Abstract
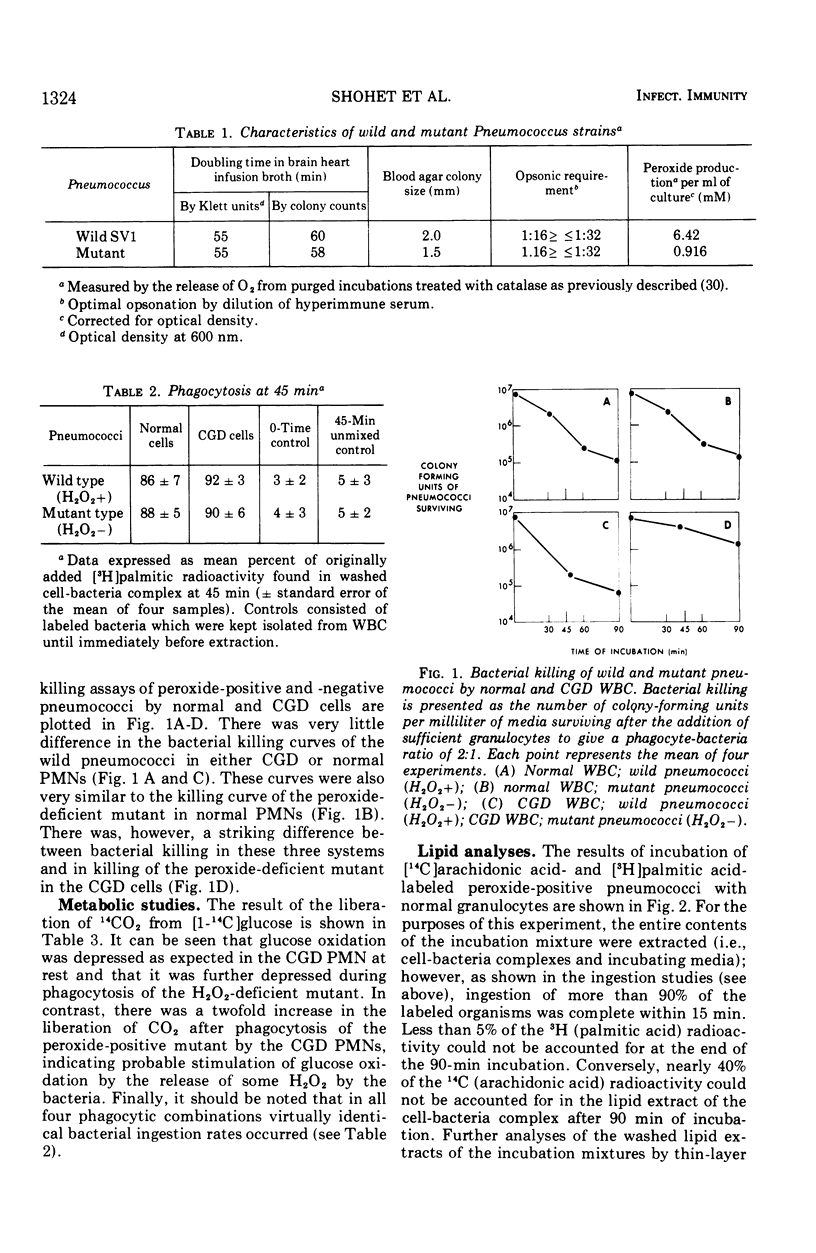
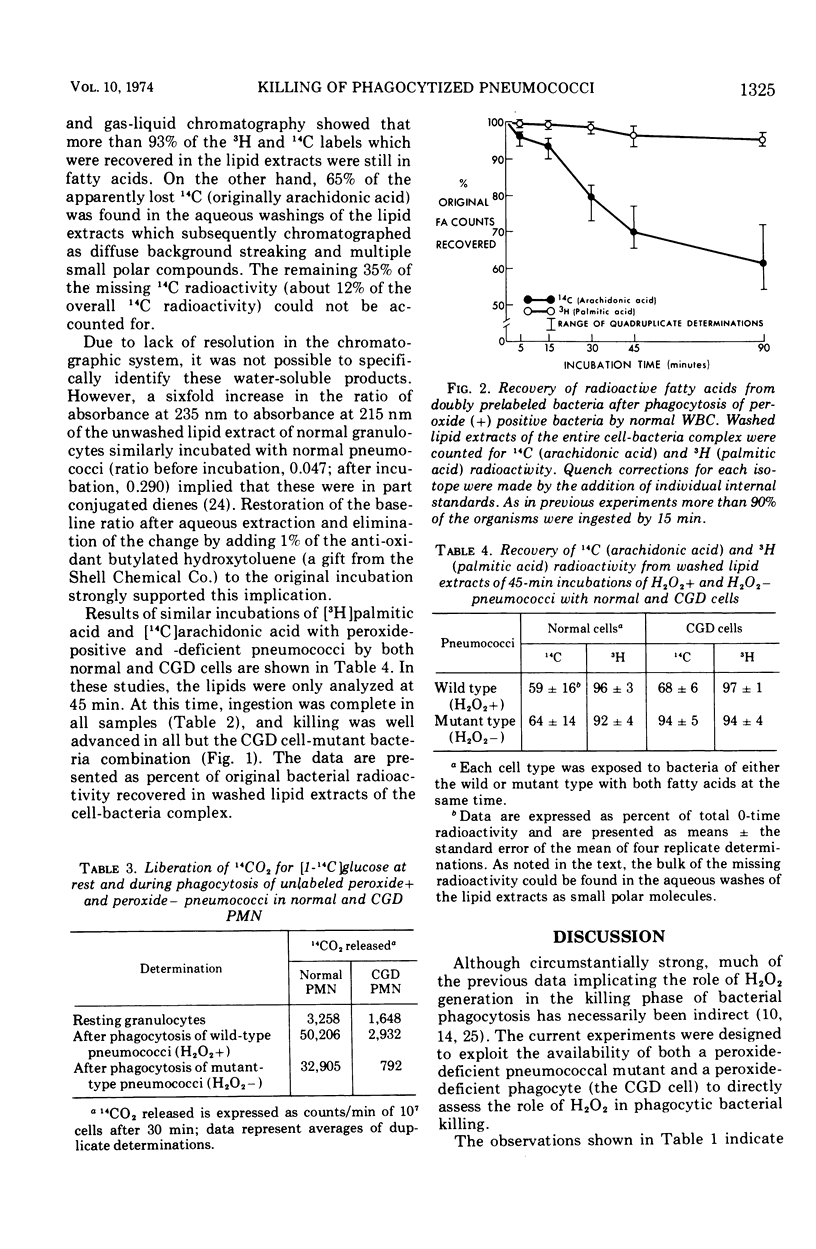
To directly examine the role of hydrogen peroxide in the killing of bacteria after ingestion by granulocytes, we have studied some of the events of phagocytosis of a mutant strain of pneumococci which is relatively deficient in peroxide production. The hydrogen peroxide-deficient pneumococci and the otherwise identical wild type were grown with [14C]arachidonic and [3H]palmitic acid labels to label their lipids with unsaturated and saturated fatty acids, respectively. They were then incubated with both normal and chronic granulomatous disease granulocytes. The rates of ingestion and bacterial killing and the stability of fatty acids in the cell-bacteria complex were followed. Radioactive carbon dioxide released from glucose was also independently followed to measure glucose oxidation. Ingestion was similar for all cell-bacteria combinations. Chronic granulomatous disease cells killed the peroxide-positive wild pneumococci much more effectively (20-fold) than the peroxide-deficient mutant. Normal cells killed both peroxide-positive and -negative strains effectively. A considerable loss of [14C]arachidonic acid (∼40%) consistent with lipid peroxidation of this unsaturated fatty acid was observed in all normal cells and in chronic granulomatous disease cells with peroxide-positive pneumococci. However, no loss of [14C]arachidonic acid occurred in chronic granulomatous disease cells with the peroxide-deficient pneumococci. No loss tritiated palmitic acid occurred in any cell-bacteria combination. Glucose oxidation was impaired in the chronic granulomatous disease cells in comparison to normal cells at rest and was especially impaired in chronic granulomatous disease cells ingesting the peroxide-deficient mutant pneumococci. This defect was partially corrected after phagocytosis of the peroxide-positive strain. These data directly support the hypothesis that bacterial killing is partially dependent upon an intact peroxide-generating system in the leukocyte-bacteria complex. Moreover, they indicate that bacterial lipid peroxidation is associated with the generation of peroxide during phagocytosis. Finally, they suggest that such peroxidation may contribute to effective phagocytic bacterial killing.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUSTRIAN R., BERNHEIMER H. P. Simultaneous production of two capsular polysaccharides by pneumococcus. I. Properties of a pneumococcus manifesting binary capsulation. J Exp Med. 1959 Oct 1;110:571–584. doi: 10.1084/jem.110.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Gilman N., Karnovsky M. L. Respiration and glucose oxidation in human and guinea pig leukocytes: comparative studies. J Clin Invest. 1970 Apr;49(4):692–700. doi: 10.1172/JCI106281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Nathan D. G., Karnovsky M. L. Correction of metabolic deficiencies in the leukocytes of patients with chronic granulomatous disease. J Clin Invest. 1970 May;49(5):865–870. doi: 10.1172/JCI106305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Nathan D. G. Leukocyte oxidase: defective activity in chronic granulomatous disease. Science. 1967 Feb 17;155(3764):835–836. doi: 10.1126/science.155.3764.835. [DOI] [PubMed] [Google Scholar]
- COHN Z. A., MORSE S. I. Interactions between rabbit polymorphonuclear leucocytes and staphylococci. J Exp Med. 1959 Sep 1;110:419–443. doi: 10.1084/jem.110.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. F., Lund D. B., Richardson T. Essential fatty acids and glucose permeability of lecithin membranes. Biochim Biophys Acta. 1971 Jan 5;225(1):89–95. doi: 10.1016/0005-2736(71)90287-2. [DOI] [PubMed] [Google Scholar]
- DAHLE L. K., HILL E. G., HOLMAN R. T. The thiobarbituric acid reaction and the autoxidations of polyunsaturated fatty acid methyl esters. Arch Biochem Biophys. 1962 Aug;98:253–261. doi: 10.1016/0003-9861(62)90181-9. [DOI] [PubMed] [Google Scholar]
- Dodge J. T., Phillips G. B. Composition of phospholipids and of phospholipid fatty acids and aldehydes in human red cells. J Lipid Res. 1967 Nov;8(6):667–675. [PubMed] [Google Scholar]
- ELSBACH P., RIZACK M. A. ACID LIPASE AND PHOSPHOLIPASE ACTIVITY IN HOMOGENATES OF RABBIT POLYMORPHONUCLEAR LEUKOCYTES. Am J Physiol. 1963 Dec;205:1154–1158. doi: 10.1152/ajplegacy.1963.205.6.1154. [DOI] [PubMed] [Google Scholar]
- Elsbach P., Goldman J., Patriarca P. Phospholipid metabolism by phagocytic cells. VI. Observations on the fate of phospholipids of granulocytes and ingested Escherichia coli during phagocytosis. Biochim Biophys Acta. 1972 Sep 7;280(1):33–44. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fong K. L., McCay P. B., Poyer J. L., Keele B. B., Misra H. Evidence that peroxidation of lysosomal membranes is initiated by hydroxyl free radicals produced during flavin enzyme activity. J Biol Chem. 1973 Nov 25;248(22):7792–7797. [PubMed] [Google Scholar]
- HIRSCH J. G., COHN Z. A. Degranulation of polymorphonuclear leucocytes following phagocytosis of microorganisms. J Exp Med. 1960 Dec 1;112:1005–1014. doi: 10.1084/jem.112.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes B., Good R. A. Laboratory models of chronic granulomatous disease. J Reticuloendothel Soc. 1972 Aug;12(2):216–237. [PubMed] [Google Scholar]
- Holmes B., Quie P. G., Windhorst D. B., Good R. A. Fatal granulomatous disease of childhood. An inborn abnormality of phagocytic function. Lancet. 1966 Jun 4;1(7449):1225–1228. doi: 10.1016/s0140-6736(66)90238-8. [DOI] [PubMed] [Google Scholar]
- Jacobs A. A., Paul B. B., Strauss R. R., Sbarra A. J. The role of the phagocyte in host-parasite interactions. 23. Relation of bactericidal activity to peroxidase-associated decarboxylation and deamination. Biochem Biophys Res Commun. 1970 Apr 24;39(2):284–289. doi: 10.1016/0006-291x(70)90791-6. [DOI] [PubMed] [Google Scholar]
- Jensen M. S., Bainton D. F. Temporal changes in pH within the phagocytic vacuole of the polymorphonuclear neutrophilic leukocyte. J Cell Biol. 1973 Feb;56(2):379–388. doi: 10.1083/jcb.56.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Baehner R. L. Improvement of leukocyte bactericidal activity in chronic granulomatous disease. Blood. 1970 Mar;35(3):350–355. [PubMed] [Google Scholar]
- Johnston R. B., Jr, Klemperer M. R., Alper C. A., Rosen F. S. The enhancement of bacterial phagocytosis by serum. The role of complement components and two cofactors. J Exp Med. 1969 Jun 1;129(6):1275–1290. doi: 10.1084/jem.129.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Iodination of bacteria: a bactericidal mechanism. J Exp Med. 1967 Dec 1;126(6):1063–1078. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J., White L. R. Iodination defect in the leukocytes of a patient with chronic granulomatous disease of childhood. N Engl J Med. 1969 Feb 27;280(9):460–466. doi: 10.1056/NEJM196902272800902. [DOI] [PubMed] [Google Scholar]
- Klein R. A. The detection of oxidation in liposome preparations. Biochim Biophys Acta. 1970 Sep 8;210(3):486–489. doi: 10.1016/0005-2760(70)90046-9. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infection. J Clin Invest. 1969 Aug;48(8):1478–1488. doi: 10.1172/JCI106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. O., STUMPF P. K. Fat metabolism in higher plants. XII. alpha-Oxidation of long chain fatty acids. J Biol Chem. 1959 Oct;234:2548–2554. [PubMed] [Google Scholar]
- Mason R. J., Stossel T. P., Vaughan M. Lipids of alveolar macrophages, polymorphonuclear leukocytes, and their phagocytic vesicles. J Clin Invest. 1972 Sep;51(9):2399–2407. doi: 10.1172/JCI107052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D. R., Morrison M. The arrangement of proteins in the human erythrocyte membrane. Biochem Biophys Res Commun. 1970 Jul 27;40(2):284–289. doi: 10.1016/0006-291x(70)91007-7. [DOI] [PubMed] [Google Scholar]
- Pitt J., Bernheimer H. P. Role of peroxide in phagocytic killing of pneumococci. Infect Immun. 1974 Jan;9(1):48–52. doi: 10.1128/iai.9.1.48-52.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quie P. G., White J. G., Holmes B., Good R. A. In vitro bactericidal capacity of human polymorphonuclear leukocytes: diminished activity in chronic granulomatous disease of childhood. J Clin Invest. 1967 Apr;46(4):668–679. doi: 10.1172/JCI105568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohet S. B. Changes in fatty acid metabolism in human leukemic granulocytes during phagocytosis. J Lab Clin Med. 1970 Apr;75(4):659–672. [PubMed] [Google Scholar]
- Shohet S. B., Nathan D. G., Karnovsky M. L. Stages in the incorporation of fatty acids into red blood cells. J Clin Invest. 1968 May;47(5):1096–1108. doi: 10.1172/JCI105799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipski V. P., Peterson R. F., Barclay M. Quantitative analysis of phospholipids by thin-layer chromatography. Biochem J. 1964 Feb;90(2):374–378. doi: 10.1042/bj0900374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welton A. F., Aust S. D. Lipid peroxidation during enzymatic iodination of rat liver endoplasmic reticulum. Biochem Biophys Res Commun. 1972 Nov 1;49(3):661–666. doi: 10.1016/0006-291x(72)90462-7. [DOI] [PubMed] [Google Scholar]
- Zeya H. I., Spitznagel J. K. Arginine-rich proteins of polymorphonuclear leukocyte lysosomes. Antimicrobial specificity and biochemical heterogeneity. J Exp Med. 1968 May 1;127(5):927–941. doi: 10.1084/jem.127.5.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gier J., Mandersloot J. G., van Deenen L. L. Lipid composition and permeability of liposomes. Biochim Biophys Acta. 1968 Jun 11;150(4):666–675. doi: 10.1016/0005-2736(68)90056-4. [DOI] [PubMed] [Google Scholar]


