Abstract
Objective
The primary target cells for HIV infection in the genital tract are CD4 T-cells expressing CCR5 on the surface. Alterations in genital tract T-cells expressing CCR5 could impact human immunodeficiency virus (HIV) acquisition risk. We hypothesized that when compared to baseline, use of a hormonal intrauterine device (IUD) would alter HIV target cells (primarily CCR5+ CD4 cells) in the female genital tract more than a non-hormonal IUD.
Study Design
Thirty-four healthy, HIV negative women age 18-40 seeking an IUD for contraception were randomized to receive a levonorgestrel IUD or a copper T380A IUD. A parallel group of 8 control women not needing contraception was also enrolled. Genital tract mucosal immune cell populations collected by cervical cytobrush and endometrial biopsy before and two months after IUD placement were analyzed by flow cytometry. Mean differences in cell number and percent expressing receptors from baseline to follow-up were evaluated using paired Student’s t-tests.
Results
Neither IUD altered the number of T-cells within the upper and lower genital tracts. Levonorgestrel IUD users had a decrease in T-cells expressing the HIV co-receptor CCR5 in the endometrium and cervix after two months of use compared with baseline. There was a decrease in activated endometrial T-cells in levonorgestrel IUD users and a decrease in activated cervical T-cells in copper IUD users after two months of IUD use compared with baseline.
Conclusions
Women using IUDs have reduced expression of the CCR5 HIV co-receptor on T-cells in the endometrium and cervix compared to expression prior to IUD placement. These findings suggest that susceptibility to HIV infection would not be increased by IUD use.
Keywords: CCR5, HIV, hormonal contraception, IUD, T-cells
Introduction
Combating the spread of HIV is a major global goal,1 achievement of which could be accelerated by the eventual development of highly effective dual protection methods that prevent both sexual acquisition of HIV and unwanted pregnancy. There is currently a tension between HIV prevention and family planning because emerging data suggest some hormonal contraceptives, particularly injectable progestins, may increase risk of HIV acquisition and transmission.2-6 Long acting reversible contraceptives (LARC), which include IUDs and implants, are more effective than combined oral contraceptive pills (COCs) and DMPA and offer a significant reduction or complete elimination of systemic exposure to exogenous hormones compared to these methods. IUDs are the most commonly used reversible contraceptive worldwide7 and are regaining popularity in the United States; currently approximately 8.5% of American women who use contraception choose an IUD.8 Observational data from women living in high HIV incidence areas have included few IUD users. Thus, the available data cannot provide reliable estimates of HIV acquisition risk associated with IUD use. 3
HIV acquisition and sexual transmission are dependent on the immune environment of the female genital tract9,10 and may be under hormonal regulation. Endogenous sex hormones vary through the menstrual cycle and exogenous hormonal exposure occurs commonly with contraceptive use, which may influence mucosal immune cellular populations.11
Mucosal CD4 T lymphocytes in the vagina and cervix are thought to be the primary targets for sexual transmission of HIV to women.12-16 HIV infects discrete subsets of CD4 T cells, which express phenotypic receptors and co-receptors necessary for HIV to gain intracellular access.17-20 Antigen-presenting cells, such as mucosal dendritic cells, monocytes and macrophages (CD14+ cells) may help transport HIV from the surface to underlying target cells.21
CCR5 is expressed on genital tract T-cells22-25 and is the predominant target co-receptor for initial HIV infection.18 CCR5 expression is enhanced by sex hormones as ex-vivo studies that have demonstrated stimulation of CCR5 expression within explanted cervical tissue when incubated in media containing progesterone.23 In vivo, increased CCR5 expression in the setting of increased progesterone may contribute to the observed increased risk of HIV acquisition during pregnancy.26-30 However, there are limited data on lymphocyte changes with use of COCs31 and DMPA;32-34 there are no data on genital tract immune cell populations within reproductive tract mucosa of women using other contraceptive methods, including IUDs.
We hypothesized that genital tract immune cell populations, particularly CCR5+ T-cells, would be increased from baseline two months after initiating intrauterine contraception with a levonorgestrel (LNG) IUD containing 52mg LNG more than after initiation of use of a non-hormonal copper IUD (Cu-IUD). We hypothesized that concentrated progestin exposure in the genital tract would recruit HIV target cells to the area. We tested this hypothesis by examining immune cellular populations in upper and lower genital tract samples obtained immediately before and two months after IUD insertion in healthy women randomized to receive a LNG-IUD or Cu-IUD. Because IUDs are placed directly into the uterus, we hypothesized that the greatest impact on genital lymphocytes would be observed in T-cells recovered from endometrial biopsies.
Materials and Methods
We performed a randomized study of women initiating intrauterine contraception plus a parallel control group of women not at risk of pregnancy due to heterosexual abstinence or prior surgical sterilization. The primary objective was to assess the impact of IUD initiation on T-cells in the upper and lower genital tract. The University of Pittsburgh Institutional Review Board approved this study. All participants were enrolled at the Center for Family Planning Research, Magee-Womens Hospital of the University of Pittsburgh Medical Center and signed informed consent before study participation.
Forty-two women, age 18-40 years, were enrolled, including 34 women seeking an IUD for contraception and 8 women not seeking contraception who comprised an observational control group. Eligible women were healthy, HIV negative, non-pregnant and had regular menstrual cycles. All enrolled study participants were free of genital tract infection on screening exam, including rapid testing for Trichomonas vaginalis (OSOM, Sekisui Diagnostics, Lexington, MA), yeast vaginitis, symptomatic bacterial vaginosis by Amsel’s criteria,35 and abnormal inflammation (>10 WBC/hpf on wet mount). Women were excluded if within 60 days of enrollment they: 1) used any hormonal or intrauterine contraceptive; 2) were pregnant or breastfeeding; 3) underwent any genital tract procedure (including biopsy); 4) were diagnosed with any genital tract infection; 5) had a new sexual partner. Exclusion criteria included use of DMPA within 10 months of enrollment; use of oral or vaginal antibiotics, oral or vaginal steroids, or any vaginal product except tampons (such as spermicide, microbicide, douche, antifungal, steroid, or hormone) within 30 days of enrollment; having a contraindication to IUD use or an allergy to any component of the IUDs; or having a prior malignancy of the cervix or uterus. Women in the control group had to be not at risk of pregnancy defined as heterosexually abstinent or surgically sterile.
Screening also included urine pregnancy testing, collection of blood to rule out HIV infection and collection of cervical swabs for detection of Neisseria gonorrhoeae and Chlamydia trachomatis by nucleic acid amplification testing (NAAT, Gen-Probe, San Diego, CA). One participant was found to be ineligible after enrollment due to chlamydial infection and a second participant withdrew from the study after IUD insertion; both were replaced to maintain the targeted sample size.
Participants were enrolled immediately after screening if eligible that day, or returned for enrollment on a day when no vaginal bleeding was present. Day of menses at the time of enrollment was recorded. Participants were asked to refrain from any vaginal or anal intercourse for 1 week prior to sample collection at both visits. The 34 women who were seeking an IUD for contraception were randomized 1:1 to receive either a LNG-IUD (Mirena®, Bayer HealthCare Pharmaceuticals, Wayne, NJ) or CopperT380A IUD (ParaGard®, Teva Pharmaceuticals, Sellersville, PA). At the time of randomization, the study investigator opened the next sequentially numbered, opaque, sealed envelope containing the group assignment of LNG-IUD or Cu-IUD. A statistician not involved with the clinical conduct of the study prepared the envelopes using computer-generated random allocations in permutated blocks. The IUD was inserted per standard clinical practice at the enrollment visit immediately following the collection of all study samples. All laboratory personnel were masked to clinical status of participants including randomization to IUD type.
Genital tract samples were collected at enrollment and 8-week follow-up visits. Endocervical specimens were obtained by inserting a cytobrush (Cooper Surgical, Trumbull, CT) into the cervical os, rotating 360°, and placing the cytobrush in 4mL RPMI-1640 medium supplemented with 25mM HEPES, L-glutamine, and 10% fetal bovine serum (tRPMI). The ectocervix and endocervix were cleansed with chlorhexidine solution (Hibiclens, Mölnlycke Health Care, Norcross, GA) and dried with a sterile swab. Endometrial aspiration biopsies (Pipelle®, Cooper Surgical) were obtained with care not to touch the aspirator to the vaginal walls or the ectocervix. Adequacy of the sample was visually assessed by the clinician obtaining the biopsy. Endometrial samples were transported in the aspirator to the laboratory and extruded under sterile conditions. All samples were transported to the laboratory for processing within 30 minutes of collection.
Endometrial biopsies were weighed and then washed 3-4 times with phosphate buffered saline without calcium and magnesium (PBS) (Mediatech, Manassas, VA). The biopsies were minced using sterile scissors and placed in digest buffer containing 20mL RPMI-1640, 1mg/mL collagenase D (Roche Ltd, Nutley, NJ), and 2000U/mL DNase I (0.5μl/ml) (New England Biosciences, Ipswich, MA). Agitation of the tissue in digest buffer was limited to 15 minutes at ~300rpm at 37°C to maintain cell surface marker integrity.36,37 The transport vial containing the endocervical cytobrush was vortexed and washed with tRPMI to dislodge cells from the cytobrush. Both biopsy and cytobrush collected cells were filtered through a 40μm nylon cell sieve (Becton Dickenson, Franklin Lakes, NJ) to obtain single cell suspensions.
Endometrial biopsies additionally underwent density gradient centrifugation to remove dead cells, red blood cells, and other debris.36 The digested and filtered endometrial cells were re-suspended in 5mL 36% Histopaque® (Sigma-Aldrich, St. Louis, MO) in RPMI (Mediatech) then layered over 4.5mL undiluted Histopaque® and under 500uL PBS. This tube was centrifuged at 600× g for 30 minutes with no brake; lymphocytes were recovered from the interface. Recovered cells of both specimen types were then washed by centrifugation in RPMI and re-suspended in 1mL PBS. Using Trypan blue (Sigma-Aldrich) exclusion criteria,38 viable cell yields were obtained manually with a hemocytometer.
Cell suspensions were adjusted to 1×106 cells/mL in PBS and 1ml was stained for viability using LIVE/DEAD® Fixable Aqua Dead Cell Stain (Invitrogen, Carlsbad, CA) and incubated for 25 minutes at room temperature protected from light. Cells were washed once with 1ml of Flow Cytometry Staining Buffer (FACs) (eBioscience, San Diego, CA) by centrifugation for 5 minutes at 400× g, and stained with fluorochrome-conjugated antibodies (BD Biosciences, San Jose, CA) specific for the following cell surface markers: CD45 (FITC), CD3 (PerCP), CD8 (APC-H7), CD4 (PacificBlue), CD195 (CCR5)(APC), CD69 (PE), and CD14 (PE-Cy7). Cells were incubated for 25 minutes at room temperature protected from light, washed with 1mL FACs buffer by centrifugation for 5 minutes at 400× g, and fixed in 1% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA). Stained samples were stored at 4°C and flow cytometric analysis was conducted no later than 24 hours after fixation.
Lymphocyte populations were analyzed using a BD™ LSR II flow cytometer (BD Biosciences). To compensate for spectral overlap, single color compensation was applied specific for each flourochrome-conjugate used. T-cell populations were identified using forward and side scatter and fluorescent-minus-one (FMO) controls were utilized to assist in defining gate positions. Two senior laboratory technicians trained in advanced flow cytometry independently reviewed and agreed upon the gating parameters for each sample.
The sample size for this study was calculated based on available published data indicating the mean percent expression of CCR5 on cervical CD4 cells among women not using contraception was 48 +/−4% with a standard deviation of +/−7% for women using COCs.31 Assuming the standard deviation of the mean change in the percent expression would be no greater than +/−8%, a sample size of 10 would have 90% power to detect at least a 20% difference (set to be greater than one standard deviation for mean change) in the percent expression of CCR5 on CD4 measured prior to and two months after IUD placement, using a paired t-test evaluated at the 0.05 two-sided significance level. The enrollment target was increased to 16 participants per group to account for potential loss to follow-up, post-randomization ineligibility, inadequate specimen quality, and the plan to evaluate additional cell populations.
Data were analyzed using FACS DIVA software v.6.2 (BD Biosciences) and FlowJo software v.10.0.5 (Tree Star Inc., Ashland, OR). The gating strategy for all populations can be seen in Figure 1. Cell numbers from biopsy specimens were normalized per gram of tissue. Cytobrush collected cells were reported as “cells per cytobrush.” The cell numbers quantified were log10 transformed for analysis and presentation. Expression of CCR5 and CD69 was reported as the percentage of parent population expressing these cell surface markers. Each participant served as her own control by using the baseline visit as the control normal and assessing change at the two month follow-up. Statistical analysis was performed using SPSS® Statistical software version 20.0 (IBM Corporation, Armonk, NY) and statistical tests were evaluated at the 2-sided 0.05 significance level. Differences in enrollment characteristics between the groups were assessed using one-way analysis of variance, Kruskal-Wallis, and Fisher’s exact tests, where appropriate. Differences in levels of expression from baseline to follow-up were evaluated using paired Student’s t-tests.
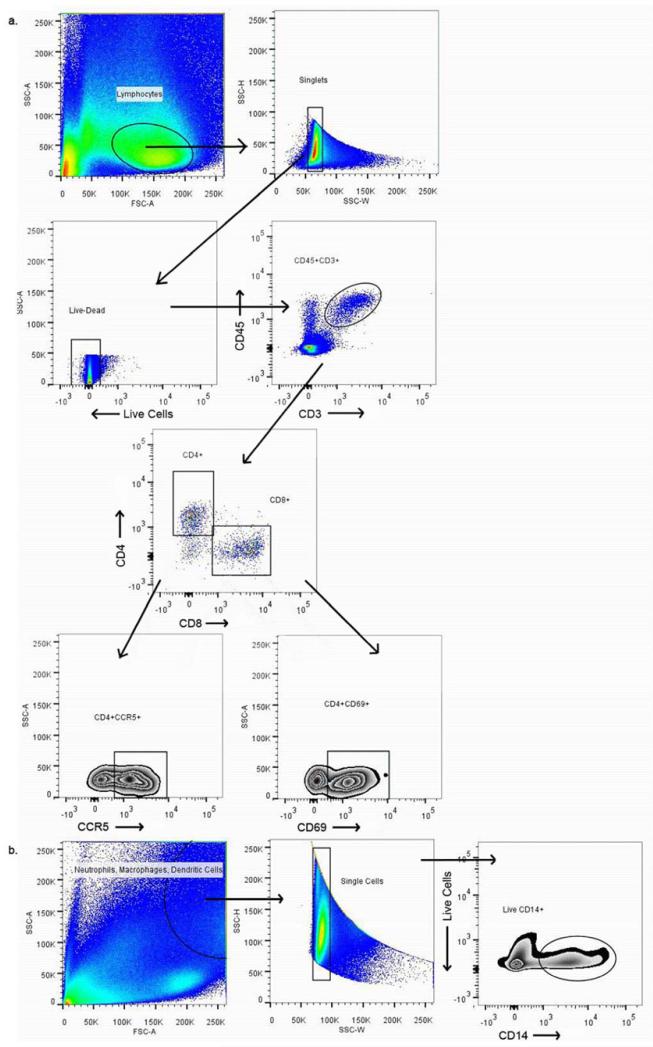
Figure 1. Gating strategy used to identify populations of interest.
a.) Live single cells were identified from the lymphocyte population. CD4 and CD8 T-cells were identified from CD45CD3+ populations and CCR5 and CD69 positive cells were identified from both CD4 and CD8 cells. b.) Single cells were identified from the neutrophil, macrophage, dendritic cell populations and live CD14 cells were gated from this population of single cells.
Results
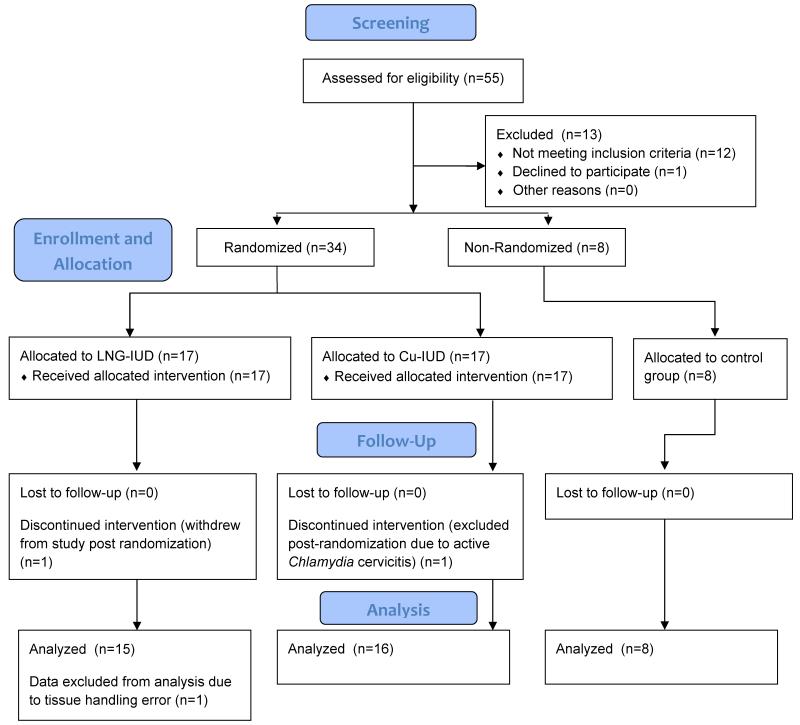
Between December 2010 and July 2011, 42 women were enrolled in the study (Fig. 2). Demographic information was not significantly different among the groups, including phase of menstrual cycle at the time of enrollment (Table 1). All of the sample data from one participant randomized to the LNG-IUD group were excluded from analysis due to inadequate specimen quality following a tissue handling error. The endometrial biopsies were performed with a single pass for 78/82 (95%) biopsies. The remaining 4 biopsies required two attempts to obtain an adequate sample.
Figure 2. CONSORT flow diagram.
Table 1. Demographics.
| Control (N=8) | LNG-IUD (N=15) |
Cu-IUD (N=16) | P-value | |
|---|---|---|---|---|
| Age, years | 27.6 ± 6.0 | 25.4 ± 6.2 | 26.9 ± 3.8 | 0.58* |
| Body Mass Index, kg/m2 | 25.8 ± 7.4 | 27.3 ± 8.3 | 27.3 ± 7.9 | 0.88* |
| Gravidity | 0 (0, 4) | 0 (0, 4) | 1 (0, 2) | 0.72† |
| Hispanic | 1 (12.5%) | 1 (6.7%) | 0 | 0.50† |
| Race | 0.29‡ | |||
| White | 5 (62.5%) | 12 (80.0%) | 15 (93.8%) | |
| Black | 1 (12.5%) | 2 (13.3%) | 1 (6.2%) | |
| Asian | 1 (12.5%) | 1 (6.7%) | 0 | |
| Marital Status | 0.82‡ | |||
| Single | 7 (87.5%) | 14 (93.3%) | 13 (81.2%) | |
| Married | 1 (12.5%) | 1 (6.7%) | 3 (18.8%) | |
| Education | 0.26‡ | |||
| High school graduate/GED | 2 (25.0%) | 9 (60.0%) | 6 (37.5%) | |
| College graduate | 6 (75.0%) | 6 (40.0%) | 10 (62.5%) | |
| Insurance | 0.69‡ | |||
| None | 2 (25.0%) | 1 (6.7%) | 2 (12.5%) | |
| Private | 5 (62.5%) | 13 (86.7%) | 12 (75.0%) | |
| Public | 1 (12.5%) | 1 (6.7%) | 2 (12.5%) | |
| Menstrual Cycle Day | 0.52‡ | |||
| Follicular phase (1-14) | 4 (50.0%) | 4 (26.7%) | 6 (37.5%) | |
| Luteal phase (15-28) | 4 (50.0%) | 11 (73.3%) | 10 (62.5%) | |
| Nugent Score ≤3 | 5 (62.5%) | 13 (86.7%) | 11 (68.8%) | 0.40‡ |
Data presented as mean ± standard deviation or n (%)
P-value from one-way analysis of variance
P-value from Kruskal-Wallis test
P-value from Fisher’s Exact test
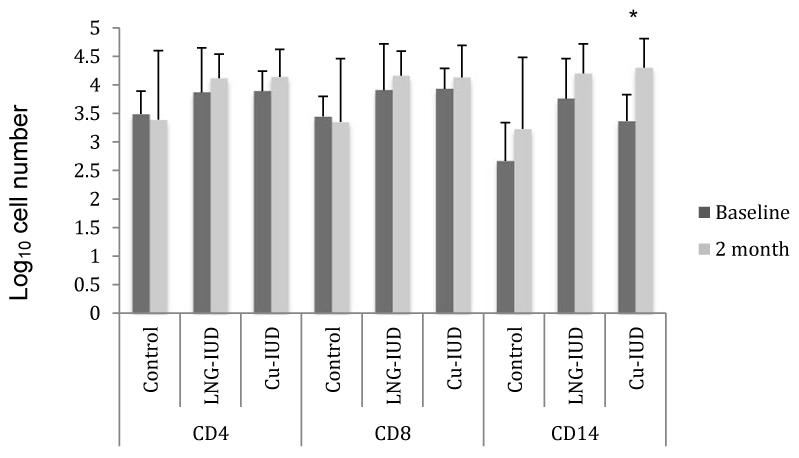
Two months after IUD insertion, there was no statistically significant change from baseline in the number of CD4+ or CD8+ T-cells in the endometrium (Fig. 3) or cervix (data not shown) amongst women using hormonal and non-hormonal IUDs. The number of CD14+ immune cells (macrophages, neutrophils, and dendritic cells) in the endometrium significantly increased two months after Cu-IUD placement (log10 3.4→4.3, p<0.001); among women receiving a LNG-IUD, the increase was less marked and not statistically significant (log10 3.8→4.2, p=0.06).
Figure 3. Immune Cell Number in Endometrial Biopsy Specimens.
The data represent the mean log10 transformed number of cells per gram of tissue weight.
LNG-IUD= levonorgestrel IUD
Cu-IUD= Copper IUD
*p<0.001
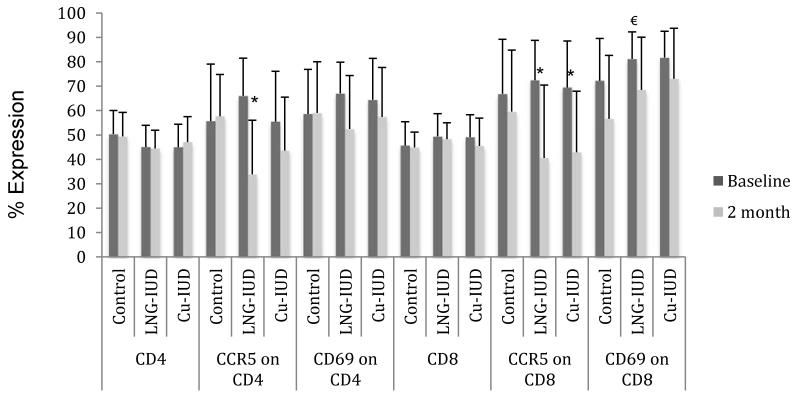
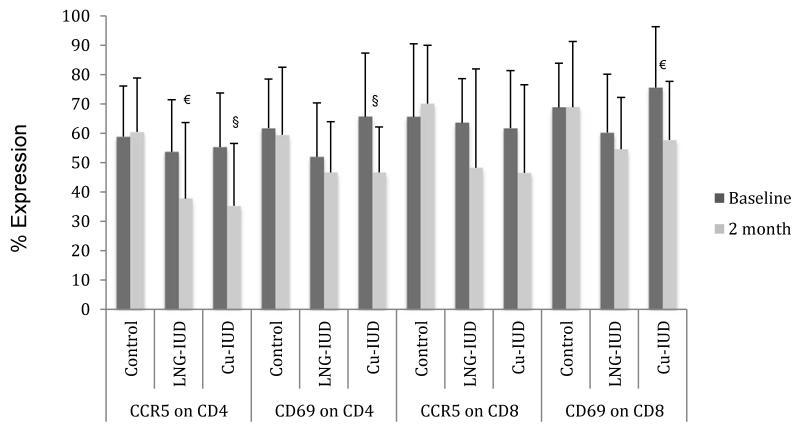
Within the endometrium, the percentage of CD4+ and CD8+ T-cells expressing the CCR5 HIV co-receptor significantly decreased from baseline levels two months after LNG-IUD insertion (66%→34%, p<0.001 and 72%→41%, p<0.005 respectively) (Fig. 4a). CCR5 expression also significantly decreased from baseline levels on endometrial CD8+ T-cells two months following Cu-IUD insertion (70%→43%, p<0.005).
Figure 4. Percent of T-cells expressing CCR5 and CD69.
LNG-IUD= levonorgestrel IUD
Cu-IUD= Copper IUD
*p<0.005
§p<0.01
€p<0.05
Within the cervix, CCR5 co-receptor expression on endocervical CD4+ T-cells was significantly diminished from baseline levels two months after initiation of an IUD (54%→38%, p<0.05 for LNG-IUD and 55%→35%, p<0.01 for Cu-IUD) (Fig. 4b). There was a decreased, but non-significant, expression of the CCR5 co-receptor on cervical CD8+ T-cells as compared to baseline in women randomized to Cu-IUD (62%→47%, p=0.06).
T-cell activation was assessed by measuring the percentage of T-cells expressing CD69. Endometrial cells from women using the LNG-IUD had significantly decreased CD69 on CD8+ T-cells (81%→68%, p<0.05) as compared to baseline; there was also a non-significant decrease in activation of CD4+ T-cells as compared to baseline (67%→52%, p=0.06) (Fig. 4a). In women who received a Cu-IUD, there was no significant change over two months in the activation of T-cells within the endometrium; however in the cervix there was a significant decrease in the percentage of activated CD4+ and CD8+ T-cells as compared to baseline (66%→47%, p<0.01 and 76%→58%, p<0.05 respectively) (Fig. 4b).
No significant changes were observed over time in any of these parameters among the control women who did not receive an IUD.
Comment
Although there is an incomplete understanding of factors that can increase or decrease CCR5 and CD69 expression on genital T-cells, CCR5 expression appears to increase predominantly with viral46 and parasitic infections.47-48 Expression of both CD6939-40 and CCR541-43 on various immune cells appear to be increased with exposure to pro-inflammatory cytokines and chemokines, and such an inflammatory milieu in the genital tract has been prospectively associated with increased HIV acquisition risk.44-45 Conversely, CD69 and CCR5 expression on lymphocytes may be decreased with exposure to steroids49-51 and antibiotics.52 Based on suggestions that injectable progestins may increase susceptibility to HIV infection, we had hypothesized that local progestin delivery with a LNG-IUD would have a greater impact on genital immune cells compared with exposure to a non-hormonal Cu-IUD, altering HIV susceptibility. Surprisingly, there was no change in the number of T-cells, the percentage of T-cells expressing HIV-co-receptor CCR5 was reduced, and the activation state of the T-cells was either reduced or unchanged within the upper and lower genital tracts two months after initiation of either a hormonal or non-hormonal IUD. No statistically significant T-cell changes, including CCR5 and CD69 expression, occurred among women in the parallel control group. Taken together these data suggest IUD use (either hormonal or copper) do not induce a pro-inflammatory milieu in the genital tract and would not increase HIV transmission risk.
We evaluated immune cell populations simultaneously in the upper and lower genital tracts of women initiating IUD use in order to assess a range of sexually exposed mucosa since the primary site(s) of sexual transmission remains unknown. Furthermore, the Cu-IUD has long been purported to create an inflammatory reaction within the endometrium as part of its mechanism of contraceptive action, however we did not find evidence of such. We found no increase in activation of T-cells as measured by CD69 expression in the endometrium of IUD users. We did find a statistically significant increase in macrophages, neutrophils and dendritic cells in the endometrium of Cu-IUD users as measured by CD14, and a similar, but not significant, increase in these cells among the LNG-IUD users. Interestingly, these data suggest differential alterations in immune cellular populations after CU-IUD insertion, with an increase in innate immune cells (CD14+) and a decrease in lymphocyte activation. Importantly, innate immune cells at mucosal surfaces, particularly macrophages and dendritic cells, may also play a significant role in HIV transmission by capturing and transporting HIV virions to susceptible T-cells.
There are few studies to date that have evaluated immune cells from freshly collected upper reproductive tract tissue surrounding IUD use. Studies using in situ genital tract immune cells generally use tissue collected at surgery from heterogeneous patients having procedures for a variety of pathologic conditions and with a mean age of greater than 40 years.53-55 Given evidence that age modifies the relationship between contraceptive use and risk of HIV acquisition with younger women at greater risk,6 performing biopsies rather than using surgical specimens allows investigation of the endometrial immune cells of younger healthy women. Since most studies evaluating genital tract immune cells to date have been performed with cytobrush collected cervical cells, we also chose to include cytobrush-collected cells in the present study. More work is needed to better understand the correlation between cells recovered by brush compared with tissue biopsy cells.
In order to minimize the effects of natural variation in cellular populations over time and with respect to sexual practices, we used a pair-wise comparison study design such that women acted as their own controls. The 100% follow-up of study participants in this study contributed to the strength of this analysis. One limitation of the present study is the single follow-up visit and the brief two-month evaluation time. A strength of the present study was the inclusion of a control group of women who were followed in parallel, since this group of women had no statistically significant changes in immune cell populations over time, suggesting that the changes observed among the women initiating IUD use was not due to normal variability of these cell populations over time.
Given the low probability of HIV transmission per sexual exposure to an HIV-infected partner, more research is needed to characterize the HIV-target cells present in the female genital tract and how their numbers, activation status and co-receptor expression relate to HIV susceptibility. Further studies are needed that directly compare genital CD4+ T-cell subsets and antigen presenting cells in women initiating the full range of hormonal and non-hormonal contraceptives, including DMPA, to better understand the range of cellular alterations and to learn which changes, if any, are important determinants of susceptibility to HIV. Furthermore, a better understanding of the endometrial and cervical immune effects of Cu-IUD use, particularly in the context of enrolling Cu-IUD users as non-hormonal contraceptive ’controls’ for larger trials designed to understand HIV risk with contraceptive use, are urgently needed.
In conclusion, this study of reproductive-aged women initiating IUD use demonstrated that women using the LNG-IUD had decreased numbers of CD4 cells expressing the HIV co-receptor CCR5, in both the endocervix and the endometrium, compared to baseline suggesting that the numbers of HIV targets in the cervix and endometrium would be decreased following initiation of this hormonal IUD. Women using the Cu-IUD had a similar decrease in CD4 cells expressing the CCR5 receptor in the cervix, suggesting that use of either type of IUD is associated with changes in T-cell populations in the female genital tract that are not suggestive of an increased risk of HIV acquisition. Given that the HIV-target cell populations were largely decreased in the genital tract with IUD use, a hypothesis could be generated that IUD use, particularly LNG-IUD use may be somewhat protective for HIV acquisition. The direct effect of these IUD-induced changes on actual cellular susceptibility either increased, decreased, or unchanged, has yet to be evaluated.
Acknowledgments
Sources of support: An anonymous foundation and The Bill & Melinda Gates Foundation
The findings reported herein were presented in part at the 12th Congress of the International Society for Immunology of Reproduction (ISIR) hosted by the American Society for Reproductive Immunology (ASRI) in Boston, MA, May 28-June 1, 2013.
This study was conducted and performed at the Center for Family Planning Research at Magee-Womens Hospital of the University of Pittsburgh Medical Center located in Pittsburgh, PA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: SLA, MDC, and SLH are consultants for Merck, Whitehouse Station, NJ and MDC receives research funding from Merck. MDC and BAC receive research funding from Medicines360, San Francisco, CA. BAC receives research funding from Bayer, Leverkusen, Germany and Evofem, San Diego, CA. The remaining authors report no conflicts of interest.
References
- 1.United Nations Millennium Development Goals Report. New York: 2011. [Google Scholar]
- 2.Heffron R, Donnell D, Rees H, et al. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. The Lancet Infectious Diseases. 2012;12:19–26. doi: 10.1016/S1473-3099(11)70247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morrison CS, Turner AN, Jones LB. Highly effective contraception and acquisition of HIV and other sexually transmitted infections. Best Pract Res Clin Obstet Gynaecol. 2009;23:263–84. doi: 10.1016/j.bpobgyn.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Morrison CS, Skoler-Karpoff S, Kwok C, et al. Hormonal contraception and the risk of HIV acquisition among women in South Africa. AIDS. 2012;26:497–504. doi: 10.1097/QAD.0b013e32834fa13d. [DOI] [PubMed] [Google Scholar]
- 5.Morrison CS, Richardson BA, Mmiro F, et al. Hormonal contraception and the risk of HIV acquisition. AIDS. 2007;21:85–95. doi: 10.1097/QAD.0b013e3280117c8b. [DOI] [PubMed] [Google Scholar]
- 6.Morrison CS, Chen PL, Kwok C, et al. Hormonal contraception and HIV acquisition: reanalysis using marginal structural modeling. AIDS. 2010;24:1778–81. doi: 10.1097/QAD.0b013e32833a2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Population Reference Bureau Family Planning Worldwide Report. Washington DC: 2013. [Google Scholar]
- 8.Finer LB, Jerman J, Kavanaugh ML. Changes in use of long-acting contraceptive methods in the United States, 2007-2009. Fertil Steril. 2012;98:893–7. doi: 10.1016/j.fertnstert.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaul R, Pettengell C, Sheth PM, et al. The genital tract immune milieu: an important determinant of HIV susceptibility and secondary transmission. Journal of Reproductive Immunology. 2008;77:32–40. doi: 10.1016/j.jri.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature. 2010;464:217–23. doi: 10.1038/nature08757. [DOI] [PubMed] [Google Scholar]
- 11.Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol Rev. 2005;206:306–35. doi: 10.1111/j.0105-2896.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 12.Greenhead P, Hayes P, Watts PS, Laing KG, Griffin GE, Shattock RJ. Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. J Virol. 2000;74:5577–86. doi: 10.1128/jvi.74.12.5577-5586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta P, Collins KB, Ratner D, et al. Memory CD4(+) T cells are the earliest detectable human immunodeficiency virus type 1 (HIV-1)-infected cells in the female genital mucosal tissue during HIV-1 transmission in an organ culture system. J Virol. 2002;76:9868–76. doi: 10.1128/JVI.76.19.9868-9876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hladik F, Sakchalathorn P, Ballweber L, et al. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity. 2007;26:257–70. doi: 10.1016/j.immuni.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu Q, Frank I, Williams V, et al. Blockade of attachment and fusion receptors inhibits HIV-1 infection of human cervical tissue. J Exp Med. 2004;199:1065–75. doi: 10.1084/jem.20022212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saba E, Grivel JC, Vanpouille C, et al. HIV-1 sexual transmission: early events of HIV-1 infection of human cervico-vaginal tissue in an optimized ex vivo model. Mucosal Immunol. 2010;3:280–90. doi: 10.1038/mi.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kader M, Wang X, Piatak M, et al. Alpha4(+)beta7(hi)CD4(+) memory T cells harbor most Th-17 cells and are preferentially infected during acute SIV infection. Mucosal Immunol. 2009;2:439–49. doi: 10.1038/mi.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorry PR, Ancuta P. Coreceptors and HIV-1 pathogenesis. Curr HIV/AIDS Rep. 2011;8:45–53. doi: 10.1007/s11904-010-0069-x. [DOI] [PubMed] [Google Scholar]
- 19.Douek DC, Brenchley JM, Betts MR, et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417:95–8. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 20.Cicala C, Martinelli E, McNally JP, et al. The integrin α4β7 forms a complex with cell-surface CD4 and defines a T-cell subset that is highly susceptible to infection by HIV-1. Proceedings of the National Academy of Sciences. 2009;106:20877–82. doi: 10.1073/pnas.0911796106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peressin M, Proust A, Schmidt S, et al. Efficient transfer of HIV-1 in trans and in cis from Langerhans dendritic cells and macrophages to autologous T lymphocytes. AIDS. 2014 doi: 10.1097/QAD.0000000000000193. [DOI] [PubMed] [Google Scholar]
- 22.Rottman JB, Ganley KP, Williams K, Wu L, Mackay CR, Ringler DJ. Cellular localization of the chemokine receptor CCR5. Correlation to cellular targets of HIV-1 infection. Am J Pathol. 1997;151:1341–51. [PMC free article] [PubMed] [Google Scholar]
- 23.Patterson BK, Landay A, Andersson J, et al. Repertoire of chemokine receptor expression in the female genital tract: implications for human immunodeficiency virus transmission. Am J Pathol. 1998;153:481–90. doi: 10.1016/S0002-9440(10)65591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hladik F, Lentz G, Delpit E, McElroy A, McElrath MJ. Coexpression of CCR5 and IL-2 in Human Genital But Not Blood T Cells: Implications for the Ontogeny of the CCR5+ Th1 Phenotype. The Journal of Immunology. 1999;163:2306–13. [PubMed] [Google Scholar]
- 25.Yeaman GR, Howell AL, Weldon S, et al. Human immunodeficiency virus receptor and coreceptor expression on human uterine epithelial cells: regulation of expression during the menstrual cycle and implications for human immunodeficiency virus infection. Immunology. 2003;109:137–46. doi: 10.1046/j.1365-2567.2003.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheffield JS, Wendel GD, Jr., McIntire DD, Norgard MV. The effect of progesterone levels and pregnancy on HIV-1 coreceptor expression. Reprod Sci. 2009;16:20–31. doi: 10.1177/1933719108325510. [DOI] [PubMed] [Google Scholar]
- 27.Gray RH, Li X, Kigozi G, et al. Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study. Lancet. 2005;366:1182–8. doi: 10.1016/S0140-6736(05)67481-8. [DOI] [PubMed] [Google Scholar]
- 28.Morrison CS, Wang J, Van Der Pol B, Padian N, Salata RA, Richardson BA. Pregnancy and the risk of HIV-1 acquisition among women in Uganda and Zimbabwe. AIDS. 2007;21:1027–34. doi: 10.1097/QAD.0b013e3280f00fc4. [DOI] [PubMed] [Google Scholar]
- 29.Reid SE, Dai JY, Wang J, et al. Pregnancy, contraceptive use, and HIV acquisition in HPTN 039: relevance for HIV prevention trials among African women. J Acquir Immune Defic Syndr. 2010;53:606–13. doi: 10.1097/QAI.0b013e3181bc4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mugo NR, Heffron R, Donnell D, et al. Increased risk of HIV-1 transmission in pregnancy: a prospective study among African HIV-1-serodiscordant couples. AIDS. 2011;25:1887–95. doi: 10.1097/QAD.0b013e32834a9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prakash M, Kapembwa MS, Gotch F, Patterson S. Oral contraceptive use induces upregulation of the CCR5 chemokine receptor on CD4(+) T cells in the cervical epithelium of healthy women. J Reprod Immunol. 2002;54:117–31. doi: 10.1016/s0165-0378(01)00125-5. [DOI] [PubMed] [Google Scholar]
- 32.Chandra N, Thurman AR, Anderson S, et al. Depot medroxyprogesterone acetate increases immune cell numbers and activation markers in human vaginal mucosal tissues. AIDS Res Hum Retroviruses. 2013;29:592–601. doi: 10.1089/aid.2012.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huijbregts RP, Helton ES, Michel KG, et al. Hormonal contraception and HIV-1 infection: medroxyprogesterone acetate suppresses innate and adaptive immune mechanisms. Endocrinology. 2013;154:1282–95. doi: 10.1210/en.2012-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huijbregts RP, Michel KG, Hel Z. Effect of progestins on immunity: medroxyprogesterone but not norethisterone or levonorgestrel suppresses the function of T cells and pDCs. Contraception. 2014 doi: 10.1016/j.contraception.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74:14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 36.Flynn L, Carton J, Byrne B, Kelehan P, O’Herlihy C, O’Farrelly C. Optimisation of a technique for isolating lymphocyte subsets from human endometrium. Immunol Invest. 1999;28:235–46. doi: 10.3109/08820139909060858. [DOI] [PubMed] [Google Scholar]
- 37.Abuzakouk M, Feighery C, O’Farrelly C. Collagenase and Dispase enzymes disrupt lymphocyte surface molecules. J Immunol Methods. 1996;194:211–6. doi: 10.1016/0022-1759(96)00038-5. [DOI] [PubMed] [Google Scholar]
- 38.Strober W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol. 2001 doi: 10.1002/0471142735.ima03bs21. Appendix 3:Appendix 3B. [DOI] [PubMed] [Google Scholar]
- 39.Atzeni F, Schena M, Ongari AM, et al. Induction of CD69 activation molecule on human neutrophils by GM-CSF, IFN-gamma, and IFN-alpha. Cellular immunology. 2002;220:20–9. doi: 10.1016/s0008-8749(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 40.Nopp A, Lundahl J, Hallden G. Quantitative, rather than qualitative, differences in CD69 upregulation in human blood eosinophils upon activation with selected stimuli. Allergy. 2000;55:148–56. doi: 10.1034/j.1398-9995.2000.00363.x. [DOI] [PubMed] [Google Scholar]
- 41.Croitoru-Lamoury J, Guillemin GJ, Boussin FD, et al. Expression of chemokines and their receptors in human and simian astrocytes: evidence for a central role of TNF alpha and IFN gamma in CXCR4 and CCR5 modulation. Glia. 2003;41:354–70. doi: 10.1002/glia.10181. [DOI] [PubMed] [Google Scholar]
- 42.Kroll-Palhares K, Silverio JC, Silva AA, et al. TNF/TNFR1 signaling up-regulates CCR5 expression by CD8+ T lymphocytes and promotes heart tissue damage during Trypanosoma cruzi infection: beneficial effects of TNF-alpha blockade. Memorias do Instituto Oswaldo Cruz. 2008;103:375–85. doi: 10.1590/s0074-02762008000400011. [DOI] [PubMed] [Google Scholar]
- 43.Wong JL, Berk E, Edwards RP, Kalinski P. IL-18-primed helper NK cells collaborate with dendritic cells to promote recruitment of effector CD8+ T cells to the tumor microenvironment. Cancer research. 2013;73:4653–62. doi: 10.1158/0008-5472.CAN-12-4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levinson P, Kaul R, Kimani J, et al. Levels of innate immune factors in genital fluids: association of alpha defensins and LL-37 with genital infections and increased HIV acquisition. AIDS. 2009;23:309–17. doi: 10.1097/QAD.0b013e328321809c. [DOI] [PubMed] [Google Scholar]
- 45.Mlisana K, Naicker N, Werner L, et al. Symptomatic vaginal discharge is a poor predictor of sexually transmitted infections and genital tract inflammation in high-risk women in South Africa. J Infect Dis. 2012;206:6–14. doi: 10.1093/infdis/jis298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanchooli J, Sanadgol N, Kazemi Arababadi M, Kennedy D. CCR5 plays important roles in hepatitis B infection. Viral Immunol. 2014;27:2–6. doi: 10.1089/vim.2013.0067. [DOI] [PubMed] [Google Scholar]
- 47.Chachage M, Podola L, Clowes P, et al. Helminth-Associated Systemic Immune Activation and HIV Co-receptor Expression: Response to Albendazole/Praziquantel Treatment. PLoS neglected tropical diseases. 2014;8:e2755. doi: 10.1371/journal.pntd.0002755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rojas-Dotor S, Perez-Ramos J, Gimenez-Scherer JA, Blanco-Favela F, Rico-Rosillo G. Effect of the monocyte locomotion inhibitory factor (MLIF) produced by E. histolityca on cytokines and chemokine receptors in T CD4+ lymphocytes. Biological research. 2009;42:415–25. [PubMed] [Google Scholar]
- 49.Attanasio R, Gust DA, Wilson ME, Meeker T, Gordon TP. Immunomodulatory effects of estrogen and progesterone replacement in a nonhuman primate model. Journal of clinical immunology. 2002;22:263–9. doi: 10.1023/a:1019997821064. [DOI] [PubMed] [Google Scholar]
- 50.Guo W, Li P, Zhao G, Fan H, Hu Y, Hou Y. Glucocorticoid receptor mediates the effect of progesterone on uterine natural killer cells. Am J Reprod Immunol. 2012;67:463–73. doi: 10.1111/j.1600-0897.2012.01114.x. [DOI] [PubMed] [Google Scholar]
- 51.Vassiliadou N, Tucker L, Anderson DJ. Progesterone-induced inhibition of chemokine receptor expression on peripheral blood mononuclear cells correlates with reduced HIV-1 infectability in vitro. J Immunol. 1999;162:7510–8. [PubMed] [Google Scholar]
- 52.Singh M, Singh P, Vaira D, Amand M, Rahmouni S, Moutschen M. Minocycline Attenuates HIV-1 Infection and Suppresses Chronic Immune Activation in Humanized NOD/LtsZ-scidIL-2Rgamma mice. Immunology. 2014 doi: 10.1111/imm.12246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pudney J, Quayle AJ, Anderson DJ. Immunological microenvironments in the human vagina and cervix: mediators of cellular immunity are concentrated in the cervical transformation zone. Biol Reprod. 2005;73:1253–63. doi: 10.1095/biolreprod.105.043133. [DOI] [PubMed] [Google Scholar]
- 54.Kaldensjö T, Petersson P, Tolf A, Morgan G, Broliden K, Hirbod T. Detection of Intraepithelial and Stromal Langerin and CCR5 Positive Cells in the Human Endometrium: Potential Targets for HIV Infection. PLoS ONE. 2011;6:e21344. doi: 10.1371/journal.pone.0021344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patel MV, Ghosh M, Fahey JV, Wira CR. Uterine epithelial cells specifically induce interferon-stimulated genes in response to polyinosinic-polycytidylic acid independently of estradiol. PLoS ONE. 2012;7:e35654. doi: 10.1371/journal.pone.0035654. [DOI] [PMC free article] [PubMed] [Google Scholar]