Abstract
Background
Chronic pain is the most common and disabling feature of endometriosis. Surgical excision of endometriosis lesions provides relief but pain relapse is common. Studies in a preclinical model of endometriosis might help to unravel the role of the ectopic lesions as the source of pain. Thus, we evaluated the impact of lesion excision on mechanical hyperalgesia in a preclinical model of endometriosis pain.
Methods
Endometriosis was induced by implanting autologous uterine tissue onto the gastrocnemius muscle. Surgical excision or aspiration drainage of the cystic lesion was performed at different times post-implant and mechanical nociceptive thresholds assessed at the site of the lesion.
Results
Lesions at 2, 8 and 16 weeks post-implant, produced mechanical hyperalgesia of similar magnitude (n = 6/group). Excision of lesions (n = 6/group) produced a longer inhibition, with a magnitude and time-course depending on the timing of excision. Excision at 2 and 8 weeks produced a rapid onset marked attenuation of hyperalgesia, which returned to pre-excision values by postsurgical week 3. In contrast, excision of the lesion at 16 weeks produced a peak of inhibition of hyperalgesia 2 weeks post-excision, but then the inhibition was sustained. Aspiration of fluid from cysts in the lesions briefly attenuated mechanical hyperalgesia (n = 6/group).
Conclusions
In this preclinical model we demonstrate that endometriosis pain is alleviated by surgical excision of the ectopic lesion or drainage of its cysts, providing support for the clinical observation that endometriosis pain is dependent on the ongoing presence of the lesions.
Keywords: Chronic pelvic pain, mechanical hyperalgesia, nociceptors, ectopic endometrium
1. Introduction
Chronic pain is a prominent feature of endometriosis, a common gynecological disease affecting approximately 10% of women in their reproductive years (Giudice, 2010). Expressed as dysmenorrhea, dyschezia, dysuria or dyspareunia, endometriosis pain has an enormous impact on personal and interpersonal aspects of quality of life (Falcone and Lebovic, 2011; Giudice, 2010), which also affects gainful employment and produces high costs related to health care (Falcone and Lebovic, 2011; Simoens et al., 2012). Medical therapy can offer some pain relief but side effects can be marked, and pain relapse is often observed shortly after the cessation of therapy (Giudice, 2010; Sinaii et al., 2007; Streuli et al., 2013). Surgical interventions aimed to treat endometriosis pain are diverse and include the drainage of cystic lesions (Aboulghar et al., 1991; Acien et al., 2010; de Crespigny et al., 1989; Mesogitis et al., 2000), ablation (Diwadkar and Falcone, 2011), excision (Barton-Smith et al., 2006; Diwadkar and Falcone, 2011) or vaporization (Barton-Smith et al., 2006; Diwadkar and Falcone, 2011) of lesions, or section of branches of nerves innervating lesions (Barton-Smith et al., 2006; Berlanda et al., 2010; Diwadkar and Falcone, 2011), providing different degrees of pain relief. Surgical therapies are, however, often followed by recurrence of pain (Barton-Smith et al., 2006; Guo, 2009). Thus, while excision of lesions by laparoscopic surgery may provide clinically meaningful pain relief (Jacobson et al., 2009), relapse occurs in up to 50% of patients (Berlanda et al., 2010; Guo, 2009; Vercellini et al., 2009). Furthermore, in some cases, after several surgical interventions – including hysterectomy – recurrence is observed and can be associated with worse endometriosis pain (Berlanda et al., 2010; Matalliotakis et al., 2003; Vercellini et al., 2009). Importantly, many criteria related to the surgical treatment of endometriosis pain such as the selection of patients, whether interventions should be radical or conservative, or if adjunct of add-back therapy is necessary, are still a matter of debate (Koninckx et al., 2013; Stratton et al., 2008; Vercellini et al., 2009). This indicates that an important lack of knowledge about the basis of endometriosis pain precludes the application of mechanism-based criteria for decisions regarding surgical therapy. This lack of knowledge is not only related to the heterogeneity of the clinical presentation and recurrence of endometriosis pain after surgery (Guo, 2009) and to a poor correlation between structural features of the lesions and perceived pain (Giudice, 2010; Stratton and Berkley, 2011), but also to the lack of appropriate animal models allowing mechanism-based studies of endometriosis pain. We have previously shown that rats implanted with autologous uterine tissue on the gastrocnemius muscle develop cystic lesions, that infiltrate the underlying muscle, and persistent mechanical hyperalgesia, reminiscent of human endometriosis pain (Alvarez et al., 2012). Here we explored whether surgical excision and drainage attenuate mechanical hyperalgesia and the duration of such attenuation in rats with this endometriosis-like lesion.
2. Material and methods
2.1 Animals
Adult female Sprague Dawley rats (220–240 g; Charles River, Hollister, CA, USA) were used in these experiments. They were housed in the Animal Care Facility at the University of California San Francisco, under environmentally controlled conditions (lights on 07:00–19:00 h; room temperature 21–23°C) with food and water available ad libitum. Upon completion of experiments, rats were euthanized by carbon dioxide asphyxia followed by bilateral thoracotomy. Animal care and use conformed to NIH guidelines (NIH Guide for the Care and Use of Laboratory Animals). The University of California San Francisco Committee on Animal Research approved all experimental protocols. Concerted effort was made to minimize number and suffering of experimental animals.
2.2 Measurement of mechanical hyperalgesia
Mechanical nociceptive threshold in the implanted gastrocnemius muscle was quantified using a digital force transducer (Chatillon DFI2; Amtek Inc., Largo, FL, USA) with a custom-made 7 mm-diameter probe (Alvarez et al., 2012); the use of a probe with a tip diameter ≥ 2.6 mm allows reliable measurements of mechanical nociceptive threshold in subcutaneous tissue, even when overlying cutaneous hyperalgesia is present (Nasu et al., 2010). Rats were lightly restrained in a cylindrical acrylic holder with lateral slats allowing for easy access to the hind limbs and application of the force transducer probe to the site of implantation in the belly of the gastrocnemius muscle. The nociceptive threshold was defined as the force (mN) required to produce a flexion reflex in the hind leg. Baseline withdrawal threshold was defined as the mean of 3 readings taken at 5-min intervals and hyperalgesia values were calculated as a percentage of the baseline withdrawal threshold.
2.3 Determination of estrous cycle phase
The phase of the estrous cycle was assessed pre-operatively, as previously reported (Alvarez et al., 2012). Immediately after anesthesia induction for surgical implant of ectopic endometrium, 20 μl of 0.9% NaCl was flushed 3–4 times into the vaginal cavity. The resulting fluid was then placed onto a slide and observed unstained at 100 × magnification. The diagnostic criteria used to determine the estrous cycle stage was based on cellular type predominance as reported by Marcondes and colleagues (Marcondes et al., 2002).
2.4 Surgical induction of endometriosis
The model of surgically-induced muscle endometriosis used here has been previously described in detail (Alvarez et al., 2012). Briefly, female rats were pre-medicated with a mixture of ketamine hydrochloride (Putney, Portland, ME) and xylazine (AnaSed®, Lloid Laboratories, Shenandoah, IA) and anesthesia was maintained with isoflurane (Phoenix Pharmaceuticals, St. Joseph, MO). The right dorsal paravertebral area was infiltrated with 0.25% bupivacaine (Marcaine®, Hospira, Lake Forest, IL) and, under aseptic conditions, an incision approximately 2 cm in length was performed and to expose and isolate the right uterine horn. After ligature of uterine blood vessels, a 1 cm segment was removed and immediately placed in a Petri dish containing 0.9% NaCl. The musculature of the dorsal abdominal wall was closed with single crossed stitches and the skin incision closed with horizontal mattress stitches using 5-0 nylon. The excised uterine tissue was measured with a millimeter scale and opened longitudinally; a full thickness 3 × 3 mm square of uterine tissue was then removed and kept in physiologic saline. The implant was performed through an incision in the biceps femoris muscle allowing exposure of the underlying gastrocnemius muscle. The square of uterine tissue was sutured to the surface of the gastrocnemius muscle applying three to four single stitches using 5-0 nylon with the endometrial portion of the uterine tissue contacting the gastrocnemius muscle. After checking for hemostasis, the b. femoris muscle and the skin incisions were sutured separately with single stitches. The sham surgical procedure was similar but the uterus was left intact and not implanted on the surface of the gastrocnemius. Surgical procedures were performed regardless of the estrous cycle status of the rats.
2.5 Surgical excision of endometriosis lesions
Rats were pre-medicated with a ketamine hydrochloride-xylazine mixture and carprofen (5 mg/kg, sc; Rimadyl®, Pfizer Animal Health, New York, NY) and anesthetized with isoflurane. The skin of the implanted calf was prepared for aseptic surgery and a 1 cm incision performed at the site of the implant. The underlying stitches placed on the biceps femoris muscle were removed, allowing exposure of the lesion in the implanted gastrocnemius muscle. Using a surgical microscope the margins of the lesion were identified and the fluid content of the cystic lesion aspirated. Thereafter, the roof of the cystic lesion was removed allowing the exposure of the stitches placed laterally in the base of the implant. These stitches were removed and the visible base of the lesion was dissected free from the underlying gastrocnemius muscle. Care was taken to remove as much of the lesion tissue while avoiding damage to the external saphenous vein as well as gastrocnemius vascular and nerve supply. After checking for hemostasis, the b. femoris muscle and the skin incisions were sutured separately with single stitches using 5-0 nylon. Rats were allowed to recover alone from anesthesia, receiving a daily injection of carprofen for the next 3 days after the operation; sutures were removed 7 days after surgery.
2.6 Fluid drainage from cysts in the endometriosis lesions
The drainage of fluid from cysts in the ectopic endometrial lesion was performed by percutaneous puncture using a 29-gauge × ½″ needle attached to a 1 ml insulin syringe (Becton & Dickinson, Franklin Lakes, NJ). To facilitate the location of the uterine implant and cystic lesions, rats were briefly anesthetized with 2.5% isoflurane and the belly of the implanted gastrocnemius muscle was explored by palpation. The tip of the needle was then inserted into the center of the implant and slowly advanced until a decrease in resistance was experienced. The needle was then kept in this position and a gentle aspiration of fluid was performed. The needle was withdrawn and the site of puncture compressed to allow additional fluid to flow from the cyst. The skin puncture site was marked with a fine-tip indelible ink pen, so that the same mechanical nociceptive threshold of the lesion site in the muscle could be repeatedly tested.
2.7 Statistical analysis
Group data are expressed as mean ± SEM of n independent observations. Statistical comparisons were made using GraphPad Prism 5.0 statistical software (GraphPad Software, Inc., La Jolla, CA, USA). The paired Student’s t-test (two tailed) was used to compare mechanical nociceptive threshold pre/post-surgical induction of endometriosis pain model and to compare changes in mechanical nociceptive threshold pre/post-drainage of the endometriosis-like lesions. The effect of the estrous cycle phase at the moment of surgical implant was analyzed by means of a one-way analysis of variance (ANOVA). The effect of surgical excision of cystic lesions at different times after implant was analyzed by means of a two-way repeated measures ANOVA followed by Bonferroni’s multiple comparisons test. P < 0.05 was considered statistically significant.
3. Results
3.1 Induction of endometriosis and measurement of hyperalgesia
All rats recovered uneventfully from surgery. Fourteen days after surgery, close inspection and palpation of the operated gastrocnemius muscle revealed the presence of a well-delineated tumescent area at the implant site in all uterine tissue-implanted rats, regardless of their preoperative estrous cycle status (Alvarez et al., 2012). At this time point, rats exhibited a marked decrease in nociceptive threshold (i.e., mechanical hyperalgesia) compared to preoperative baseline (2530 ± 5.8 mN versus 1281 ± 40 mN, n = 25, P < 0.0001, Fig. 1A). To evaluate whether this mechanical hyperalgesia was related to the rat’s estrous cycle stage at the moment of surgical implant, individual mechanical threshold values on day 14 after surgery were matched to their respective stages diagnosed immediately prior to surgery. No significant differences in the amplitude of the mechanical hyperalgesia were observed among the rats operated in proestrus (n = 7), estrus (n = 7), metestrus (n = 4) or diestrus (n = 7) (P > 0.05, Fig. 1B). Lesions at post-implant times 2, 8 and 16 weeks, did not exhibit significant differences in the magnitude of mechanical hyperalgesia at the site of endometriosis lesion (all ~ 50% decrease in mechanical nociceptive threshold, n = 6/group, P > 0.05, Fig. 1C).
Figure 1.
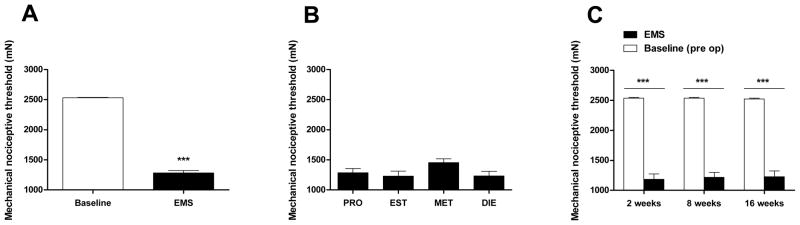
Implant of ectopic uterine tissue produces persistent mechanical hyperalgesia. (A) Compared to baseline values, the surgical implant of autologous uterine tissue onto the gastrocnemius muscle (EMS) produced a marked decrease in mechanical nociceptive threshold at the implant site (n = 25). (B) Two weeks after surgical implant of uterine tissue, rats exhibited a marked decrease in mechanical nociceptive threshold, regardless the estrous stage at the moment of surgery (PRO, proestrus [n = 7]; EST, estrus [n = 7]; MET, metestrus [n = 4]; DIE, diestrus [n = 7]). (C) After 2, 8 or 16 weeks post-surgery (n = 6/group), rats exhibited a comparable decrease in mechanical nociceptive threshold at the site of implant (solid bars), compared to pre-surgical baseline (open bars). *** P < 0.0001
3.2 Surgical excision of lesions
To excise endometriosis lesions, rats were pre-medicated and anesthetized for surgical excision of the cystic lesions (see material and methods). Rats recovered uneventfully from surgery, mechanical nociceptive thresholds were then obtained every week for 5 consecutive weeks. One week after excision, rats with times after implant of 2 and 8 weeks exhibited maximum attenuation of mechanical hyperalgesia (−56.7 ± 2.4% pre versus −21.9 ± 2.7% post-surgery, n = 6, P < 0.0001; −52.1 ± 3% pre versus −28.5 ±3.9% post-surgery, n = 6, P < 0.0001, respectively, Fig. 3A,B). In contrast, while rats with a time after implant of 16 weeks exhibit attenuation of hyperalgesia (−50.8 ± 3.7% pre versus −26.9 ± 3.8% post-surgery, n = 6, P < 0.0001; Fig. 3C), it only reached maximal attenuation of mechanical hyperalgesia 2 weeks after excision (−11.3 ± 2.4% post-surgery, n = 6, P < 0.0001; Fig. 3C). While rats with excision of implants 2 and 8 weeks prior returned to pre-surgical level of hyperalgesia by week 3 (−56.9 ± 1.9%, n = 6, P > 0.05; −44.4 ± 3.4%, n = 6, P > 0.05, respectively, Fig. 3A,B), rats with time after implant of 16 weeks exhibited significant attenuation of mechanical hyperalgesia for at least 5 weeks after surgical excision (−34.5 ± 1.3%, n = 6, P < 0.0001; Fig. 3C).
Figure 3.

Effects of surgical excision of endometriotic-like lesions on primary mechanical hyperalgesia. The excision of cystic lesions at 2 (A) or 8 (B) weeks after surgical implant of uterine tissue produced a short lasting decrease in mechanical hyperalgesia. In contrast, the excision of cystic lesions at 16 weeks (C) after surgical implant produced a persistent diminution of mechanical hyperalgesia. N=6/group; ** P < 0.01; *** P < 0.001
3.3 Drainage of cyst fluid
Rats were anesthetized and the site of their implant palpated. The aspiration and compression procedure allowed extraction of 10–40 μl/lesion of a reddish-brown to amber fluid. Before and one hour after this procedure mechanical nociceptive threshold was measured. When compared to pre-drainage values, rats implanted 2 and 8 weeks prior exhibited an increase in mechanical thresholds (−53.2 ± 3.7% versus −44.7 ± 3.4%, n = 6, P = 0.0313; and −52.1 ± 3% versus −41.2 ± 2.5%, n = 6, P = 0.0313, respectively, Fig. 2A,B). In contrast, mechanical hyperalgesia was not significantly modified in rats with a time after implant of 16 weeks (−50.8 ± 3.8% versus −44.6 ± 1.7%, n = 6, P = 0.093, respectively, Fig. 2C). Twenty four hours after drainage, the mechanical nociceptive threshold had returned to pre-drainage levels (data not shown).
Figure 2.

Effects of aspiration drainage of fluid from cysts in the endometriosis lesions on primary mechanical hyperalgesia. Drainage of fluid from cysts, 2 (A) or 8 (B), but not 16 (C), weeks (n = 6/group) after surgical implant of uterine tissue onto the gastrocnemius muscle produced a decrease in mechanical hyperalgesia. n.s., non-significant; * P < 0.05
4. Discussion
Given the heterogeneity of its clinical presentation and the lack of adequate methods of non-invasive monitoring of disease progression, animal models of endometriosis are important tools for the study of underlying pain mechanisms. We have previously provided morphological, behavioral, electrophysiological and pharmacological evidence showing that the ectopic placement of autologous uterine tissue onto the gastrocnemius muscle produces pain which is reminiscent to that observed clinically in patients with endometriosis (Alvarez et al., 2012). The main advantage of this model is that it allows direct exploration of primary mechanical hyperalgesia, the mechanism underlying the major symptoms experienced by women with endometriosis: dyspareunia, dyschezia and dysuria (Giudice, 2010).
We observed that the stage of the estrous cycle at the moment of the surgical implant of ectopic uterine tissue does not play a role in the development of lesions, the induction of mechanical hyperalgesia or its magnitude. This not only represents an obvious technical advantage over other models of endometriosis – where surgical procedures are made only at specific stages of the estrous cycle (McAllister et al., 2009) – but also suggests that, at least in the initial phases of lesion growth, ectopically placed uterine tissue has the potential to form endometriosis-like lesions regardless of the influence of ovarian sex hormone levels. Instead, other mediators involved in early development of neo-angiogenesis, such as leptin (Asante and Taylor, 2011; Styer et al., 2008) and vascular endothelial growth factor (VEGF) (Asante and Taylor, 2011), seem to play a critical role in the establishment of endometriosis lesions.
While the successful implant of the uterine tissue may have been due to the ovarian sexual steroids, the marked mechanical hyperalgesia observed regardless of estrous cycle phase at the moment of implant, indicates that the mechanisms involved in the induction of pain also evolve independent of the ovarian sex hormone influence on the receptive tissue, in this case the gastrocnemius muscle. This is consistent with the lack of change in the magnitude of the mechanical hyperalgesia induced by endometriosis-like lesions in different stages of the estrous cycle (Alvarez et al., 2012).
Rats that underwent excision of their lesions exhibited a marked decrease in mechanical hyperalgesia at the site of implant, one week later. This indicates that this primary hyperalgesia depends on endometriosis-like lesions, which is consistent with the pain relief observed after laparoscopic excision of lesions in patients (Jacobson et al., 2009). These results contrast with a previous study showing a delayed diminution of hyperalgesia after surgical excision of endometriosis-like lesions (McAllister et al., 2009). This is probably due to the fact that those studies evaluated pain by outcome measures that do not involve structures affected by ectopic lesions (i.e., secondary hyperalgesia) (Berkley et al., 2001; McAllister et al., 2009). Indeed, mechanical hyperalgesia is observed only at 4–5 weeks after the implant of uterine tissue in these models of endometriosis pain (McAllister et al., 2012), suggesting that the development of central sensitization is needed for the responses to be measured. In line with this, rats 16 weeks post-implant also exhibit a more delayed peak to postsurgical diminution of their mechanical hyperalgesia (see Fig. 3C).
With regard to the role of time after implant on the outcome of the surgical excision procedure, rats operated at shorter times after implant of uterine tissue exhibited a shorter duration reversal of the primary hyperalgesia. In contrast, rats submitted to surgical excision at advanced time after implant exhibited the most marked and persistent reversal of hyperalgesia. This is consistent with clinical studies reporting better pain relief in cases of advanced endometriosis compared to early disease (Milingos et al., 2006). Besides, several studies have identified a younger age at surgery as a risk factor for recurrence after surgical excision of endometriosis lesions (Sengoku et al., 2013) (for a review see Guo, 2009 (Guo, 2009)), which is also the case in our experimental design (i.e. being that all groups of rats were implanted at the same age those with more time after implant are also older).
Chronic lesions may take longer to reach maximal antihyperalgesia after excision probably because these lesions are related to a marked central sensitization (He et al., 2010). Indeed, successful surgical treatment of these lesions reverses such central sensitization (He et al., 2010). These observations suggest that endometriosis-like lesions not only produce input-dependent primary hyperalgesia but also central sensitization, which appears at latter stages of lesion growth. This is also a likely reason why models based on measurements of secondary hyperalgesia take longer before pain can be detected (McAllister et al., 2012).
Our finding that, regardless of the time after implant, there was a recurrence of chronic pain after the excision of the endometriosis lesion, is reminiscent of the recurrence of pelvic pain observed in many patients after surgical procedures aimed to remove endometriosis lesions (Guo, 2009; Vercellini et al., 2009). However, it is still an open question as to whether recurrence is due to an incomplete resection of (re-growth of lesions from remaining endometrial cells) or to a true disease recurrence (i.e., establishment of new of lesions). Retrograde menstruation is the most widely accepted theory for the origin of intra-abdominal endometriosis lesions. However, while more than 80% of women have retrograde menstruation only a fraction exhibit endometriosis (Giudice, 2010), making it unlikely as a source of postsurgical recurrence. Indeed, recurrent endometriosis pain and lesions are observed in post-menopausal women (Fatemi et al., 2005; Rosa-e-Silva et al., 2008; Takayama et al., 1998) and recurrence or persistence of endometriosis pain is observed in up to 15% women after hysterectomy (Berlanda et al., 2010), including in post-menopausal patients (Rosa-e-Silva et al., 2008).
The incomplete resection of lesions and their re-growth from remaining endometrial cells in the gastrocnemius muscle is also a plausible explanation for the recurrence of pain. Indeed, full elimination of endometrial cells by currently used surgical techniques is extremely unlikely. Furthermore, the surgical procedure could stimulate by itself the growth of new lesions: low oxygen levels are known to trigger the expression of mediators involved in angiogenesis of endometriosis lesions (Rocha et al., 2013), suggesting that the removal of the vascular supply during surgical excision of endometriosis lesions may induce the release of angiogenic factors by remaining endometrial cells and the regrowth of residual endometrial tissue. And, some important angiogenic factors observed in endometriosis lesions, such as endothelin-1 (Van Langendonckt et al., 2008) and VEGF (Donnez et al., 1998; Li et al., 2013), are pro-algesic (Joseph et al., 2011; Maeda et al., 2009; Zhang et al., 2008).
The drainage of fluid from the cysts in the endometriosis lesions produced a small, albeit significant, reduction of mechanical hyperalgesia in lesions times 2 and 8 weeks post-implant. Increased hydrostatic pressure in the cystic lesions, where glands cannot excrete their fluid to the uterine cavity, as they normally do, is presumably involved in the mechanical hyperalgesia observed at these time points. Painful symptoms associated with such an increased pressure are also observed in abscesses (Kessler et al., 2012), dental pulpitis (Heyeraas and Berggreen, 1999), acute glaucoma (Dargin and Lowenstein, 2008) or benign intracranial hypertension (Galgano and Deshaies, 2013), which are also readily responsive to appropriate drainage interventions. Importantly, pro-algesic inflammatory mediators are known to be present in the fluid in endometriotic cysts (Darai et al., 2003; Zhang et al., 2008), suggesting that the effect of drainage is due to the decompression of a structure innervated by sensitized nociceptors. Indeed, the drainage of cysts in endometriosis patients also provides some pain relief observed immediately after the procedure (Aboulghar et al., 1991; Mesogitis et al., 2000). In line with our results endometriosis pain, although milder, persists or recurs after drainage of fluid from lesion cysts (Acien et al., 2010; Mesogitis et al., 2000). We have no explanation for the lack of significant effect of drainage of cystic fluid in rats at 16 weeks post-implant, though it might be due to an inability to adequately drain fluid from a multi-cystic lesion. And, given the trend to a diminution in the hyperalgesia exhibited by these rats, the effect of drainage at 16 weeks may have also been limited by the sample size.
In conclusion we provide evidence that endometriosis pain is alleviated by surgical excision of the ectopic endometrial tissue, dependent on time post implant and dependent on pressure in the cysts in the endometriosis lesion. These results indicate that endometriosis pain is an input-dependent phenomenon related to the presence of ectopic uterine tissue lesions.
What’s already known about this topic?
Endometriosis is a chronic painful condition thought to arise from ectopic endometrium.
What does this study add?
In this study we demonstrate in a rat preclinical model of endometriosis that excision of the lesions attenuated pain.
Acknowledgments
Funding source: This work was supported by the National Institutes of Health (NIH).
Footnotes
Conflict of interest: None declared.
Author Contributions
PA. and J.D.L. designed experiments; P.A. performed experiments. PA. and J.D.L. analyzed the data. P.A., L.C.G. and J.D.L. wrote the manuscript. All authors discussed the results and commented on the manuscript.
References
- Aboulghar MA, Mansour RT, Serour GI, Rizk B. Ultrasonic transvaginal aspiration of endometriotic cysts: an optional line of treatment in selected cases of endometriosis. Hum Reprod. 1991;6:1408–1410. doi: 10.1093/oxfordjournals.humrep.a137279. [DOI] [PubMed] [Google Scholar]
- Acien P, Velasco I, Acien M, Quereda F. Treatment of endometriosis with transvaginal ultrasound-guided drainage and recombinant interleukin-2 left in the cysts: a third clinical trial. Gynecologic and obstetric investigation. 2010;69:203–211. doi: 10.1159/000270901. [DOI] [PubMed] [Google Scholar]
- Alvarez P, Chen X, Hendrich J, Irwin JC, Green PG, Giudice LC, Levine JD. Ectopic uterine tissue as a chronic pain generator. Neuroscience. 2012;225:269–282. doi: 10.1016/j.neuroscience.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asante A, Taylor RN. Endometriosis: the role of neuroangiogenesis. Annual review of physiology. 2011;73:163–182. doi: 10.1146/annurev-physiol-012110-142158. [DOI] [PubMed] [Google Scholar]
- Barton-Smith P, Ballard K, Kent ASH. Endometriosis: A general review and rationale for surgical therapy. Reviews in Gynaecological and Perinatal Practice. 2006;6:168–176. [Google Scholar]
- Berkley KJ, Cason A, Jacobs H, Bradshaw H, Wood E. Vaginal hyperalgesia in a rat model of endometriosis. Neuroscience letters. 2001;306:185–188. doi: 10.1016/s0304-3940(01)01906-1. [DOI] [PubMed] [Google Scholar]
- Berlanda N, Vercellini P, Fedele L. The outcomes of repeat surgery for recurrent symptomatic endometriosis. Current opinion in obstetrics & gynecology. 2010;22:320–325. doi: 10.1097/GCO.0b013e32833bea15. [DOI] [PubMed] [Google Scholar]
- Darai E, Detchev R, Hugol D, Quang NT. Serum and cyst fluid levels of interleukin (IL) -6, IL-8 and tumour necrosis factor-alpha in women with endometriomas and benign and malignant cystic ovarian tumours. Hum Reprod. 2003;18:1681–1685. doi: 10.1093/humrep/deg321. [DOI] [PubMed] [Google Scholar]
- Dargin JM, Lowenstein RA. The painful eye. Emergency medicine clinics of North America. 2008;26:199–216. viii. doi: 10.1016/j.emc.2007.10.001. [DOI] [PubMed] [Google Scholar]
- de Crespigny LC, Robinson HP, Davoren RA, Fortune D. The ‘simple’ ovarian cyst: aspirate or operate? British journal of obstetrics and gynaecology. 1989;96:1035–1039. doi: 10.1111/j.1471-0528.1989.tb03377.x. [DOI] [PubMed] [Google Scholar]
- Diwadkar GB, Falcone T. Surgical management of pain and infertility secondary to endometriosis. Seminars in reproductive medicine. 2011;29:124–129. doi: 10.1055/s-0031-1272474. [DOI] [PubMed] [Google Scholar]
- Donnez J, Smoes P, Gillerot S, Casanas-Roux F, Nisolle M. Vascular endothelial growth factor (VEGF) in endometriosis. Hum Reprod. 1998;13:1686–1690. doi: 10.1093/humrep/13.6.1686. [DOI] [PubMed] [Google Scholar]
- Falcone T, Lebovic DI. Clinical management of endometriosis. Obstetrics and gynecology. 2011;118:691–705. doi: 10.1097/AOG.0b013e31822adfd1. [DOI] [PubMed] [Google Scholar]
- Fatemi HM, Al-Turki HA, Papanikolaou EG, Kosmas L, De Sutter P, Devroey P. Successful treatment of an aggressive recurrent post-menopausal endometriosis with an aromatase inhibitor. Reproductive biomedicine online. 2005;11:455–457. doi: 10.1016/s1472-6483(10)61140-6. [DOI] [PubMed] [Google Scholar]
- Galgano MA, Deshaies EM. An update on the management of pseudotumor cerebri. Clinical neurology and neurosurgery. 2013;115:252–259. doi: 10.1016/j.clineuro.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Giudice LC. Clinical practice. Endometriosis. The New England journal of medicine. 2010;362:2389–2398. doi: 10.1056/NEJMcp1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo SW. Recurrence of endometriosis and its control. Human reproduction update. 2009;15:441–461. doi: 10.1093/humupd/dmp007. [DOI] [PubMed] [Google Scholar]
- He W, Liu X, Zhang Y, Guo SW. Generalized hyperalgesia in women with endometriosis and its resolution following a successful surgery. Reprod Sci. 2010;17:1099–1111. doi: 10.1177/1933719110381927. [DOI] [PubMed] [Google Scholar]
- Heyeraas KJ, Berggreen E. Interstitial fluid pressure in normal and inflamed pulp. Critical reviews in oral biology and medicine : an official publication of the American Association of Oral Biologists. 1999;10:328–336. doi: 10.1177/10454411990100030501. [DOI] [PubMed] [Google Scholar]
- Jacobson TZ, Duffy JM, Barlow D, Koninckx PR, Garry R. Laparoscopic surgery for pelvic pain associated with endometriosis. The Cochrane database of systematic reviews. 2009:CD001300. doi: 10.1002/14651858.CD001300.pub2. [DOI] [PubMed] [Google Scholar]
- Joseph EK, Gear RW, Levine JD. Mechanical stimulation enhances endothelin-1 hyperalgesia. Neuroscience. 2011;178:189–195. doi: 10.1016/j.neuroscience.2011.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler DO, Krantz A, Mojica M. Randomized trial comparing wound packing to no wound packing following incision and drainage of superficial skin abscesses in the pediatric emergency department. Pediatric emergency care. 2012;28:514–517. doi: 10.1097/PEC.0b013e3182587b20. [DOI] [PubMed] [Google Scholar]
- Koninckx PR, Ussia A, Donnez J, Stepanian A, Adamyan L, Wattiez A. The title is misleading: an opinion paper is not a consensus paper. Hum Reprod. 2013 doi: 10.1093/humrep/det281. [DOI] [PubMed] [Google Scholar]
- Li YZ, Wang LJ, Li X, Li SL, Wang JL, Wu ZH, Gong L, Zhang XD. Vascular endothelial growth factor gene polymorphisms contribute to the risk of endometriosis: an updated systematic review and meta-analysis of 14 case-control studies. Genetics and molecular research : GMR. 2013;12:1035–1044. doi: 10.4238/2013.April.2.20. [DOI] [PubMed] [Google Scholar]
- Maeda T, Kiguchi N, Kobayashi Y, Ikuta T, Ozaki M, Kishioka S. Leptin derived from adipocytes in injured peripheral nerves facilitates development of neuropathic pain via macrophage stimulation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13076–13081. doi: 10.1073/pnas.0903524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Brazilian journal of biology = Revista brasleira de biologia. 2002;62:609–614. doi: 10.1590/s1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- Matalliotakis IM, Mahutte NG, Goumenou AG, Arici A. Twenty-year history of endometriosis-associated pelvic pain: too much surgery or not enough? American journal of obstetrics and gynecology. 2003;188:1103–1104. doi: 10.1067/mob.2003.24. [DOI] [PubMed] [Google Scholar]
- McAllister SL, Dmitrieva N, Berkley KJ. Sprouted innervation into uterine transplants contributes to the development of hyperalgesia in a rat model of endometriosis. PloS one. 2012;7:e31758. doi: 10.1371/journal.pone.0031758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister SL, McGinty KA, Resuehr D, Berkley KJ. Endometriosis-induced vaginal hyperalgesia in the rat: role of the ectopic growths and their innervation. Pain. 2009;147:255–264. doi: 10.1016/j.pain.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesogitis S, Antsaklis A, Daskalakis G, Papantoniou N, Michalas S. Combined ultrasonographically guided drainage and methotrexate administration for treatment of endometriotic cysts. Lancet. 2000;355:1160. doi: 10.1016/S0140-6736(00)02071-7. [DOI] [PubMed] [Google Scholar]
- Milingos S, Protopapas A, Kallipolitis G, Drakakis P, Loutradis D, Liapi A, Antsaklis A. Endometriosis in patients with chronic pelvic pain: is staging predictive of the efficacy of laparoscopic surgery in pain relief? Gynecologic and obstetric investigation. 2006;62:48–54. doi: 10.1159/000092023. [DOI] [PubMed] [Google Scholar]
- Nasu T, Taguchi T, Mizumura K. Persistent deep mechanical hyperalgesia induced by repeated cold stress in rats. Eur J Pain. 2010;14:236–244. doi: 10.1016/j.ejpain.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Rocha AL, Reis FM, Taylor RN. Angiogenesis and endometriosis. Obstetrics and gynecology international. 2013;2013:859619. doi: 10.1155/2013/859619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa-e-Silva JC, Carvalho BR, de Barbosa HF, Poli-Neto OB, Rosa-e-Silva AC, Candido-dos-Reis FJ, Nogueira AA. Endometriosis in postmenopausal women without previous hormonal therapy: report of three cases. Climacteric : the journal of the International Menopause Society. 2008;11:525–528. doi: 10.1080/13697130802490256. [DOI] [PubMed] [Google Scholar]
- Sengoku K, Miyamoto T, Horikawa M, Katayama H, Nishiwaki K, Kato Y, Kawanishi Y, Saijo Y. Clinicopathologic risk factors for recurrence of ovarian endometrioma following laparoscopic cystectomy. Acta obstetricia et gynecologica Scandinavica. 2013;92:278–284. doi: 10.1111/aogs.12051. [DOI] [PubMed] [Google Scholar]
- Simoens S, Dunselman G, Dirksen C, Hummelshoj L, Bokor A, Brandes I, Brodszky V, Canis M, Colombo GL, DeLeire T, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. 2012;27:1292–1299. doi: 10.1093/humrep/des073. [DOI] [PubMed] [Google Scholar]
- Sinaii N, Cleary SD, Younes N, Ballweg ML, Stratton P. Treatment utilization for endometriosis symptoms: a cross-sectional survey study of lifetime experience. Fertility and sterility. 2007;87:1277–1286. doi: 10.1016/j.fertnstert.2006.11.051. [DOI] [PubMed] [Google Scholar]
- Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Human reproduction update. 2011;17:327–346. doi: 10.1093/humupd/dmq050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton P, Sinaii N, Segars J, Koziol D, Wesley R, Zimmer C, Winkel C, Nieman LK. Return of chronic pelvic pain from endometriosis after raloxifene treatment: a randomized controlled trial. Obstetrics and gynecology. 2008;111:88–96. doi: 10.1097/01.AOG.0000297307.35024.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli I, de Ziegler D, Santulli P, Marcellin L, Borghese B, Batteux F, Chapron C. An update on the pharmacological management of endometriosis. Expert opinion on pharmacotherapy. 2013;14:291–305. doi: 10.1517/14656566.2013.767334. [DOI] [PubMed] [Google Scholar]
- Styer AK, Sullivan BT, Puder M, Arsenault D, Petrozza JC, Serikawa T, Chang S, Hasan T, Gonzalez RR, Rueda BR. Ablation of leptin signaling disrupts the establishment, development, and maintenance of endometriosis-like lesions in a murine model. Endocrinology. 2008;149:506–514. doi: 10.1210/en.2007-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K, Zeitoun K, Gunby RT, Sasano H, Carr BR, Bulun SE. Treatment of severe postmenopausal endometriosis with an aromatase inhibitor. Fertility and sterility. 1998;69:709–713. doi: 10.1016/s0015-0282(98)00022-3. [DOI] [PubMed] [Google Scholar]
- Van Langendonckt A, Donnez J, Defrere S, Dunselman GA, Groothuis PG. Antiangiogenic and vascular-disrupting agents in endometriosis: pitfalls and promises. Molecular human reproduction. 2008;14:259–268. doi: 10.1093/molehr/gan019. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Barbara G, Abbiati A, Somigliana E, Vigano P, Fedele L. Repetitive surgery for recurrent symptomatic endometriosis: what to do? European journal of obstetrics, gynecology, and reproductive biology. 2009;146:15–21. doi: 10.1016/j.ejogrb.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Zhang G, Dmitrieva N, Liu Y, McGinty KA, Berkley KJ. Endometriosis as a neurovascular condition: estrous variations in innervation, vascularization, and growth factor content of ectopic endometrial cysts in the rat. American journal of physiology Regulatory, integrative and comparative physiology. 2008;294:R162–171. doi: 10.1152/ajpregu.00649.2007.. [DOI] [PMC free article] [PubMed] [Google Scholar]


