and social or cognitive function in infants or children. Based on the idea that statistical learning might be input domain specific, visual statistical learning may be more strongly associated with non-verbal abilities, just as auditory statistical learning has been associated with language development. Additionally, many elements of social interaction are governed by unspoken rules that are not explicitly taught. The implicit learning of the rules of social interaction has been proposed to serve as the foundation for social intuition and adaptation to ones social environment (Lieberman, 2000). As a result, visual statistical learning may be associated with social behavior.
In the present study we sought to characterize visual statistical learning in ASD through the development of an event related potential (ERP) paradigm modified from the Kirkham et al. (2002) study described previously. By relying on electrophysiological rather than behavioral responses to stimuli, the use of ERPs provides us with the opportunity to study cognitive domains in lower functioning children and younger children whose behavioral repertoire may be limited or obscured by other factors such as language delay, cognitive impairment or inattention. This methodology also allows us to quantify the neural processing of stimuli, both in the robustness (amplitude of the components) and speed (latency of components) of the response. These variables may allow us to identify differences in neural mechanisms that underlie aberrant behavior in children with ASD. Our sample consisted of preschool-age children on the autism spectrum with a wide range of non-verbal and verbal abilities, and typically developing, age-matched children. In order to better characterize the heterogeneity within our ASD sample, we also analyzed the relations between social and cognitive function and visual statistical learning in both the TD and the ASD group.
Our first hypothesis was that learning could be measured by EEG responses, namely by the differential neural response to stimuli that varied only in their familiarity. In other words, if children did implicitly learn the identity of the shape pairs, the robustness or speed of response to the expected shapes would differ from the response to those objects they did not expect to see. Secondly, we hypothesized that, as a group, young children with ASD would demonstrate an impairment in visual statistical learning, quantified by a diminished differentiation of conditions. However, based on the proposed relation between visual statistical learning and non-verbal ability, we expected to identify heterogeneity in statistical learning within the spectrum that mapped onto non-verbal IQ and social function.
2. Methods
2.1 Participants
Children with ASD were recruited as part of a larger study investigating predictors of treatment outcome in children enrolled in the UCLA Early Childhood Partial Hospitalization Program (ECPHP). ECPHP is an intensive intervention program for children ages 2-6 with ASD. Children are enrolled in the program for a three-month period, with comprehensive standardized clinical assessments performed before treatment initiation. All children admitted to ECPHP enter the program with a prior clinical diagnosis of ASD. Diagnoses are made through the California State Regional Center, independent clinical psychologists, child psychiatrists, or developmental pediatricians. If there are any questions about the accuracy of the diagnosis, the child is reevaluated using diagnostic assessments (Autism Diagnostic Interview-Revised, the Autism Diagnostic Observation Scale, and clinical history). All children in this study were assessed before beginning intervention in order to capture their baseline characteristics and to avoid the confounding effect of treatment on the results.
Age-matched, typically developing (TD) children from the greater Los Angeles area were recruited as controls. Recruitment was accomplished through birth records provided by Los Angeles county. Parents of children with targeted birthdates were first sent a letter of invitation to participate in the experiment. Interested parents returned a postcard and were later contacted by telephone for further screening. Based on the telephone screening questionnaire, children were excluded from the control group if they had a history of any neurological abnormalities, history of birth related complications, developmental delays, need for special services in school, diagnosis of psychiatric conditions such as ADHD, OCD or bipolar disorder, or uncorrected vision impairment.
A total of 68 children with ASD and 35 TD children were investigated with the visual statistical learning paradigm as detailed below.
2.2 Behavioral testing
Children with ASD underwent standardized behavioral testing through ECPHP prior to their entry into the program. Cognitive and language assessments varied based on the ability of the child. Therefore, only standard scores were used to facilitate comparison across assessments. Assessments included the Mullen Scales of Early Learning (MSEL; Mullen, 1995), the Wechsler Preschool and Primary Scale of Intelligence-3 (WPPSI-3; Wechsler, 2002), the Preschool Language Scale-3 (PLS-3; Zimmerman, Steiner, & Pond, 1992), Clinical Evaluation of Language Fundamentals-4 (CELF-4; Semel, Wiig, & Secord, 2003), and the Vineland Adaptive Behavior Scales-II (VABS-II; Sparrow, Cicchetti, & Balla, 2005). All TD children were assessed at the time of their EEG visit, with assessments including the MSEL, the Differential Abilities Scale-II (DAS-II; Elliott, 2007) and the VABS-II. From these measures, standard scores for full scale IQ, non-verbal IQ (NVIQ), verbal IQ (VIQ), expressive language, receptive language, and adaptive social function were calculated for each child and used for analysis.
We were particularly interested in the relations between cognitive subdomains (verbal and non-verbal) and statistical learning in order to determine if there was evidence of domain specificity. For children who were tested with the WPPSI-3 or the DAS-II, a NVIQ score was automatically calculated from the protocol specific subscores. Based on prior literature, for children who were administered the MSEL, a NVIQ was calculated using the average of the Visual Reception (VR) subscale T score and the Fine Motor (FM) subscale T score (Akshoomoff, 2006). This T score was then converted to a standard score. Several studies and articles have supported the convergent validity of the WPPSI-3 with other cognitive assessments, such as the MSEL and the DAS-II, thereby justifying the combination of assessments through standard scores (Bishop, Guthrie, Coffing, & Lord, 2011; Kasari, Freeman, & Paparella, 2006).
2.3 EEG visual statistical learning paradigm (Figure 1)
Figure 1. Statistical learning paradigm.
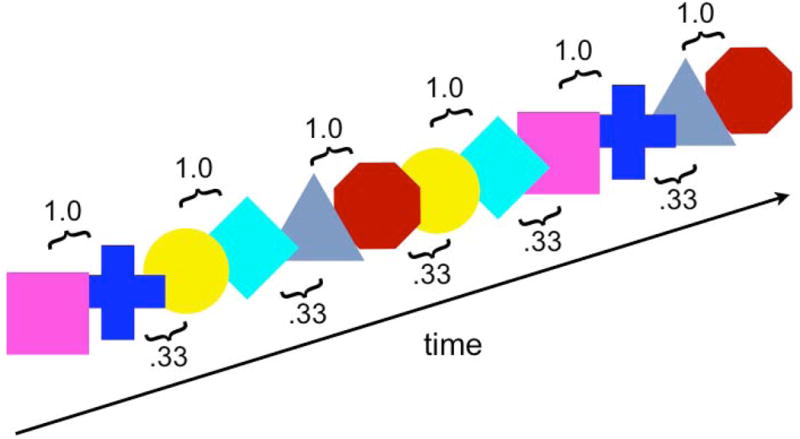
The paradigm consisted of a modified version of the habituation phase of the Kirkham et al. (2002) visual statistical learning task. Six colored shapes (turquoise square, blue cross, yellow circle, pink diamond, green triangle, and red octagon) were presented one at a time in a continuous “stream” in the center of a computer monitor, looming from 3 to 6 cm in height. In the exposure, or learning phase, three pairs of shapes were presented ten times each in random order. Shape pairing was randomized for each child. The initial member of the shape pair always predicted the next member, and the next stimulus pair was constrained to be the initial member of one of the three allowable pairs. There were repetition constraints, with one pair only allowed to repeat twice in a row. The test phase followed the exposure phase. In an oddball paradigm format, 90% of the trials in the test phase consisted of the standard learned shape pairs (“expected” condition), and 10% of trials consisted of a shape 1 followed by an unmatching, or “oddball” shape 2. The oddball condition resulted in only a total of 20 possible trials, which would yield a very low signal to noise ratio and, often, an inadequate number of acceptable trials given the apriori data quality threshold of 10 trials per condition (see methods section for details). Therefore, we used the “transitional probability object” (or pair) as a proxy for the unexpected condition. The transitional probability object was defined as a shape 2 preceding the next pair's shape 1, with this probability of this pair being 0.33. Hence, although placed in the setting of an oddball paradigm, the analysis included an unexpected stimulus that occurred 33% of the time and, therefore, not a true oddball. Each stimulus shape was presented for 500 ms with an interstimulus interval of 500-750 ms. In the exposure phase, 30 shapes were presented (10 random repetitions of each pair), while in the ensuing test phase a minimum of 100 and maximum of 200 stimuli were presented. The number of stimuli presented in the test phase depended on the child's mood and ability to focus on the task.
2.4 EEG procedure
Prior to the testing session, parents of participants were interviewed regarding their child's preferences and interests. The information was used to cater the experimental setting to the child's needs and to make the session as comfortable and enjoyable as possible. For instance, during netting, children were shown a favorite video or allowed to play a specific game. They also were provided with a preferred snack to be eaten prior to the start of the paradigm. In addition, parents were provided with an EEG training net prior to the session to desensitize their children to the net. This training net mimics the tightness and feel of the 128-electrode Geodesic Sensor Net (Electrical Geodesics, Inc., Eugene, OR) used to collect EEG data, with plastic pedestals replacing the actual sensors and sponges.
ERPs were recorded while the child was seated on a child-sized seat approximately 65 cm in front of the monitor, in a sound-attenuated dark room. Stimuli were presented using E-Prime 2.0 software (Psychology Software Tools) on a 24-inch monitor with 1080 pixel resolution. Careful measures were taken to ensure that only trials attended to by the child were included in analysis. During testing, a video of the child was recorded, and off-line coding of visual attention to the screen was performed by an assistant blinded to the child's diagnosis. In order to ensure that all children included in the analysis were adequately exposed to the shape pairs in the learning phase, only children who attended to at least 75% of the exposure phase were included in the analysis. Additionally, during testing, trials in which the child was not watching the screen were marked during recording and rejected during post-processing. Finally, although the paradigm was shortened if the child became fussy or lost interest in the session, a minimum of 100 trials (50 in each condition) were required to include the data in further analysis.
2.5 Processing of EEG data
Continuous EEG was recorded using a 128-channel HydroCel Geodesic Sensor Net with impedances kept below 100 kOhms in all electrodes. Raw EEG data were referenced online to vertex (Cz). The electrical signal was amplified with a 0.1 to 100 Hz band pass, digitized at 250 Hz, and stored on a computer drive before being processed offline using NetStation 4.4.5 software (Electrical Geodesics, Inc.). EEG was digitally filtered using a 0.3 to 50 Hz bandpass filter, segmented into 1000 ms epochs starting at 100 ms before stimulus onset, and baseline corrected using mean voltage during the 100 ms pre-stimulus baseline period.
Data were then processed using an automated artifact detection tool that rejected channels if the amplitude difference (max-min) was greater than 150 mV. The purpose of this automated detection is to remove channels that have grossly noisy data, usually from excessive electrode movement, net manipulation or drift. Following this automatic artifact detection, each trial was visually inspected (SSJ and CK) to remove any remaining channels that contained EMG, eye-blink, or eye-movement artifacts from further analysis. Trials with evidence of eye blinks or saccades during the stimulus presentation were rejected based on the assumption that the child was not looking at the visual stimulus of interest during the eye blink or movement. Additionally, trials with more than 15% bad channels were rejected. Bad channels in the data of trials containing fewer than 15% bad electrodes were replaced using a spherical spline interpolation algorithm (Srinivasan, Nunez, Tucker, Silberstein, & Cadusch, 1996) The data were then averaged for each participant and re-referenced to an average reference.
After artifact rejection, only subjects with more than 10 good trials per condition were accepted for further analysis. Although there was variability across subjects with regards to total number of good trials, the choice of using a minimum threshold of 10 trials per condition is based on extensive prior literature using this convention, with the convention based on the finding that once a signal threshold is crossed, adding more trials does not significantly change the signal to noise ratio (de Haan & Nelson, 1999). However, we did want to confirm that the number of good trials did not significantly differ by condition within each subject. We therefore performed paired samples t-tests comparing the percent good trials in the expected condition to the percent good trials in the unexpected condition. There was no significant difference in the percent good trials between expected (M=49.3, SD=19.5) and unexpected (M=50.2, SD=20.0) conditions [t(68)=-1.32, p=0.193] across subjects.
After artifact detection and rejection, data from 45 children (66%) with ASD and 23 TD children (66%) were considered acceptable for further analysis. Based on the methods described above, the participants whose data were rejected included those with (1) inadequate attention to the exposure phase, (2) insufficient trials presented or watched in the exposure phase, or (3) insufficient number of acceptable trials per condition (<10) due to artifact and noise. The success rate of 66% is higher than that reported in studies of developmental populations (for review see Jeste & Nelson, 2009), and it reflects the rigorous efforts made to prepare children for their EEG visit.
Grand average ERPs were created for the 45 children with ASD and 23 TD children with acceptable data. Components were first chosen based on our hypotheses about waveforms generated from visual learning paradigms, particularly those with variations in level of expectancy of stimuli (such as traditional oddball paradigms) as well as paradigms focused on category recognition. We emphasize that the study was not a typical oddball paradigm because it required a learning or exposure phase and because the analysis centered on the differentiation of expected from transitional probabilities, not the oddball condition. Because of the novelty of this paradigm, both in the cognitive domain and population being studied, we also selected components based on visual inspection of the data in both groups. Based on prior literature, we expected to find a frontal P300, which represents attention to salient information, with the amplitude modulated by the improbability of a target stimulus (Picton, 1992). We also anticipated a frontal Nc, a component most robustly described in infant studies to indicate attention to encoded information (de Haan & Nelson, 1997, 1999; Reynolds & Richards, 2005). Finally, because this was a visual paradigm, we expected to elicit a posterior P1 component, representing early visual processing of information. Upon visual inspection, we also detected a very robust early frontal negativity, occurring between 100-250 msec, which we called the N1. Somewhat in line with the classic N100, which is found in spatial cueing tasks as a marker of early attention to visual stimuli, we interpreted the N1 to signify early category recognition (Coull, 1998). Regions of interest were generated with clusters of electrodes in right, middle and left frontal regions for the N1, P300, and Nc and in right, middle and left posterior electrodes for the P1 (see Figure 2). Time windows for the components were chosen based on prior literature and based on visual inspection of data from both groups, with a window wide enough to include the peaks for each component in every participant. Peak amplitude was calculated for the components in the following windows as follows: N1 (100-250 ms), P300 (190-350 ms), and P1 (90-230 ms). Because of its diffuse and broad morphology, for the Nc, a mean, rather than peak, amplitude was calculated, with a window of 450-750 ms (de Haan & Nelson, 1999). Because we analyzed a mean amplitude for the Nc we did not analyze a latency, as there would be no peak to use for latency measurement. Additionally, difference scores for each variable were calculated using the difference of the unexpected value from the expected value. For instance, N1 amplitude difference = N1 amplitude expected – N1 amplitude unexpected.
Figure 2. Electrode groupings.

3. Results
3.1 Behavioral data
Based on independent samples t-tests, there was no statistically significant difference in the age of participants. Average age was 54.2 months in the TD group (range 29.2 to 75.2 months) and 54.2 months in the ASD group (range 28.8 to 71.6 months) [t(66)=-0.11, p=0.99]. There were statistically significant differences between groups in full scale IQ, NVIQ, VIQ, receptive and expressive language, and adaptive measures of social and communication function, with lower scores in the ASD group across domains. Means, ranges, and standard deviations are presented for all behavioral variables in Table 1. As expected, and of particular interest to our study, there was much more phenotypic heterogeneity in the ASD sample, with wider ranges in each of the cognitive and developmental measures of interest.
Table 1. Behavioral results.
| Measure | TD | ASD | P value |
|---|---|---|---|
| Age (months) | 54.2 (26.6-72.1; SD 11.8) | 54.2 (29.2-75.2; SD 13.1) | 0.99 |
| Receptive Language* | 114.8 (86-151; SD 16.6) | 80.7 (50-127; SD 23.4) | <0.001 |
| Expressive Language* | 117.8 (100-137; SD 10.8) | 80.4 (50-138; SD 24.3) | <0.001 |
| Non-verbal IQ* | 110 (94-149; SD 10.9) | 81.8 (51-125; SD 24.0) | <0.001 |
| Verbal IQ* | 116.3 (96-141; SD 11.9) | 80.6 (50-133; SD 23.5) | <0.001 |
| Full scale IQ* | 117.1 (101-149; SD 11.8) | 77.7 (49-123; SD 24.3) | <0.001 |
| Vineland communication* | 116.4 (100-139; SD 11.6) | 76.4 (40-104; SD 16.1) | <0.001 |
| Vineland social function* | 113.7 (50-138; SD 11.2) | 66.1 (42-100; SD 11.4) | <0.001 |
Standard scores, with means, ranges and standard deviations
3.2 ERP data: ASD vs. TD: whole group analysis
There were no group differences in the proportion of time spent attending to the stimuli, nor in the percent of accepted trials per condition (see figure 3). Both groups attended to the stimuli for more than 75% of the presentation, and both groups generated approximately 50% acceptable trials per condition. The first analysis performed was a three-way ANOVA. Within subjects factors included condition (expected, unexpected) and region (right, middle, left) and between subjects factors included group (ASD, TD). Given the relatively wide age range in our groups, we included age as a covariate in the ANOVA. ERPs by group are shown in figure 4. There was a significant condition effect across groups in the N1 amplitude [f(1,65)=(4.96), p=0.029] and in the Nc amplitude [f(1,65)=(5.60), p=0.021]. On post-hoc analysis, the N1 and the Nc were more negative to the expected condition. There was also a significant region effect across groups in the N1 amplitude, with the mid frontal region (-7.21 mV) showing a significantly lower N1 amplitude than right frontal (-6.71 mV) and left frontal (-6.70 mV). There were no significant condition, group, or region effects in the P300 or P1 components.
Figure 3. Percent accepted trials and percent looking time during task.
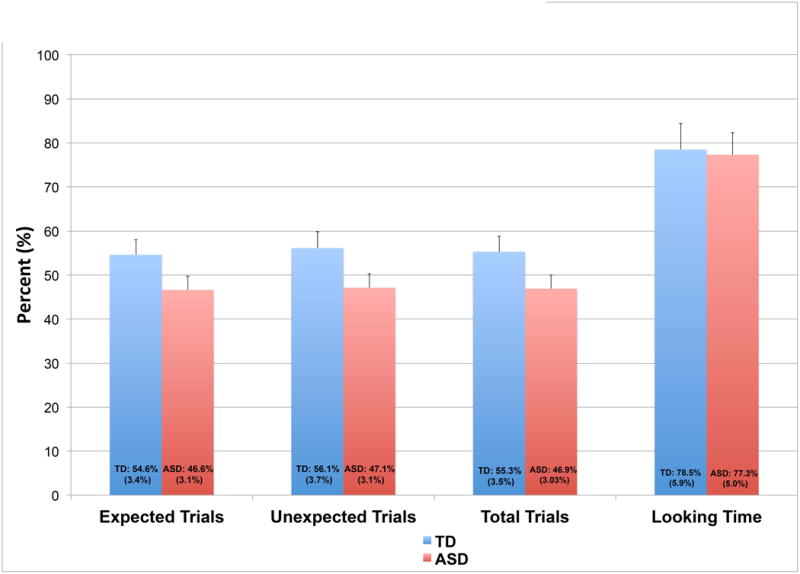
Figure 4. ERP correlates of learning by region and group.
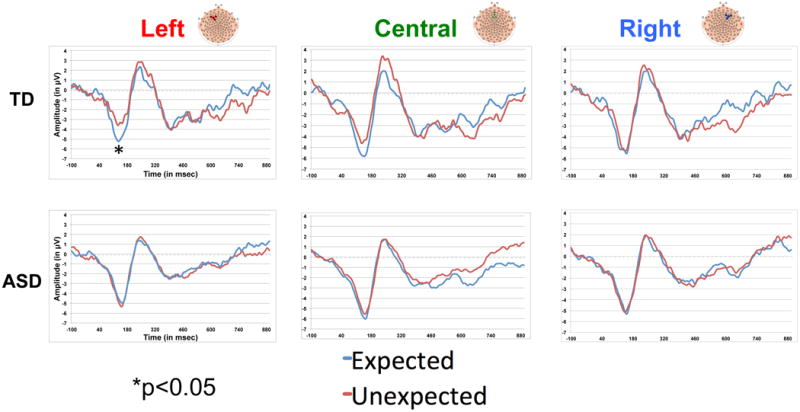
In order to better characterize the differentiation of conditions within each group, we performed paired samples t-tests to compare the N1, P300, P1 peak amplitude and latency, and the Nc mean amplitude, by condition. In the TD group, learning was evidenced by the following: (1) a significant condition difference in the left N1 amplitude (TD: Expected: -6.54 mV, Unexpected: -5.36 mV; p=0.043) and a (2) trend towards a difference in the P300 amplitude across regions (TD: Expected: 4.18 mV, Unexpected: 4.94 mV; p=0.09). The ASD group, as a whole, showed no evidence of differentiation of conditions based on the N1, P1, P300, or Nc components. (For comparison to the TD group, ASD means were as follows: Left N1 amplitude: Expected: -7.07 mV, Unexpected: -7.10 mV; p=0.95; Across regions P300 amplitude: Expected: -3.73 mV, Unexpected: 3.78 mV; p=0.91).
ASD vs. TD: age effects
Given the fact that condition differences in the N1 and Nc were found across groups when age was controlled, we explored the correlation between age and N1 and Nc difference scores. The goal was to investigate whether there were trends in learning based on age of the participants, given the relatively wide age range of the sample. We collapsed across groups because the groups were age matched. There was a significant negative correlation between age and N1 amplitude difference across regions (R=-0.238, p=0.015) and Nc amplitude difference across regions (R=-0.289, p=0.017), suggesting that younger children showed an overall more robust learning based on differentiation of conditions.
ASD vs. TD: looking time effects
There were no differences between groups in attention to the task, as quantified by time spent looking at the screen (see Figure 3; ASD: M=78.5%; TD: M=77.3%). Looking time did not correlate with learning, based on ERP difference scores for the N1, P300, P1 peak amplitude or latency. However, there was a positive correlation between Nc mean amplitude difference and looking time (R=0.33, p=0.021), suggesting a larger Nc to expected compared to unexpected in those with longer looking times. There was no significant correlation between age and looking time.
3.3 ERP data: Subgroup analyses within ASD
Covarying by NVIQ, VIQ, socialization
By performing the full group analysis, we established not only the feasibility of the paradigm, but we also showed that TD children do differentiate the conditions, both in early category recognition and in later allocation of attention to different categories of stimuli. We also showed that age may play an important role, with younger children showing more robust differentiation of objects despite similar looking times. One of our overarching goals was to better determine whether subtle differences in learning could be identified within the ASD group. We hypothesized that cognitive and adaptive function would correspond with and possibly inform different patterns of statistical learning. Given the literature in visual statistical learning in infancy and the proposition that it may be domain specific, we were most interested in the relationship between visual statistical learning and non-verbal cognitive function as compared with the association with language. We repeated the 3-way ANOVA controlling for (1) NVIQ, (2) adaptive social function and then (3) VIQ. Thus, a total of three additional analyses were performed. With NVIQ as a covariate, there was a significant group by condition interaction for the left N1 amplitude (f(1,64)=4.40, p=0.040). With socialization as a covariate, there was a significant group by condition interaction for the left P300 amplitude (f(1,48)=8.04, p=0.007). There was no group × condition effect with VIQ as a covariate.
NVIQ and N1 amplitude
To further understand the role of NVIQ in learning, Pearson correlations were estimated using NVIQ and N1 amplitude difference. As expected, due to lack of phenotypic variability, no significant correlation was found between N1 amplitude difference and NVIQ in the TD group. However, in the ASD group there was a positive correlation approaching significance between NVIQ and N1 amplitude difference (r=0.283, p=0.06; See figure 5). In an effort to characterize functional differences based on NVIQ, we then split the ASD sample by the mean NVIQ of the participants (NVIQ=82), and conducted an independent samples t-test by group. We used the mean NVIQ as our split because it best captured the range of IQ scores in our data while staying true to the common cutoffs used in the distinction of “low” and “high” functioning children with ASD (75 or 80). There was a significant difference in N1 amplitude difference score between high NVIQ ASD and low NVIQ ASD (t(1,43)=-2.4, p=0.02), as well as between high NVIQ ASD and TD (t(1,37)=-2.4, p=0.02). The high NVIQ ASD group showed a distinctive pattern of learning, with a larger N1 to the unexpected condition. Of note, children with the maximum and minimum N1 amplitude differences had relatively low numbers of good trials (less than 25). Given the wide range in number of good trials in our data, we ran additional sensitivity analysis to adjust for possible heteroscedasticity due to good number of trials (Stahl, Parise, Hoehl, & Striano, 2010). Findings matched results from t-tests by group, where separate variance components are estimated for low, medium and high good number of trials in a mixed effects model (See Figure 6).
Figure 5. NVIQ and N1 amplitude difference correlation (ASD).

Figure 6. N1 amplitude difference by NVIQ grouping.

Adaptive social function and P300 amplitude
Pearson correlations were estimated using adaptive social scores and P300 amplitude difference. Again, no correlation was found in the TD group, but in the ASD group there was a significant positive correlation (r=0.388, p=0.012; see Figure 7). To further explore this finding, we divided the ASD group by the mean socialization score (social = 66) and independent samples t-tests were performed with P300 amplitude difference as the variable of interest. There was a significant difference in P300 amplitude difference between “high social” ASD and “low social” ASD [t(1,40)=-2.38, p=0.022], as well as between “high social” ASD and TD [t(1,26)=-2.67, p=0.015]. In a similar pattern to that seen with the N1 amplitude difference, the “high social” ASD group showed a distinctive pattern of learning with a larger P300 to the expected condition. Sensitivity analysis adjusting for good number of trials, as performed above, revealed consistent results (See Figure 8).
Figure 7. Socialization and P300 amplitude difference correlation.
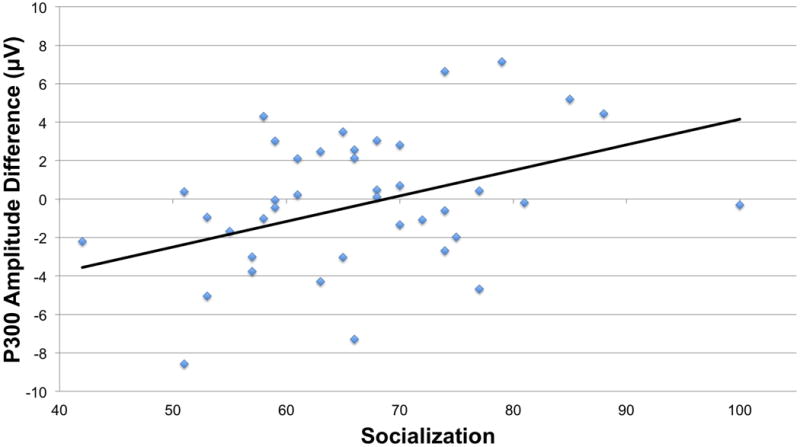
Figure 8. P300 amplitude difference by socialization grouping.

4. Discussion
We investigated the electrophysiological correlates of visual statistical learning in a young, heterogeneous population of children with ASD, compared to age-matched typically developing children. This is the first study to characterize visual statistical learning in children using electrophysiology as a means to identify subtle differences in the processing of visual patterns that may not be captured by behavior. This study is multifaceted in that it first establishes an electrophysiological correlate of visual statistical learning, and it then investigates this domain in children with ASD, with particular attention to the relation between visual statistical learning and non-verbal cognition and social behavior. Our results show the following: (1) In this passive viewing paradigm, visual pattern learning can occur with the presentation of repeated shape pairs, with more robust learning found in younger ages and in typically developing children; (2) Within the ASD group, there does exist heterogeneity in visual statistical learning; (3) Upon further exploration of this heterogeneity, we found evidence of an association between visual statistical learning and both non-verbal IQ and social function in children with ASD, not found in verbal IQ, that may represent an adaptive cognitive mechanism; and (4) Children with higher non-verbal cognitive abilities and social function demonstrated a pattern of statistical learning that was distinct from the pattern seen in typically developing children, begging the question of whether a behavioral correlate of learning would have, in fact, shown “intact” learning despite distinctive EEG profiles.
We defined learning as a differential neural response to the expected and unexpected conditions in the test phase. In other words, we hypothesized that if the participants learned the three shape pairs in the exposure phase, they would show a differential response between the transitional probability within a matching pair (“expected” probability 1.0) and the transitional probability between pairs (“unexpected” probability 0.33). We focused on the following components: early recognition of stimuli (frontal N1) and attention to stimuli based on level of expectation (P300 and Nc). Here, the term N1 was used to describe the early frontal negativity robustly produced through this statistical learning paradigm, and we suggest that it represents early visual discrimination of categories of stimuli. While somewhat comparable, it is not the equivalent of the traditional visual N100, an occipital component elicited in studies of visual-spatial selective attention, in which stimuli that are attended to elicit a larger (more negative) occipital N100 than unattended stimuli (Vogel & Luck, 2000). The P300 is traditionally assessed using a classic oddball paradigm, with a larger P300 elicited by events representing a low-probability category, even in the absence of explicit instructions to categorize the conditions (McCarthy & Donchin, 1981). We also anticipated a frontal Nc, a late negativity that represents attention to encoded information (de Haan & Nelson, 1997, 1999; Reynolds & Richards, 2005).
The component most representative of learning in this task was the early negativity, the N1. In the entire sample, we found a larger (more negative) response to the expected condition, suggesting that overall, children allocated more attention to the stimulus that was anticipated. This electrophysiological correlate of this learning was inversely associated with age across groups, as young children demonstrated greater N1 and Nc amplitude differences. Notably, this age effect was not modulated by overt attention to the task, as there were no correlations between age and looking time in this paradigm. Although not captured by looking time, it is still possible that the paradigm was not challenging or interesting enough for our older subjects and, therefore, while learning occurred, the ERP correlates of attention attenuated over the course of the paradigm. In order to better define changes in learning over development, two future studies will include the investigation of this domain in younger ages as well as the modification of the pattern towards increasing complexity. Ideally, analysis of individual trial data over the course of the exposure and test phase could be used to demonstrate the timing of maximum learning, or condition differentiation. However, due to the limited number of trials available in data, such analysis was unable to be performed reliably in this sample.
To our knowledge, this study provides the first demonstration of visual statistical learning in typically developing children. Our findings imply that visual statistical learning of sequential information may be continuous across development, having been observed in newborn and older infants (Bulf et al., 2011; Kirkham et al., 2002), and adults (Fiser & Aslin, 2002a; Turke-Browne, Isola, Scholl, & Treat, 2008). In addition, our findings provide an important complement to the literature on auditory statistical learning, which confirms developmental continuity from infancy (Saffran et al., 1996), through childhood (Arciuli & Simpson, 2011; Evans, Saffran, & Robe-Torres, 2009; Saffran, Newport, & Aslin, 1997), and into adulthood (Saffran, 2002; Saffran et al. 1997). Our findings also suggest that our electrophysiological methods may serve as a vital tool for examining visual statistical learning. In particular, this methodology holds particular promise in the investigation of developmental continuity because our methods do not require a behavioral response, and (presumably) can be used with participants at any age. To be clear, however, current evidence regarding developmental continuity of visual statistical learning must be considered tentative. Unlike experiments that examined auditory statistical learning, many of which required participants to endorse (e.g., with looking times or verbally) trained vs. novel strings of items, our ERP methods do not require any behavioral response, and the differences in ERPs that are revealed may stem from a distinct set of learning processes. It is notable as well that learning visual sequences is quite difficult for infants. For example, recent developmental studies with typically developing participants revealed limits in young infants' identification of transitional probabilities (Slone & Johnson, 2013) and rule-governed patterns (Johnson et al., 2009) in visual sequences, two cognitive skills that are more readily revealed in infants when auditory sequences are used as stimuli (Aslin, Saffran, & Newport, 1998; Marcus, Fernandes, & Johnson, 2007).
We found evidence of domain specificity of statistical learning in our children with ASD. Just as the statistical learning of language has been associated with language ability in children with ASD, we found a significant association between visual statistical learning and non-verbal cognitive abilities in our ASD group. The two non-verbal abilities that were measured included non-verbal IQ based on standardized IQ assessments as well as the adaptive social measure of the VABS-II. While social behavior cannot be entirely disentangled from language, the items on this subdomain of the VABS-II focus largely on non-verbal communication, such as imitation, physical affection, smiling, gestures towards others, and play. The lack of any correlations in the TD group most likely results from the lack of heterogeneity in IQ or social function, and a follow-up study will include children with cognitive and developmental delays.
Particularly intriguing in these findings is the directionality of differentiation found in the “high NVIQ” ASD group, as they exhibited the opposite neural response to that seen in TD children, with the lower functioning children with ASD demonstrating a minimal differentiation of the conditions. Two important points emerge from this finding. First, it again emphasizes the importance of investigating individual differences and variability within the autism spectrum. Averaged together, the ERP findings of the two groups (low and high functioning in each domain) essentially “cancel” each other out and lead to the erroneous conclusion that children with ASD show no neural evidence of visual statistical learning. Secondly, it leads us to ask whether these higher functioning children are using an alternative mechanism to distinguish distinct categories, thus representing a cognitive compensation or strength that facilitates strong non-verbal ability and adaptive social function. In our sample, the children with high NVIQ and ASD demonstrated a larger (more negative) response to the unexpected condition, as quantified by the N1 component difference, suggesting greater allocation of attention to unexpected events. Perhaps in ASD, a strength in category recognition and heightened vigilance to novel events facilitates pattern learning which, in turn, leads to better performance on the items tested in standardized assessments of non-verbal ability. It is also possible that children with inherently higher non-verbal cognitive abilities are simply able to learn patterns more easily. Whether statistical learning ability precedes non-verbal IQ or vice versa can only be determined in prospective studies of development, work that we are currently pursuing. Future studies will need to more closely investigate individual differences within this high NVIQ group and to investigate whether certain children with high learning respond more effectively to interventions using more patterned-based learning approaches.
In the socialization subgroup analysis, the high-social ASD group also showed a distinctive EEG pattern, with greater attention allocated to the expected condition as measured by P300 amplitude. Given the nature of the P300, one might expect to see a larger response to the unexpected condition, as the novel stimulus elicits greater vigilance, or attention. This “reverse” pattern may, in fact, be adaptive. It is possible that in children with ASD, where there is a global deficit in social cognition, increased vigilance to the familiar elements of ones environment actually facilitates or improves social behavior. Such a phenomenon may be particularly evident in children who are receiving interventions, such as discrete trial therapy, that reinforce learning through repetition and patterns. Whether this heightened attention to the familiar, or expected, is truly adaptive or compensatory will be investigated in younger children with ASD, prior to intervention.
Both the TD group and the low-social ASD group showed a pattern of greater attention to the unexpected condition, and both exhibited less differentiation of conditions than the high-social ASD group. As discussed earlier, it is possible that the simple patterns presented and the subsequent test phase of abstract shapes were not engaging or challenging enough for the typically developing children and, therefore, despite similar overt attention to the screen, their attention as quantified by their EEG was diminished when compared to the children with ASD. Several studies have shown that statistical learning, while automatic, can be modulated by attention, with better learning demonstrated in attended versus unattended input (Emberson, Conway, & Christiansen, 2011; Toro, Sinnett, & Soto-Faraco, 2005; Turk-Browne, Junge, & Scholl, 2005). Thus, a more complicated pattern may have yielded a larger learning response in the TD group. It is also possible that there is inherent heterogeneity in the TD group that is not captured by the parent report of social behaviors on the VABS-II, and that this heterogeneity affects the group average of their learning patterns. Overall, we would emphasize the fact that children with ASD and higher NVIQ and adaptive social abilities exhibit a distinctive pattern in the differentiation of objects based on expectancy, either in early processing of visual categories or attention to an expected event, that highlights subtle variations in cognitive ability that may translate to quantifiable phenotypic differences.
A broader, perhaps more philosophical, question that also must be raised is whether we truly define learning through these electrophysiological measures. In other words, does the differentiation of conditions in fact reflect learning, or is this phenomenon simply a marker of differences in attention or perception? We would contend that as learning is defined as an adaption to an experience, the differentiation of these shapes does reflect learning. The shapes are distinct only in their level of expectedness, as they are matched on visual qualities such as size, luminance, and overall salience. Perhaps another approach to address this question would be through the quantification of a behavioral output that demonstrates learning. For instance, participants could be asked to verbally or visually identify the shape pairs after the presentation of the pattern. One might ask, however, whether such a task would test their explicit awareness of the patterns, not their implicit learning of the sequences. Another strategy, employed in infant studies, would be to quantify looking time to discrete stimuli of novel or expected shape pairs. Unfortunately, such looking time analysis is less reliable in toddlers and preschoolers whose attention is more challenging to contain. Nevertheless, future research may benefit from a modification of the study to include a behavioral correlate. A final approach, which will be the focus of future investigation, would be to identify changes in the EEG pattern, through spectral analysis or individual trial ERPs, over the course of the experiment that could quantify the process of learning. There may be individual differences in speed of learning that inform the overall EEG patterns in each condition. A longer exposure phase with a larger sample of clean trials will be required for such a study. However, we would contend that this current study and its findings raise important questions to consider for future studies using electrophysiology to truly characterize the process of learning in developmental populations, both typical and atypical.
Several limitations in this study have laid the foundation for continued studies of this domain. First, the novelty of this EEG paradigm and lack of normative data for these electrophysiological measures require replication, which is ongoing. Secondly, given our hypotheses about non-verbal cognitive ability and overall domain specificity of statistical learning, an IQ-matched control group will better address the question of whether differences in learning are truly specific to ASD or are more reflective of overall cognitive function, particularly in the non-verbal domain. Next, while the VABS-II represents a well-validated measure of social function, it is a parent report and, therefore, can be susceptible to reporting bias. An analysis of the association between statistical learning and scores on a clinician-administered measures of social function, such as the Autism Diagnostic Observation Schedule (ADOS), will be informative moving forward.
Research in autism, both in behavior and biomarkers, has been increasingly focused on capturing individual and subgroup differences within the spectrum, recognizing the tremendous clinical variability in presentation and outcome in children with ASD (Lenroot & Yeung, 2013). We investigated the heterogeneity within our ASD sample by taking a dimensional approach, linking EEG markers with clinical measures. Based on these results, an ongoing study is investigating the association between statistical learning and response to specific behavioral interventions in children with ASD, with particular focus on interventions centered on visual pattern learning.
Through this work we have demonstrated electrophysiological evidence of heterogeneity in statistical learning in ASD, heterogeneity that maps onto non-verbal cognition and adaptive social function. Continued work in this domain will focus on understanding individual differences in learning within ASD, with the ultimate goal of designing tailored interventions that target specific learning profiles.
References
- Akshoomoff N. Use of the Mullen Scales of Early Learning for the assessment of young children with Autism Spectrum Disorders. Child Neuropsychol. 2006;12(4-5):269–277. doi: 10.1080/09297040500473714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association & American Psychiatric Association. Diagnostic and statistical manual of mental disorders : DSM-5. 5th. Washington, DC: American Psychiatric Association; 2013. Task Force on DSM-V. [Google Scholar]
- Arciuli J, Simpson IC. Statistical learning in typically developing children: The role of age and speed of stimulus presentation. Developmental Science. 2011;14:464–473. doi: 10.1111/j.1467-7687.2009.00937.x. [DOI] [PubMed] [Google Scholar]
- Aslin RN, Saffran JR, Newport EL. Computation of conditional probability statistics by 8-month-old infants. Psychological Science. 1998;9:321–324. [Google Scholar]
- Barnes KA, Howard JH, Jr, Howard DV, Gilotty L, Kenworthy L, Gaillard WD, et al. Intact implicit learning of spatial context and temporal sequences in childhood autism spectrum disorder. Neuropsychology. 2008;22(5):563–570. doi: 10.1037/0894-4105.22.5.563. [DOI] [PubMed] [Google Scholar]
- Bishop SL, Guthrie W, Coffing M, Lord C. Convergent validity of the Mullen Scales of Early Learning and the differential ability scales in children with autism spectrum disorders. Am J Intellect Dev Disabil. 2011;116(5):331–343. doi: 10.1352/1944-7558-116.5.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J, Aczel B, Jimenez L, Kaufman SB, Grant KP. Intact implicit learning in autism spectrum conditions. Q J Exp Psychol (Hove) 2010;63(9):1789–1812. doi: 10.1080/17470210903536910. [DOI] [PubMed] [Google Scholar]
- Bulf H, Johnson SP, Valenza E. Visual statistical learning in the newborn infant. Cognition. 2011;121(1):127–132. doi: 10.1016/j.cognition.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Cleeremans A. Consciousness: the radical plasticity thesis. Prog Brain Res. 2008;168:19–33. doi: 10.1016/S0079-6123(07)68003-0. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Squire LR. Preserved learning and retention of pattern-analyzing skill in amnesia: dissociation of knowing how and knowing that. Science. 1980;210(4466):207–210. doi: 10.1126/science.7414331. [DOI] [PubMed] [Google Scholar]
- Coull JT. Neural correlates of attention and arousal: insights from electrophysiology, functional neuroimaging and psychopharmacology. Prog Neurobiol. 1998;55(4):343–361. doi: 10.1016/s0301-0082(98)00011-2. [DOI] [PubMed] [Google Scholar]
- de Haan M, Nelson CA. Recognition of the mother's face by six-month-old infants: a neurobehavioral study. Child Dev. 1997;68(2):187–210. [PubMed] [Google Scholar]
- de Haan M, Nelson CA. Brain activity differentiates face and object processing in 6-month-old infants. Dev Psychol. 1999;35(4):1113–1121. doi: 10.1037//0012-1649.35.4.1113. [DOI] [PubMed] [Google Scholar]
- Elliott CD. Differential ability scales. 2nd. San Antonio, TX: Harcourt Assessment; 2007. [Google Scholar]
- Emberson LL, Conway CM, Christiansen MH. Timing is everything: changes in presentation rate have opposite effects on auditory and visual implicit statistical learning. Q J Exp Psychol (Hove) 2011;64(5):1021–1040. doi: 10.1080/17470218.2010.538972. [DOI] [PubMed] [Google Scholar]
- Evans JL, Saffran JR, Robe-Torres K. Statstical learning in children with specific language impairment. Journal of Speech, Language, and Hearing Research. 2009;52:321–335. doi: 10.1044/1092-4388(2009/07-0189). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiser J, Aslin RN. Unsupervised statistical learning of higher-order spatial structures from visual scenes. Psychol Sci. 2001;12(6):499–504. doi: 10.1111/1467-9280.00392. [DOI] [PubMed] [Google Scholar]
- Fiser J, Aslin RN. Statistical learning of higher-order temporal structure from visual shape sequences. J Exp Psychol Learn Mem Cogn. 2002a;28(3):458–467. doi: 10.1037//0278-7393.28.3.458. [DOI] [PubMed] [Google Scholar]
- Fiser J, Aslin RN. Statistical learning of new visual feature combinations by infants. Proc Natl Acad Sci U S A. 2002b;99(24):15822–15826. doi: 10.1073/pnas.232472899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidley-Larson JC, Mostofsky SH. Evidence that the pattern of visuomotor sequence learning is altered in children with autism. Autism Res. 2008;1(6):341–353. doi: 10.1002/aur.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon B, Stark S. Procedural Learning of a Visual Sequence in Individuals with Autism. Focus on Autism and Other Developmental Disabilities. 2007;22(14-22) [Google Scholar]
- Howard DV, Howard JH, Jr, Japikse K, DiYanni C, Thompson A, Somberg R. Implicit sequence learning: effects of level of structure, adult age, and extended practice. Psychol Aging. 2004;19(1):79–92. doi: 10.1037/0882-7974.19.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeste SS, Nelson CA., 3rd Event related potentials in the understanding of autism spectrum disorders: an analytical review. J Autism Dev Disord. 2009;39(3):495–510. doi: 10.1007/s10803-008-0652-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SP, Fernandes KJ, Frank MC, Kirkham NZ, Marcus GF, Rabagliati H, Slemmer JA. Abstract rule learning for visual sequences in 8- and 11-month-olds. Infancy. 2009;14:2–18. doi: 10.1080/15250000802569611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasari C, Freeman S, Paparella T. Joint attention and symbolic play in young children with autism: a randomized controlled intervention study. J Child Psychol Psychiatry. 2006;47(6):611–620. doi: 10.1111/j.1469-7610.2005.01567.x. [DOI] [PubMed] [Google Scholar]
- Kirkham NZ, Slemmer JA, Johnson SP. Visual statistical learning in infancy: Evidence for a domain general learning mechanism. Cognition. 2002;83:B35–B42. doi: 10.1016/s0010-0277(02)00004-5. [DOI] [PubMed] [Google Scholar]
- Kirkham NZ, Slemmer JA, Richardson DC, Johnson SP. Location, location, location: development of spatiotemporal sequence learning in infancy. Child Dev. 2007;78(5):1559–1571. doi: 10.1111/j.1467-8624.2007.01083.x. [DOI] [PubMed] [Google Scholar]
- Klinger LG, Dawson G. Prototype formation in autism. Dev Psychopathol. 2001;13(1):111–124. doi: 10.1017/s0954579401001080. [DOI] [PubMed] [Google Scholar]
- Komatsu S, Naito M, Fuke T. Age-Related and Intelligence-Related DIfferences in Implicit Memory: Effects of Generation on a Word-Fragment Completion Test. Journal of Experimental Child Psychology. 1996;62(2):151–172. doi: 10.1006/jecp.1996.0026. [DOI] [PubMed] [Google Scholar]
- Lieberman MD. Intuition: A social cognitive neuroscience approach. Psychol Bull. 2000;126(1):109–37. doi: 10.1037/0033-2909.126.1.109. [DOI] [PubMed] [Google Scholar]
- Marcus GF, Fernandes KJ, Johnson SP. Infant rule learning facilitated by speech. Psychological Science. 2007;18:387–391. doi: 10.1111/j.1467-9280.2007.01910.x. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Donchin E. A metric for thought: a comparison of P300 latency and reaction time. Science. 1981;211(4477):77–80. doi: 10.1126/science.7444452. [DOI] [PubMed] [Google Scholar]
- Mitchell DB. Implicit and explicit memory for pictures: Multiple views across the lifespan. In: G P, Masson MEJ, editors. Implicit memory: New directions in cognition, development and neuropsychology. Hillsdale, NJ: Erlbaum; 1993. pp. 171–190. [Google Scholar]
- Molesworth CJ, Bowler DM, Hampton JA. The prototype effect in recognition memory: intact in autism? J Child Psychol Psychiatry. 2005;46(6):661–672. doi: 10.1111/j.1469-7610.2004.00383.x. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Goldberg MC, Landa RJ, Denckla MB. Evidence for a deficit in procedural learning in children and adolescents with autism: implications for cerebellar contribution. J Int Neuropsychol Soc. 2000;6(7):752–759. doi: 10.1017/s1355617700677020. [DOI] [PubMed] [Google Scholar]
- Mullen EM. Mullen Scales of Early Learning: AGS Edition. Circle Pines, MN: American Guidance Service; 1995. [Google Scholar]
- Pelucchi B, Hay JF, Saffran JR. Statistical learning in a natural language by 8-month-old infants. Child Dev. 2009;80(3):674–685. doi: 10.1111/j.1467-8624.2009.01290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrig P, Perrig WJ. Implicit and explicit memory in mentally retarded, learning disabled, and normal children. Swiss Journal of Psychology. 1995;54:77–86. [Google Scholar]
- Perruchet P, Pacton S. Implicit learning and statistical learning: one phenomenon, two approaches. Trends Cogn Sci. 2006;10(5):233–238. doi: 10.1016/j.tics.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Picton TW. The P300 wave of the human event-related potential. J Clin Neurophysiol. 1992;9(4):456–479. doi: 10.1097/00004691-199210000-00002. [DOI] [PubMed] [Google Scholar]
- Reber PJ, Squire LR. Parallel brain systems for learning with and without awareness. Learn Mem. 1994;1(4):217–229. [PubMed] [Google Scholar]
- Reynolds GD, Richards JE. Familiarization, attention, and recognition memory in infancy: an event-related potential and cortical source localization study. Dev Psychol. 2005;41(4):598–615. doi: 10.1037/0012-1649.41.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romberg AR, Saffran JR. Statistical learning and language acquisition. Wiley Interdiscip Rev Cogn Sci. 2010;1(6):906–914. doi: 10.1002/wcs.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseberry S, Richie R, Hirsh-Pasek K, Golinkoff RM, Shipley TF. Babies catch a break: 7- to 9-month-olds track statistical probabilities in continuous dynamic events. Psychol Sci. 2011;22(11):1422–1424. doi: 10.1177/0956797611422074. [DOI] [PubMed] [Google Scholar]
- Saffran JR. Constraints on statistical language learning. Journal of Memory and Language. 2002;47:172–196. [Google Scholar]
- Saffran JR, Aslin RN, Newport EL. Statistical learning by 8-month-old infants. Science. 1996;274(5294):1926–1928. doi: 10.1126/science.274.5294.1926. [DOI] [PubMed] [Google Scholar]
- Saffran JR, Johnson EK, Aslin RN, Newport EL. Statistical learning of tone sequences by human infants and adults. Cognition. 1999;70(1):27–52. doi: 10.1016/s0010-0277(98)00075-4. [DOI] [PubMed] [Google Scholar]
- Saffran JR, Newport EL, Aslin RN. Incidental language learning: Listening (and learning) out of the corner of your ear. Psychological Science. 1997;8:101–105. [Google Scholar]
- Scott-Van Zeeland AA, McNealy K, Wang AT, Sigman M, Bookheimer SY, Dapretto M. No neural evidence of statistical learning during exposure to artificial languages in children with autism spectrum disorders. Biol Psychiatry. 2010;68(4):345–351. doi: 10.1016/j.biopsych.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semel E, Wiig EH, Secord WA. Clinical evaluation of language fundamentals. 4th. Toronto, Canada: Psychological Corporation; 2003. [Google Scholar]
- Slone LK, Johnson SP. Statistical learning in visual sequences: Infants' sensitivity to conditional probabilities. 2013 Manuscript submitted for publication. [Google Scholar]
- Sparrow SS, Cicchetti DV, Balla DA. Vineland adaptive behavior scales. 2nd. Livonia, MN: Pearson Assessments; 2005. [Google Scholar]
- Srinivasan R, Nunez PL, Tucker DM, Silberstein RB, Cadusch PJ. Spatial sampling and filtering of EEG with spline laplacians to estimate cortical potentials. Brain Topogr. 1996;8(4):355–366. doi: 10.1007/BF01186911. [DOI] [PubMed] [Google Scholar]
- Stahl D, Parise E, Hoehl S, Striano T. Eye contact and emotional face processing in 6-month-old infants: advanced statistical methods applied to event-related potentials. Brain Dev. 2010;32(4):305–317. doi: 10.1016/j.braindev.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Toro JM, Sinnett S, Soto-Faraco S. Speech segmentation by statistical learning depends on attention. Cognition. 2005;97(2):B25–34. doi: 10.1016/j.cognition.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Travers BG, Klinger MR, Mussey JL, Klinger LG. Motor-linked implicit learning in persons with autism spectrum disorders. Autism Res. 2010;3(2):68–77. doi: 10.1002/aur.123. [DOI] [PubMed] [Google Scholar]
- Turk-Browne NB, Junge J, Scholl BJ. The automaticity of visual statistical learning. J Exp Psychol Gen. 2005;134(4):552–564. doi: 10.1037/0096-3445.134.4.552. [DOI] [PubMed] [Google Scholar]
- Turk-Brown NB, Isola PJ, Scholl BJ, Treat TA. Multidimensional visual statistical learning. Journal of Experimental Psychology: Learning, Memory, & Cognition. 2008;34:399–407. doi: 10.1037/0278-7393.34.2.399. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Luck SJ. The visual N1 component as an index of a discrimination process. Psychophysiology. 2000;37(2):190–203. [PubMed] [Google Scholar]
- Wechsler D. Wechsler Preschool and Primary Scale of Intelligence. 3rd. San Antonio, TX: Psychological Corporation; 2002. [Google Scholar]
- Wu R, Gopnik A, Richardson DC, Kirkham NZ. Infants learn about objects from statistics and people. Dev Psychol. 2011;47(5):1220–1229. doi: 10.1037/a0024023. [DOI] [PubMed] [Google Scholar]
- Wyatt BS, Conners FA. Implicit and explicit memory in individuals with mental retardation. Am J Ment Retard. 1998;102(5):511–526. doi: 10.1352/0895-8017(1998)102<0511:iaemii>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Zimmerman IL, Steiner VG, Pond RE. Preschool Language Scale–3. San Antonio, TX: 1992. [Google Scholar]


