Abstract
At every point in the lifespan, the brain balances malleable processes representing neural plasticity that promote change with homeostatic processes that promote stability. Whether a child develops typically or with brain injury, his or her neural and behavioral outcome is constructed through transactions between plastic and homeostatic processes and the environment. In clinical research with children in whom the developing brain has been malformed or injured, behavioral outcomes provide an index of the result of plasticity, homeostasis, and environmental transactions. When should we assess outcome in relation to age at brain insult, time since brain insult, and age of the child at testing? What should we measure? Functions involving reacting to the past and predicting the future, as well as social-affective skills, are important. How should we assess outcome? Information from performance variability, direct measures and informants, overt and covert measures, and laboratory and ecological measures should be considered. In whom are we assessing outcome? Assessment should be cognizant of individual differences in gene, socio-economic status (SES), parenting, nutrition, and interpersonal supports, which are moderators that interact with other factors influencing functional outcome.
Keywords: Plasticity, Neuropsychological Assessment, Neurodevelopmental Disorder, Childhood Acquired Brain Injury
Introduction
At every point in the lifespan, the brain balances malleable processes representing neural plasticity that promote change with homeostatic processes that promote stability (Dennis, Spiegler, et al., 2013). Whether a child develops typically or with brain injury, behavior is actively and progressively constructed through transactions between plastic and homeostatic processes and the environment (Piaget, 1954; Johnson, 1997).
In children in whom the developing brain has been malformed or injured, behavioral outcomes provide an index of the result of plasticity, homeostasis, and environmental transactions. At a theoretical level, outcome in these children represents complex processes. Childhood brain insult occurs in the context of ongoing neural and behavioral development; however, the mechanisms that propel developmental change also increase vulnerability of the immature brain to adverse outcome. The effects of childhood brain insult are seen in the entire trajectory of development, not only from single time points. Children have a wide range of possible outcome trajectories that affect not only skill deficits, but also delayed effects and developmental arrests (Dennis, 1988), and degeneration, all of which can vary with different skills.
At a practical level, assessment of outcome in children with brain insult involves four issues:
When should we assess outcome in relation to age at brain insult, time since brain insult, and the current age of the child?
What should we measure to assess outcome in children with brain injury? As well as global outcome measures such as IQ and content domains involving language, space, and number, functions involving reacting to the past and predicting the future, as well as social-affective skills, are important.
How should we assess outcome in children with brain injury? Information from performance variability, direct measures and parent or teacher reports, overt and covert measures, laboratory and ecological and social measures need to be considered.
In whom do we assess outcome? Individual differences in risk and resilience constitute moderators that interact with other factors to affect outcome. Individual differences in genetic factors, socio-economic status (SES), family conflicts and interpersonal supports, parenting, and nutrition are all important influences on functional outcome.
Modeling Age and Development
Age is a factor in the expression of plasticity and homeostasis, even though the young age plasticity privilege (the belief that the younger the age and/or immaturity of the organism, the greater the brain plasticity) has been overstated (Dennis, Spiegler, et al., 2013). Understanding the role of age in outcome requires models relating age and function, where trajectory includes both intercepts (age markers such as age at insult and age at outcome assessment, time intervals such as time since insult), and slopes (e.g., the angle of the curve relating outcome performance and development). Similar endpoints may have different origins and trajectories and developmental change may be non-linear. Although polynomial modeling may be one alternative to the common assumption of linear change, other ways to characterize change include modeling of potential discontinuities or of trajectories that are in relative stasis with rarer bursts of change, similar to what has been described as punctuated equilibrium (Eldredge and Gould, 1972). Changes are also likely to vary across different measures and at both individual and population levels. Ideally, developmental change involves within-subjects, longitudinal observations, although the data discussed below include longitudinal and cross-sectional information.
When to Assess
Deciding when to assess outcome, and the meaning of outcome measures at different points in development, is a challenge for children in whom developmental processes unfold at the same time as the effects of brain insult.
Atypical development
Some plastic changes in the brain are driven by the expression of genetic developmental programs, others by experience, trauma, or lesions. In his discussion of the term, plasticity, Paillard (1976, translation 2008) distinguished genetic plasticity, the structural malleability of a system during its development, and adaptive plasticity, the capacity of a mature system to change its structure and expand its behavioral repertoire.
In some disorders, the brain develops with significant dysmorphologies, hypoplasias, and altered connectivity, due to anomalies of gene (e.g., Down Syndrome, Ullrich-Turner Syndrome, Klinefelter Syndrome); neural tube closure (e.g., spina bifida meningomyelocele); hormone biosynthesis (e.g., congenital adrenal hyperplasia); and prenatal environment (e.g. fetal alcohol syndrome). These also include conditions defined by abnormal behavior (e.g., autism, attention deficit hyperactivity disorder [ADHD], dyslexia). In other conditions, brain structure and function are compromised later in the course of brain development due to infectious, traumatic, vascular, neoplastic, degenerative, or other forms of pathology. Early-onset disorders have no period of normal brain and behavioral development against which age-expectations can be referenced, so questions concern how well dysmorphic substrates can support skill development. Later-onset conditions, varying with age at onset and time since injury, concern how brain insult affects pre-injury skill maintenance as well as ongoing skill development.
Trajectories for children with brain insult
A variety of terms have been used for outcome impairments in children. The term developmental lag describes impairments relative to age (Satz et al., 1981); however, the term lag presupposes that the function will eventually approximate normative benchmarks, which has rarely been demonstrated for children with brain insult, either longitudinally or cross-sectionally. We use the term deficit to refer to observable impairments in function relative to age peers, leaving open the issue of whether the function ever becomes age-appropriate.
In the examples below, we have plotted theoretical models relating age and development. We recognize that ‘development’ may involve a relatively short period of time within an outcome assessment, a longer time period (e.g., from time of acquired injury to two years later), or a very long swathe of the lifespan (e.g., from birth to mid-life). The examples are drawn from both brain and behavior, which may be non-isomorphic even within the same disorder. The trajectory for one skill may be different for that from other skills, even within the same content domain. For example, a left parietooccipital lesion in the first year of life preserves lower-level vision at age 12, but disrupts high-level function involving object selectivity for faces, words, and objects (Hu et al., 2013). The examples are drawn from the published literature, but are sometimes clearer in a clinical research study or clinical trial, where both age-adjusted and raw scores are available to make decisions about new skill development.
Typical development (solid lines in Fig. 1) proceeds from conception to maturity with no major disruption of brain development, brain function, behavior, or their relations. Outcome is assessed early in development (Early Intercept #1), later in development (Mature Intercept #2), and by the rate of development between #1 and #2 (Developmental Slope #3, which is positive).
Figure 1.

Model of typical development (solid line) and brain disorders (dashed line). Models A-D include two time points (Early Intercept #1, Mature Intercept #2), and one slope (Developmental Slope #3). Models E-J include four additional time points (Early intercept #1, Mature intercept #2, Injury Intercept #4, Recovery Intercept #6), and four additional slopes (Developmental Slope #3, Loss Slope #5, Recovery Slope #7, and New Skills Slope #8). Intercepts are shown in regular font, slopes in italics; star represents timing of insult.
In early-onset disorders (dotted lines in Fig. 1, Models A-D), compromised primary brain formation reshapes the relation of brain development to brain function and behavioral outcome. Outcome is measured by Intercepts 1 and 2, and Slope 3. Trajectory A shows no deficit and new development. Trajectories B – D show deficits but different developmental slopes (stable and positive in Example B, zero in Example C, and negative in Example D).
Trajectory B (Early Deficit + New Development) involves age-related neurocognitive deficits that are manifest consistently over some developmental epoch. New skills are acquired and, relative to typically developing peers, individuals with this profile have similar, positive Slope 3 but lower Intercepts 1 and 2. Individuals with Ulrich-Turner syndrome have spatial deficits relative to peers in childhood and young adulthood samples (Ross et al., 1995; Romans et al., 1998). Individuals with Williams-Beuren syndrome have more severe, stable neurocognitive deficits that persist into young adult life (Howlin et al., 1998). Both children and adults with spina bifida meningomyelocele have difficulties in upper limb function (Dennis et al., 2009; Jewell et al., 2010) and mathematics (e.g., Barnes et al., 2006; Dennis and Barnes, 2002; Dennis, Berch, and Mazzocco, 2009).
Trajectory C (Early Deficit + Arrested Development) represents neurocognitive deficits that increase over development, relative to peers but not to self. Intercept 1 is lower than typically developing peers, but because Slope 3 is zero, Intercept 2 is the same as Intercept 1. Individuals with spina bifida meningomyelocele have arrested white matter development in the inferior longitudinal fasciculus; between 8-16 years of age, they show either no change or increased diffusivity on diffusion tensor imaging (DTI), whereas transverse diffusivity decreases with age in their typically developing peers (Hasan et al., 2008). Individuals with Rett syndrome (a disordered expression of MeCP2 gene, a transcriptional repressor for chromatin remodeling) have a severe neuromotor deficit and slowed developmental rate (Hagberg, 2002).
Trajectory D (Early Deficit + Degeneration) represents neurocognitive deficits that increase with age relative to both self and peers. Intercept 1 is lower than typically developing peers, and because Slope 3 is negative, Intercept 2 is lower than Intercept 1. Neurocognitive function in individuals with cerebral degenerative disorders (e.g., Tay-Sachs or Gaucher disease) declines with age (Noronha, 1974). The dotted line may be inflected, as when degeneration sets in after a period of ongoing skill development. Individuals with Down syndrome have relatively stable neurocognitive deficits until mid-life, when many develop dementia (Lott et al., 2012; Margallo-Lana et al., 2007).
In some children, brain insult occurs after a period of normal primary brain development (dotted lines in Fig. 1, Models E-J). Because children with later-onset injury typically have some measured or historical information about pre-injury development, delayed effects (what Kennard identified as growing into a deficit, discussed in Dennis, 2010) can be identified, whereby deficits not apparent at the time of the injury emerge with increasing time since injury, or as the brain matures (Luciana, 2003). Functional outcome is measured by Intercepts 1 and 2, and Slope 3, but also by indices of the effect of acquired lesion on loss and recovery of previously developed skills and new skill development. Specific measures are: impact of injury on skills existing at time of the insult (Injury Intercept #4), rate of loss of existing skills (Loss Slope #5), level of recovery of existing skills (Recovery Intercept #6), rate of recovery of skills attained prior to injury (Recovery Slope #7), and acquisition rate for new skills (New Skills Slope #8).
Trajectory E (Delayed Acquired Deficit) involves no immediate loss of previously acquired skills, but a widening gap with age-peers as development proceeds because of decreased velocity of skill growth. Children with mild TBI as preschoolers display increasing cognitive-behavioral deficits over ages 7-13 years (McKinlay et al., 2010). Children with TBI show performance monitoring deficits that increase with time since injury (Ornstein et al., 2009). Children treated with cranial radiation for posterior fossa medulloblastoma show increasingly poor attention and memory over the 6 years after treatment (Palmer et al., 2013). Children with medulloblastoma show an age-related decline in IQ after radiation treatment; relative to peers, however, their raw scores increase with time, albeit more slowly, showing a failure to acquire new skills at an appropriate rate (Palmer et al., 2001).
Trajectory F (Acquired Deficit + No/Attenuated Recovery + New Development) involves post-injury deficits, attenuated recovery, and/or a lower rate of skill development. Children with TBI show deficits in affective speech prosody (Tonks et al., 2008), and low-SES children after TBI show a slowed rate of new speech prosody improvement in the first two years after injury, with the difference between TBI and comparison groups increasing with time since injury (Schmidt et al., 2010).
Trajectory G (Acquired Deficit + No Recovery + Arrested Development) involves post-injury deficits, no recovery to pre-injury levels, and a flat development curve thereafter. Children with a younger age at radiation treatment show an immediate post-treatment loss of function that reaches a plateau around 6 years after treatment (Palmer et al., 2003).
Trajectory H (Acquired Deficit + Recovery + New Development) involves post-injury deficits that improve, and slower skill development thereafter. After childhood TBI, the corpus callosum is damaged, but there is microstructure regrowth in the form of increased fractional anisotropy and fiber density over the 15 months post-injury that do not approximate pre-injury levels (Wu et al., 2010; Fig. 2).
Figure 2.

At 3 months the loss of fiber tracts indicates early degeneration which could be primary, reflecting neuronal cell death and Wallerian degeneration, or secondary to retrograde and/or anterograde degeneration specific to axonal injury and shearing. By 18 months fiber tracts are still reduced in number and level of dispersion, but some have either reconstituted, remyelinated or maturational changes have occurred in existing aggregate axonal tracts. As a consequence of neural degeneration from traumatic brain injury, it takes hours to several days to begin to detect degenerative effects with neuroimaging techniques like MRI, but maximal changes typically peak in the 3-6 month post-injury timeframe. As seen in this case thinning of corpus callosum tracts as a consequence of TBI is evident by 3 months and although the 18 month follow-up shows some difference, the overall pattern of loss of tracts retains the frontal distribution. Dynamic changes that occur after that 3-6 month timeframe likely reflect aspects of compensatory changes and neural plasticity involving connectivity via different tracts and networks. As explicitly shown in this figure, if a tract is lost as a consequence of trauma, it may be permanently absent (note some of the gaps in fiber tracts are permanently reduced and no different at 18 months). As such, interhemispheric transfer of information must flow via alternate connections.
Trajectory I (Acquired Deficit + Recovery + Arrested Development) involves post-injury deficits that improve to level of pre-injury function, but that do not develop thereafter. In a set of twins, one of whom had a left hemisphere arteritic stroke at age 6, post-injury immediate, severe syntactic deficits improved to their level at the time of the stroke, but, compared to the co-twin, failed to develop thereafter (Hetherington and Dennis, 2004).
Trajectory J (Acquired Deficit + Recovery + Degeneration) involves post-injury deficits that improve, although not fully, and that are accompanied by early degenerative changes. The corpus callosum white matter changes after TBI improve over time, but in the 15 months after the injury, begin to show degenerative change in the form of volume loss (Wu et al., 2010).
Using trajectories to compare disorders
Plotting developmental trajectories has a descriptive function. In addition, it facilitates comparisons within and across disorders.
Children with ADHD have difficulty sustaining attention (Seidel and Joschko, 1990), and, over time, show increased reaction time (RT) and commit more omission errors, as in Trajectory D (Anderson et al., 2006; Brewer et al., 2001; but see Huang-Pollock and Nigg, 2003). In children with SBM, in contrast, RT does not increase over time, as in Trajectory B (Brewer et al., 2001; Swartwout et al., 2008).
Age at onset of diagnosis and treatment may produce different trajectories. In children with cranial radiation for medulloblastoma, a younger age at diagnosis and treatment results in an initial decrement and then stability (Trajectory G), whereas an older age produces delayed effects (Trajectory E; Palmer et al., 2003). Different acquired etiologies may involve distinct developmental trajectories for the same function. For example, working memory follows trajectory E for children radiated for brain tumors (Redmond et al., 2013) and trajectory F in children after TBI (Levin et al., 2004; Sesma et al., 2008). Myelination is disturbed in both spina bifida meningomyelocele, and childhood TBI. In a direct comparison across disorder types, myelination in spina bifida meningomyelocele shows an early deficit and arrested development (Trajectory C; Hasan et al., 2008), whereas in TBI it demonstrates both microstructure regrowth and early neurodegeneration (Trajectory H and J; Wu et al., 2010).
Multiple insults
For some children, brain development is subject to more than one insult. Outcome measures may reflect the effects of multiple insults, treatment toxicity, or hydrocephalus.
Early and later insults
Children with pre-morbid learning disabilities who sustain a TBI exhibit poorer memory and attention than those with TBI but no prior learning disabilities (Farmer et al., 2002). In children with ADHD, a subsequent TBI is associated with increased behavior problems (Yeates et al., 2005). Children with pre-morbid untreated ADHD exhibit more ADHD symptoms after TBI than do treated children with ADHD (Levin et al., 2007) and recovery after mild TBI is prolonged for children with pre-injury ADHD (Bonfield et al., 2013).
Multiple concussions
Sport-related concussions are a useful model for multiple insults. Compared to those with no concussion or a single concussion, adolescent athletes with a history of multiple concussions show more cognitive morbidity (Belanger et al., 2010), reporting changes in memory, mental status, and recovery time (Collins et al., 2002; Gaetz et al., 2000; Guskiewicz et al., 2003; Iverson et al., 2004). Whether the number and timing of concussive events (are three concussions equally disruptive when they are sustained closer together or further apart in time?) affects outcome remains to be understood. Some evidence suggests that children with a history of a previous concussion, particularly recent or multiple, are at increased risk for prolonged symptoms after a subsequent concussion (Eisenberg et al., 2013).
Treatment toxicity
Some childhood brain conditions require adjuvant treatments (most commonly, radiation and chemotherapy) that add to cognitive morbidity. Some of these treatment toxicities may result in an early onset of cognitive degenerative processes in adult life (Edelstein et al., 2011). An informative comparison is that between two posterior fossa brain tumors, medulloblastoma and subtentorial astrocytoma, that both generally involve the cerebellar vermis (Hopyan, Laughlin and Dennis, 2010) but require different adjuvant treatments. Astrocytomas are treated with surgery alone while malignant medulloblastomas are treated with surgery, craniospinal radiation, and sometimes chemotherapy (Stavrou et al., 2001). Comparison of outcome in children treated for astrocytomas and medulloblastomas reveals that radiation has a negative effect on outcome, consistent with its association with vascular and demyelinating neuropathology (Cohen and Duffner, 1994). Functional outcome for astrocytoma is better than that for medulloblastoma (Roncadin et al., 2008).
Chemotherapy for acute lymphoblastic leukemia produces cognitive morbidity (Conklin et al., 2012; Janzen and Spiegler, 2008; Moleski, 2000), although, compared to radiation effects, the cognitive effects of chemotherapy-only protocols are less global and less functionally impairing (Janzen and Spiegler, 2008; Buizer, et al., 2009). Higher intensity chemotherapy produces poorer outcome (Conklin et al., 2012).
Hydrocephalus
Hydrocephalus is common in childhood brain disorders. What does hydrocephalus contribute to cognitive morbidity, beyond that conferred by the brain insult?
Hydrocephalus occurs in individuals with spina bifida meningomyelocele, many of whom require a ventricular shunt shortly after birth to divert excess CSF to control ventriculomegaly (Raimondi, 1994). The variable expression of hydrocephalus over development provides a means of investigating what hydrocephalus contributes to cognitive morbidity. Using shunt status together with neuroimaging as a proxy for hydrocephalus, Hampton et al. (2011) found that different shunt histories (ongoing, arrested, or no hydrocephalus) in children with spina bifida meningomyelocele produced a similar outcome pattern, but different levels of performance. Whether a child is shunted depends in part on the progression of ventriculomegaly, which is associated with the core brain malformations of spina bifida meningomyelocele, so outcomes are a product of both brain malformations and hydrocephalus. Memory, numeracy, independence, and employment are negatively related to number of lifetime shunt revisions in young adults with spina bifida myelomeningocele (Dennis and Barnes, 2002; Dennis et al., 2007; Hetherington et al, 2006; Hunt et al., 1999).
Comparison of different neuropathologies with shared hydrocephalus also provides a way of evaluating the effects of hydrocephalus. Hampton et al. (2013) compared neuropsychological profiles in children with shunted hydrocephalus secondary to aqueduct stenosis, a rare form of congenital hydrocephalus, and spina bifida myelomeningocele, a common form of congenital hydrocephalus. Cerebellar abnormalities (in the whole spina bifida myelomeningocele group and in a subset of the aqueduct stenosis group), rather than hydrocephalus, produced compromised cognitive and motor function. Treble-Barna, Kulesz, Dennis, & Fletcher (2014) found that aqueduct stenosis was not associated with deficits in attention orienting, in contrast with spina bifida myelomeningocele, where attention deficits were apparent and related to the severity of midbrain dysmorphology.
Brain tumors are often associated with hydrocephalus. Within a diverse brain tumor group treated with radiation, hydrocephalus is associated with poorer outcome (Conklin et al., 2013). In survivors of childhood medulloblastoma, all treated with surgery, radiation, and chemotherapy, shunt placement for hydrocephalus after tumor resection is associated with poorer neurocognitive outcome (Hardy et al., 2008). Increased intracranial pressure and hydrocephalus are two of a number of pre- and perioperative risk factors for long-term neuropsychological impairment in a tumor type, cerebellar astrocytoma, not treated with radiotherapy (Aarsen et al., 2004; Ater et al., 1996; Ronning et al., 2005; Yule et al., 2001).
Hydrocephalus affects level of function in congenital conditions like spina bifida and aqueduct stenosis, even though the functional profile seems set by the brain dysmorphologies. Increased lifetime shunt revisions changes the level of function, if not its profile, in young adults with spina bifida. In brain tumors, hydrocephalus is a risk factor for poorer outcome.
The when - Summary
The question of when to assess outcome is complex, and often dictated by practical rather than theoretical considerations. Nevertheless, to interpret outcome measures, it is important to identify where in the trajectory of development and brain insult the assessment has occurred.
What To Assess
Traditional neuropsychological assessments include indices of global outcome, such as IQ, as well as measures of specific cognitive domains, such as language, space, and number. An assessment also needs to include social cognition, both cold (concerned about thinking about the social world) and hot (concerned with emotion and social influence). Important targets of an outcome assessment are also regulator-homeostatic functions, whereby children react to past information with working memory, inhibitory control, and reward sensitivity, and predict the future by processes such as performance monitoring and prospective memory. Many of these skills are interrelated: the ability to inhibit irrelevant information is important for working memory (Lavie et al., 2004; Sakai et al., 2002), while maintenance of relevant information is important for inhibitory control (Kane and Engle, 2003); prospective memory difficulties are correlated with working memory and inhibitory control impairments (Kerns and Price, 2001; McCauley and Levin, 2004); reward improves inhibitory control (Sinopoli, Schachar and Dennis, 2011) and prospective memory (Kvavilashvili et al., 2007; Somerville et al., 1983).
Reacting to the past: Working memory, inhibitory control, and reward sensitivity
Working memory, a resource for holding and manipulating information (Baddeley, 1992), increases from childhood through adolescence (Gathercole et al., 2004; Luciana et al., 2005; Roncadin et al., 2007) and supports many cognitive and academic skills (Jonides, 1995), including language comprehension (Hanten, Levin, and Song, 1999), literacy (Gathercole and Baddeley, 1997), and arithmetic (Bull and Scerif, 2001). Working memory impairments are found in genetic disorders such as Williams Syndrome (Rhodes et al., 2011) and 22q11.2 deletion syndrome (Azuma et al., 2009), ADHD (Dowson et al., 2004), childhood TBI (Dennis et al., 2009), and brain tumors (Conklin, 2012).
Working memory tasks generally utilize static information (e.g., digits and letters), yet the everyday use of working memory requires activating and manipulating dynamic social information, such as relationships and knowledge of beliefs and intentions. Researchers have recently begun to examine the behavioral and neural correlates of social working memory (Meyer and Lieberman, 2012) in typically developing individuals. It is not known whether working memory for digits and letters, and that for social information, are similarly affected by childhood brain insult.
The term inhibitory control encompasses a variety of interrelated self-regulatory processes (Sinopoli and Dennis, 2012) that allow for adaptive interactions within the environment. Different forms of inhibitory control are refined throughout childhood and adolescence (Sinopoli, Schachar and Dennis, 2012).
Stopping, or cancelling an ongoing action, is often estimated via the latency to stop or interrupt an ongoing action (Logan, 1994). Cancellation is deficient in children with ADHD (e.g., Schachar et al., 2007; Sinopoli, Schachar and Dennis. 2011), and also in children who develop de novo symptoms of ADHD following TBI (LeBlanc et al., 2005). In the latter group, less severe cancellation deficits persist (LeBlanc et al., 2005; Sinopoli, Schachar and Dennis, 2011). Restraining or withholding a response is another form of inhibitory control in which children reach adult levels of competence around age 12 (Levin et al., 1991; Liston et al., 2006). Children with ADHD show restraint inhibition deficits (Schachar et al., 2007; Metin, Roeyers, et al., 2012), unlike children with ADHD emerging after TBI (Sinopoli, Schachar and Dennis, 2011). Inhibitory control deficits vary with the etiology of the brain disorder and with time since injury in acquired TBI. This underscores the issue of timing of outcome assessments.
Reward sensitivity refers to acts of self-regulation that often occur in response to perceived or actual reward or punishment. Children with ADHD, who are generally disinhibited, exhibit difficulties delaying or waiting to receive a reward (termed “delay intolerance,” Sonuga-Barke, 2002). Rewards for successful inhibition decrease the deficits in both cancellation and restraint in children with ADHD (Sinopoli, Schachar and Dennis, 2011, but see Haenlein and Caul, 1987). Punishments may promote self-regulation in children with ADHD (Luman et al., 2005).
Predicting the future: movement prediction, performance monitoring, and prospective memory
The cerebellum has a role in cognition (Schmahmann, 2010) that involves movement prediction as well as reaction. Anticipatory control loops originated for on-line control of action, but gradually came to predict distal and abstract consequences of actions (Koziol et al., 2013). The predictive and reactive aspects of cerebellar motor control may be dissociated. For example, individuals with spina bifida meningomyelocele, who have significant cerebellar dysmorphologies (Juranek et al., 2010; Fig. 3) show preserved motor learning, but impaired motor functions requiring predictive signals and precise calibration of the temporal features of movement (Dennis et al., 2010). Assessing motor prediction is important, even if not often done, because many disorders of the brain involve cerebellar pathology (e.g., Fernandez et al., 2013).
Figure 3.

Cerebellar macrostructure in spina bifida meningomyelocele (see Juranek et al, 2010). Parcellation shows a four-compartment model (one white matter and three principally gray matter) of the cerebellum. 1) Corpus medullare (light blue): central white matter and output nuclei; 2) Anterior lobe (green) lobules I-V, bounded by the most posterior point of fourth ventricle, corpus medullare, and primary fissure; 3) Superior posterior lobe (dark blue): lobe VI and crus I of VIIA, bounded by primary fissure, corpus medullare, and horizontal fissure; 4) Inferior posterior lobe (khaki): crus II of VIIA, VIIB, VIII, IX, X, bounded by the most posterior point of the fourth ventricle, corpus medullare, and horizontal fissure. The total cerebellar volume is smaller. However, the configurative is distinctive to spina bifida. After correcting for total cerebellum volume, and relative to controls, the spina bifida group shows:
- posterior lobe significantly smaller
- corpus medullare not different
- anterior lobe enlarged.
- Cerebellar volume deficits in SBM group involves a reconfiguration involving anterior lobe enlargement and posterior lobe reduction.
On a moment-to-moment basis, task-related behavior depends on performance monitoring, including error monitoring and correction (Rabbitt and Rodgers, 1977; Krusch et al., 1996; Logan, 1985). Detecting performance failure, which continues to develop through adolescence (Tamnes et al., 2013), helps adjust future actions to optimize task success. Deficient perforance monitoring has been documented in children with ADHD (Schachar et al., 2004) and children after TBI (Dennis et al., 1996; Hanten et al., 2004; Ornstein et al., 2009). Performance monitoring is negatively related to time since childhood TBI (Ornstein et al., 2009.
Prospective memory is the act of remembering to perform an intended action in the future (Birenbaum, 1930; Craik, 1986; Kerns, 2000; Lewin, 1961; McCauley & Levin, 2004; Yang, Chan, & Shum, 2011). It may be event-based (the presence of an external cue prompts the performance of an intention), time-based (remembering to complete an intention at a specific time), or activity-based (remembering to carry out a specific intention after an activity is completed; McCauley and Levin, 2004). At age 2, toddlers can remind a caregiver to get ice cream (Somerville et al., 1983), and prospective memory improves into adolescence (Kerns, 2000; Maylor et al., 2010; Yang et al., 2011). Prospective memory may be poor in individuals with spina bifida meningomyelocele even when other forms of memory are not deficient (Dennis et al, 2007; Dennis et al, 2010). Prospective memory is impaired in ADHD (Kerns and Price, 2001) and TBI (McCauley and Levin, 2004; Ward et al., 2004; Ward et al., 2007).
Outcome assessments typically involve multiple measures of retrospective memory, with prospective memory tasks being few, unstandardized, variable, and of uncertain validity (Kvavilashvili, 1992; Kerns, 2000). Nevertheless, prospective memory is an important function, because lack of future orientation disrupts an individual's self-care, community, occupational activities, and overall independence (Shum, Levin, and Chan, 2011).
Social cognition, hot and cold
Children's ability to identify, produce, and regulate emotions; to consider other people's perspectives, beliefs, and intentions; and to solve interpersonal problems are critical determinants of their social adjustment (Yeates et al., 2007). One component of social cognition is theory of mind (ToM), which involves mentalizing (Frith and Frith, 2003), the ability to think about mental states in oneself and others and to use this information to understand what other people know and predict how they will act. This cognitive theory of mind is the “cold” component of social cognition, on which psychopaths perform well (Blair, 2008). Two other “hot” components of social cognition involve affect as well as cognition (Shamay-Tsoory and Aharon-Peretz, 2007; Hein and Singer, 2008). One involves affective theory of mind, the use and understanding of affective information, such as that in facial expressions, and the regulation of emotion expression according to the needs and beliefs of the viewer. Conative theory of mind involves exerting influence on what someone else feels, through the use of ironic or empathic communication (Dennis, Simic, Agostino, et al., 2013).
One reason for evaluating both “cold” and “hot” social cognition is that the perturbation threshold for “cold” theory of mind may be higher than that for “hot” theory of mind. Only severe TBI disturbs cognitive theory of mind, but even mild–moderate TBI can disrupt affective and conative theory of mind (Dennis, Simic, Agostino, et al., 2013). Another is that social cognition, especially hot social cognition, is related to concurrent social adjustment (Yeates et al., 2013).
The what - Summary
Neuropsychological outcome should involve both content domains and regulatory-homeostatic skills, as in the example in Table 1.
Table 1.
Neuropsychological outcome assessment including three content domains (language, space and number, social cognition) and two regulatory-homeostatic processes: those like working memory, inhibitory control, and reward sensitivity concerned with reacting to the past, and those like motor prediction, prospective memory, and performance monitoring concerned with predicting the future. Table 1 does not attempt to prescribe specific tests. While available tasks to measure categorical and coordinate space are both elegant and brief (Wiedenbauer and Jansen-Osmann's (2006) virtual reality paradigms), the space domain might be assessed with existing face recognition (a form of categorical perception) and line orientation (a form of coordinate space) tasks.
| REGULATORY-HOMEOSTATIC FUNCTIONS |
| Reacting to the past |
| Working memory |
| Inhibitory control |
| Reward sensitivity |
| Predicting the future |
| Motor prediction |
| Prospective memory |
| Performance monitoring |
| CONTENT DOMAINS |
| Language |
| Phonological awareness |
| Verbal fluency |
| Reading fluency |
| Space and number |
| Categorical space |
| Coordinate space |
| Number fluency |
| Social cognition |
| Cognitive theory of mind |
| Affective theory of mind |
| Conative theory of mind |
How To Assess
How to assess might seem obvious, but it is not. Sources of information include means and performance variability; direct and informant reports; laboratory and ecological measures; latent function; and phenocopies and fault tolerance.
Performance variability
Historically, the performance mean or average has dominated outcome measures, with intraindividual variability being viewed as random error, measurement unreliability, or noise. However, individual performance fluctuations, which often correlate negatively with mean performance (Hultsch et al., 2008), may be more sensitive than mean performance to cognitive deficits (Lövdén et al., 2007). Significant intraindividual variability is characteristic of individuals with neurodevelopmental and acquired brain disorders. Those with sports concussion demonstrate substantial intraindividual variation (Rabinowitz and Arnett, 2013). Poor formation and supply of lactate may produce inefficient and variable performance in ADHD and other white matter disorders (Russell et al., 2006).
Variability is manifested in several forms, including intra-individual variability across tests/scores/domains, and intra-individual variability across time. We have mainly described the latter form, but there is value in considering all forms of performance variability as data in outcome assessments to provide a more sensitive index of cognitive deficits.
Direct and informant reports
Direct assessments of children with brain disorder require examiners or observers to objectively assess child traits or parent or environmental characteristics by cognitive and achievement tests; observations of children's classroom behaviors, interactions with peers or caregivers, and the home environment; or information obtained through record reviews. These assessments can measure specific skills, or laboratory analogues of everyday functions like driving or medication management in adults (Heaton et al., 2004; Schultheis, Hillary, and Chute, 2003) or zoo trips in pediatric samples (Lawrence et al., 2004). Direct assessments control measurement methods and conditions, unlike informant-based assessments that rely on reports from persons familiar with the child or family. Examples are parent, teacher, or self-reports of child behavior symptoms, and clinical interviews. Informant-based assessments permit efficient collection of information that is typically inaccessible to outside observers and that offers insight into how children function in their everyday environments. Interview-based assessments of adaptive behavior yield results about everyday functions and can be done by telephone for individuals of varying levels of cognitive functioning (Fletcher, 2013; Ris et al., 2008). However, informant impressions may be subject to observer bias, which can sometimes be addressed through structured interviews (Sparrow et al., 2005). The modest correlations between different informants may signal contextual variations in child functioning but may also reflect observer characteristics (Farooqi et al., 2007; Williams, McGee, Anderson, and Silva, 1989).
Post-injury performance on cognitive testing after childhood TBI is only weakly related with behavior ratings (Fletcher et al., 1990; Schwartz et al., 2003). Performance assessments and ratings of executive function are poorly associated in clinical groups (Anderson et al., 2002; McAuley et al., 2010; Toplak et al., 2009; Toplak, West, and Stanovich, 2013). Other studies document the utility of specific cognitive measures as predictors of informant-based assessments of behavior or educational outcomes (Litt et al., 2012; Miller and Donders, 2003; Scott et al., 2012; Taylor, Hack, and Klein, 1998; Taylor et al., 2006; Willcutt et al., 2005; Yeates et al., 2004). Some studies find that informant-based measures are more closely associated with clinical diagnoses or adaptive behavior skills than are psychometric tests (Ris et al., 2008). Others show that tests are more sensitive to disease factors (Anderson et al., 2002; Fastenau et al., 2009; Levin et al., 2004; MacAllister et al., 2012; Taylor and Schatschneider, 1992; Toplak et al., 2009).
Direct and informant measures make independent contributions to predicting outcomes (Arnett et al., 2013; Heaton et al., 2004) and so provide complementary information (Isquith, Roth, and Gioia, 2013), so both are useful in evaluating children with brain disorders within a framework of multiple levels of influence on outcomes (Masten, 2001; Sameroff, 2010; Yeates et al., 2007).
Laboratory and ecological measures
Functional skills are instantiated in real environments, not only under laboratory conditions. Neuropsychological instruments originally designed in the 1930s and 1940s to detect adult brain pathology are now used to address social, vocational, and functional outcomes in both adults and children (Rabin, Burton, and Barr, 2007), although most remain disconnected from environmental function.
The relation between test performance and everyday function in children is not well understood (Olson, Jacobson, and Van Oot, 2013). Adults with spina bifida meningomyelocele have impaired upper limb motor skills under conditions of ecological challenge, such as counting backwards (Dennis et al., 2009). In children with TBI, the ability to identify reasons for action is better than the ability to demonstrate them (Dennis et al., 1998).
Latent function
Most functional outcome measures are overt. Functional outcome may be also measured by creating special conditions for studying impaired skills under which latent function becomes apparent. Sometimes the brain registers information that the patient cannot report (Simon et al., 2011). Individuals with hemispherectomy for infantile seizures discriminate textures with the hand ipsilateral to the hemispherectomy (although not with the contralateral hand). The contralateral hand, however, has some latent function because it can identify textures provide that the ipsilateral hand selects the target (Kohn and Dennis, 1974).
In blindsight, individuals with visual field loss can process visual information in their blind visual field, even when they are unaware of seeing anything (Cowey and Stoerig, 1991; Weiskranz et al., 1974). Individuals with hemispherectomy for early seizures show homonymous hemianopsia along a vertical meridian and claim to see nothing in the hemianopic field, which, however, has latent visual function (Sharpe et al., 1979). Infants with cerebral hemispherectomy can fixate targets in the blind visual field although their optokinetic nystagmus is asymmetric (Braddick et al., 1992). Blindfield pupil responses are similar to the sighted field, although attenuated in amplitude (Sahraie et al., 2013).
Phenocopies and fault tolerance
The same behavioral outcomes may represent phenocopies, in which similar outcomes reflect different genetic, neural, and cognitive mechanisms. For instance, acquired attention deficits may represent a phenocopy of the behavior exhibited by children with the developmental form of the disorder. Poor inhibitory control is a signature deficit in ADHD (e.g., Haenlein and Caul, 1987; Lipszyc and Schachar, 2010; Schachar et al., 2007; Sonuga-Barke, 2002) and includes impairment in both cancellation (interrupting an ongoing response) and restraint (withholding a response before initiating it). ADHD after TBI, however, involves cancellation but not restraint deficits (Sinopoli, Schachar and Dennis, 2011). Inhibitory control deficits improve over time more in ADHD after TBI than in developmental ADHD (Leblanc et al., 2005). Methylphenidate improves hyperactivity-impulsiveness in children with ADHD after TBI, although improvement occurs acutely and is less robust than that in children with developmental ADHD (Jin and Schachar, 2004).
Related to phenocopies is the concept of fault tolerance (Taylor and Zwaan, 2009; 2012). With multiple routes to outcome, deficits in one route need not be associated with deficits in another. Fault tolerance studies help identify the spatial perception deficits (e.g., Dennis et al., 2002) in children with brain insult. There are two different spatial relations between observers and objects (Kosslyn, 1987; Saneyoshi and Michimata, 2009). Categorical relations specify discrete spatial relationships (objects, feature groupings, faces), or locatives (e.g., above, below, left, right); coordinate relations specify precise spatial relations by coordinate metrics (e.g., “the line and the dot are 2 cm apart”). Typically developing children master both spatial relations, but children with spina bifida meningomyelocele master only one: they can navigate a virtual reality task using spatial landmarks (a form of categorical perception) but not spatial coordinates (Wiedenbauer and Jansen-Osmann, 2006).
The how – Summary
Traditional measures of outcome are based on means and direct performance. However, important information about outcome may also be derived from intraindividual variability, informant reports, covert measures, and analyses of phenocopies and fault tolerance.
Whom to Assess: Individual Differences in Reserve, Risk and Resilience
Individual differences in brain injury
Measuring outcomes in clinical research studies requires awareness of normal and lesion-related individual differences in children's brains. Even in apparently healthy individuals complex molecular, synaptic and pathway differences ensure that no two brains are ever structurally identical (Fig. 4).
Figure 4.
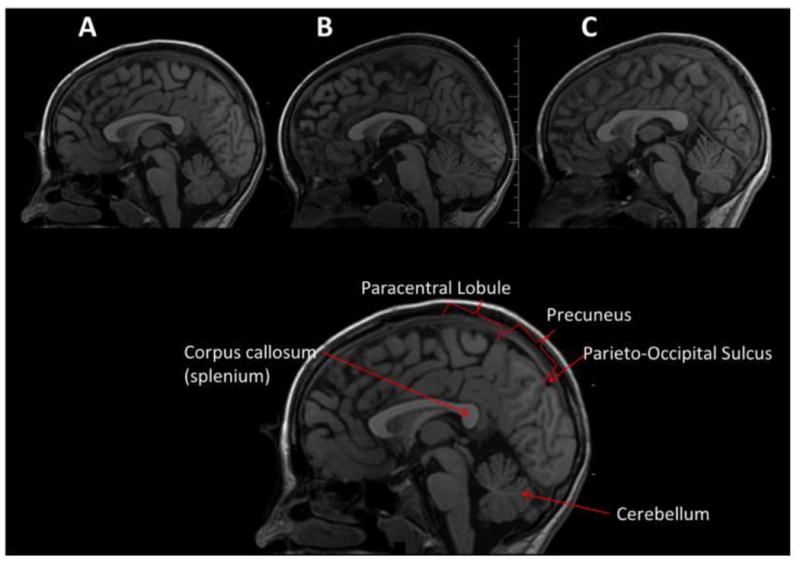
Mid-sagittal view of the brain from three typically developing children of approximately the same age and SES, who served as orthopedically injured controls in the Social Outcome in Brain Injury in Kids (SOBIK) study (see Bigler et al., 2013; Dennis et al., 2012). The cutting plane is identical in all three at the level of the aqueduct of Sylvius, a traditional midline marker. While similar in general appearance, each brain is unique in shape, size and morphology. For example, a prominent posterior corpus callosum with a rather bulbous splenium is evident in A and C, but not B. The cerebellum in B is substantially larger than in A or C. The bottom figure is the same as “A” and used to label regions of interest for comparison to show the heterogeneity in brain morphology. Note the individual differences: the unique shape of the corpus callosum, the different gyral patterns within identifiable regions like the paracentral lobule and precuneus, and the distinctiveness of the parieto-occipital sulcus.
No two physical circumstances involving childhood TBI are ever identical. In an auto-pedestrian accident, the speed and type of the vehicle, the road circumstances, position of the child when struck are all unique. Even in animal models of TBI where identical equipment and procedures are used along with identical genetic strains of animals, the exact lesion cannot be consistently replicated across animals (Statler et al., 2008).
Whether the condition that induces the brain injury be neoplastic, infections, inflammatory or vascular, each condition unfolds within morphologically and functionally unique individual brains. Even in vascular disorders with a common etiology, the uniqueness of the microvasculature of the brain is as complex as microstructural environment to which it supplies blood.
Cognitive and brain reserve
The same burden of brain insult can affect outcome differently depending on cognitive and brain reserve, a construct that attempts to account for individual variability in plasticity and outcome (Dennis et al., 2007; Katzman, 1993; Satz, 1993; Stern, 2002). Reserve is a function of factors that affect the brain, disease, and individual learning and social experiences.
Stress and resilience
Individual differences in outcome may be related to variation in stress reactivity. The COMT gene, located on chromosome 22 codes for the enzyme catechol-O-methyltransferase (COMT). COMT degrades catecholamine neurotransmitters such as dopamine and thereby regulates their availability in the synapse. COMT is associated with several allelic variants, the best studied of which is Val158Met: a normal variant of COMT at position 158 results in a valine to methionine mutation. The Val158 variant degrades dopamine up to four times the rate of Met158 (Lachman et al., 1996), resulting in significantly greater synaptic dopamine for Met158 carriers. Under normal conditions, Met158 carriers exhibit stronger cognitive performance than Val158 carriers (Bruder et al., 2005; Diamond et al., 2004; Jaspar et al., 2013). However, the faster Val158 carriers have a performance advantage under stress conditions (Yeh et al., 2009) because they are able to normalize the excess prefrontal dopamine faster than Met158 carriers (Hernaus et al., 2013). Stress may enhance dopaminergic transmission and produce better performance in Val158 carriers, but has the opposite effects in Met158 carriers.
Stress during development may promote resilience (Lyons and Macrì, 2011), the ability to handle stressors more efficiently (DiCorcia and Tronick, 2011). Ideally, daily stress activates adaptive behavioral and physiologic systems that increase the capacity to cope with more intense stressors. Whether stress promotes resilience may depend on how well the individual phenotype is tuned to the forecasted adult environment (Macrì, 2013). In children with brain insult, the tuning of the individual stress phenotype to the environment may be more easily perturbed, but whether and how stress and resilience is different in typically developing children and children with brain insult remains to be understood. In measuring outcome, it is recognized that stress reactivity and genetic susceptibilities moderate the effects of family factors on health and development (Caspi et al., 2003; Leve et al., 2010; Moffitt, Caspi, and Rutter, 2005; Obradovic et al., 2010).
Socio-economic, parenting, and family factors
Socio-economic status
SES influences a variety of cognitive and behavioral outcome measures in typically developing children. For example, SES differences are found in neural processing even when children are equated for performance levels.
SES also predicts outcomes after childhood brain disorder
For instance, SES accounts for significant variance in cognition and psychosocial outcomes among children with spina bifida (Fletcher et al., 2005), effects that are stronger than those attributable to ethnicity (Swartwout et al., 2010). The role of SES in outcome for children with TBI is complex. SES is related to outcome in children with TBI (Taylor et al, 2002), but SES and injury mechanism are also related, making SES differences intrinsic to injury groups based on TBI severity (Dennis, Simic, Bigler, et al., 2013). Epidemiological studies show that the risk of TBI, particularly those linked to motorized vehicles, is highest for children of lower SES and minority status (Brown, 2010; Howard, Joseph, and Natale, 2005; Langlois, Rutland-Brown, and Thomas, 2005; McKinlay et al., 2010; Parslow et al., 2005; Yates et al., 2006).
Parenting and family factors
SES has indirect effects on functional outcome that are mediated by factors such as parenting (Hackman, Farah, and Meaney, 2009). More proximal parenting and family factors influence cognitive, academic, and behavioral outcomes, including less family stress and dysfunction, greater stability in family structure and residence and greater regularity of contact with both parents, lower levels of parental psychological distress, and home environments that are more child-oriented and invested in literacy development (Gross et al., 2001; McClelland, Kessenich, and Morrison, 2003). Parenting characteristics associated with better developmental progress include warmth, responsiveness, and support for child initiatives (Bernier, Carlson, and Whipple, 2010; Landry et al., 2002; Lomax-Bream et al., 2007; Mahoney, Weeden, and Perales, 2004). Interventions fostering these characteristics promote better development (Landry, Smith, and Swank, 2006; Neville et al., 2013). More severe and prolonged family stress or deprivation, especially early in life, has adverse consequences for cognition and behavior, as well as for health and brain development (Hostinar et al., 2012; Shonkoff, 2010). Child nutrition is a family factor related to cognitive development, especially relevant in resource-limited settings (Kitsao-Wekulo et al., 2013).
The effects of proximal family factors are partially independent of SES, pointing to the importance of environmental variations within families of similar levels of education or economic standing (Lomax-Bream et al., 2007; Taylor et al., 1998). Parenting and family factors also have associations with developmental outcomes that are independent of biological risks and that relate to development in distinct ways (Taylor et al., 1998). Family factors may have different effects on different motor and cognitive outcomes (Dennis et al., 2006; Farah et al., 2008; Laucht, Esser, and Schmidt, 1997; Taylor et al., 1998). Extreme disruptions in the family environment may have selective neurocognitive effects (Pollak et al., 2010).
Children with high-risk preterm birth, epilepsy, and TBI (Gross et al., 2001; Fastenau et al., 2004; Landry et al., 1997, 1998; Taylor et al., 1998, 1999) may be more vulnerable to family and parenting adversity than typically developing peers. To be sure, neurologically compromised children are less able to take advantage of environmental supports (Bendersky & Lewis, 1995; Hack et al., 1992; Treyvaud et al., 2012).
Parenting and family factors are related not only to child outcomes at a given age but to developmental change. Higher quality parenting is related to more rapid growth in children's skills with advancing age, and higher parenting stress and more parent distress to age-related increases in child behavior problems (Friedman et al., 2004; Landry et al., 1997, 1998, 2006). Family factors also moderate differences in developmental change between children with neurodevelopmental disabilities and their peers. Recovery from TBI in children is more likely in those from more advantaged families (Fay et al., 2009; Taylor et al., 2002). However, multiple factors are associated with developmental change, including the child's potential to benefit from environmental advantage. In a study of outcomes of very low birth weight infants, Taylor et al. (2004) observed slower growth in some cognitive abilities from ages 7-14 years in children at greater environment advantage, as defined by higher socioeconomic status and resources or lower family stressors. Although counterintuitive, this pattern may reflect a diminished capacity of these children to benefit from environmental advantage, perhaps related to their attainment (at least by age 7) of a kind of biological ceiling or “upper limit” that constrains further developmental gains. Developmental change in children with brain insults is further complicated by bidirectional relations between child and family (Friedman et al., 2004; Taylor et al., 2001).
The whom - Summary
Individual differences in brain development and cognitive outcome have long been recognized, but only more recently have these been viewed as more than error variance. Individual differences in reserve, risk, and resilience should be considered in assessing outcome.
7. Discussion
Plasticity is complex, subject to homeostatic regulation, environmentally contingent, and plays out in different trajectories over age and development. This paper has considered when, what, how, and whom to measure in clinical research assessments of outcome. This Discussion will concern five issues: the need for genuinely developmental evaluations over the lifespan involving both longitudinal and cross-sectional observations; the need to avoid both Type I and Type II errors in assessing outcome; the importance of real-time and ecological evaluations; the importance of understanding endogenous and exogenous moderators that interact with biology and experience to shape outcome; and the need for a brief but comprehensive assessment of functional outcome.
For children with brain disorders, a single point on a developmental curve will not necessarily indicate outcome, whether the deficit is stable or increasing, if there is ongoing development or not, or whether there is recovery or not. While assessment at a single time point is, of necessity, limited in evaluating outcome, and while it is theoretically desirable to favor longitudinal curves over individual time points (Karmiloff-Smith, 2010), both types of information are important (Raz and Lindenberger, 2013). The practical issues involved in conducting longitudinal assessments are daunting, so perhaps a modest step would be to devote less assessment time to IQ and semantic knowledge tasks and more to integrating test scores with historical information about earlier points in development.
The value of cross-sectional studies conducted over development should not be underrated. Information about children with brain insult can be supplemented with studies of adult outcomes to provide proxies for developmental trajectories. The demonstration of similar social cognitive deficits in children with TBI (Dennis, Simic, Bigler, et al., 2013) and adult survivors of childhood TBI (McLellan and McKinlay, 2013) indicates how social impairments after childhood TBI persist into adult life.
A Type I error, or false positive, involves the incorrect rejection of a true null hypothesis, and the conclusion that a deficit exists when in fact it does not. A Type II error, or false negative, involves the failure to reject a false null hypothesis, and the conclusion that no deficit exists when in fact it does. Both Type I and Type II errors arise from the limitations of a traditional outcome assessment, heavily weighted towards IQ testing and redundant measures of semantic memory. Consistently, IQ scores have been shown to be relatively insensitive to most of the spectrum of TBI severity at the same time as deficits are apparent in other neurocognitive functions, such as social cognition (e.g., Dennis et al., 2012). We have illustrated the importance of evaluating covert as well as overt function in order to avoid Type I errors, by which an outcome is falsely deemed to be deficient when the actual deficits are more limited. We have also illustrated the importance of a comprehensive assessment of outcome in order to avoid Type II errors, by which an outcome is falsely deemed to be age-appropriate when it is actually deficient or truncated.
Children function in real time and in a real world in which they must react to the past and predict the future. Most tests of outcome have a limited real-time component, and, even in those that do so, the signal contained in performance variations over time is not always utilized to contrast deficits in clinical groups with poor overall performance. Ecological function is difficult to test in a laboratory, one-on-one setting. Nevertheless, many tests of outcome bear little relation to the functional demands of a child's life, so it is important that outcome assessments attempt to evaluate ecological function. Adaptive behavior assessments may be especially useful for enhancing ecological validity.
Reward-mediated motivational processes, stress, and environmental factors affect brain and behavioral development through effects on neuromodulatory processes (Li, 2013). Outcome is determined, not only by factors operative at the time of the assessment, but also by the child's life. This includes endogenous factors such as genetic contributions to stress and testing and exogenous factors in the children's family and environment.
The bulk of a typical outcome assessment concerns retrieving static information. Functions concerned with dynamics predicting the future need to be part of an assessment because they indicate the ability to maintain regulatory homeostasis. A child who has average IQ and vocabulary but is unable to react to the past or change future actions based on need has content but no regulatory homeostasis. This is especially important because poor outcomes in a range of childhood brain disorders are associated with a dysequilibrium between developmental plasticity and regulatory homeostasis (Dennis, Spiegler, et al, 2013).
Many current outcome protocols are repetitive, redundant, and disconnected from function in real environments. Because many skills not traditionally (or well) assessed ought to be part of a more modern outcome evaluation, an assessment might be deemed to be unrealistically lengthy. Not so. An assessment of outcome – which, in theory, could be quite brief – needs not only to include new tasks, but also to omit traditional tasks that are difficult to interpret or are based on outdated cognitive models with no theoretical framework guiding test selection. To be sure, many of the test domains proposed have been studied primarily in the context of developmental research studies. Adopting new tools to measure new constructs, tools that can track development over long periods of time requires appropriate time and resources for test construction. The job of formulating a useful developmental outcome assessment is important for children with brain insult throughout the lifespan, because accurate assessment of functional outcome is necessary to make inferences, not only about the level, rate, and trajectory of development in the face of brain insult, but also about constructs such as plasticity, recovery, and restitution of function.
Acknowledgments
Supported in part by National Institute of Child Health and Human Development Grants P01 HD35946 & P01 HD35946-06,“Spina Bifida: Cognitive and Neurobiological Variability” and byNational Institutes of Health Grant 1RO1 HD04946, “Social Outcomes in Pediatric Traumatic Brain Injury.” We thank Beverly Andres for help with manuscript preparation and Laura Janzen for assistance with the brain tumor literature.
Contributor Information
Maureen Dennis, Program in Neurosciences and Mental Health, The Hospital for Sick Children, Toronto; Department of Surgery, Faculty of Medicine, University of Toronto; Music and Health Research Collaboratory, Faculty of Music, University of Toronto.
Brenda J. Spiegler, Department of Psychology, The Hospital for Sick Children, Toronto; Department of Pediatrics, Faculty of Medicine, University of Toronto
Nevena Simic, Program in Neurosciences and Mental Health, The Hospital for Sick Children, Toronto.
Katia J. Sinopoli, Department of Psychology, Division of Neurology, The Hospital for Sick Children, Toronto
Amy Wilkinson, Program in Neurosciences and Mental Health, The Hospital for Sick Children, Toronto; Department of Psychology, University of Toronto.
Keith Owen Yeates, Department of Pediatrics, The Ohio State University, and The Research Institute at Nationwide Children's Hospital, Columbus, Ohio.
H. Gerry Taylor, Department of Pediatrics, Case Western Reserve University, and Rainbow Babies & Children's Hospital, Cleveland, Ohio.
Erin D. Bigler, Departments of Psychology and Neuroscience, Brigham Young University, Provo UT; Department of Psychiatry, University of Utah, Salt Lake City UT
Jack M. Fletcher, Department of Psychology, University of Houston, Houston, Texas
References
- Aarsen FK, Van Dongen HR, Paquier PF, Van Mourik M, Catsman-Berrevoets CE. Long-term sequelae in children after cerebellar astrocytoma surgery. Neurology. 2004;62(8):1311–1316. doi: 10.1212/01.wnl.0000120549.77188.36. [DOI] [PubMed] [Google Scholar]
- Anderson VA, Anderson P, Northam E, Jacobs R, Mikiewicz O. Relationships between cognitive and behavioral measures of executive function in children with brain disease. Child Neuropsychol. 2002;8(4):231–240. doi: 10.1076/chin.8.4.231.13509. [DOI] [PubMed] [Google Scholar]
- Anderson V, Anderson D, Anderson P. Comparing attentional skills in children with acquired and developmental central nervous system disorders. J Int Neuropsychol Soc. 2006;12(4):519–531. doi: 10.1017/s135561770606067x. [DOI] [PubMed] [Google Scholar]
- Arnett AB, Peterson RL, Kirkwood MW, Taylor HG, Stancin T, Brown TM, et al. Behavioral and cognitive predictors of educational outcomes in pediatric traumatic brain injury. J Int Neuropsychol Soc. 2013;19(8):881–889. doi: 10.1017/S1355617713000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ater JL, Moore BD, 3rd, Francis DJ, Castillo R, Slopis J, Copeland DR. Correlation of medical and neurosurgical events with neuropsychological status in children at diagnosis of astrocytoma: utilization of a neurological severity score. J Child Neurol. 1996;11(6):462–469. doi: 10.1177/088307389601100610. [DOI] [PubMed] [Google Scholar]
- Azuma R, Daly EM, Campbell LE, Stevens AF, Deeley Q, Giampietro V, et al. Visuospatial working memory in children and adolescents with 22q11.2 deletion syndrome; an fMRI study. J Neurodev Disord. 2009;1(1):46–60. doi: 10.1007/s11689-009-9008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A. Working memory. Science. 1992;255(5044):556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Barnes MA, Wilkinson M, Khemani E, Boudesquie A, Dennis M, Fletcher JM. Arithmetic processing in children with spina bifida: Calculation accuracy, strategy use, and fact retrieval fluency. J Learn Disabil. 2006;39(2):174–187. doi: 10.1177/00222194060390020601. [DOI] [PubMed] [Google Scholar]
- Belanger HG, Spiegel E, Vanderploeg RD. Neuropsychological performance following a history of multiple self-reported concussions: a meta-analysis. J Int Neuropsychol Soc. 2010;16(2):262–267. doi: 10.1017/S1355617709991287. [DOI] [PubMed] [Google Scholar]
- Bendersky M, Lewis M. Effects of intraventricular hemorrhage and other medical and environmental risks on multiple outcomes at age three years. J Dev Behav Pediatr. 1995;16(2):89–96. [PubMed] [Google Scholar]
- Bernier A, Carlson SM, Whipple N. From external regulation to self-regulation: early parenting precursors of young children's executive functioning. Child Dev. 2010;81(1):326–339. doi: 10.1111/j.1467-8624.2009.01397.x. [DOI] [PubMed] [Google Scholar]
- Bigler ED, Abildskov TJ, Petrie J, Farrer TJ, Dennis M, Simic N, et al. Heterogeneity of brain lesions in pediatric traumatic brain injury. Neuropsychology. 2013;27(4):438–451. doi: 10.1037/a0032837. [DOI] [PubMed] [Google Scholar]
- Birenbaum G. Das vergessen einer vorahme (Forgetting a future intention) Psychologische Forschung. 1930;13:218–284. [Google Scholar]
- Blair RJ. Fine cuts of empathy and the amygdala: dissociable deficits in psychopathy and autism. Q J Exp Psychol (Hove) 2008;61(1):157–170. doi: 10.1080/17470210701508855. [DOI] [PubMed] [Google Scholar]
- Bonfield CM, Lam S, Lin Y, Greene S. The impact of attention deficit hyperactivity disorder on recovery from mild traumatic brain injury. J Neurosurg Pediatr. 2013;12(2):97–102. doi: 10.3171/2013.5.PEDS12424. [DOI] [PubMed] [Google Scholar]
- Braddick O, Atkinson J, Hood B, Harkness W, Jackson G, Vargha-Khadem F. Possible blindsight in infants lacking one cerebral hemisphere. Nature. 1992;360(6403):461–463. doi: 10.1038/360461a0. [DOI] [PubMed] [Google Scholar]
- Brewer VR, Fletcher JM, Hiscock M, Davidson KC. Attention processes in children with shunted hydrocephalus versus attention deficit-hyperactivity disorder. Neuropsychology. 2001;15(2):185–198. doi: 10.1037//0894-4105.15.2.185. [DOI] [PubMed] [Google Scholar]
- Brown RL. Epidemiology of injury and the impact of health disparities. Curr Opin Pediatr. 2010;22(3):321–325. doi: 10.1097/MOP.0b013e3283395f13. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Keilp JG, Xu H, Shikhman M, Schori E, Gorman JM, et al. Catechol-O-methyltransferase (COMT) genotypes and working memory: associations with differing cognitive operations. Biol Psychiatry. 2005;58(11):901–907. doi: 10.1016/j.biopsych.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Buizer AI, de Sonneville LM, Veerman AJ. Effects of chemotherapy on neurocognitive function in children with acute lymphoblastic leukemia: a critical review of the literature. Pediatr Blood Cancer. 2009;52(4):447–454. doi: 10.1002/pbc.21869. [DOI] [PubMed] [Google Scholar]
- Bull R, Scerif G. Executive functioning as a predictor of children's mathematics ability: inhibition, switching, and working memory. Dev Neuropsychol. 2001;19(3):273–293. doi: 10.1207/S15326942DN1903_3. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cohen ME, Duffner PK. Brain tumors in children: principles of diagnosis and treatment. J Child Neurol. 1994;10(5):415–416. [Google Scholar]
- Collins MW, Lovell MR, Iverson GL, Cantu RC, Maroon JC, Field M. Cumulative effects of concussion in high school athletes. Neurosurgery. 2002;51(5):1175–1179. doi: 10.1097/00006123-200211000-00011. [DOI] [PubMed] [Google Scholar]
- Conklin HM, Ashford JM, Di Pinto M, Vaughan CG, Gioia GA, Merchant TE, et al. Computerized assessment of cognitive late effects among adolescent brain tumor survivors. J Neurooncol. 2013;113(2):333–340. doi: 10.1007/s11060-013-1123-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin HM, Krull KR, Reddick WE, Pei D, Cheng C, Pui CH. Cognitive outcomes following contemporary treatment without cranial irradiation for childhood acute lymphoblastic leukemia. J Natl Cancer Inst. 2012;104(18):1386–1395. doi: 10.1093/jnci/djs344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowey A, Stoerig P. The neurobiology of blindsight. Trends Neurosci. 1991;14(4):140–145. doi: 10.1016/0166-2236(91)90085-9. [DOI] [PubMed] [Google Scholar]
- Craik FIM. A functional account of age differences in memory. In: Klix F, Hagendorf H, editors. Human memory and cognitive capabilities: Mechanisms and performances. Amsterdam: Elsevier-North-Holland; 1986. pp. 409–422. [Google Scholar]
- Dennis M. Clinical Neuropsychology and Brain Function: Research, Measurement and Practice (Master Lecture Series, Volume 7) Washington: American Psychological Association; 1988. Language and the young damaged brain; pp. 85–123. [Google Scholar]
- Dennis M. Margaret Kennard (1899-1975): not a ‘principle’ of brain plasticity but a founding mother of developmental neuropsychology. Cortex. 2010;46(8):1043–1059. doi: 10.1016/j.cortex.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, Agostino A, Roncadin C, Levin H. Theory of mind depends on domain-general executive functions of working memory and cognitive inhibition in children with traumatic brain injury. J Clin Exp Neuropsychol. 2009;31(7):835–847. doi: 10.1080/13803390802572419. [DOI] [PubMed] [Google Scholar]
- Dennis M, Barnes M. Math and numeracy in young adults with spina bifida and hydrocephalus. Dev Neuropsychol. 2002;21(2):141–155. doi: 10.1207/S15326942DN2102_2. [DOI] [PubMed] [Google Scholar]
- Dennis M, Barnes MA, Donnelly RE, Wilkinson M, Humphreys RP. Appraising and managing knowledge: Metacognitive skills after childhood head injury. Dev Neuropsychol. 1996:77–103. [Google Scholar]
- Dennis M, Berch DB, Mazzocco MM. Mathematical learning disabilities in special populations: phenotypic variation and cross-disorder comparisons. Dev Disabil Res Rev. 2009;15(1):80–89. doi: 10.1002/ddrr.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, Fletcher JM, Rogers T, Hetherington R, Francis DJ. Object-based and action-based visual perception in children with spina bifida and hydrocephalus. J Int Neuropsychol Soc. 2002;8(1):95–106. [PubMed] [Google Scholar]
- Dennis M, Jewell D, Drake J, Misakyan T, Spiegler B, Hetherington R, et al. Prospective, declarative, and nondeclarative memory in young adults with spina bifida. J Int Neuropsychol Soc. 2007;13(2):312–323. doi: 10.1017/S1355617707070336. [DOI] [PubMed] [Google Scholar]
- Dennis M, Landry SH, Barnes M, Fletcher JM. A model of neurocognitive function in spina bifida over the life span. J Int Neuropsychol Soc. 2006;12(2):285–296. doi: 10.1017/S1355617706060371. [DOI] [PubMed] [Google Scholar]
- Dennis M, Salman MS, Jewell D, Hetherington R, Spiegler BJ, MacGregor DL, et al. Upper limb motor function in young adults with spina bifida and hydrocephalus. Childs Nerv Syst. 2009;25(11):1447–1453. doi: 10.1007/s00381-009-0948-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, Salman MS, Juranek J, Fletcher JM. Cerebellar motor function in spina bifida meningomyelocele. Cerebellum. 2010;9(4):484–498. doi: 10.1007/s12311-010-0191-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, Simic N, Agostino A, Taylor HG, Bigler ED, Rubin K, et al. Irony and empathy in children with traumatic brain injury. J Int Neuropsychol Soc. 2013;19(3):338–348. doi: 10.1017/S1355617712001440. [DOI] [PubMed] [Google Scholar]
- Dennis M, Simic N, Bigler ED, Abildskov T, Agostino A, Taylor HG, et al. Cognitive, affective, and conative theory of mind (ToM) in children with traumatic brain injury. Dev Cogn Neurosci. 2013;5C:25–39. doi: 10.1016/j.dcn.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, Simic N, Gerry Taylor H, Bigler ED, Rubin K, Vannatta K, et al. Theory of mind in children with traumatic brain injury. J Int Neuropsychol Soc. 2012;18(5):908–916. doi: 10.1017/S1355617712000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, Spiegler BJ, Juranek JJ, Bigler ED, Snead OC, Fletcher JM. Age, plasticity, and homeostasis in childhood brain disorders. Neurosci Biobehav Rev. 2013 doi: 10.1016/j.neubiorev.2013.09.010. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A, Briand L, Fossella J, Gehlbach L. Genetic and neurochemical modulation of prefrontal cognitive functions in children. Am J Psychiatry. 2004;161(1):125–132. doi: 10.1176/appi.ajp.161.1.125. [DOI] [PubMed] [Google Scholar]
- DiCorcia JA, Tronick E. Quotidian resilience: exploring mechanisms that drive resilience from a perspective of everyday stress and coping. Neurosci Biobehav Rev. 2011;35(7):1593–1602. doi: 10.1016/j.neubiorev.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Dowson JH, McLean A, Bazanis E, Toone B, Young S, Robbins TW, et al. Impaired spatial working memory in adults with attention-deficit/hyperactivity disorder: comparisons with performance in adults with borderline personality disorder and in control subjects. Acta Psychiatr Scand. 2004;110(1):45–54. doi: 10.1111/j.1600-0447.2004.00292.x. [DOI] [PubMed] [Google Scholar]
- Edelstein K, Spiegler BJ, Fung S, Panzarella T, Mabbott DJ, Jewitt N, et al. Early aging in adult survivors of childhood medulloblastoma: long-term neurocognitive, functional, and physical outcomes. Neuro Oncol. 2011;13(5):536–545. doi: 10.1093/neuonc/nor015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg MA, Andrea J, Meehan W, Mannix R. Time interval between concussions and symptom duration. Pediatrics. 2013;132(1):8–17. doi: 10.1542/peds.2013-0432. [DOI] [PubMed] [Google Scholar]
- Eldredge N, Gould SJ. Punctuated equilibria: an alternative to phyletic gradualism. In: Schopf TJM, editor. Models in Paleobiology. San Francisco: Freeman Cooper, Reprinted in N Eldredge Time frames; Princeton: Princeton Univ. Press; 1972. pp. 82–115.pp. 193–223. 1985. [Google Scholar]
- Farah MJ, Betancourt L, Shera DM, Savage JH, Giannetta JM, Brodsky NL, et al. Environmental stimulation, parental nurturance and cognitive development in humans. Dev Sci. 2008;11(5):793–801. doi: 10.1111/j.1467-7687.2008.00688.x. [DOI] [PubMed] [Google Scholar]
- Farmer JE, Kanne SM, Haut JS, Williams J, Johnstone B, Kirk K. Memory functioning following traumatic brain injury in children with premorbid learning problems. Dev Neuropsychol. 2002;22(2):455–469. doi: 10.1207/S15326942DN2202_2. [DOI] [PubMed] [Google Scholar]
- Farooqi A, Hagglof B, Sedin G, Gothefors L, Serenius F. Mental health and social competencies of 10- to 12-year-old children born at 23 to 25 weeks of gestation in the 1990s: a Swedish national prospective follow-up study. Pediatrics. 2007;120(1):118–133. doi: 10.1542/peds.2006-2988. [DOI] [PubMed] [Google Scholar]
- Fastenau PS, Johnson CS, Perkins SM, Byars AW, deGrauw TJ, Austin JK, et al. Neuropsychological status at seizure onset in children: risk factors for early cognitive deficits. Neurology. 2009;73(7):526–534. doi: 10.1212/WNL.0b013e3181b23551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fastenau PS, Shen J, Dunn DW, Perkins SM, Hermann BP, Austin JK. Neuropsychological predictors of academic underachievement in pediatric epilepsy: moderating roles of demographic, seizure, and psychosocial variables. Epilepsia. 2004;45(10):1261–1272. doi: 10.1111/j.0013-9580.2004.15204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay TB, Yeates KO, Wade SL, Drotar D, Stancin T, Taylor HG. Predicting longitudinal patterns of functional deficits in children with traumatic brain injury. Neuropsychology. 2009;23(3):271–282. doi: 10.1037/a0014936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez VG, Stuebing K, Juranek J, Fletcher JM. Volumetric analysis of regional variability in the cerebellum of children with dyslexia. Cerebellum. 2013;12(6):906–915. doi: 10.1007/s12311-013-0504-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JM. Alternative approaches to outcomes assessment: Beyond psychometric tests. Pediatr Blood Cancer. 2013 doi: 10.1002/pbc.24824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JM, Copeland K, Frederick JA, Blaser SE, Kramer LA, Northrup H, et al. Spinal lesion level in spina bifida: a source of neural and cognitive heterogeneity. J Neurosurg. 2005;102(3 Suppl):268–279. doi: 10.3171/ped.2005.102.3.0268. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Ewing-Cobbs L, Miner ME, Levin HS, Eisenberg HM. Behavioral changes after closed head injury in children. J Consult Clin Psychol. 1990;58(1):93–98. doi: 10.1037//0022-006x.58.1.93. [DOI] [PubMed] [Google Scholar]
- Friedman D, Holmbeck GN, Jandasek B, Zukerman J, Abad M. Parent functioning in families of preadolescents with spina bifida: longitudinal implications for child adjustment. J Fam Psychol. 2004;18(4):609–619. doi: 10.1037/0893-3200.18.4.609. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philos Trans R Soc Lond B Biol Sci. 2003;358(1431):459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz M, Goodman D, Weinberg H. Electrophysiological evidence for the cumulative effects of concussion. Brain Inj. 2000;14(12):1077–1088. doi: 10.1080/02699050050203577. [DOI] [PubMed] [Google Scholar]
- Gathercole SE, Baddeley AD. Sense and sensitivity in phonological memory and vocabulary development: a reply to Bowey (1996) J Exp Child Psychol. 1997;67(2):290–294. doi: 10.1006/jecp.1997.2391. [DOI] [PubMed] [Google Scholar]
- Gathercole SE, Pickering SJ, Ambridge B, Wearing H. The structure of working memory from 4 to 15 years of age. Dev Psychol. 2004;40(2):177–190. doi: 10.1037/0012-1649.40.2.177. [DOI] [PubMed] [Google Scholar]
- Gross SJ, Mettelman BB, Dye TD, Slagle TA. Impact of family structure and stability on academic outcome in preterm children at 10 years of age. J Pediatr. 2001;138(2):169–175. doi: 10.1067/mpd.2001.111945. [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM, McCrea M, Marshall SW, Cantu RC, Randolph C, Barr W, et al. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290(19):2549–2555. doi: 10.1001/jama.290.19.2549. [DOI] [PubMed] [Google Scholar]
- Hack M, Breslau N, Aram D, Weissman B, Klein N, Borawski-Clark E. The effect of very low birth weight and social risk on neurocognitive abilities at school age. J Dev Behav Pediatr. 1992;13(6):412–420. [PubMed] [Google Scholar]
- Hackman DA, Farah MJ. Socioeconomic status and the developing brain. Trends Cogn Sci. 2009;13(2):65–73. doi: 10.1016/j.tics.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenlein M, Caul WF. Attention deficit disorder with hyperactivity: a specific hypothesis of reward dysfunction. J Am Acad Child Adolesc Psychiatry. 1987;26(3):356–362. doi: 10.1097/00004583-198705000-00014. [DOI] [PubMed] [Google Scholar]
- Hagberg B. Clinical manifestations and stages of Rett syndrome. Ment Retard Dev Disabil Res Rev. 2002;8(2):61–65. doi: 10.1002/mrdd.10020. [DOI] [PubMed] [Google Scholar]
- Hampton LE, Fletcher JM, Cirino P, Blaser S, Kramer LA, Dennis M. Neuropsychological profiles of children with aqueductal stenosis and spina bifida myelomeningocele. J Int Neuropsychol Soc. 2013;19(2):127–136. doi: 10.1017/S1355617712001117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton LE, Fletcher JM, Cirino PT, Blaser S, Kramer LA, Drake J, et al. Hydrocephalus status in spina bifida: an evaluation of variations in neuropsychological outcomes. J Neurosurg Pediatr. 2011;8(3):289–298. doi: 10.3171/2011.6.PEDS10584. [DOI] [PubMed] [Google Scholar]
- Hanten G, Dennis M, Zhang L, Barnes M, Roberson G, Archibald J, et al. Childhood head injury and metacognitive processes in language and memory. Dev Neuropsychol. 2004;25(1-2):85–106. doi: 10.1080/87565641.2004.9651923. [DOI] [PubMed] [Google Scholar]
- Hanten G, Levin HS, Song JX. Working memory and metacognition in sentence comprehension by severely head-injured children: A preliminary study with implications for rehabilitation. Dev Neuropsychol. 1999;16(3):393–414. [Google Scholar]
- Hardy KK, Bonner MJ, Willard VW, Watral MA, Gururangan S. Hydrocephalus as a possible additional contributor to cognitive outcome in survivors of pediatric medulloblastoma. Psychooncology. 2008;17(11):1157–1161. doi: 10.1002/pon.1349. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Eluvathingal TJ, Kramer LA, Ewing-Cobbs L, Dennis M, Fletcher JM. White matter microstructural abnormalities in children with spina bifida myelomeningocele and hydrocephalus: a diffusion tensor tractography study of the association pathways. J Magn Reson Imaging. 2008;27(4):700–709. doi: 10.1002/jmri.21297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004;10(3):317–331. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- Hein G, Singer T. I feel how you feel but not always: the empathic brain and its modulation. Curr Opin Neurobiol. 2008;18(2):153–158. doi: 10.1016/j.conb.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Hernaus D, Collip D, Lataster J, Ceccarini J, Kenis G, Booij L, et al. COMT Val158Met genotype selectively alters prefrontal [18F]fallypride displacement and subjective feelings of stress in response to a psychosocial stress challenge. PLoS One. 2013;8(6):e65662. doi: 10.1371/journal.pone.0065662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington R, Dennis M. Plasticity for recovery, plasticity for development: cognitive outcome in twins discordant for mid-childhood ischemic stroke. Child Neuropsychol. 2004;10(2):117–128. doi: 10.1080/09297040490911122. [DOI] [PubMed] [Google Scholar]
- Hetherington R, Dennis M, Barnes M, Drake J, Gentili F. Functional outcome in young adults with spina bifida and hydrocephalus. Childs Nerv Syst. 2006;22(2):117–124. doi: 10.1007/s00381-005-1231-4. [DOI] [PubMed] [Google Scholar]
- Hopyan T, Laughlin S, Dennis M. Emotions and their cognitive control in children with cerebellar tumors. J Int Neuropsychol Soc. 2010;16(6):1027–1038. doi: 10.1017/S1355617710000974. [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Stellern SA, Schaefer C, Carlson SM, Gunnar MR. Associations between early life adversity and executive function in children adopted internationally from orphanages. Proc Natl Acad Sci U S A. 2012;109:17208–17212. doi: 10.1073/pnas.1121246109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard I, Joseph JG, Natale JE. Pediatric traumatic brain injury: do racial/ethnic disparities exist in brain injury severity, mortality, or medical disposition? Ethn Dis. 2005;15(4 Suppl 5):S5-51–56. [PubMed] [Google Scholar]
- Howlin P, Davies M, Udwin O. Cognitive functioning in adults with Williams syndrome. J Child Psychol Psychiatry. 1998;39(2):183–189. [PubMed] [Google Scholar]
- Hu S, Jin H, Chen Z, Mo L, Liu J. Failure in developing high-level visual functions after occipitoparietal lesions at an early age: A case study. Cortex. 2013 doi: 10.1016/j.cortex.2013.07.009. [DOI] [PubMed] [Google Scholar]
- Huang-Pollock CL, Nigg JT. Searching for the attention deficit in attention deficit hyperactivity disorder: the case of visuospatial orienting. Clin Psychol Rev. 2003;23(6):801–830. doi: 10.1016/s0272-7358(03)00073-4. [DOI] [PubMed] [Google Scholar]
- Hultsch DF, Strauss E, Hunter MA, MacDonald SWS. Intraindividual variability, cognition, and aging. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. 3rd. New York: Psychology Press; 2008. pp. 491–556. [Google Scholar]
- Hunt GM, Oakeshott P, Kerry S. Link between the CSF shunt and achievement in adults with spina bifida. J Neurol Neurosurg Psychiatry. 1999;67(5):591–595. doi: 10.1136/jnnp.67.5.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isquith PK, Roth RM, Gioia G. Contribution of rating scales to the assessment of executive functions. Appl Neuropsychol Child. 2013;2(2):125–132. doi: 10.1080/21622965.2013.748389. [DOI] [PubMed] [Google Scholar]
- Iverson GL, Gaetz M, Lovell MR, Collins MW. Cumulative effects of concussion in amateur athletes. Brain Inj. 2004;18(5):433–443. doi: 10.1080/02699050310001617352. [DOI] [PubMed] [Google Scholar]
- Janzen LA, Spiegler BJ. Neurodevelopmental sequelae of pediatric acute lymphoblastic leukemia and its treatment. Dev Disabil Res Rev. 2008;14(3):185–195. doi: 10.1002/ddrr.24. [DOI] [PubMed] [Google Scholar]
- Jaspar M, Genon S, Muto V, Meyer C, Manard M, Dideberg V, et al. Modulating effect of COMT genotype on the brain regions underlying proactive control process during inhibition. Cortex. 2013 doi: 10.1016/j.cortex.2013.1006.1003. In press. [DOI] [PubMed] [Google Scholar]
- Jewell D, Fletcher JM, Mahy CE, Hetherington R, MacGregor D, Drake JM, et al. Upper limb cerebellar motor function in children with spina bifida. Childs Nerv Syst. 2010;26(1):67–73. doi: 10.1007/s00381-009-0991-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Schachar R. Methylphenidate treatment of attention-deficit/hyperactivity disorder secondary to traumatic brain injury: a critical appraisal of treatment studies. CNS Spectr. 2004;9(3):217–226. doi: 10.1017/s1092852900009019. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Developmental cognitive neuroscience. Cambridge, Mass: Blackwell Publishers; 1997. [Google Scholar]
- Jonides J. Invitation to Cognitive Science: Thinking. Cambridge, MA: MIT Press; 1995. Working memory and thinking; pp. 215–265. [Google Scholar]
- Juranek J, Dennis M, Cirino PT, El-Messidi L, Fletcher JM. The cerebellum in children with spina bifida and Chiari II malformation: Quantitative volumetrics by region. Cerebellum. 2010;9(2):240–248. doi: 10.1007/s12311-010-0157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. Working-memory capacity and the control of attention: the contributions of goal neglect, response competition, and task set to Stroop interference. J Exp Psychol Gen. 2003;132(1):47–70. doi: 10.1037/0096-3445.132.1.47. [DOI] [PubMed] [Google Scholar]
- Karmiloff-Smith A. Neuroimaging of the developing brain: taking “developing” seriously. Hum Brain Mapp. 2010;31(6):934–941. doi: 10.1002/hbm.21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzman R. Education and the prevalence of dementia and Alzheimer's disease. Neurology. 1993;43(1):13–20. doi: 10.1212/wnl.43.1_part_1.13. [DOI] [PubMed] [Google Scholar]
- Kerns KA. The CyberCruiser: an investigation of development of prospective memory in children. J Int Neuropsychol Soc. 2000;6(1):62–70. doi: 10.1017/s1355617700611074. [DOI] [PubMed] [Google Scholar]
- Kerns KA, Price KJ. An investigation of prospective memory in children with ADHD. Child Neuropsychol. 2001;7(3):162–171. doi: 10.1076/chin.7.3.162.8744. [DOI] [PubMed] [Google Scholar]
- Kitsao-Wekulo P, Holding P, Taylor HG, Abubakar A, Kvalsvig J, Connolly K. Nutrition as an important mediator of the impact of background variables on outcome in middle childhood. Frontiers in Human Neuroscience. 2013;7:713. doi: 10.3389/fnhum.2013.00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn B, Dennis M. Somatosensory functions after cerebral hemidecortication for infantile hemiplegia. Neuropsychologia. 1974;12(1):119–130. doi: 10.1016/0028-3932(74)90033-5. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM. Seeing and imagining in the cerebral hemispheres: a computational approach. Psychol Rev. 1987;94(2):148–175. [PubMed] [Google Scholar]
- Koziol LF, Budding D, Andreasen N, D'Arrigo S, Bulgheroni S, Imamizu H, et al. Consensus Paper: The Cerebellum's Role in Movement and Cognition. Cerebellum. 2013 doi: 10.1007/s12311-013-0511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krusch DA, Klorman R, Brumaghim JT, Fitzpatrick PA, Borgstedt AD, Strauss J. Methylphenidate slows reactions of children with attention deficit disorder during and after an error. J Abnorm Child Psychol. 1996;24(5):633–650. doi: 10.1007/BF01670104. [DOI] [PubMed] [Google Scholar]
- Kvavilashvili L. Remembering intentions: A critical review of existing experimental paradigms. Applied Cognitive Psychology. 1992;6(6):507–524. [Google Scholar]
- Kvavilashvili L, Kyle FE, Messer DJ. The development of prospective memory in children: Methodological issues, empirical findings and future directions. In: Kliegel M, McDaniel MA, Einstein GO, editors. Prospective memory: Cognitive, neuroscience, developmental, and applied perspectives. New York: Psychology Press; 2007. pp. 113–140. [Google Scholar]
- Lachman HM, Morrow B, Shprintzen R, Veit S, Parsia SS, Faedda G, et al. Association of codon 108/158 catechol-O-methyltransferase gene polymorphism with the psychiatric manifestations of velo-cardio-facial syndrome. Am J Med Genet. 1996;67(5):468–472. doi: 10.1002/(SICI)1096-8628(19960920)67:5<468::AID-AJMG5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Landry SH, Miller-Loncar CL, Smith KE, Swank PR. The role of early parenting in children's development of executive processes. Dev Neuropsychol. 2002;21(1):15–41. doi: 10.1207/S15326942DN2101_2. [DOI] [PubMed] [Google Scholar]
- Landry SH, Smith KE, Miller-Loncar CL, Swank PR. Predicting cognitive-language and social growth curves from early maternal behaviors in children at varying degrees of biological risk. Dev Psychol. 1997;33(6):1040–1053. doi: 10.1037//0012-1649.33.6.1040. [DOI] [PubMed] [Google Scholar]
- Landry SH, Smith KE, Miller-Loncar CL, Swank PR. The relation of change in maternal interactive styles to the developing social competence of full-term and preterm children. Child Dev. 1998;69(1):105–123. [PubMed] [Google Scholar]
- Landry SH, Smith KE, Swank PR. Responsive parenting: establishing early foundations for social, communication, and independent problem-solving skills. Dev Psychol. 2006;42(4):627–642. doi: 10.1037/0012-1649.42.4.627. [DOI] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Thomas KE. The incidence of traumatic brain injury among children in the United States: differences by race. J Head Trauma Rehabil. 2005;20(3):229–238. doi: 10.1097/00001199-200505000-00006. [DOI] [PubMed] [Google Scholar]
- Laucht M, Esser G, Schmidt MH. Developmental outcome of infants born with biological and psychosocial risks. J Child Psychol Psychiatry. 1997;38(7):843–853. doi: 10.1111/j.1469-7610.1997.tb01602.x. [DOI] [PubMed] [Google Scholar]
- Lavie N, Hirst A, de Fockert JW, Viding E. Load theory of selective attention and cognitive control. J Exp Psychol Gen. 2004;133(3):339–354. doi: 10.1037/0096-3445.133.3.339. [DOI] [PubMed] [Google Scholar]
- Lawrence V, Houghton S, Douglas G, Durkin K, Whiting K, Tannock R. Executive function and ADHD: a comparison of children's performance during neuropsychological testing and real-world activities. J Atten Disord. 2004;7(3):137–149. doi: 10.1177/108705470400700302. [DOI] [PubMed] [Google Scholar]
- Leblanc N, Chen S, Swank PR, Ewing-Cobbs L, Barnes M, Dennis M, et al. Response inhibition after traumatic brain injury (TBI) in children: impairment and recovery. Dev Neuropsychol. 2005;28(3):829–848. doi: 10.1207/s15326942dn2803_5. [DOI] [PubMed] [Google Scholar]
- Leve LD, Kerr DC, Shaw D, Ge X, Neiderhiser JM, Scaramella LV, et al. Infant pathways to externalizing behavior: evidence of genotype x environment interaction. Child Dev. 2010;81(1):340–356. doi: 10.1111/j.1467-8624.2009.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin HH, Culhane KA, Hartmann J, Evankovich K, Mattson AJ. Developmental changes in performance on tests of purported frontal lobe functioning. Dev Neuropsychol. 1991;7:377–395. [Google Scholar]
- Levin H, Hanten G, Max J, Li X, Swank P, Ewing-Cobbs L, et al. Symptoms of attention-deficit/hyperactivity disorder following traumatic brain injury in children. J Dev Behav Pediatr. 2007;28(2):108–118. doi: 10.1097/01.DBP.0000267559.26576.cd. [DOI] [PubMed] [Google Scholar]
- Levin HS, Zhang L, Dennis M, Ewing-Cobbs L, Schachar R, Max J, et al. Psychosocial outcome of TBI in children with unilateral frontal lesions. J Int Neuropsychol Soc. 2004;10(3):305–316. doi: 10.1017/S1355617704102129. [DOI] [PubMed] [Google Scholar]
- Lewin K. Intention, will, and nee. In: Shipley T, editor. Classics in psychology. New York: Philosophical Library; 1961. pp. 1234–1288. Original work published 1926. [Google Scholar]
- Li SC. Neuromodulation and developmental contextual influences on neural and cognitive plasticity across the lifespan. Neurosci Biobehav Rev. 2013 doi: 10.1016/j.neubiorev.2013.07.019. [DOI] [PubMed] [Google Scholar]
- Lipszyc J, Schachar R. Inhibitory control and psychopathology: a meta-analysis of studies using the stop signal task. J Int Neuropsychol Soc. 2010;16(6):1064–1076. doi: 10.1017/S1355617710000895. [DOI] [PubMed] [Google Scholar]
- Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, et al. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cereb Cortex. 2006;16(4):553–560. doi: 10.1093/cercor/bhj003. [DOI] [PubMed] [Google Scholar]
- Litt JS, Taylor HG, Margevicius S, Schluchter M, Andreias L, Hack M. Academic achievement of adolescents born with extremely low birth weight. Acta Paediatr. 2012;101(12):1240–1245. doi: 10.1111/j.1651-2227.2012.02790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD. On the ability to inhibit simple thoughts and actions: II. Stop-signal studies of repetition priming. Journal of Experimental Psychology. 1985;11(4):675–691. [Google Scholar]
- Logan GD. On the ability to inhibit thought and action: A users' guide to the stop signal paradigm. In: Dagenbach D, Carr TH, editors. Inhibitory processes in attention, memory, and language. San Diego, CA, US: Academic Press; 1994. pp. 189–239. [Google Scholar]
- Lomax-Bream LE, Taylor HB, Landry SH, Barnes MA, Fletcher JM, Swank P. Role of early parenting and motor skills on development in children with spina bifida. Journal of Applied Developmental Psychology. 2007;28(3):250–263. [Google Scholar]
- Lott IT, Doran E, Nguyen VQ, Tournay A, Movsesyan N, Gillen DL. Down syndrome and dementia: seizures and cognitive decline. J Alzheimers Dis. 2012;29(1):177–185. doi: 10.3233/JAD-2012-111613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lövdén M, Li SC, Shing YL, Lindenberger U. Within-person trial-to-trial variability precedes and predicts cognitive decline in old and very old age: longitudinal data from the Berlin Aging Study. Neuropsychologia. 2007;45(12):2827–2838. doi: 10.1016/j.neuropsychologia.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Luciana M. Cognitive development in children born preterm: implications for theories of brain plasticity following early injury. Dev Psychopathol. 2003;15(4):1017–1047. doi: 10.1017/s095457940300049x. [DOI] [PubMed] [Google Scholar]
- Luciana M, Conklin HM, Hooper CJ, Yarger RS. The development of nonverbal working memory and executive control processes in adolescents. Child Dev. 2005;76(3):697–712. doi: 10.1111/j.1467-8624.2005.00872.x. [DOI] [PubMed] [Google Scholar]
- Luman M, Oosterlaan J, Sergeant JA. The impact of reinforcement contingencies on AD/HD: a review and theoretical appraisal. Clin Psychol Rev. 2005;25(2):183–213. doi: 10.1016/j.cpr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Macri S. Resilience and adaptive aspects of stress in neurobehavioral development. Neurosci Biobehav Rev. 2011;35(7):1451. doi: 10.1016/j.neubiorev.2010.09.004. [DOI] [PubMed] [Google Scholar]
- MacAllister WS, Bender HA, Whitman L, Welsh A, Keller S, Granader Y, et al. Assessment of executive functioning in childhood epilepsy: the Tower of London and BRIEF. Child Neuropsychol. 2012;18(4):404–415. doi: 10.1080/09297049.2011.613812. [DOI] [PubMed] [Google Scholar]
- Macri S. On the incongruity between developmental plasticity and methodological rigidity. Front Behav Neurosci. 2013;6:93. doi: 10.3389/fnbeh.2012.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney G, Wheeden CA, Perales F. Relationship of preschool special education outcomes to instructional practices and parent-child interaction. Res Dev Disabil. 2004;25(6):539–558. doi: 10.1016/j.ridd.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Margallo-Lana ML, Moore PB, Kay DW, Perry RH, Reid BE, Berney TP, et al. Fifteen-year follow-up of 92 hospitalized adults with Down's syndrome: incidence of cognitive decline, its relationship to age and neuropathology. J Intellect Disabil Res. 2007;51(Pt. 6):463–477. doi: 10.1111/j.1365-2788.2006.00902.x. [DOI] [PubMed] [Google Scholar]
- Masten AS. Ordinary magic. Resilience processes in development. Am Psychol. 2001;56(3):227–238. doi: 10.1037//0003-066x.56.3.227. [DOI] [PubMed] [Google Scholar]
- Maylor EA, Logie RH. A large-scale comparison of prospective and retrospective memory development from childhood to middle age. Q J Exp Psychol (Hove) 2010;63(3):442–451. doi: 10.1080/17470210903469872. [DOI] [PubMed] [Google Scholar]
- McAuley T, Chen S, Goos L, Schachar R, Crosbie J. Is the behavior rating inventory of executive function more strongly associated with measures of impairment or executive function? J Int Neuropsychol Soc. 2010;16(3):495–505. doi: 10.1017/S1355617710000093. [DOI] [PubMed] [Google Scholar]
- McCauley SR, Levin HS. Prospective memory in pediatric traumatic brain injury: a preliminary study. Dev Neuropsychol. 2004;25(1-2):5–20. doi: 10.1080/87565641.2004.9651919. [DOI] [PubMed] [Google Scholar]
- McClelland MM, Kessenich M, Morrison FJ. Pathways to early literacy: the complex interplay of child, family, and sociocultural factors. Adv Child Dev Behav. 2003;31:411–447. doi: 10.1016/s0065-2407(03)31010-9. [DOI] [PubMed] [Google Scholar]
- McKinlay A, Grace RC, Horwood LJ, Fergusson DM, MacFarlane MR. Long-term behavioural outcomes of pre-school mild traumatic brain injury. Child Care Health Dev. 2010;36(1):22–30. doi: 10.1111/j.1365-2214.2009.00947.x. [DOI] [PubMed] [Google Scholar]
- McLellan T, McKinlay A. Sensitivity to emotion, empathy and theory of mind: adult performance following childhood TBI. Brain Inj. 2013;27(9):1032–1037. doi: 10.3109/02699052.2013.794965. [DOI] [PubMed] [Google Scholar]
- Metin B, Roeyers H, Wiersema JR, van der Meere J, Sonuga-Barke E. A meta-analytic study of event rate effects on Go/No-Go performance in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2012;72(12):990–996. doi: 10.1016/j.biopsych.2012.08.023. [DOI] [PubMed] [Google Scholar]
- Meyer ML, Lieberman MD. Social working memory: neurocognitive networks and directions for future research. Front Psychol. 2012;3:571. doi: 10.3389/fpsyg.2012.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LJ, Donders J. Prediction of educational outcome after pediatric traumatic brain injury. Rehabilitation Psychology. 2003;48(4):237. [Google Scholar]
- Moffitt TE, Caspi A, Rutter M. Strategy for investigating interactions between measured genes and measured environments. Arch Gen Psychiatry. 2005;62(5):473–481. doi: 10.1001/archpsyc.62.5.473. [DOI] [PubMed] [Google Scholar]
- Moleski M. Neuropsychological, neuroanatomical, and neurophysiological consequences of CNS chemotherapy for acute lymphoblastic leukemia. Arch Clin Neuropsychol. 2000;15(7):603–630. [PubMed] [Google Scholar]
- Neville HJ, Stevens C, Pakulak E, Bell TA, Fanning J, Klein S, et al. Family-based training program improves brain function, cognition, and behavior in lower socioeconomic status preschoolers. Proc Natl Acad Sci U S A. 2013;110(29):12138–12143. doi: 10.1073/pnas.1304437110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obradovic J, Bush NR, Stamperdahl J, Adler NE, Boyce WT. Biological sensitivity to context: the interactive effects of stress reactivity and family adversity on socioemotional behavior and school readiness. Child Dev. 2010;81(1):270–289. doi: 10.1111/j.1467-8624.2009.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson K, Jacobson KK, Van Oot P. Ecological validity of pediatric neuropsychological measures: current state and future directions. Appl Neuropsychol Child. 2013;2(1):17–23. doi: 10.1080/21622965.2012.686330. [DOI] [PubMed] [Google Scholar]
- Ornstein TJ, Levin HS, Chen S, Hanten G, Ewing-Cobbs L, Dennis M, et al. Performance monitoring in children following traumatic brain injury. J Child Psychol Psychiatry. 2009;50(4):506–513. doi: 10.1111/j.1469-7610.2008.01997.x. [DOI] [PubMed] [Google Scholar]
- Palmer SL, Armstrong C, Onar-Thomas A, Wu S, Wallace D, Bonner MJ, et al. Processing speed, attention, and working memory after treatment for medulloblastoma: an international, prospective, and longitudinal study. J Clin Oncol. 2013;31(28):3494–3500. doi: 10.1200/JCO.2012.47.4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer SL, Gajjar A, Reddick WE, Glass JO, Kun LE, Wu S, et al. Predicting intellectual outcome among children treated with 35-40 Gy craniospinal irradiation for medulloblastoma. Neuropsychology. 2003;17(4):548–555. doi: 10.1037/0894-4105.17.4.548. [DOI] [PubMed] [Google Scholar]
- Palmer SL, Goloubeva O, Reddick WE, Glass JO, Gajjar A, Kun L, et al. Patterns of intellectual development among survivors of pediatric medulloblastoma: a longitudinal analysis. J Clin Oncol. 2001;19(8):2302–2308. doi: 10.1200/JCO.2001.19.8.2302. [DOI] [PubMed] [Google Scholar]
- Parslow RC, Morris KP, Tasker RC, Forsyth RJ, Hawley CA. Epidemiology of traumatic brain injury in children receiving intensive care in the UK. Arch Dis Child. 2005;90(11):1182–1187. doi: 10.1136/adc.2005.072405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piaget J. The construction of reality in the child. New York: Basic Books; 1954. [Google Scholar]
- Pollak SD, Nelson CA, Schlaak MF, Roeber BJ, Wewerka SS, Wiik KL, et al. Neurodevelopmental effects of early deprivation in postinstitutionalized children. Child Dev. 2010;81(1):224–236. doi: 10.1111/j.1467-8624.2009.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitt P, Rodgers B. What does a man do after he makes an error? An analysis of response programming. Quarterly Journal of Experimental Psychology. 1977;19:727–743. [Google Scholar]
- Rabin LA, Burton LA, Barr WB. Utilization rates of ecologically oriented instruments among clinical neuropsychologists. Clin Neuropsychol. 2007;21(5):727–743. doi: 10.1080/13854040600888776. [DOI] [PubMed] [Google Scholar]
- Rabinowitz AR, Arnett PA. Intraindividual cognitive variability before and after sports-related concussion. Neuropsychology. 2013;27(4):481–490. doi: 10.1037/a0033023. [DOI] [PubMed] [Google Scholar]
- Raimondi AJ. A unifying theory for the definition and classification of hydrocephalus. Childs Nerv Syst. 1994;10(1):2–12. doi: 10.1007/BF00313578. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U. Life-span plasticity of the brain and cognition: From questions to evidence and back. Neurosci Biobehav Rev. 2013 doi: 10.1016/j.neubiorev.2013.10.003. Editorial. [DOI] [PubMed] [Google Scholar]
- Redmond KJ, Mahone EM, Terezakis S, Ishaq O, Ford E, McNutt T, et al. Association between radiation dose to neuronal progenitor cell niches and temporal lobes and performance on neuropsychological testing in children: a prospective study. Neuro Oncol. 2013;15(3):360–369. doi: 10.1093/neuonc/nos303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes SM, Riby DM, Fraser E, Campbell LE. The extent of working memory deficits associated with Williams syndrome: exploration of verbal and spatial domains and executively controlled processes. Brain Cogn. 2011;77(2):208–214. doi: 10.1016/j.bandc.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Ris MD, Beebe DW, Armstrong FD, Fontanesi J, Holmes E, Sanford RA, et al. Cognitive and adaptive outcome in extracerebellar low-grade brain tumors in children: a report from the Children's Oncology Group. J Clin Oncol. 2008;26(29):4765–4770. doi: 10.1200/JCO.2008.17.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romans SM, Stefanatos G, Roeltgen DP, Kushner H, Ross JL. Transition to young adulthood in Ullrich-Turner syndrome: neurodevelopmental changes. Am J Med Genet. 1998;79(2):140–147. [PubMed] [Google Scholar]
- Roncadin C, Dennis M, Greenberg ML, Spiegler BJ. Adverse medical events associated with childhood cerebellar astrocytomas and medulloblastomas: natural history and relation to very long-term neurobehavioral outcome. Childs Nerv Syst. 2008;24(9):995–1002. doi: 10.1007/s00381-008-0658-9. [DOI] [PubMed] [Google Scholar]
- Roncadin C, Pascual-Leone J, Rich JB, Dennis M. Developmental relations between working memory and inhibitory control. J Int Neuropsychol Soc. 2007;13(1):59–67. doi: 10.1017/S1355617707070099. [DOI] [PubMed] [Google Scholar]
- Ronning C, Sundet K, Due-Tonnessen B, Lundar T, Helseth E. Persistent cognitive dysfunction secondary to cerebellar injury in patients treated for posterior fossa tumors in childhood. Pediatr Neurosurg. 2005;41(1):15–21. doi: 10.1159/000084860. [DOI] [PubMed] [Google Scholar]
- Ross JL, Stefanatos G, Roeltgen D, Kushner H, Cutler GB., Jr Ullrich-Turner syndrome: neurodevelopmental changes from childhood through adolescence. Am J Med Genet. 1995;58(1):74–82. doi: 10.1002/ajmg.1320580115. [DOI] [PubMed] [Google Scholar]
- Russell VA, Oades RD, Tannock R, Killeen PR, Auerbach JG, Johansen EB, et al. Response variability in attention-deficit/hyperactivity disorder: a neuronal and glial energetics hypothesis. Behav Brain Funct. 2006;2:30–54. doi: 10.1186/1744-9081-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahraie A, Trevethan CT, Macleod MJ, Urquhart J, Weiskrantz L. Pupil response as a predictor of blindsight in hemianopia. Proc Natl Acad Sci U S A. 2013;110(45):18333–18338. doi: 10.1073/pnas.1318395110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Rowe JB, Passingham RE. Active maintenance in prefrontal area 46 creates distractor-resistant memory. Nat Neurosci. 2002;5(5):479–484. doi: 10.1038/nn846. [DOI] [PubMed] [Google Scholar]
- Sameroff A. A unified theory of development: a dialectic integration of nature and nurture. [Addresses] Child Dev. 2010;81(1):6–22. doi: 10.1111/j.1467-8624.2009.01378.x. [DOI] [PubMed] [Google Scholar]
- Saneyoshi A, Michimata C. Lateralized effects of categorical and coordinate spatial processing of component parts on the recognition of 3D non-nameable objects. Brain Cogn. 2009;71(3):181–186. doi: 10.1016/j.bandc.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Satz P. Brain reserve capacity on symptom onset after brain injury: a formulation and review of evidence for threshold theory. Neuropsychology. 1993;7(3):273. [Google Scholar]
- Satz P, Fletcher JM, Clark W, Morris R. Lag, deficit, delay and rate constructs in learning disability: A re-examination. In: Ansara A, Geschwind N, Galaburda A, Albert M, Gartrell N, editors. Sex differences in dyslexia. New York: Orton Dyslexia Society; 1981. pp. 129–150. [Google Scholar]
- Schachar RJ, Chen S, Logan GD, Ornstein TJ, Crosbie J, Ickowicz A, et al. Evidence for an error monitoring deficit in attention deficit hyperactivity disorder. J Abnorm Child Psychol. 2004;32(3):285–293. doi: 10.1023/b:jacp.0000026142.11217.f2. [DOI] [PubMed] [Google Scholar]
- Schachar R, Logan GD, Robaey P, Chen S, Ickowicz A, Barr C. Restraint and cancellation: multiple inhibition deficits in attention deficit hyperactivity disorder. J Abnorm Child Psychol. 2007;35(2):229–238. doi: 10.1007/s10802-006-9075-2. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. The role of the cerebellum in cognition and emotion: personal reflections since 1982 on the dysmetria of thought hypothesis, and its historical evolution from theory to therapy. Neuropsychol Rev. 2010;20(3):236–260. doi: 10.1007/s11065-010-9142-x. [DOI] [PubMed] [Google Scholar]
- Schmidt AT, Hanten GR, Li X, Orsten KD, Levin HS. Emotion recognition following pediatric traumatic brain injury: longitudinal analysis of emotional prosody and facial emotion recognition. Neuropsychologia. 2010;48(10):2869–2877. doi: 10.1016/j.neuropsychologia.2010.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheis MT, Hillary F, Chute DL. The neurocognitive driving test: applying technology to the assessment of driving ability following brain injury. Rehabilitation Psychology. 2003;48(4):275–280. [Google Scholar]
- Schwartz L, Taylor HG, Drotar D, Yeates KO, Wade SL, Stancin T. Long-term behavior problems following pediatric traumatic brain injury: prevalence, predictors, and correlates. J Pediatr Psychol. 2003;28(4):251–264. doi: 10.1093/jpepsy/jsg013. [DOI] [PubMed] [Google Scholar]
- Scott MN, Taylor HG, Fristad MA, Klein N, Espy KA, Minich N, et al. Behavior disorders in extremely preterm/extremely low birth weight children in kindergarten. J Dev Behav Pediatr. 2012;33(3):202–213. doi: 10.1097/DBP.0b013e3182475287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel WT, Joschko M. Evidence of difficulties in sustained attention in children with ADHD. J Abnorm Child Psychol. 1990;18(2):217–229. doi: 10.1007/BF00910732. [DOI] [PubMed] [Google Scholar]
- Sesma HW, Slomine BS, Ding R, McCarthy ML. Executive functioning in the first year after pediatric traumatic brain injury. Pediatrics. 2008;121(6):e1686–1695. doi: 10.1542/peds.2007-2461. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Aharon-Peretz J. Dissociable prefrontal networks for cognitive and affective theory of mind: a lesion study. Neuropsychologia. 2007;45(13):3054–3067. doi: 10.1016/j.neuropsychologia.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Sharpe JA, Lo AW, Rabinovitch HE. Control of the saccadic and smooth pursuit systems after cerebral hemidecortication. Brain. 1979;102(2):387–403. doi: 10.1093/brain/102.2.387. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP. Building a new biodevelopmental framework to guide the future of early childhood policy. Child Dev. 2010;81(1):357–367. doi: 10.1111/j.1467-8624.2009.01399.x. [DOI] [PubMed] [Google Scholar]
- Shum D, Levin H, Chan RC. Prospective memory in patients with closed head injury: a review. Neuropsychologia. 2011;49(8):2156–2165. doi: 10.1016/j.neuropsychologia.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Simon SR, Khateb A, Darque A, Lazeyras F, Mayer E, Pegna AJ. When the brain remembers, but the patient doesn't: converging fMRI and EEG evidence for covert recognition in a case of prosopagnosia. Cortex. 2011;47(7):825–838. doi: 10.1016/j.cortex.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Sinopoli KJ, Dennis M. Inhibitory control after traumatic brain injury in children. International Journal of Developmental Neuroscience. 2012;30(3):207–215. doi: 10.1016/j.ijdevneu.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinopoli KJ, Schachar R, Dennis M. Traumatic brain injury and secondary attention-deficit/hyperactivity disorder in children and adolescents: the effect of reward on inhibitory control. J Clin Exp Neuropsychol. 2011;33(7):805–819. doi: 10.1080/13803395.2011.562864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinopoli KJ, Schachar R, Dennis M. Reward improves cancellation and restraint inhibition across childhood and adolescence. Dev Psychol. 2012;47(5):1479–1489. doi: 10.1037/a0024440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville SC, Wellman HM, Cultice JC. Young children's deliberate reminding. The Journal of genetic psychology. 1983;143(1):87–96. [Google Scholar]
- Sonuga-Barke EJ. Psychological heterogeneity in AD/HD--a dual pathway model of behaviour and cognition. Behav Brain Res. 2002;130(1-2):29–36. doi: 10.1016/s0166-4328(01)00432-6. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Cicchetti DV, Balla DA. Vineland Adaptive Behavior Scales-II. San Antonio TX: Pearson; 2005. [Google Scholar]
- Statler KD, Swank S, Abildskov T, Bigler ED, White HS. Traumatic brain injury during development reduces minimal clonic seizure thresholds at maturity. Epilepsy Res. 2008;80(2-3):163–170. doi: 10.1016/j.eplepsyres.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavrou T, Bromley CM, Nicholson HS, Byrne J, Packer RJ, Goldstein AM, et al. Prognostic factors and secondary malignancies in childhood medulloblastoma. J Pediatr Hematol Oncol. 2001;23(7):431–436. doi: 10.1097/00043426-200110000-00008. [DOI] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8(3):448–460. [PubMed] [Google Scholar]
- Swartwout MD, Cirino PT, Hampson AW, Fletcher JM, Brandt ME, Dennis M. Sustained attention in children with two etiologies of early hydrocephalus. Neuropsychology. 2008;22(6):765–775. doi: 10.1037/a0013373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartwout MD, Garnaat SL, Myszka KA, Fletcher JM, Dennis M. Associations of ethnicity and SES with IQ and achievement in spina bifida meningomyelocele. J Pediatr Psychol. 2010;35(9):927–936. doi: 10.1093/jpepsy/jsq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes CK, Walhovd KB, Torstveit M, Sells VT, Fjell AM. Performance monitoring in children and adolescents: A review of developmental changes in the error-related negativity and brain maturation. Dev Cogn Neurosci. 2013;6C:1–13. doi: 10.1016/j.dcn.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor GH, Hack M, Klein NK. Attention deficits in children with< 750 gm birth weight. Child Neuropsychol. 1998;4(1):21–34. doi: 10.1076/0929-7049(200003)6:1;1-B;FT049. [DOI] [PubMed] [Google Scholar]
- Taylor HG, Klein N, Drotar D, Schluchter M, Hack M. Consequences and risks of <1000 g birth weight for neuropsychological skills, achievement, and adaptive functioning. J Dev Behav Pediatr. 2006;27(6):459–469. doi: 10.1097/00004703-200612000-00002. [DOI] [PubMed] [Google Scholar]
- Taylor HG, Klein N, Minich NM, Hack M. Long-term family outcomes for children with very low birth weights. Arch Pediatr Adolesc Med. 2001;155(2):155–161. doi: 10.1001/archpedi.155.2.155. [DOI] [PubMed] [Google Scholar]
- Taylor HG, Klein N, Schatschneider C, Hack M. Predictors of early school age outcomes in very low birth weight children. J Dev Behav Pediatr. 1998;19(4):235–243. doi: 10.1097/00004703-199808000-00001. [DOI] [PubMed] [Google Scholar]
- Taylor HG, Minich NM, Klein N, Hack M. Longitudinal outcomes of very low birth weight: neuropsychological findings. J Int Neuropsychol Soc. 2004;10(2):149–163. doi: 10.1017/S1355617704102038. [DOI] [PubMed] [Google Scholar]
- Taylor HG, Schatschneider C. Child neuropsychological assessment: A test of basic assumptions. The Clinical Neuropsychologist. 1992;6(3):259–275. [Google Scholar]
- Taylor HG, Yeates KO, Wade SL, Drotar D, Klein SK, Stancin T. Influences on first-year recovery from traumatic brain injury in children. Neuropsychology. 1999;13(1):76–89. doi: 10.1037//0894-4105.13.1.76. [DOI] [PubMed] [Google Scholar]
- Taylor HG, Yeates KO, Wade SL, Drotar D, Stancin T, Minich N. A prospective study of short- and long-term outcomes after traumatic brain injury in children: behavior and achievement. Neuropsychology. 2002;16(1):15–27. doi: 10.1037//0894-4105.16.1.15. [DOI] [PubMed] [Google Scholar]
- Taylor LJ, Zwaan RA. Action in cognition: The case of language. Language and cognition. 2009;1(1):45–58. [Google Scholar]
- Taylor LJ, Zwaan RA. Fault-tolerant comprehension. Language and Action in Cognitive Neuroscience. 2012;145 [Google Scholar]
- Tonks J, Williams WH, Frampton I, Yates P, Wall SE, Slater A. Reading emotions after childhood brain injury: case series evidence of dissociation between cognitive abilities and emotional expression processing skills. Brain Inj. 2008;22(4):325–332. doi: 10.1080/02699050801968303. [DOI] [PubMed] [Google Scholar]
- Toplak ME, Bucciarelli SM, Jain U, Tannock R. Executive functions: performance-based measures and the behavior rating inventory of executive function (BRIEF) in adolescents with attention deficit/hyperactivity disorder (ADHD) Child Neuropsychol. 2009;15(1):53–72. doi: 10.1080/09297040802070929. [DOI] [PubMed] [Google Scholar]
- Toplak ME, West RF, Stanovich KE. Practitioner review: do performance-based measures and ratings of executive function assess the same construct? J Child Psychol Psychiatry. 2013;54(2):131–143. doi: 10.1111/jcpp.12001. [DOI] [PubMed] [Google Scholar]
- Treble A, Juranek J, Stuebing KK, Dennis M, Fletcher JM. Functional significance of atypical cortical organization in spina bifida myelomeningocele: relations of cortical thickness and gyrification with IQ and fine motor dexterity. Cereb Cortex. 2013;23(10):2357–2369. doi: 10.1093/cercor/bhs226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treble-Barna A, Kulesz PA, Dennis M, Fletcher JM. Covert orienting in three etiologies of congenital hydroephalus: the effect of midbrain and posterior fossa dysmorphology. J Int Neuropsychol Neuropsychol. 2014;20(X):X–XX. doi: 10.1017/S1355617713001501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treyvaud K, Inder TE, Lee KJ, Northam EA, Doyle LW, Anderson PJ. Can the home environment promote resilience for children born very preterm in the context of social and medical risk? J Exp Child Psychol. 2012;112(3):326–337. doi: 10.1016/j.jecp.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Ward H, Shum D, Dick B, McKinlay L, Baker-Tweney S. Interview study of the effects of paediatric traumatic brain injury on memory. Brain Injury. 2004;18(5):471–495. doi: 10.1080/02699050310001646107. [DOI] [PubMed] [Google Scholar]
- Ward H, Shum D, McKinlay L, Baker S, Wallace G. Prospective memory and pediatric traumatic brain injury: effects of cognitive demand. Child Neuropsychol. 2007;13(3):219–239. doi: 10.1080/09297040600910003. [DOI] [PubMed] [Google Scholar]
- Weiskrantz L, Warrington EK, Sanders MD, Marshall J. Visual capacity in the hemianopic field following a restricted occipital ablation. Brain. 1974;97(4):709–728. doi: 10.1093/brain/97.1.709. [DOI] [PubMed] [Google Scholar]
- Wiedenbauer G, Jansen-Osmann P. Spatial knowledge of children with spina bifida in a virtual large-scale space. Brain Cogn. 2006;62(2):120–127. doi: 10.1016/j.bandc.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Will B, Dalrymple-Alford J, Wolff M, Cassel JC. Reflections on the use of the concept of plasticity in neurobiology. Translation and adaptation by Bruno Will, John Dalrymple-Alford, Mathieu Wolff and Jean-Christophe Cassel from J. Paillard, J Psychol 1976;1:33-47. Behav Brain Res. 2008;192(1):7–11. doi: 10.1016/j.bbr.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biological Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Williams S, McGee R, Anderson J, Silva PA. The structure and correlates of self-reported symptoms in 11-year-old children. J Abnorm Child Psychol. 1989;17(1):55–71. doi: 10.1007/BF00910770. [DOI] [PubMed] [Google Scholar]
- Wu TC, Wilde EA, Bigler ED, Li X, Merkley TL, Yallampalli R, et al. Longitudinal changes in the corpus callosum following pediatric traumatic brain injury. Dev Neurosci. 2010;32(5-6):361–373. doi: 10.1159/000317058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang TX, Chan RC, Shum D. The development of prospective memory in typically developing children. Neuropsychology. 2011;25(3):342–352. doi: 10.1037/a0022239. [DOI] [PubMed] [Google Scholar]
- Yates PJ, Williams WH, Harris A, Round A, Jenkins R. An epidemiological study of head injuries in a UK population attending an emergency department. J Neurol Neurosurg Psychiatry. 2006;77(5):699–701. doi: 10.1136/jnnp.2005.081901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates KO, Armstrong K, Janusz J, Taylor HG, Wade S, Stancin T, et al. Long-term attention problems in children with traumatic brain injury. J Am Acad Child Adolesc Psychiatry. 2005;44(6):574–584. doi: 10.1097/01.chi.0000159947.50523.64. [DOI] [PubMed] [Google Scholar]
- Yeates KO, Bigler ED, Abildskov T, Dennis M, Gerhardt CA, Vannatta K, et al. Social competence in pediatric traumatic brain injury from brain to behavior. Clinical Psychological Science 2013 [Google Scholar]
- Yeates KO, Bigler ED, Dennis M, Gerhardt CA, Rubin KH, Stancin T, et al. Social outcomes in childhood brain disorder: a heuristic integration of social neuroscience and developmental psychology. Psychol Bull. 2007;133(3):535–556. doi: 10.1037/0033-2909.133.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates KO, Swift E, Taylor HG, Wade SL, Drotar D, Stancin T, et al. Short- and long-term social outcomes following pediatric traumatic brain injury. J Int Neuropsychol Soc. 2004;10(3):412–426. doi: 10.1017/S1355617704103093. [DOI] [PubMed] [Google Scholar]
- Yeh TK, Chang CY, Hu CY, Yeh TC, Lin MY. Association of catechol-O-methyltransferase (COMT) polymorphism and academic achievement in a Chinese cohort. Brain Cogn. 2009;71(3):300–305. doi: 10.1016/j.bandc.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Yule SM, Hide TA, Cranney M, Simpson E, Barrett A. Low grade astrocytomas in the West of Scotland 1987-96: treatment, outcome, and cognitive functioning. Arch Dis Child. 2001;84(1):61–64. doi: 10.1136/adc.84.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]


