Abstract
The recognition memory tasks, novel object and novel object location, have been beneficial to neuroendocrine research concerning the effects of gonadal and adrenal hormones on cognitive function. This review discusses the advantages of these tasks in comparison with other learning and memory tasks. Experiments conducted across a number of laboratories show that gonadal hormones, both estradiol and testosterone, promote memory while the adrenal hormone, corticosterone, impairs memory. The effects of these steroid hormones on spine density in the prefrontal cortex and hippocampus are also briefly presented. Overall, results show that these steroid hormones are potent modulators of memory consolidation in rodent models.
Keywords: Corticosterone, estradiol, object recognition, object placement, recognition memory, testosterone
1. Introduction
Use of the recognition memory tasks, novel object and novel object location, have been beneficial to neuroendocrine research concerning effects of gonadal, adrenal and other hormones on cognitive function. Hormones, in comparison to most drugs, exert wide ranging effects in brain areas and can affect psychological performance parameters like affective state, sensory-perception and motor activity. Thus, delineating hormonal effects on performance parameters from mnemonic effects in cognitive tasks is often difficult. Since recognition tasks do not rely on either positive or negative reinforcements, the influence of psychological performance parameters is greatly lessened. In addition, the tasks can be applied in a post training paradigm which measures memory consolidation. The current review focuses on use of recognition memory tasks to demonstrate that gonadal and adrenal hormones are potent modulators of memory in rodent subjects and provides some information on the mechanisms for the changes.
2. Application of recognition memory tasks
2.1. Rational for use
In order to mitigate possible confounding influences of task requirements, experience, reinforcements and psychological performance variables in assessing hormonal effects on memory, our lab and others have adopted the use of recognition memory tasks to investigate hormonal effects on learning and memory (1-3). Most memory tasks utilize positive (food or water) or negative (shock or fear of drowning) reinforcements which can influence performance. Hormones can influence performance parameters like affect (arousal, anxiety, mood motivation), regulatory mechanisms (thirst, hunger, body weight, composition, temperature), sensory-perception (vision, audition, olfaction, gustation, touch, attention, proprioception, nociception) and motor ability (activity, balance, skill) (4). Thus, tasks with positive or negative reinforcements are sensitive to effects of psychological performance parameters. Recognition memory tasks instead utilize the curiosity, novelty seeking and exploratory nature of most rodents. Rats will readily explore new or novel objects and are more likely to explore a new object or an object in a new location than one previously explored previously. Instituting a delay period between the first exploration of an object and when subjects are presented the same, known object and a new object, allows for assessment of memory for the known object. In addition, recognition memory tasks require minimal learning which allows for measuring hormone effects on memory without confounding effects of learning. However, possible changes in some performance parameters such as anxiety and motor activity cannot be ruled out in performance of recognition tasks. The contribution of these parameters can be assessed during the task itself (see below) and by use of other tasks such as open field and elevated plus maze to independently assess the effects of a specific treatment on anxiety and activity (5). A further caveat is that hormones may increase the preference for novelty, not mnemonic processes. This possibility cannot be ruled out for chronic hormone treatments, but acute, post-training applications of estradiol, either subcutaneously or directly into the hippocampus, indicate that estrogens enhance memory consolidation (see section 3.2).
2.2 Protocols
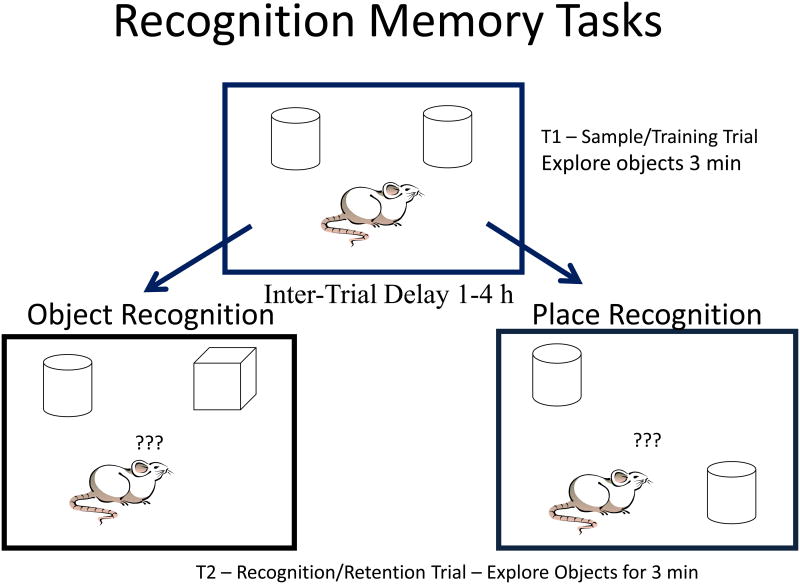
Variations in protocols for recognition memory tasks exist. We conduct recognition memory tests as shown schematically in Figure 1. Rats are allowed three minutes to explore two identical objects on an open field in the sampling or training trial (T1). After 1 to 4 h, subjects are returned to the field for testing in the recognition/retention trial (T2). As shown in the bottom portion of Figure 1, one of the identical objects can be replaced with a new object, which is termed the object recognition (OR) task or one object can be moved to a new location, which is termed the object placement (OP) task. Object placement is a spatial memory task like radial arm maze and Morris water maze (6). In both tasks, the time spent exploring at the new object/location and at the old object/location is recorded. Spending significantly more time exploring at the new object/location as compared to the old object/location indicates that the rat discriminates between old and new configurations, i.e. remembers the old object/location. If subjects spend similar amounts of time exploring the new object/location and old object/location, poor memory function is indicated. Recognition memory results can also be reported using an exploration ratio (time exploring new/time exploring old + new) where a ratio of 0.5 indicates chance (poor memory) and ratios higher than 0.5 indicate that subjects remember and significantly discriminate between the objects or locations. Ratios less than 0.5 indicate perseverative behavior, seeking the known. Perseveration is rare in young adult rats, but is present in aged rats and mice [8,64]. We also utilize extensive habituation of subjects to the task before testing in order to eliminate effects of acute stress and anxiety. Subjects are first allowed to explore the field without objects for 5 min., and then objects are placed on the field and subjects receive object recognition trials with 1 min, 1 h and 2 h inter-trial delays. The following week, object placement tests with 1, 2 and 4 h delays are given. New objects are used in all trials, and we also give vehicle injections during some trials in order to habituate to this acute stress. Testing, with 4 h delays, begins either the day following the last habituation or three days later in order to account for weekends.
Figure 1. Schematic of recognition memory task protocols.
A rat is shown on the open field with objects. Reprinted with permission from Frontiers in Neuroendocrinology (45).
2.3. General Performance on the tasks
It is our experience that most adult rats are able to readily discriminate in object recognition with a 4 hr inter-trial delay (5, 7-11), and others show significant discriminations up to 24 and 48 h (12,13). Object placement, on the other hand, appears more difficult for rodents, and significant discriminations after delays longer than 4 h are not common (14). Differences in task demands may account for performance differences between the two versions of the task. Cognitive load for spatial memory in object placement is greater than non-spatial object recognition (15, 16). Objects can be encoded and discriminated through multiple sensory modalities (eg vision and tactile) and using a variety of cues such as the size, shape, color and textures while discrimination of location of objects involves abstract categorizations and use of “cognitive maps”. The type of objects used and the size of the field may also impact on the ability of subjects to discriminate (7).
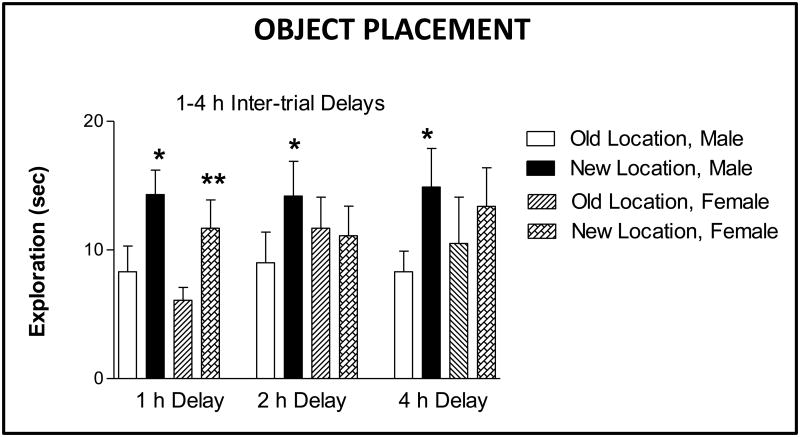
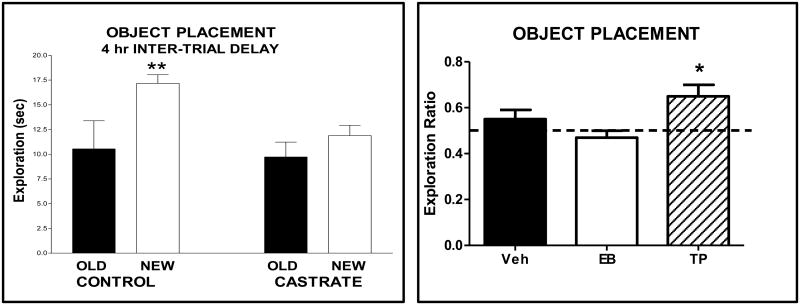
It should also be noted that sex differences are found in ability to perform object placement but not object recognition and should be taken into account in experimental designs. As shown in Figure 2, males significantly discriminate between objects at old and new locations at 1, 2 and 4 h inter-trial delays while females can only typically significantly discriminate at a 1 h inter-trial delay. This observation is consistent with better performance of males as compared to females in other spatial memory tasks such as radial arm maze and water maze (4, 17). However, treatment of females with hormones such as estradiol (see figure 3) enables significant discriminations in object placement testing at 4 h inter-trial delays. It should also be noted that we sometimes find performance differences between cohorts of subjects such that some males may not discriminate at 4 h and some females may discriminate at 2 hr. Thus, habituating subjects to the tasks also serves as an opportunity to assess subjects' abilities in order to plan experiments.
Figure 2. Sex differences in object placement.
Bars represent the mean time (± S.E.M.) exploring the object at the old and new locations for male and female rats in the recognition trial (T2) for inter-trial delays of 1, 2 and 4 h. ANOVA showed a significant difference in time spent at the locations (old and new) and a significant interaction, sex x object effect. ** P < 0.01, * P < 0.05 (paired t-test) within each group. Data adapted from Bisagno et al (10).
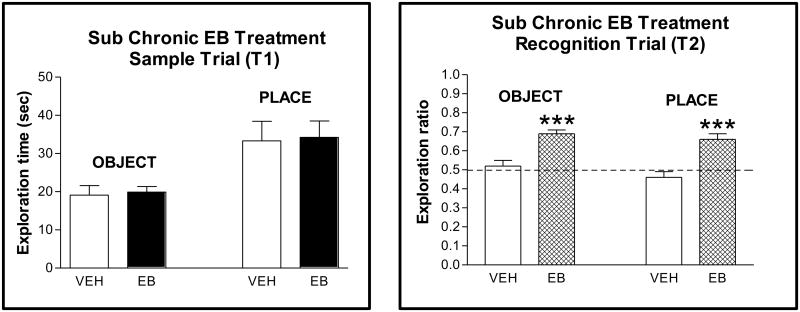
Figure 3.
Effects of sub chronic EB treatment on object and place recognition.
A. In the sample trial (T1), exploration times (± S.E.M.) for the object and place tests are shown in vehicle- and EB-treated subjects (n=9/group). No significant differences. (B) In the recognition trial (T2), given 4 h post T1, entries are ratios (new/old + new) (± S.E.M.) of time spent exploring each object and objects in each location for vehicle- and EB-treated subjects. Dotted line at 0.5 indicates spending the same amount of time exploring new and old objects or locations. *** p < 0.001 (paired t-test within each group of old vs new). Figure reprinted with permission from Neurobiology of Learning and Memory (5).
3. Estrogens enhance learning and memory
3.1 Chronic Treatments with estrogens
My laboratory and others have documented cognitive changes in female rodent models in relation to gonadal hormone status including ovariectomy, estrus cyclicity, pregnancy and multiparity, estrapause and aging (18-22). Earlier studies utilized spatial memory tasks such as radial arm maze, Morris water maze and T- maze to show that estradiol administration improves performance of ovariectomized rats (23-27). Differences in performance of tasks were also found across the estrous cycle (28-32), and estradiol enhances memory in object recognition and placement in OVX rats when it is given in a paradigm that simulates the levels and secretion pattern during diestrous and pro-estrous morning (33). It should be emphasized that changes are small and not all studies reported positive effects, but at this time, the preponderance of studies report estradiol enhances learning and memory. It is important to note whether studies examined learning vs memory as differential effects of estrogens have been reported (See ref. 20 for discussion). In addition, effects of estradiol on response learning, mediated by the striatum, have not been well studied, but estradiol may impair this type of learning in females (34,35). The dose and the duration of estradiol treatment is also critical because effects do not always follow a traditional dose-response curve (16) and higher doses sometimes cause impairments in performance of some memory tasks (18). Moreover, the history of the subjects is important as environmental enrichment can mitigate estrogenic effects (20). Nonetheless, there was still some concern that enhancements in cognition derived from indirect effects of estradiol on performance parameters and not on mnemonic processes. The application of recognition memory tasks was invaluable in further evaluating this idea.
We gave estradiol benzoate (long acting form of estradiol) or vehicle to ovariectomized rats via SC injection (50 ug/kg) for two days and assessed object recognition or object placement memory 48 h after the last estradiol injection (5). As shown in Figure 3A, treatments did not alter exploration of objects in the sample trial. The lack of differences in exploring the objects suggests that estradiol did not alter overall activity or anxiety for object exploration. In contrast, during the recognition trial, estradiol treated rats had significantly higher exploration ratios than vehicle treated rats, approximately 0.50 vs 0.72. Thus, estradiol treatment appeared to enhance memory. Nonetheless, it might still be argued that changes in activity or anxiety may have contributed. We therefore tested the same estradiol treatment regimen for activity and anxiety effects on the open field and for anxiety effects on the elevated plus maze. No differences between vehicle and estradiol treated rats were noted suggesting that estradiol, at least at the dose and duration given, did enhance memory (5). However, hormonal effects on novelty preference cannot be ruled out. Consistent with estradiol's enhancing effects in ovariectomized females, ovariectomy is associated with a loss in the ability to significantly discriminate in recognition tasks (36). Interestingly, object recognition is affected before object placement, 1 vs 4 weeks following ovariectomy.
Chronic effects of estrogens on memory and other estrogen-dependent functions occur through genomic mediation, and there are currently two known estrogen receptors: estrogen receptor α (ERα) and estrogen receptor β (ERβ). Which of the receptors mediates the changes in recognition memory was assessed using the ERα specific agonist PPT and the ERβ specific agonists DPN and C19. Given in the same regimen as estradiol, only the ERβ agonists enhanced object recognition and object placement. Similar results have been reported by others (37,38); however, other studies indicate that both receptors may mediate changes in memory (14,39,40). Thus, it remains unclear at this time whether one or both of the estrogen receptors mediate chronic changes in memory function.
3.2. Acute Treatments with Estrogens
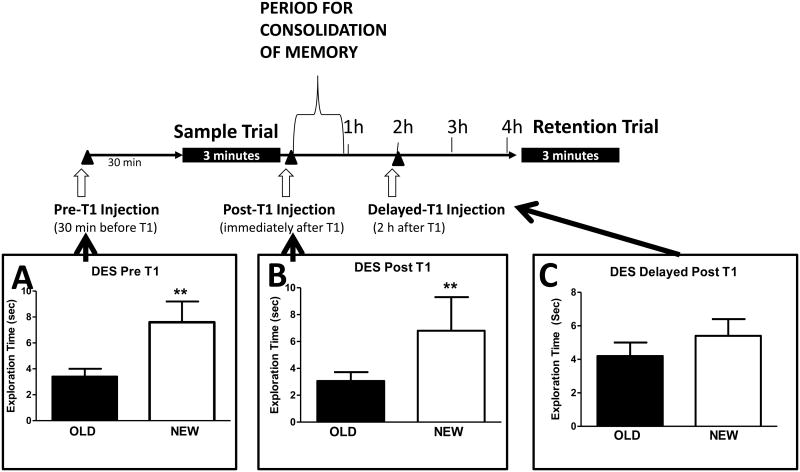
Substantial evidence now exists that estrogen receptors are also present outside of the cell nucleus in membranes on the cell body, axons, spines, presynaptic terminals and near post-synaptic neurotransmitter receptors (41-43) in a number of brain regions including the medial PFC and the hippocampus. Thus, it is possible for rapid effects of estrogens mediated through signal transduction pathways in these brain areas important for cognition. Several laboratories have investigated whether estrogens might rapidly activate learning and memory processes. For these studies, we adopted the post training protocol for assessing memory as advanced by McGaugh and colleagues (44). In this protocol treatments are given after subjects acquire information such as having explored objects in recognition tasks (T1 Trial) or searched for the submerged platform in water maze tasks. Following the training trial, new information requires consolidation, and drugs or hormones could influence memory storage processes during the immediate period following training. It was found that subjects receiving injections of some drugs like amphetamine or noradrenergic agonists showed better retention of information from the training trial (44). Moreover, the post-training injections had a time dependency, only treatments given within 1-2 h after training/sampling trials enhanced memory. Treatments given later in the inter-trial delay were ineffective. Thus, an important advantage of post training protocols is that consolidation enhancements after immediate, but not delayed, post-trial injections rule out the possibility that drug or hormonal enhancing effects derive from non-mnemonic effects during learning since the drugs are given after the learning trial (44). Work by several labs using post-training injections in recognition memory tasks shows that estradiol enhances consolidation of memory (5,9,11-14,45,46). Figure 4 shows that diethylstilbestrol (DES), a synthetic estrogen, enhances object recognition when given 30 min before the sample trial and immediately following the sample trial but not when given 2 h after the sample trial. Thus, DES enhances memory consolidation. The dose-response curves for the estrogen effects are inverted U curves consistent with membrane mediated effects, and lower doses of estradiol require administration within 45 min after T1 (16). Other studies show that immediate, post-sample trial enhancements in OR in OVX mice are present 48 h following estradiol (See 46 for details). In addition, 17β-estradiol-cyclodextrin (does not pass through cell membranes) applied directly to the dorsal hippocampus enhances object memory suggesting that estrogenic activation within the hippocampus is sufficient to enhance memory for objects in a post sample trial paradigm (12-14). Packard et al (45) reported that post training injections of estradiol, either IP or intra-hippocampally, enhanced Morris water maze performance 24 h later.
Figure 4.
Timeline for memory consolidation and effects of DES given before and after sample trial on consolidation.
Work by McGaugh (42) has shown that memories are consolidated during the first 1-2 hours following a sample or training trial. Drug or hormone treatments can enhance or impair memory during this window for treatment even when retention is tested up to 48 hours later. If treatments are given later than 2 h post T1, they do not affect memory consolidation.
A. DES, 15 ug/kg, was given 30 min before T1, and object recognition was enhanced 4 h later. B. DES, 15 ug/kg, was given immediately after T1, and object recognition was enhanced 4 h later.
C. DES, 15 ug/kg, was given 2h after T1, and object recognition was not enhanced 4 h later.
** P < 0.01. Figure reprinted by permission from Frontiers in Neuroendocrinology (45) and data redrawn from Luine et al (9)
3.3. Effects of Estrogens on Spine Density
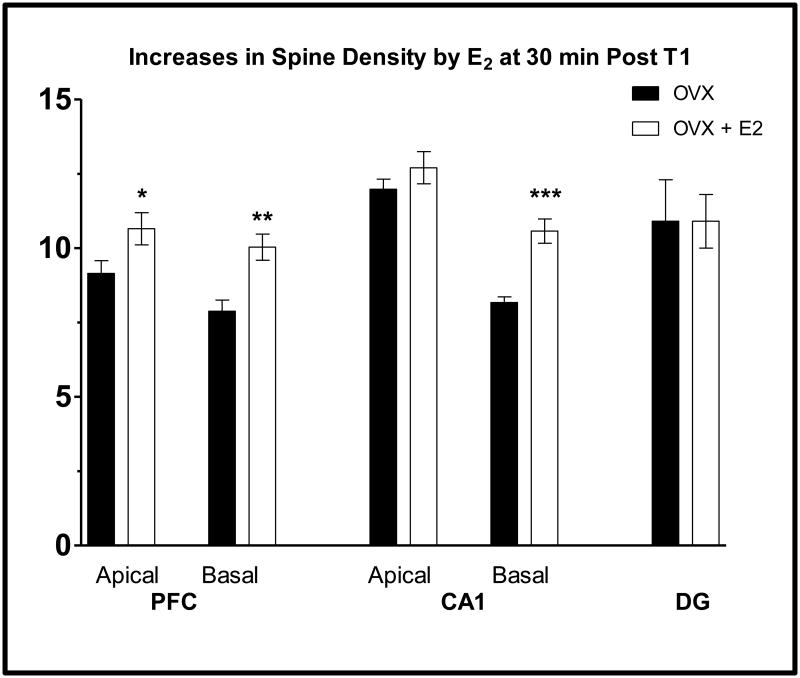
Both chronic and acute treatments with estradiol are associated with increased dendritic spine densities in the prefrontal cortex and CA1 subfield of the hippocampus (11,21,22,47). Ovariectomized rats were given a sample trial for recognition memory testing, immediately injected with vehicle of 20 μg/kg of 17β and sacrificed 30 min later. Spine densities were measured after Golgi impregnation (Figure 5). In CA1, estradiol administration increased basal spine density by 29% compared to control ovariectomized rats but did not alter apical spine density. Spine densities in the dentate gyrus were not altered by estradiol administration. In the PFC, treatment resulted in a 27% increase in basal dendrites and a 16% increase in apical dendrites. Thus, estradiol leads to a rapid and large increase in spine density in both areas known to be important for memory function and at a time when memory consolidation is known to occur. Therefore, increased spine density may contribute to enhancements in memory consolidation by estrogens.
Figure 5.
Acute estrogen treatments increase spine density.
Spine density is shown in areas of brain from Ovx rats given corn oil or 20 μg/kg of estradiol immediately following T1 and sacrificed 30 min later. Entries are mean ± SEM. ** p < 0.01, * p < 0.05 (t-test). PFC and CA1 data from Inagaki et al. (11) and DG data from Lama and Luine, unpublished.
Overall, experiments show that estrogen enhances recognition memory after chronic treatments. In addition, estrogens also rapidly enhance memory consolidation of recognition memory tasks and other memory tasks within approximately one hour. Use of recognition memory tasks has been critical in establishing that estradiol acts on mnemonic processes to enhance memory.
4. Effects of Androgens
Androgens have not been as extensively investigated in relation to cognitive function as estrogens, but several studies show that castration impairs and chronic treatments with testosterone propionate to castrate rats enhance radial arm maze, Y-maze and T-maze performance (48-51), but, as in females, dose and duration of dose appear critical, and effects maybe different on learning vs memory. For recognition memory tasks, our results are similar to Aubele et al (52); we found that castrated rats did not significantly discriminate between old and new locations (Figure 6) or between old and new objects. Castrated rats, treated in a regimen like ovariectomized rats (see 3.1), received testosterone (500 ug) or estradiol benzoate (50 ug/kg) for two days and were tested two days later. Testosterone treated, but not castrated vehicle or estradiol benzoate treated male rats, could significantly discriminate between locations in the object placement task. Thus, testosterone enhances recognition memory in males, but more studies at different hormones doses and treatment intervals need to be conducted to substantiate these and other reported results.
Figure 6. Effects of castration and gonadal hormone replacement on object placement.
A. Male rats were castrated or sham castrated (Control) and 4 weeks later received object placement testing with a 4 h inter-trial delay. Bars represent the mean time (± S.E.M.) exploring the object at the old and new locations. ** P < 0.01 (Paired t-test). B. Castrate rats received vehicle, estradiol benzoate (EB) 50 ug/kg or testosterone propionate for two days and tested for object placement two days after the injection. ANOVA F=4.19, P < 0.04. * P< 0.05 (LSD). Luine and Saens, unpublished.
5. Effects of Corticosteroid
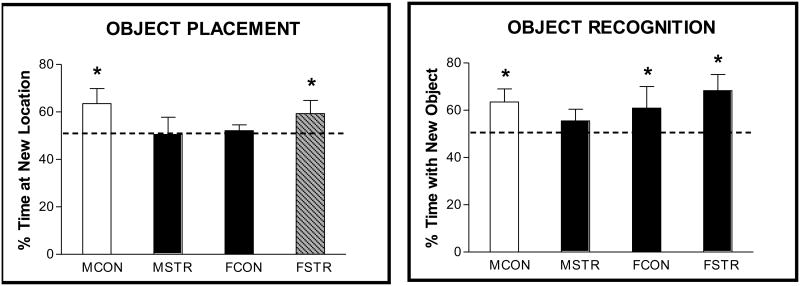
The deleterious nature of chronic stress, characterized by sustained increases in the adrenal steroid corticosteroid in rats (cortisol in humans), has been documented in animal models and humans and includes, but is not limited to, fatigue, myopathy, ulcers, decreases in immune function and impaired cognitive function. My lab was the first to report that 21 days of restraint stress for 6 h/day impaired the performance of male rats on the radial arm maze (53). Stressed males made significantly more errors, fewer correct choices and earlier mistakes in completing the eight arm choices than unstressed males. Stress dependent impairments in these and other spatial memory tasks in males have now been reported. For example, Conrad et al (54) reported that 21 days of restraint stress impaired spatial memory on the Y-maze and Kitraki et al (55) demonstrated that male rats were impaired on the water maze following 21 days of daily restraint. These results remained somewhat questionable for some investigators because it was possible that the debilitating effects of stress might have contributed to the poor performance of the stressed rats on the strenuous radial arm maze and water maze and that the subjects were not cognitively impaired. Thus, as in the gonadal hormone studies, we applied recognition memory tasks to rats that were given daily restraint stress for 21 days. Experiments were conducted in both males and females. First, exploration times during the sample trial were not significantly different between control and stressed rats (data not shown; 8). As shown for the recognition trial of object placement in Figure 6A, control males significantly discriminated between the old and new locations with an exploration ratio of approximately 0.62 while stressed males did not. Consistent with results seen in Figure 2, control females did not significantly discriminate in the object placement task with a 4 h delay, but unlike stressed males, stressed females significantly discriminated between locations, an exploration ratio of approximately 0.60. Thus, results in males after stress in this spatial task were consistent with results of previous spatial tasks; stress is associated with impaired learning and memory. The results of stress in females were novel, but enhancements in memory following chronic stress have since been confirmed in the water maze, radial arm maze and Y-Maze (17). Interestingly, stress decreased anxiety in the males and increased it in the females (8,17). Thus, neither activity nor anxiety changes appear to contribute to the stress-dependent changes in recognition memory.
A somewhat different pattern of effects after stress emerged in object recognition (Figure 6B). As expected, control males and females significantly discriminated between old and new objects. Stressed males did not discriminate between old and new objects, but stressed females did. Thus, as in object placement, effects of stress are different in females as compared to males. It is beyond the scope of the current review, but circulating (17) and intra-neurally derived (56) estradiol appears to protect females against the deleterious effects of chronic stress.
Conclusions
Application of the recognition memory tasks, object recognition and placement, have been invaluable in neuroendocrine research. Experiments conducted across a number of laboratories show that gonadal hormones promote memory while adrenal hormones impair memory. In the future, use of hormonal manipulations along with these tasks may help to unravel the basic mechanisms involved in the acquisition, consolidation and retrieval of memories.
Figure 7. Effect of 21 days of chronic restraint stress on recognition memory.
Male and female rats served as control (MCON, FCON) or received daily restraint for 6 h/day (MSTR, FSTR). A. Object Placement - In the recognition trial (T2), given 4 h post T1, bars are ratios (new/old + new) (± S.E.M.) of time spent exploring objects in each location for control and stressed subjects. Data analyzed by a paired t-test within each group. **P < 0.05, * P < 0.05. Dotted line at 0.5 indicates spending the same amount of time exploring new and old objects or locations. B. Object Recognition – Subjects tested and analyzed as in A. Data redrawn from (8).
Highlights.
The use of recognition memory tasks in neuroendocrine research is reviewed
Advantages of recognition tasks as compared to other memory tasks are discussed
Gonadal hormones enhance while adrenal hormones impair memory
Increases in dendritic spine density may contribute to enhancements of memory
Acknowledgments
R25-GM-60665 (MBRS/RISE) and S06-GM-60654 (SCORE) grants from NIH; CUNY faculty and doctoral student research grants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ennaceur A, Meliani K. A new one-trial test for neurobiological studies of memory in rats. III Spatial vs. non-spatial working memory. Behav Brain Res. 1992;51:83–92. doi: 10.1016/s0166-4328(05)80315-8. [DOI] [PubMed] [Google Scholar]
- 2.Ennaceur A, Aggleton JP. Spontaneous recognition of object configurations in rats: effects of fornix lesions. Exp Brain Res. 1994;100:85–92. doi: 10.1007/BF00227281. [DOI] [PubMed] [Google Scholar]
- 3.Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp Brain Res. 1997;113:509–519. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- 4.Luine VN, Dohanich G. Sex differences in cognitive function in Rodents. In: Becker JB, Berkley KJ, Geary N, Hampson E, Herman JP, Young EA, editors. Sex Differences in the Brain: From Genes to Behavior. Oxford University Press; 2008. pp. 227–252. [Google Scholar]
- 5.Jacome LF, Gautreaux C, Inagaki T, Mohan G, Alves S, Lubbers LS, Luine V. Estradiol and ERbeta agonists enhance recognition memory, and DPN, an ERbeta agonist, alters brain monoamines. Neurobiology of learning and memory. 2010;94:488–498. doi: 10.1016/j.nlm.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci U S A. 2004;101:14515–14520. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beck KD, Luine VN. Food deprivation modulates chronic stress effects on object recognition in male rats: role of monoamines and amino acids. Brain Res. 1999;830:56–71. doi: 10.1016/s0006-8993(99)01380-3. [DOI] [PubMed] [Google Scholar]
- 8.Beck KD, Luine VN. Sex differences in behavioral and neurochemical profiles after chronic stress: Role of housing conditions. Physiology and Behavior. 2002;75:661–73. doi: 10.1016/s0031-9384(02)00670-4. [DOI] [PubMed] [Google Scholar]
- 9.Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- 10.Bisagno V, Ferguson D, Luine VN. Chronic d-amphetamine induces sexually dimorphic effects on locomotion, recognition memory and brain monoamines. Pharmac Biochem & Behav. 2003;74:859–867. doi: 10.1016/s0091-3057(03)00017-0. [DOI] [PubMed] [Google Scholar]
- 11.Inagaki T, Frankfurt M, Luine V. Estrogen-Induced Memory Enhancements are Blocked by Acute Bisphenol A in Adult Female Rats: Role of Dendritic Spines. Endocrinology. 2012;153:3357–67. doi: 10.1210/en.2012-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J Neurosci. 2008;28:8660–7. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis MC, Kerr KM, Orr PT, Frick KM. Estradiol-induced enhancement of object memory consolidation involves NMDA receptors and protein kinase A in the dorsal hippocampus of female C57BL/6 mice. Behav Neurosci. 2008;122:716–21. doi: 10.1037/0735-7044.122.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boulware MI, Heisler JD, Frick KM. The memory-enhancing effects of hippocampal estrogen receptor activation involve metabotropic glutamate receptor signaling. J Neurosci. 2013;33:15184–15194. doi: 10.1523/JNEUROSCI.1716-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ennaceur A, Michalikova S, Bradford A, Ahmed S. Detailed analysis of the behavior of Lister and Wistar rats in anxiety, object recognition and object location tasks. Behav Brain Res. 2005;159:247–66. doi: 10.1016/j.bbr.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Inagaki T, Gautreaux C, Luine V. Acute Estrogen Treatment Facilitates Recognition Memory Consolidation and Alters Monoamine levels in Memory-related Brain Areas. Horm Behav. 2010;58:415–426. doi: 10.1016/j.yhbeh.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luine VN, Beck KD, Bowman RE, Frankfurt M, MacLusky NJ. Stress and neural function – Accounting for sex and age. J Neuroendocrinology. 2007;19:743–751. doi: 10.1111/j.1365-2826.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- 18.Dohanich GP. Gonadal steroids, learning and memory. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RI, editors. Hormones, brain and behavior. San Diego: Academic Press; 2002. pp. 265–327. [Google Scholar]
- 19.Luine V. Neuroendocrinology of memory and cognition. In: Lajtha Abel., editor. Handbook of Neurochemistry and Molecular Neurobiology. 3rd. Berlin: Springer; 2006. pp. 775–800. Blaustein Jeff D, Volume Editor: Behavioral Neurochemistry and Neuroendocrinology. [Google Scholar]
- 20.Luine VN. Sex steroids and cognitive function. J Neuroendocrinology. 2008;20:866–72. doi: 10.1111/j.1365-2826.2008.01710.x. [DOI] [PubMed] [Google Scholar]
- 21.Luine V, Frankfurt M. An Integrative Review of Estradiol Effects on Dendritic Spines and Memory over the Lifespan. In: Kahn Scott M., editor. Sex Steroid. Intech Open Access Publishing; 2012. http://www.intechopen.com/articles/show/title/an-integrative-review-of-estrogen-effects_on-memory-and-dendritic-spines-over-the-lifespan. [Google Scholar]
- 22.Luine V, Frankfurt M. Interactions between estradiol, BDNF and dendritic spines in promoting memory. Neuroscience. 2013;239:34–45. doi: 10.1016/j.neuroscience.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luine V, Rodriguez M. Effects of estradiol on radial arm maze performance of young and aged rats. Behavioral & Neural Biology. 2012;62:230–236. doi: 10.1016/s0163-1047(05)80021-4. [DOI] [PubMed] [Google Scholar]
- 24.Luine V, Richards ST, Wu VY, Beck K. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Horm Behav. 1998;34:149–162. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- 25.Daniel JM, Fader AJ, Spencer AL, Dohanich GP. Estrogen enhances performance of female rats during acquisition of a radial arm maze task. Horm Behav. 1997;32:217–225. doi: 10.1006/hbeh.1997.1433. [DOI] [PubMed] [Google Scholar]
- 26.Dohanich G, Fader AF, Javorsky DJ. Estrogen and estrogen-progesterone treatments counteract the effect of scopolamine on reinforced T-maze alternation in female rats. Behav Neurosci. 1994;108:988–992. doi: 10.1037//0735-7044.108.5.988. [DOI] [PubMed] [Google Scholar]
- 27.Sandstrom N, Williams C. Spatial memory retention is enhanced by acute and continuous estradiol replacement. Hormones and Behavior. 2004;45:128–135. doi: 10.1016/j.yhbeh.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 28.Frye C, Duffy C, Walf A. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiology of Learning and Memory. 2007;88:208–16. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walf A, Rhodes M, Frye C. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiology of Learning and Memory. 2006;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frick KM, Berger-Sweeney J. Spatial reference memory and neocortical neurochemistry vary with the estrous cycle in C57Bl/6 mice. Behav Neurosci. 2201;115:229–237. doi: 10.1037/0735-7044.115.1.229. [DOI] [PubMed] [Google Scholar]
- 31.Frye CA. Estrus-associated decrements in a water maze task are limited to acquisition. Physiol Behav. 1995;57:5–14. doi: 10.1016/0031-9384(94)00197-d. [DOI] [PubMed] [Google Scholar]
- 32.Warren SG, Juraska JM. Spatial and nonspatial learning across the rat estrous cycle. Behav Neurosci. 1997;111:259–266. doi: 10.1037//0735-7044.111.2.259. [DOI] [PubMed] [Google Scholar]
- 33.Scharfman E, Hintz TM, Gomez J, Stormes KA, Barouk S, Malthankarhatak GH, McCloskey DP, Luine VN, Maclusky NJ. Changes in hippocampal function of ovariectomized rats after sequential low doses of estradiol to simulate the preovulatory estrogen surge. Eur J Neurosci. 2007;26:2595–612. doi: 10.1111/j.1460-9568.2007.05848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zurkovsky L, Serio SJ, Korol DL. Intra-striatal estradiol in female rats impairs reaponse learning within two hours of treatment. Horm Behav. 2011;60:470–7. doi: 10.1016/j.yhbeh.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 35.Davis DM, Jacobson TK, Aliakbari S, Mizumori SJ. Differential effects of estrogen on hippocampal- and striatal-dependent learning. Neurobiology of Learning and Memory. 2005;84:132–7. doi: 10.1016/j.nlm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Wallace M, Luine V, Arellano A, Frankfurt M. Ovariectomized rats show decreased recognition memory and spine density in hiocampus and prefrontal cortex. Brain Research. 2006;1126:176–182. doi: 10.1016/j.brainres.2006.07.064. [DOI] [PubMed] [Google Scholar]
- 37.Liu F, Day M, Muñiz L, Bitran D. Activation of estrogen receptor-β regulates hippocampal synaptic plasticity and improves memory. Nature Neuroscience. 2008;11:334–343. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- 38.Walf A, Koonce C, Frye C. Estradiol or diarylpropionitrile administration to wild type, but not estrogen receptor beta knockout, mice enhances performance in the object recognition and object placement tasks. Neurobiology of Learning and Memory. 2008;89:513–21. doi: 10.1016/j.nlm.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frye C, Duffy C, Walf A. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiology of Learning and Memory. 2007;88:208–16. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hammond, Mauk R, Ninaci D, Nelson D, Gibbs R. Chronic treatment with estrogen receptor agonists restores acquisition of a spatial learning task in young ovariectomized rats. Horm Behav. 2009;56:309–314. doi: 10.1016/j.yhbeh.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blaustein JD. Cytoplasmic estrogen receptors in rat brain: immunocytochemical evidence using three antibodies with distinct epitopes. Endocrinology. 1992;131:1336–1342. doi: 10.1210/endo.131.3.1380440. [DOI] [PubMed] [Google Scholar]
- 42.Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hiocampal α estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429:355–371. [PubMed] [Google Scholar]
- 43.Towart LA, Alves SE, Znamensky V, Hayashi S, BS, Milner TA. Subcellular relationships between cholinergic terminals and estrogen receptor α in the dorsal hippocampus. J Comp Neurol. 2003;463:390–401. doi: 10.1002/cne.10753. [DOI] [PubMed] [Google Scholar]
- 44.McGaugh JL. Dissociating learning and performance: drug and hormone enhancement of memory storage. Brain Res Bull. 1989;23:339–345. doi: 10.1016/0361-9230(89)90220-7. [DOI] [PubMed] [Google Scholar]
- 45.Packard MG. Posttraining estrogen and memory modulation. Horm Behav. 1998;34:126–139. doi: 10.1006/hbeh.1998.1464. [DOI] [PubMed] [Google Scholar]
- 46.Frick KM. Building a better hormone therapy? How understanding the rapid effects of sex steroid hormones could lead to new therapeutics for age-related memory decline. Behav Neurosci. 2012;126:29–53. doi: 10.1037/a0026660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luine V, Frankfurt M. Estrogens facilitate memory processing through membrane mediated mechanisms and spine density changes. Frontiers in Neuroendocrinology. 2012;33:388–402. doi: 10.1016/j.yfrne.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Daniel JM, Winsauer PJ, Moerschbaecher JM. Castration in rats impairs performance during acquisition of a working memory task and exacerbates deficits in working memory produced by scopolamine and mecamylamine. Psychopharmac. 2003;170:294–300. doi: 10.1007/s00213-003-1537-4. [DOI] [PubMed] [Google Scholar]
- 49.Gibbs RB. Testosterone and estradiol produce different effects on cognitive performance in male rats. Horm Behav. 2005;48:268–77. doi: 10.1016/j.yhbeh.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spritzer MD, Daviau ED, Coneeny MK, Engelman SM, Prince WT, Rodriguez-Wisdom KN. Effects of testosterone on spatial learning and memory in adult male rats. Horm Behav. 2011;59:484–96. doi: 10.1016/j.yhbeh.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hawley WR, Grissom EM, Martin RC, Halmos MB, Bart CL, Dohanich GP. Testosterone modulates spatial recognition memory in male rats. Horm Behav. 2013;63:559–65. doi: 10.1016/j.yhbeh.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 52.Aubele T, Kaufman R, Montalmant F, Kritzer MF. Effects of gonadectomy and hormone replacement on a spontaneous novel object recognition task in adult male rats. Horm Behav. 2008;54:244–52.51. doi: 10.1016/j.yhbeh.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Research. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- 54.Conrad CC, Grote KD, Hobbs RJ, Ferayorni A. Sex differences in spatial and non-spatial Y-maze performance after chronic stress. Neurobiology of Learning and Memory. 2003;79:32–40. doi: 10.1016/s1074-7427(02)00018-7. [DOI] [PubMed] [Google Scholar]
- 55.Kitraki E, Kremmyda O, Youlatos D, Alexis MN, Kittas C. Gender-dependent alterations in corticosteroid receptor status and spatial performance following 21 days of restraint stress. Neuroscience. 2004;125:47–55. doi: 10.1016/j.neuroscience.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 56.Wei J, Yuen EY, Liu W, Li X X, Zhong P, Karatsoreos IN, McEwen BS, Yan Z. Estrogen protects against the detrimental effects of repeated stress on glutamatergic transmission and cognition. Mol Psychiatry. 2013 Jul 9; doi: 10.1038/mp.2013.83. Epub ahead of print. [DOI] [PubMed] [Google Scholar]