Abstract
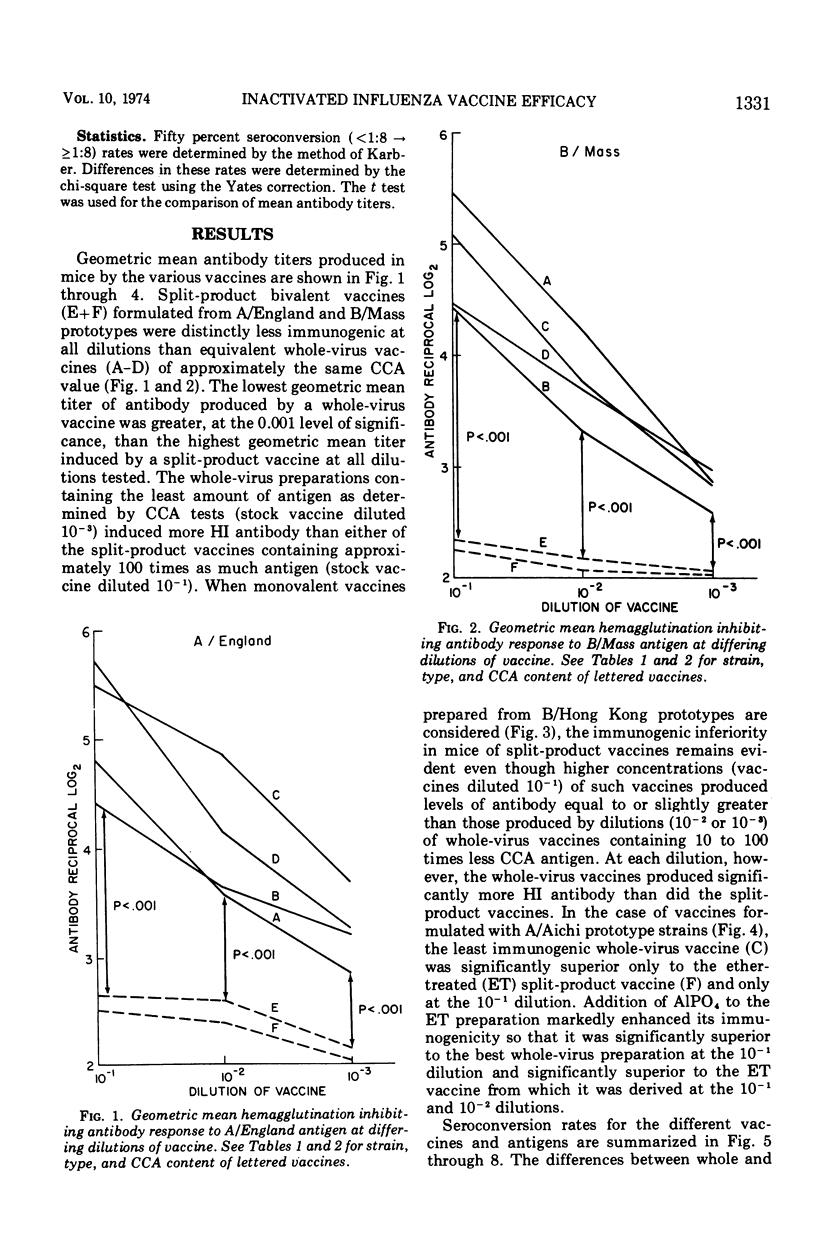
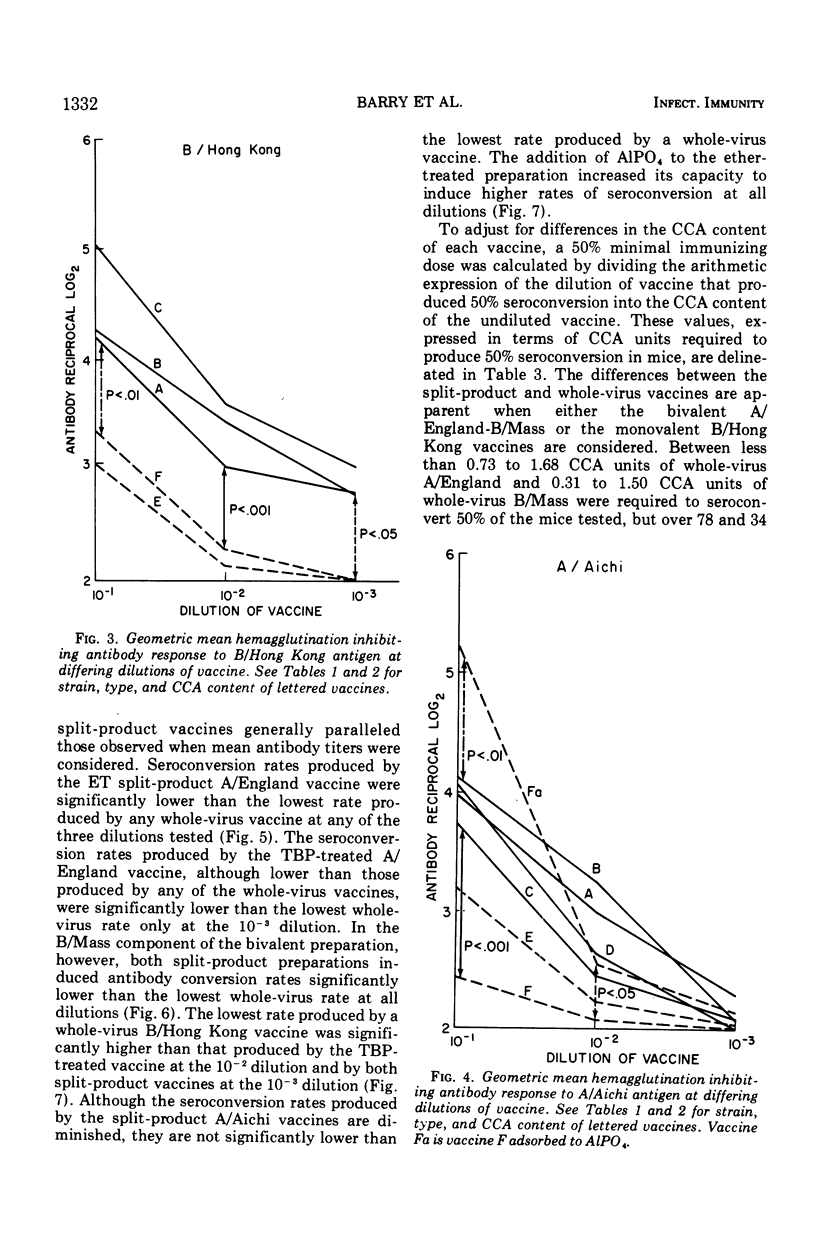
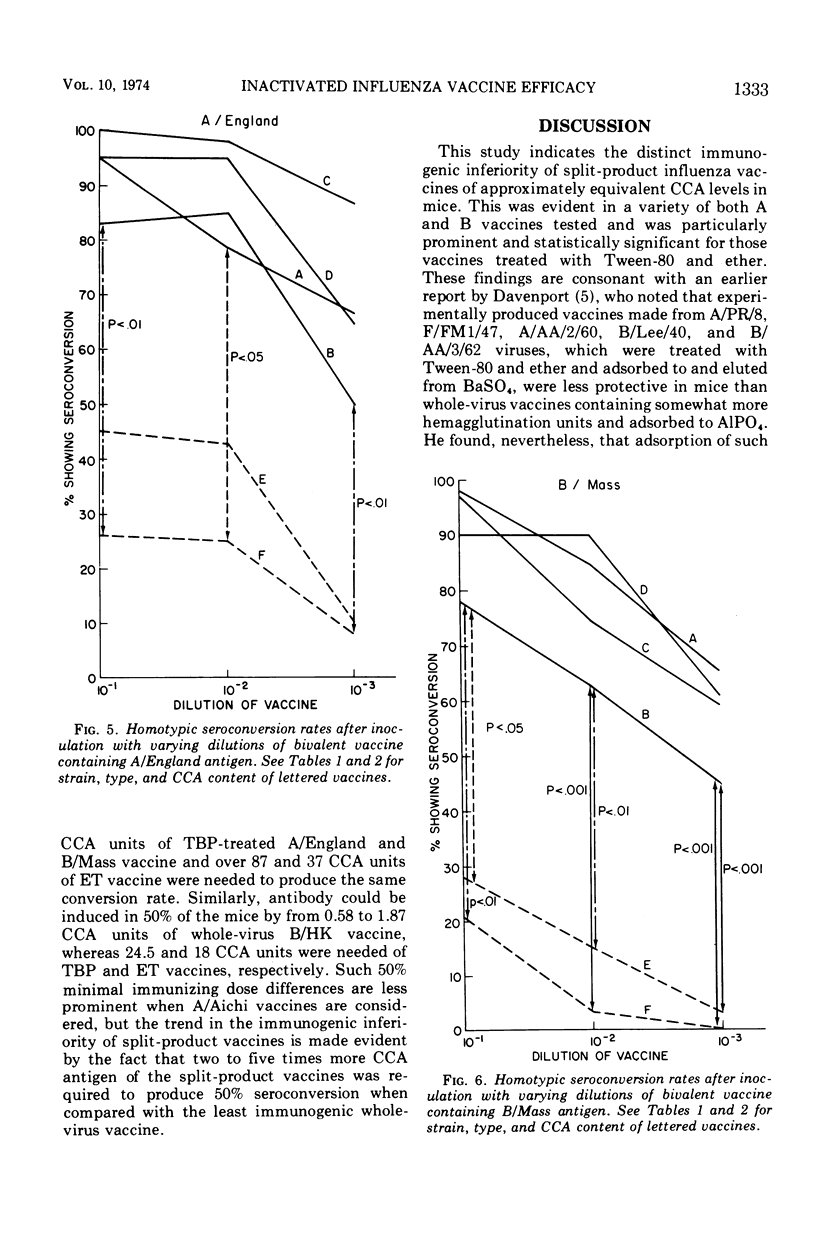
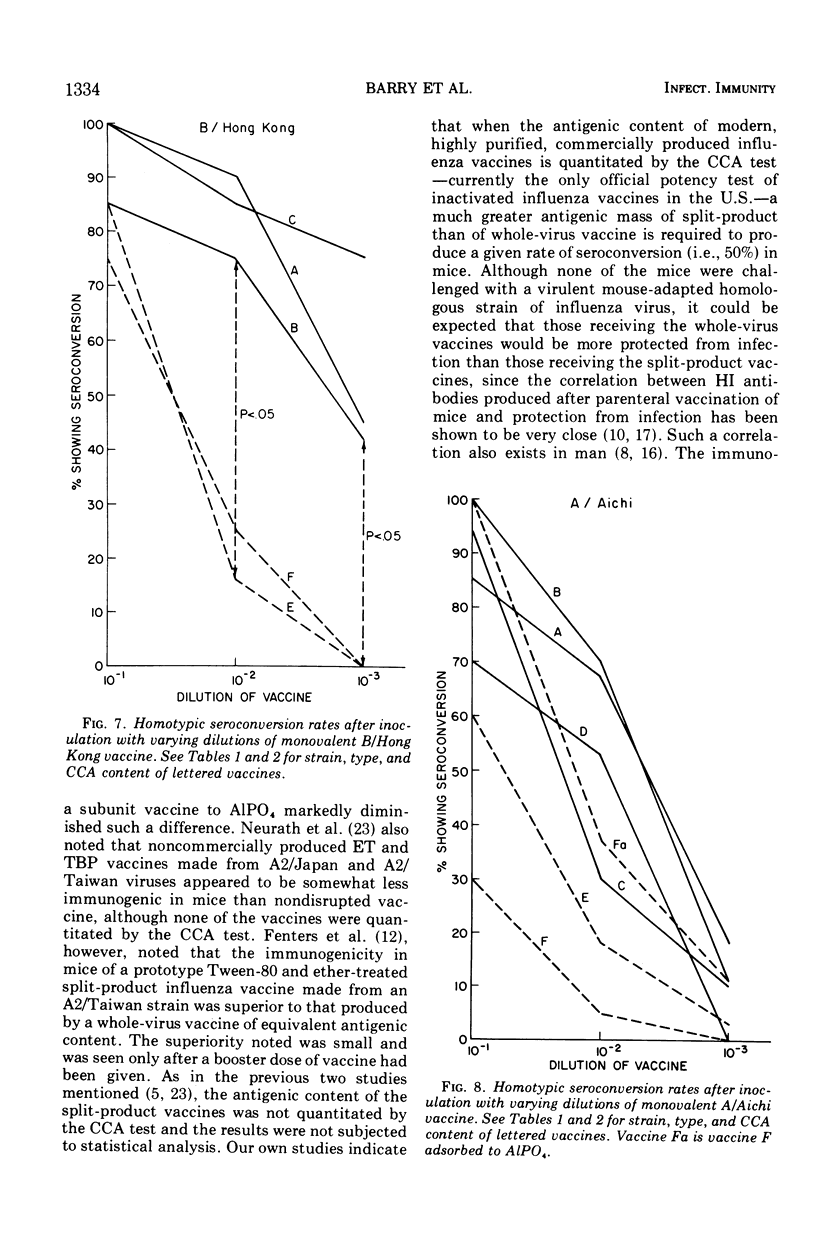
Groups of 60 to 120 mice were given a single intraperitoneal inoculation of varying dilutions of commercially prepared and licensed bivalent (A/England and B/Mass) and monovalent (A/Aichi or B/Hong Kong) inactivated influenza vaccines, and their antibody responses at 14 days were quantitated by hemagglutination inhibition tests. Split-product vaccines prepared by the treatment of A/England, B/Mass, and B/Hong Kong whole virus with Tween-80 and either tributylphosphate or ether produced significantly lower mean antibody titers than did equivalent whole-virus preparations. The rates of seroconversion (<1:8 to ≥1:8) at the various dilutions tested were also significantly reduced when these split-product vaccines were given. When the antigen content of all vaccines was quantitated by the chick cell agglutination test, between 10 and 100 times more split-product antigen than whole-virus antigen was required to produce seroconversion in 50% of the mice tested. Differences between split-product and whole-virus A/Aichi vaccines were less marked. These data point out the need to consider factors other than hemagglutinin content alone in determining the immunogenicity of inactivated influenza vaccines.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandon F. B., Barrett C. D., Jr, Hook A. E., Lease G. O. Human febrile response to influenza virus or its ether isolated hemagglutinins. Proc Soc Exp Biol Med. 1967 Jul;125(3):683–686. doi: 10.3181/00379727-125-32180. [DOI] [PubMed] [Google Scholar]
- Brandon F. B., Cox F., Lease G. O., Timm E. A., Quinn E., McLean I. W., Jr Respiratory virus vaccines. 3. Some biological properties of Sephadex-purified ether-extracted influenza virus antigens. J Immunol. 1967 Apr;98(4):800–805. [PubMed] [Google Scholar]
- Brandon F. B., Cox F., Quinn E., Timm E. A., McLean I. W., Jr Influenza immunization: clinical studies with ether-split subunit vaccines. Bull World Health Organ. 1969;41(3):629–637. [PMC free article] [PubMed] [Google Scholar]
- Cromwell H. A., Brandon F. B., McLean I. W., Jr, Sadusk J. F., Jr Influenza immunization. A new vaccine. JAMA. 1969 Nov 24;210(8):1438–1442. [PubMed] [Google Scholar]
- DAVENPORT F. M., HENNESSY A. V., BRANDON F. M., WEBSTER R. G., BARRETT C. D., Jr, LEASE G. O. COMPARISONS OF SEROLOGIC AND FEBRILE RESPONSES IN HUMANS TO VACCINATION WITH INFLUENZA A VIRUSES OR THEIR HEMAGGLUTININS. J Lab Clin Med. 1964 Jan;63:5–13. [PubMed] [Google Scholar]
- Davenport F. M. Antigenic enhancement of ether-extracted influenza virus vaccines by AIPO4. Proc Soc Exp Biol Med. 1968 Feb;127(2):587–590. doi: 10.3181/00379727-127-32748. [DOI] [PubMed] [Google Scholar]
- Eickhoff T. C. Immunization against influenza: rationale and recommendations. J Infect Dis. 1971 Apr;123(4):446–454. doi: 10.1093/infdis/123.4.446. [DOI] [PubMed] [Google Scholar]
- FAZEKAS de ST GROTH S., DONNELLEY M. Studies in experimental immunology of influenza. III. The antibody response. Aust J Exp Biol Med Sci. 1950 Jan;28(1):45–60. doi: 10.1038/icb.1950.4. [DOI] [PubMed] [Google Scholar]
- FAZEKAS de ST GROTH S., DONNELLEY M. Studies in experimental immunology of influenza. IV. The protective value of active immunization. Aust J Exp Biol Med Sci. 1950 Jan;28(1):61–75. doi: 10.1038/icb.1950.5. [DOI] [PubMed] [Google Scholar]
- Fazekas de Saint Groth, Webster R. G., Davenport F. M. The antigenic subunits of influenza viruses. I. The homologous antibody response. J Immunol. 1969 Nov;103(5):1099–1106. [PubMed] [Google Scholar]
- Fenters J. D., Yamashiroya H. M., Petzold R. F., Tolkacz V. K. Enhanced immunogenicity in mice of a purified, tween-ether-treated influenza vaccine. Appl Microbiol. 1970 Oct;20(4):544–550. doi: 10.1128/am.20.4.544-550.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy A. V., Davenport F. M. Relative antigenic potency in man of polyvalent influenza virus vaccines containing isolated hemagglutinins or intact viruses. J Immunol. 1966 Aug;97(2):235–238. [PubMed] [Google Scholar]
- Hennessy A. V., Davenport F. M. Vaccination of infants against influenza with polyvalent influenza hemagglutinin. JAMA. 1967 Jun 5;200(10):896–898. [PubMed] [Google Scholar]
- Hobson D., Curry R. L., Beare A. S., Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond) 1972 Dec;70(4):767–777. doi: 10.1017/s0022172400022610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye H. S., Dowdle W. R., McQueen J. L. Studies on inactivated influenza vaccines. I. The effect of dosage on antibody response and protection against homotypic and heterotypic influenza virus challenge in mice. Am J Epidemiol. 1969 Aug;90(2):162–169. doi: 10.1093/oxfordjournals.aje.a121060. [DOI] [PubMed] [Google Scholar]
- Maugh T. H., 2nd Influenza (II): A Persistent Disease May Yield to New Vaccines. Science. 1973 Jun 15;180(4091):1159–1215. doi: 10.1126/science.180.4091.1159. [DOI] [PubMed] [Google Scholar]
- Nakamura K. Pathogenicity and immunogenicity of various strains of influenza virus for mice. Biken J. 1965 Sep;8(3):155–165. [PubMed] [Google Scholar]
- Norrby E., Lagercrantz R., Gard S., Carlström G. Measles vaccination. 3. Serologic responses to immunization with purified hemagglutinin. Acta Paediatr Scand. 1965 Nov;54(6):581–586. doi: 10.1111/j.1651-2227.1965.tb06422.x. [DOI] [PubMed] [Google Scholar]
- Norrby E., Lagercrantz R., Gard S. Measles vaccination. V. The booster effect of purified hemagglutinin in children previously immunized with this product or formalin-killed vaccine. Acta Paediatr Scand. 1966 Jan;55(1):73–78. doi: 10.1111/j.1651-2227.1966.tb15210.x. [DOI] [PubMed] [Google Scholar]
- Perkins F. T. Control of influenza vaccine, with special reference to experience in the United Kingdom. Bull World Health Organ. 1969;41(3):554–555. [PMC free article] [PubMed] [Google Scholar]
- Phillips C. F., Phillips C. A., Hodgkin W. E., Forsyth B. R., Rubin B. A., Geraghty M. E. Killed subunit influenza vaccine in children. Pediatrics. 1973 Sep;52(3):416–419. [PubMed] [Google Scholar]
- Potter C. W., Jennings R., Marine W. M., McLaren C. Potentiation of the antibody response to inactivated A2-Hong Kong vaccines by previous heterotypic influenza virus infection. Microbios. 1973 Sep-Oct;8(30):101–110. [PubMed] [Google Scholar]
- Potter C. W., Jennings R., Rees R. C., McLaren C. Antibody response of hamsters to A2-Hong Kong virus vaccine after priming by heterotypic virus infection. Infect Immun. 1973 Aug;8(2):137–144. doi: 10.1128/iai.8.2.137-144.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben F. L., Akers L. W., Stanley E. D., Jackson G. G. Protection with split and whole virus vaccines against influenza. Arch Intern Med. 1973 Oct;132(4):568–571. [PubMed] [Google Scholar]
- Ruben F. L., Jackson G. G. A new subunit influenza vaccine: acceptability compared with standard vaccines and effect of dose on antigenicity. J Infect Dis. 1972 Jun;125(6):656–664. doi: 10.1093/infdis/125.6.656. [DOI] [PubMed] [Google Scholar]
- Rubin B. A., Pierzchala W. A., Neurath A. R. Elicitation of antibody response against influenza viruses by different viral subunit preparations. Arch Gesamte Virusforsch. 1967;20(2):268–271. doi: 10.1007/BF01241283. [DOI] [PubMed] [Google Scholar]
- Rubin R. J., Gregg M. B. "English flu"--a primer. N Engl J Med. 1973 Mar 1;288(9):467–468. doi: 10.1056/NEJM197303012880912. [DOI] [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- Schulman J. L., Kilbourne E. D. Correlated studies of a recombinant influenza-virus vaccine. II. Definition of antigenicity in experimental animals. J Infect Dis. 1971 Nov;124(5):463–472. doi: 10.1093/infdis/124.5.463. [DOI] [PubMed] [Google Scholar]
- Tauraso N. M., O'Brien T. C., Seligman E. B., Jr Problems of influenza virus vaccine standardization. Bull World Health Organ. 1969;41(3):497–506. [PMC free article] [PubMed] [Google Scholar]
- Tauraso N. M., Pedreira F. A., Spector S. L., Bernier G. M. Effect of various methods of removing non-specific inhibitors to virus hemagglutination upon serum proteins and immunoglobulins. Arch Gesamte Virusforsch. 1971;34(3):214–222. doi: 10.1007/BF01242995. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Laver W. G. Influenza virus subunit vaccines: immunogenicity and lack of toxicity for rabbits of ether- and detergent-disrupted virus. J Immunol. 1966 Apr;96(4):596–605. [PubMed] [Google Scholar]



