Abstract
Objective
To better risk stratify patients, utilizing baseline characteristics, in order to help optimize decision making for men with moderate to severe lower urinary tract symptoms (LUTS) secondary to BPH through a secondary analysis of the Medical Therapy of Prostatic Symptoms (MTOPS) trial.
Materials and Methods
After review of the literature, we identified potential baseline risk factors for BPH progression. Using bivariate tests in a secondary analysis of MTOPS data, we determined which variables retained prognostic significance. We then utilized these factors in Cox Proportional Hazard modeling to 1) more comprehensively risk stratify the study population based on pre-treatment parameters and 2) to determine which risk strata stood to benefit most from medical intervention.
Results
3047 men were followed in MTOPS for a mean of 4.5 years. We demonstrated varying risks of progression across quartiles. Baseline BPH Impact Index score, post-void residual, serum prostate specific antigen, age, AUA Symptom Index score, and maximum urinary flow rate were found to significantly correlate with overall BPH progression in multivariable analysis.
Conclusions
Utilizing baseline factors permits estimation of individual patient risk for clinical progression and the benefits of medical therapy. A novel clinical decision tool based on these analyses will allow clinicians to weigh patient-specific benefits against possible risks of adverse effects for a given patient.
Keywords: benign prostatic hyperplasia, lower urinary tract symptoms, risk factors
INTRODUCTION
Benign prostatic hyperplasia (BPH) can progress over time. Community-based studies have estimated that over 30% of men will experience clinical progression in the course of five years,1–4 predominantly characterized by increases in lower urinary tract symptom (LUTS) severity.5,6 While BPH is highly prevalent, not all men with BPH have the same risk for progression.7,8
Determining predictors of this risk has been an ongoing process over the past decade. A few trials have examined their placebo arms in order to elucidate baseline characteristics that portend worse prognoses.9,10 These efforts, however, have generally focused on a single factor, such as initiation of 5-alpha reductase inhibitors (5-ARIs) for men with enlarged prostates or elevated prostate specific antigen (PSA). In clinical practice, this may translate into focusing on an isolated variable to risk stratify when patients have multiple factors that might simultaneously influence the risk of progression and the potential benefits of therapy.
With the combined use of alpha-blockers and 5-ARIs, men can reduce their risk of BPH progression and ultimate need for invasive procedures. The Medical Therapy of Prostatic Symptoms (MTOPS) trial demonstrated clear benefit in risk reduction with this medical combination.6 Classically, clinical trials showing a positive result suggest that practitioners should adopt a “treat all” strategy. Yet, just as patient risk of progression can be variable, a given patient’s response to medical therapy may diverge from the mean, a concept known as heterogeneity of treatment effect (HTE).
It has recently been proposed11–13 that the results of clinical trials be routinely analyzed and presented in a risk stratified fashion to examine the relative and absolute effects across different risk strata, since baseline risk is a mathematical determinant of the treatment effect and can differ greatly across patients in a trial. To date, it remains unknown how the benefits of available therapies for BPH vary across patients at different progression risks, and such information could have important implications for clinical practice.
In order to better aid clinicians with decision making, we sought to risk stratify men with moderate to severe lower urinary tract symptoms (LUTS) secondary to BPH using established risk factors in a data driven model. With data from the MTOPS, we examined trial outcomes across risk strata of BPH progression in order to better define which patients are most likely to benefit from alpha-blockers, 5-ARIs, or their combination.
MATERIALS AND METHODS
We obtained original, publicly-available data from the MTOPS study. The MTOPS study was conducted by the MTOPS Investigators and supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Approval for our present analysis was provided by NIDDK and the study was approved by the Tufts Medical Center Internal Review Board.14 The design, rationale, and outcomes of the MTOPS study are described in detail elsewhere.6 In short, MTOPS was a randomized trial evaluating doxazosin, finasteride, or the combination of these medications for risk of BPH progression in men ≥50 years with AUA Symptom Index (AUA-SI) scores of 8–30 and maximal urinary flow rate (Qmax) of 4–15 mL/sec enrolled between 1993 and 1998. BPH progression, or the primary outcome, was defined as “the first occurrence of an increase over base line of at least four points in the AUA symptom score, acute urinary retention, renal insufficiency, recurrent urinary tract infection, or urinary incontinence.”6
In order to capture potential variables for risk stratification, we reviewed the current literature. Within their primary analysis, MTOPS investigators found that baseline prostate specific antigen (PSA) and baseline prostate volume significantly correlated with progression in univariate analysis.6 We identified two other models predictive of BPH progression based on 1) randomized-controlled trials with a different 5-ARI (dutasteride)8,15 and 2) expert consensus.16 These revealed additional potential risk factors: age, severe symptoms (as defined by both the BPH Impact Index17 and the AUA Symptom Index18, see Appendix 1), lower maximum urinary flow rate, and elevated post-void residual. Review of other community-based surveys and placebo-controlled trials further supported the relevance of these variables.1,2,19–23
Using Cox proportional hazards regression, we conducted univariate tests to determine which of these variables, measured at baseline, were associated with the outcome. We then utilized the factors with p-values <0.1 in a multivariate Cox model, excluding treatment assignment. Since the general practitioner does not typically assess PVR or Qmax in a primary care setting, we attempted to make our work more broadly applicable by creating a reduced model with easily measured factors (age, PSA, AUA Symptom Index, BPH Impact Index). The full and reduced Cox models were internally validated with bootstrapping to quantify optimism in model performance. We evaluated model performance with the c-statistic.24 Calibration was evaluated with a calibration plot and modified Hosmer-Lemeshow Chi-squared test for survival analysis25 and was excellent (Chi-squared p-value = 0.99).
From both the full and reduced Cox regression models, in separate analyses, we stratified the trial population into equal-sized risk quartiles. Within each risk quartile, we calculated the active drug treatment effect compared to placebo for each of the three intervention arms (i.e., doxazosin, finasteride, combination). Using Kaplan-Meier estimated failure rates after 4 years of follow-up, we calculated the absolute risk reduction (ARR) and number needed to treat (NNT) for each drug or drug combination compared to placebo as well as the combination therapy compared to each active drug individually. Heterogeneity of treatment effect was assessed by including an interaction term in the Cox regression model between the baseline linear risk predictor and treatment assignment to estimate the effect of treatment as a function of risk in BPH progression.
Finally, with results from the internally validated models, we constructed a clinical decision tool (nomogram) for care providers. Data management and regression model building were performed using SAS version 9.3 (SAS Institute, Cary NC). We used Frank Harrell’s ‘rms’ package in R software version 3.0.1 to perform the bootstrapped internal validation and generate the nomogram.
RESULTS
3047 men were followed in MTOPS for a mean of 4.5 years. They were randomized to four arms: placebo (737 subjects), doxazosin (756), finasteride (768), and combination therapy (786). 351 primary outcome events (i.e., BPH progression events) occurred in total, with 128 in the placebo arm, 85 in the doxazosin arm, 89 in the finasteride arm, and 49 in the combination arm. These were predominantly characterized as increases in symptom severity (78%), but did include acute urinary retention (12%), incontinence (9%), and recurrent urinary tract infection/urosepsis in 5 cases. Four-year overall risk of clinical progression within the placebo arm was 17%, compared to 10% in the doxazosin arm, 10% in finasteride arm, and 5% in combination arm (all previously reported p-values < 0.002).6 Baseline characteristics of the study subjects are shown in Table 1. Because collection of the variables of interest was excellent, we used complete case analysis and excluded only nine subjects from the full multivariate model and four from the reduced multivariate model.
Table 1.
Baseline characteristics of MTOPS cohort.
| Characteristic | Cohort (N=3047) |
Placebo (N=737) |
Doxazosin (N=756) |
Finasteride (N=768) |
Combination (N=786) |
|---|---|---|---|---|---|
| Age, year (range, 50–89) | 62.6 (7.3) | 62.5 (7.6) | 62.7 (7.3) | 62.6 (7.3) | 62.7 (7.1) |
| AUA Symptom Score (range, 8–35) | 16.9 (5.9) | 16.8 (6.0) | 17 (5.9) | 17.1 (6.0) | 16.8 (5.8) |
| Prostate volume, ml (range, 6.1–185.0) | 36.3 (20.1) | 35.2 (18.9) | 36.9 (21.6) | 36.9 (20.6) | 36.4 (19.2) |
| Maximal urinary flow rate, ml/sec (range, 4–15.3) | 10.5 (2.6) | 10.5 (2.7) | 10.3 (2.6) | 10.5 (2.6) | 10.6 (2.5) |
| Post-voiding residual volume, ml (range, 0–789) | 68.1 (82.9) | 69.6 (82.1) | 69.2 (88.3) | 66.2 (80.1) | 67.5 (81.2) |
| Serum PSA, ng/ml (range, 0.2–10.5) | 2.4 (2.1) | 2.3 (2.1) | 2.4 (2.2) | 2.4 (2.1) | 2.3 (2.0) |
| Serum glucose, ng/dl (range, 6–465) | 100.1 (42.5) | 100.8 (43.9) | 99.3 (41.6) | 98.6 (37.4) | 101.6 (46.4) |
| BMI, kg/m2 (range, 16.5–52.1) | 27.8 (4.2) | 27.6 (4.0) | 27.7 (3.9) | 27.9 (4.6) | 27.9 (4.2) |
| BPH Impact Index (range, 0–13) | 4.0 (2.7) | 4.0 (2.7) | 3.9 (2.7) | 4.1 (2.8) | 4.0 (2.7) |
| Primary school education only, n (%) | 120 (3.9) | 29 (3.9) | 36 (4.8) | 31 (4.0) | 24 (3.1) |
| Family history of prostate cancer, n (%) | |||||
| Yes | 416 (13.7) | 95 (12.9) | 94 (12.4) | 98 (12.8) | 129 (16.4) |
| No | 2228 (73.1) | 545 (74.0) | 546 (72.2) | 578 (75.3) | 559 (71.1) |
| Unknown | 403 (13.2) | 97 (13.2) | 116 (15.3) | 92 (12.0) | 98 (12.5) |
| Impotence, n (%) | |||||
| Yes | 747 (24.6) | 185 (25.1) | 196 (26.0) | 184 (24.0) | 182 (23.2) |
| No | 1745 (57.4) | 417 (56.7) | 423 (56.0) | 456 (59.4) | 449 (57.3) |
| Intermittent | 550 (18.1) | 134 (18.2) | 136 (18.0) | 128 (16.7) | 152 (19.4) |
| History of diabetes, n (%) | 260 (8.5) | 72 (9.8) | 59 (7.8) | 65 (8.5) | 64 (8.1) |
| History of hypertension, n (%) | 871 (28.6) | 203 (27.5) | 226 (29.9) | 212 (27.6) | 230 (29.3) |
Values are means (standard deviation) unless otherwise noted.
MTOPS = The Medical Therapy of Prostatic Symptoms; AUA = American Urological Association; PSA = prostate-specific antigen; BMI = body mass index
Hazard ratios (95% CI) for the treatment arms were as follows: doxazosin 0.54 (0.40–0.72), finasteride 0.60 (0.45–0.80), combination 0.27 (0.19–0.39)
Bivariate analysis was performed on all clinically relevant variables based on our literature review (Table 2). Variables demonstrating statistical significance were then utilized to create a parsimonious, multivariable model of BPH progression risk. Baseline BPH Impact Index (BII) (per 1 point, HR 1.12, 95% CI 1.07–1.17), post-void residual (PVR) (per 100 mL, HR 1.17, 95% CI 1.04–1.30), serum prostate specific antigen (PSA) (per 5 ng/mL, HR 1.44, 95% CI 1.14–1.82), age (per 10 yrs, HR 1.33, 95% CI 1.15–1.53), AUA Symptom Index (AUA-SI) score (per 5 points, HR 0.76, 95% CI 0.68–0.85), and maximum urinary flow rate (Qmax) (per 5 mL/sec, HR 0.74, 95% CI 0.60–0.90) were found to be significantly negatively correlated with overall BPH progression in multivariable analysis (Table 3). Internal validation after 500 bootstrap repetitions revealed an optimism-corrected c-statistic of 0.626 compared to 0.635 in the original dataset.
Table 2. Bivariate analysis: Baseline characteristics and their correlation with risk of BPH progression.
Characteristics identified within our literature search as well as a number of other factors collected within MTOPS data were analyzed. Variables are plotted with their model coefficients and hazard ratios. Standard errors are noted for each estimate.
| Variable | Estimate | SE | p-value | HR |
|---|---|---|---|---|
| Age, year | 0.0373 | 0.0072 | <.0001 | 1.038 |
| AUA Symptom Score | −0.0240 | 0.0093 | 0.0098 | 0.976 |
| Prostate volume, ml | 0.0096 | 0.0021 | <.0001 | 1.010 |
| Maximal urinary flow rate, ml/sec | −0.0587 | 0.0204 | 0.004 | 0.943 |
| Post-voiding residual volume, ml | 0.0018 | 0.0005 | 0.001 | 1.002 |
| Serum PSA, ng/ml | 0.1096 | 0.0223 | <.0001 | 1.116 |
| BPH Impact Index | 0.0452 | 0.0192 | 0.0184 | 1.046 |
| Serum glucose, ng/dl | −0.0005 | 0.0013 | 0.7126 | 1.000 |
| BMI, kg/m2 | −0.0018 | 0.0130 | 0.8930 | 0.998 |
| Primary school education only, yes vs. no | 0.3511 | 0.2420 | 0.1469 | 1.421 |
| Family history of prostate cancer | ||||
| Yes | −0.0757 | 0.1597 | 0.6357 | 0.927 |
| No | 1.00 (reference) | |||
| Unknown | 0.2040 | 0.1480 | 0.1682 | 1.226 |
| Impotence, n (%) | ||||
| Yes | 0.2117 | 0.1194 | 0.0762 | 1.236 |
| No | 1.00 (reference) | |||
| Intermittent | 0.0072 | 0.1374 | 0.9583 | 1.007 |
| History of diabetes, yes vs. no | 0.0826 | 0.1854 | 0.6561 | 1.086 |
| History of hypertension, yes vs. no | 0.0173 | 0.1186 | 0.8843 | 1.017 |
SE = standard error; HR = hazard ratio; CL = confidence limit; PSA = prostate-specific antigen; AUA = American Urological Association
Table 3. Multivariable Cox proportional hazards model.
Variables are listed with their respective hazard ratios. Prostate volume did not (by neither digital rectal exam nor transrectal ultrasound estimate) maintain significance in our multivariable model.
| Variable | Hazard Ratio | 95% Wald CL |
|---|---|---|
| Age (per 10 years) | 1.33 | 1.15, 1.53 |
| Serum PSA (per 5 ng/mL) | 1.44 | 1.14, 1.82 |
| AUA symptom score (per 5 points) | 0.76 | 0.68, 0.85 |
| BPH Impact Index (per 1 point) | 1.12 | 1.07, 1.17 |
| Maximal urinary flow rate (per 5 mL/second) | 0.74 | 0.60, 0.90 |
| Post-voiding residual volume (per 100 mL) | 1.17 | 1.04, 1.30 |
N=3036 with non-missing data for all predictors. c-statistic = 0.635
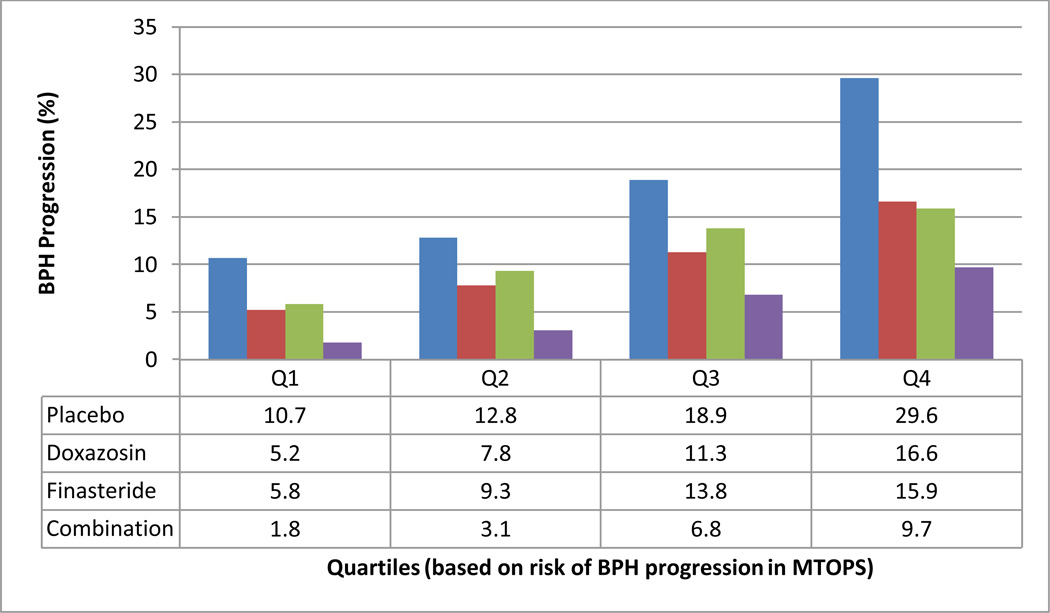
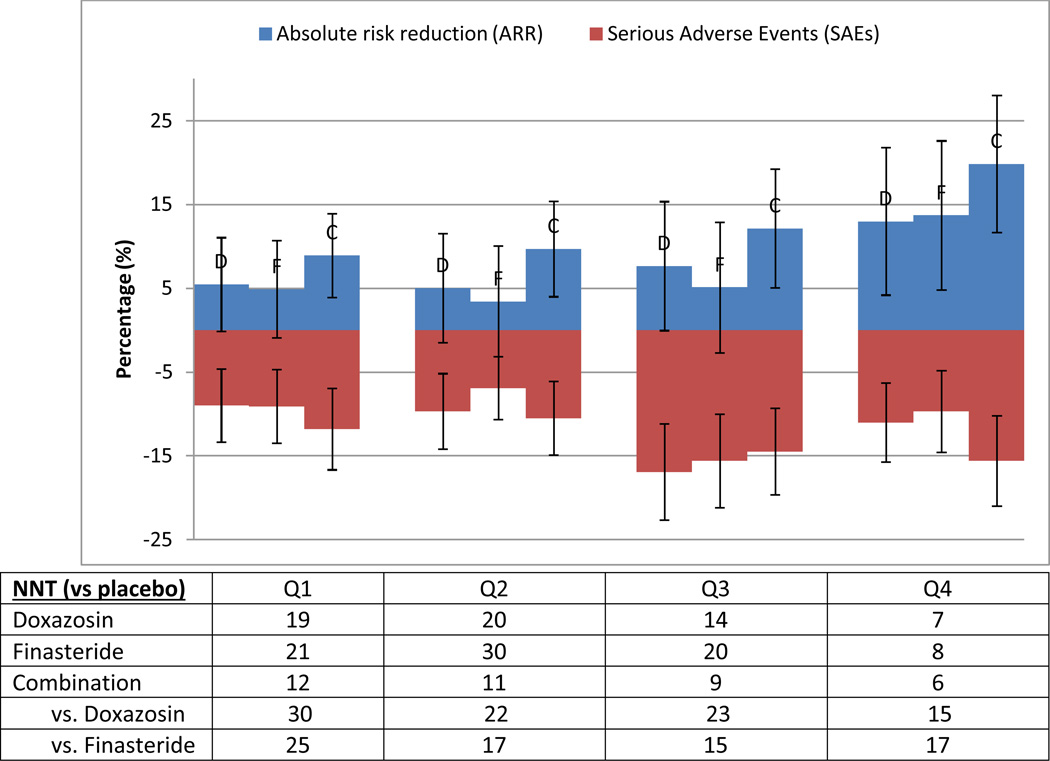
This predictive model of risk for BPH progression (c-statistic = 0.635) demonstrated varying risks of progression across quartiles (Figure 1). Formal tests of interaction between risk and treatment were not significant (data not shown), indicating that patients experienced roughly proportional benefits across risk strata from the 3 different treatments. However, the highest-risk quartile had a risk of progression approximately 300% that of the lowest within all trial arms on the absolute risk reduction (ARR) scale. Thus, the number needed to treat for those patients in the lowest quartile of risk was about three times that for patients in the highest quartile. MTOPS demonstrated a 17% overall incidence of clinical progression for the “average” patient,6 but a patient within the lowest quartile only bears a progression risk of 10.7%, while the typical highest quartile patient faces up to a 29.6% risk of progression. In order to facilitate a more direct comparison between serious adverse effects (SAEs) and therapeutic benefits, we stratified those SAEs that resulted in medication interruption or discontinuation that occurred prior to a primary outcome event within the first four years of follow-up (Figure 2). This figure demonstrates a clear increase in the ratio of the benefits to the side effects of therapy as risk increases.
Figure 1. Four-year risk of BPH progression stratified across quartiles.
Linear predictor scores from our full model were used to generate a spectrum of risk, which we divided into quartiles. This was performed for all four arms of MTOPS. The observed mean event rates for each arm within each quartile were collected based on Kaplan-Meier estimated event rates within the MTOPS trial.
***Event rates (%) are listed in the table below.
Figure 2. Medication treatment effects in terms of absolute risk compared to serious adverse events (SAEs) that resulted in therapy interruption/discontinuation.
Absolute risk reductions (with 95% CI) for the different treatment arms are calculated compared to the placebo arm and increase over quartiles of BPH progression, ordered from left to right (Q1->Q4), with each treatment arm reported (ordered as doxazosin (D), finasteride (F), combination (C) in each quartile). Serious adverse events (with 95% CI) are reported in raw percentages (listed as negative values for comparison). The number needed to treat (NNT) is derived from the absolute risk reductions in the respective groups, as listed in the table below.
ARR = absolute risk reduction compared to placebo; NNT = number needed to treat (1/ARR)
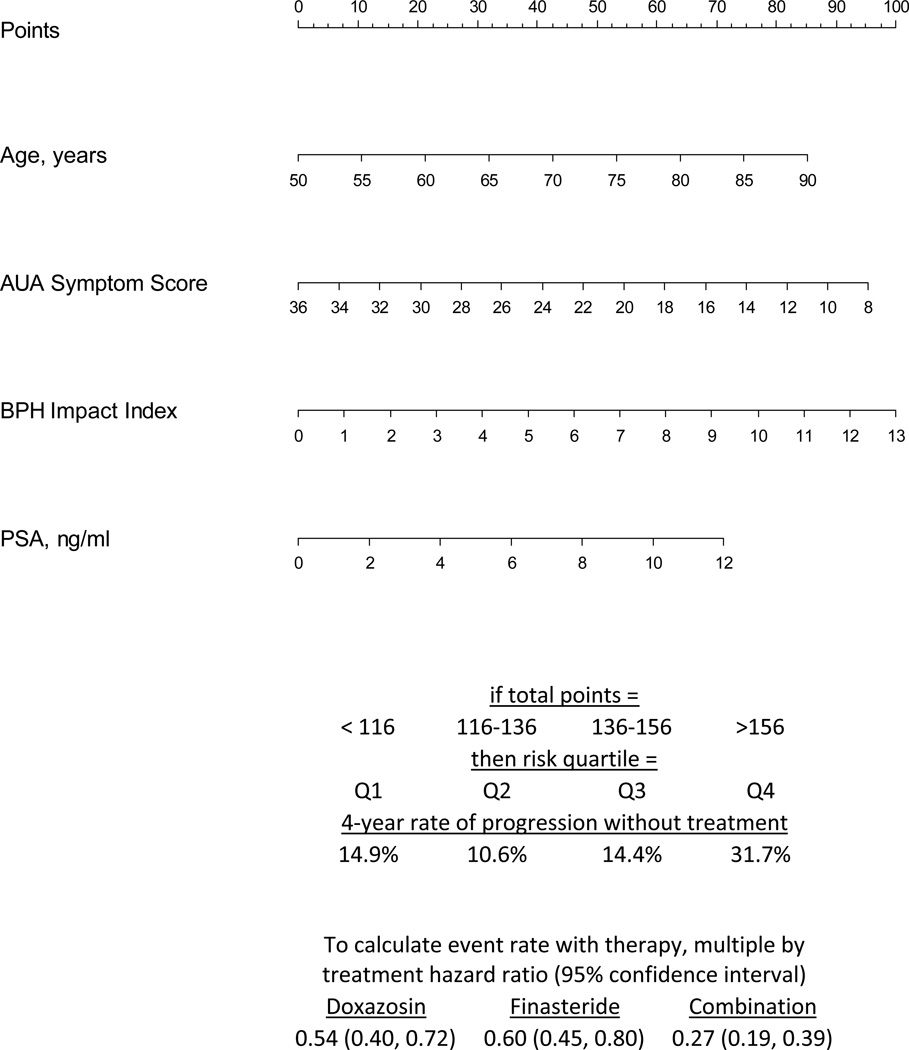
Our reduced model retained predictive value with a c-statistic of 0.623, compared to 0.635 with the full model (Table 4). This suggests that these easily attainable parameters can be utilized in a variety of clinical settings in order to predict risk of BPH progression. Clinical decision tools were then constructed from these models (see Appendix 2 and Figure 3).
Table 4. Reduced multivariable Cox proportional hazards model.
This model retains its predictive value over a similar range of decision thresholds compared to the full model above. However, this model includes variables that may be easily measured in primary care settings, allowing for broader applicability.
| Variable | Hazard Ratio | 95% Wald CL |
|---|---|---|
| Age (per 10 years) | 1.35 | 1.17, 1.56 |
| Serum PSA (per 5 ng/mL) | 1.52 | 1.21, 1.91 |
| AUA symptom score (per 5 points) | 0.79 | 0.70, 0.88 |
| BPH Impact Index (per 1 point) | 1.12 | 1.07, 1.17 |
N=3043 with non-missing data for all predictors. c-statistic= 0.623
CL = confidence limit; PSA = prostate-specific antigen; AUA = American Urological Association
Figure 3.
Reduced Model--Clinical decision tool for predicting 4-year risk of clinical BPH progression using data from MTOPS, 4 predictor regression model. In order to determine an individual’s risk of progression, draw a vertical line from each of the patient’s factors to the “Points” line above. Add the four “Points” values together to arrive at a “Total Points.” Calculate the expected event rate with therapy by applying the hazard ratios below.
DISCUSSION
While MTOPS reported an overall incidence of clinical progression of 17% in the placebo group, we demonstrated that this risk is variable among men. Whereas benefits of medical therapy for patients with a high risk of progression are clear, the benefits to patients at lower risk are less so, and may be more finely balanced with risks and sensitive to patient preferences.
Prior studies have offered a number of predictive baseline factors for the clinical progression of BPH. These have included increased symptom severity (both AUA-SI and BII), low Qmax, high PVR, high prostate volume, and high serum PSA.8,9,19,20,26 Age has also been reported to have prognostic value.6 Similar to these previous reports, we found a number of predictive characteristics, including age, serum PSA, PVR, Qmax, BII, and AUA-SI.
Of note, the AUA-SI score has a hazard ratio less than 1 in our model, indicating that higher symptoms are protective against progression. This is likely because those men with severe symptoms were less likely to progress to even worse symptoms (as detected by the AUA-SI) during the follow-up period.
In our final model, prostate volume was not necessary to yield optimal prognostic information. While seemingly in contrast to prior studies6,10,26,27 that suggested that larger prostate volumes are associated with higher risk of BPH progression, our multivariable analysis suggests that once PSA and symptom severity score are included, the information gained from knowing prostate size was no longer statistically significant in predicting BPH progression. This finding is particularly relevant in the primary care setting where the availability of prostate ultrasound to measure size is uncommon. Of note, our findings cannot be generalized to men outside the MTOPS entry criteria.
Prior analyses6,27,28 suggested that elevated serum PSA and higher total prostate volume were the strongest predictors of BPH progression. Our analysis suggests that focusing on these two factors in isolation would fail to capture the entire clinical picture for a given patient. As one can imagine, in patients who have conflicting characteristics, decisions made on a single factor may inappropriately guide therapy. Moreover, univariable analyses tend to highlight a certain threshold (e.g., a patient’s prostate is considered enlarged if it is above 30 grams), but such decision points can arbitrarily endorse treatment or no treatment again for the non-ideal candidate.
Utilizing our derived nomogram will allow clinicians to account for the key factors that have been demonstrated to predict disease progression in a large, prospective clinical trial. A patient’s age, AUA Symptom Score, BPH Impact Index score, and serum PSA can easily be collected during a clinical encounter. These values can then be translated into a “Total Points” within our nomogram, as described in Figure 3, and ultimately into a risk of progression. Clinicians can then weigh this risk of progression against the potential risk of side effects, as briefly outlined in Figure 2, and other patient-specific characteristics (e.g., patient compliance, medication costs to the patient). We hope to ultimately translate this nomogram into a user-friendly, web-based application.
Other groups have created clinical tools in an effort to help physicians most effectively employ medical therapy for BPH. Lowe et al. surveyed 12 international “experts” on BPH using over 240 hypothetical patient scenarios in order to determine the most important prognostic factors for progression. This panel concluded that symptom severity, low Qmax, and elevated PVR were the most important determinants for progression, although PSA and prostate volume were also considered significant. The authors formulated a regression model to predict risk based on the panel’s responses.16
Additionally, Slawin et al. performed secondary analysis on three 2-year, multicenter, placebo-controlled, double-blind randomized trials with dutasteride to create a clinical nomogram for the risk of acute urinary retention or BPH-related surgery.8,15 However, that data was limited to men with larger prostates (greater than 30 mL) and had a shorter follow-up time than MTOPS (2 years compared to 4.5 years). Moreover, MTOPS demonstrated, in a more inclusive sample, that progression predominantly manifests as worsening symptom severity as opposed to retention or surgery. As such, the Slawin model can assist in more specialized, second-line settings, while our model may be more widely applicable to the treatment-naïve population. Additionally, while they examined the variable risk of progression, ours remains the first study to examine combination medical therapy in a risk stratified analysis.
Clinical decisions must also consider the adverse effects of possible interventions. For those men in MTOPS who received active therapy, 27% stopped doxazosin, 24% stopped finasteride, and 18% stopped combination therapy. Medication cessation was most often because of adverse effects.6 We found that the risk of medication discontinuation was relatively stable across quartiles of progression risk, at approximately 8–15%. Clinicians can utilize our decision tool to weigh a patient’s risk of progression against this risk of discontinuation to determine if there may be a realized benefit in initiating medical therapy.
As with most statistical models, our findings may be subject to over-fitting and would benefit from external validation in another cohort. However, by basing our model on just a handful of clinically relevant variables and by having a large number of outcome events for each variable tested, overfitting is less likely to have impacted our primary conclusions, although miscalibration to the external population could affect the benefits of using the model. Additionally, for methodological reasons, we excluded therapy from our model, which lowered the c-statistic; inclusion would have greatly improved discrimination since the medications were so effective. Furthermore, while we postulated that our findings might improve effectiveness of these pharmacological interventions at the population level, there were no cost data collected in the MTOPS trial so a cost effectiveness analyses was not performed here.
CONCLUSION
The risk of BPH progression is highly variable among men. MTOPS data suggests that the benefits of medical therapy for BPH are unevenly distributed with men who were in the highest risk strata accounting for the greatest clinical benefit. Importantly, utilizing commonly available, baseline risk factors permits estimation of the patient-specific risk for clinical progression, and thus the potential for benefit. Our novel decision tool based on clinically available factors (age, AUA-SI, BII, serum PSA) may allow clinicians to better select those most likely to benefit from medical therapy for BPH and potentially inform future guidelines. Potential treatment effect can then be weighed against possible risks of adverse effects for a given patient.
Supplementary Material
ACKNOWLEDGEMENTS
Jason Nelson, MPH and David M. Kent, MD, MS had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
This manuscript was partially funded by grants 1UL1 TR001064 and U01 AA022802, both from the National Institutes of Health (NIH), as well as grant 1IP2PI000722, from the Patient-Centered Outcome Research Institute (PCORI).
The MTOPS study was conducted by the MTOPS Investigators and supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The data and samples from MTOPS reported here were supplied by the NIDDK Central Repositories. This manuscript was not prepared in collaboration with Investigators of the MTOPS study and does not necessarily reflect the opinions or views of the MTOPS study, the NIDDK Central Repositories, or the NIDDK.
Footnotes
DISCLOSURES
None of the four authors have any conflicts of interest.
REFERENCES
- 1.Flanigan RC, Reda DJ, Wasson JH, et al. 5-year outcome of surgical resection and watchful waiting for men with moderately symptomatic benign prostatic hyperplasia: a Department of Veterans Affairs cooperative study. J Urol. 1998;160(1):12–16. discussion 16-17. [PubMed] [Google Scholar]
- 2.Jacobsen SJ, Girman CJ, Lieber MM. Natural history of benign prostatic hyperplasia. Urology. 2001;58(6 Suppl 1):5–16. doi: 10.1016/s0090-4295(01)01298-5. discussion 16. [DOI] [PubMed] [Google Scholar]
- 3.Maserejian NN, Chen S, Chiu GR, et al. Treatment status and progression or regression of lower urinary tract symptoms in a general adult population sample. J Urol. 2014;191(1):107–113. doi: 10.1016/j.juro.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin S, Lange K, Haren MT, et al. Risk factors for progression or improvement of lower urinary tract symptoms in a prospective cohort of men. J Urol. 2014;191(1):130–137. doi: 10.1016/j.juro.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 5.Roehrborn CG. BPH progression: concept and key learning from MTOPS, ALTESS, COMBAT, and ALF-ONE. BJU Int. 2008;101(Suppl 3):17–21. doi: 10.1111/j.1464-410X.2008.07497.x. [DOI] [PubMed] [Google Scholar]
- 6.McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349(25):2387–2398. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 7.Anderson JB, Roehrborn CG, Schalken JA, et al. The progression of benign prostatic hyperplasia: examining the evidence and determining the risk. Eur Urol. 2001;39(4):390–399. doi: 10.1159/000052475. [DOI] [PubMed] [Google Scholar]
- 8.Slawin KM, Kattan MW. The Use of Nomograms for Selecting BPH Candidates for Dutasteride Therapy. Rev Urol. 2004;6(Suppl 9):S40–S45. [PMC free article] [PubMed] [Google Scholar]
- 9.Crawford ED, Wilson SS, McConnell JD, et al. Baseline factors as predictors of clinical progression of benign prostatic hyperplasia in men treated with placebo. J Urol. 2006;175(4):1422–1426. doi: 10.1016/S0022-5347(05)00708-1. discussion 1426-1427. [DOI] [PubMed] [Google Scholar]
- 10.Roehrborn CG, Siami P, Barkin J, et al. The influence of baseline parameters on changes in international prostate symptom score with dutasteride, tamsulosin, and combination therapy among men with symptomatic benign prostatic hyperplasia and an enlarged prostate: 2-year data from the CombAT study. Eur Urol. 2009;55(2):461–471. doi: 10.1016/j.eururo.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 11.Kent DM, Rothwell PM, Ioannidis JP, et al. Assessing and reporting heterogeneity in treatment effects in clinical trials: a proposal. Trials. 2010;11:85. doi: 10.1186/1745-6215-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorresteijn JA, Visseren FL, Ridker PM, et al. Estimating treatment effects for individual patients based on the results of randomised clinical trials. BMJ. 2011;343:d5888. doi: 10.1136/bmj.d5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burke JF, Hayward RA, Nelson JP, et al. Using internally developed risk models to assess heterogeneity in treatment effects in clinical trials. Circ Cardiovasc Qual Outcomes. 2014;7(1):163–169. doi: 10.1161/CIRCOUTCOMES.113.000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuticchia AJ, Cooley PC, Hall RD, et al. NIDDK data repository: a central collection of clinical trial data. BMC Med Inform Decis Mak. 2006;6:19. doi: 10.1186/1472-6947-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slawin KM, Kattan MW, Roehrborn CG, et al. Development of nomogram to predict acute urinary retention or surgical intervention, with or without dutasteride therapy, in men with benign prostatic hyperplasia. Urology. 2006 Jan;67(1):84–88. doi: 10.1016/j.urology.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Lowe FC, Batista J, Berges R, et al. Risk factors for disease progression in patients with lower urinary tract symptoms/benign prostatic hyperplasia (LUTS/BPH): a systematic analysis of expert opinion. Prostate Cancer Prostatic Dis. 2005;8(3):206–209. doi: 10.1038/sj.pcan.4500806. [DOI] [PubMed] [Google Scholar]
- 17.Barry MJ, Fowler FJ, Jr, O'Leary MP, et al. Measuring disease-specific health status in men with benign prostatic hyperplasia. Measurement Committee of The American Urological Association. Med Care. 1995;33(4 Suppl):AS145–AS155. [PubMed] [Google Scholar]
- 18.Barry MJ, Fowler FJ, Jr, O'Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148(5):1549–1557. doi: 10.1016/s0022-5347(17)36966-5. discussion 1564. [DOI] [PubMed] [Google Scholar]
- 19.Roehrborn CG. Definition of at-risk patients: baseline variables. BJU Int. 2006;97(Suppl 2):7–11. doi: 10.1111/j.1464-410X.2006.06098.x. discussion 21-12. [DOI] [PubMed] [Google Scholar]
- 20.Trachtenberg J. Treatment of lower urinary tract symptoms suggestive of benign prostatic hyperplasia in relation to the patient's risk profile for progression. BJU Int. 2005;95(Suppl 4):6–11. doi: 10.1111/j.1464-410X.2005.05488.x. [DOI] [PubMed] [Google Scholar]
- 21.Hong SJ, Ko WJ, Kim SI, et al. Identification of baseline clinical factors which predict medical treatment failure of benign prostatic hyperplasia: an observational cohort study. Eur Urol. 2003;44(1):94–99. doi: 10.1016/s0302-2838(03)00199-4. discussion 99-100. [DOI] [PubMed] [Google Scholar]
- 22.Kok ET, Schouten BW, Bohnen AM, et al. Risk factors for lower urinary tract symptoms suggestive of benign prostatic hyperplasia in a community based population of healthy aging men: the Krimpen Study. J Urol. 2009;181(2):710–716. doi: 10.1016/j.juro.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 23.Parsons JK. Modifiable risk factors for benign prostatic hyperplasia and lower urinary tract symptoms: new approaches to old problems. J Urol. 2007;178(2):395–401. doi: 10.1016/j.juro.2007.03.103. [DOI] [PubMed] [Google Scholar]
- 24.Steyerberg EW. Clinical Prediction Models A Practical Approach to Development, Validation, and Updating. New York, NY: Springer-Verlag New York; 2009. SpringerLink. [Google Scholar]
- 25.D’Agostino R, Nam B. Evaluation of the performance of survival analysis models: discrimination and calibration measures. Amsterdam, The Netherlands: Elsevier; 2004. [Google Scholar]
- 26.Emberton M, Zinner N, Michel MC, et al. Managing the progression of lower urinary tract symptoms/benign prostatic hyperplasia: therapeutic options for the man at risk. BJU Int. 2007;100(2):249–253. doi: 10.1111/j.1464-410X.2007.07056.x. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan SA, McConnell JD, Roehrborn CG, et al. Combination therapy with doxazosin and finasteride for benign prostatic hyperplasia in patients with lower urinary tract symptoms and a baseline total prostate volume of 25 ml or greater. J Urol. 2006;175(1):217–220. doi: 10.1016/S0022-5347(05)00041-8. discussion 220-211. [DOI] [PubMed] [Google Scholar]
- 28.Roehrborn CG, McConnell JD, Lieber M, et al. Serum prostate-specific antigen concentration is a powerful predictor of acute urinary retention and need for surgery in men with clinical benign prostatic hyperplasia. PLESS Study Group. Urology. 1999;53(3):473–480. doi: 10.1016/s0090-4295(98)00654-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.