Abstract
This Phase III, uncontrolled, open-label, multicenter study was conducted to investigate the contraceptive efficacy, bleeding pattern, and cycle control of a novel once-a-week contraceptive patch, delivering low-dose ethinyl estradiol (EE) and gestodene (GSD) at the same systemic exposure seen after oral administration of a combined oral contraceptive containing 0.02 mg EE/0.06 mg GSD. Participants were women aged 18 to 35 years, all of whom received the EE/GSD patch for 13 cycles each of 21 treatment days (one patch per week for 3 weeks) followed by a 7-day, patch-free interval. The primary efficacy variable was the occurrence of unintended pregnancies during the study period as assessed by life table analysis and the Pearl Index. Secondary efficacy variables were days with bleeding during four 90-day reference periods and during 1 treatment year, bleeding pattern, and cycle control. The Kaplan-Meier probability of contraceptive protection after 364 treatment days was 98.8% and the adjusted Pearl Index was 0.81. The percentage of participants with intracyclic bleeding/spotting decreased over time, from 11.4% to 6.8% in cycles 1 and 12, respectively. Almost all participants (range: 90.8%-97.6%) experienced withdrawal bleeding across the study period. Compliance was very high (mean: 97.9%; median: 100%). The most frequent adverse events were headache (9.5%) and application site reaction (8.5%); no clinically significant safety concerns were observed. Results suggest the EE/GSD patch is highly effective in preventing pregnancy. Menstrual bleeding pattern was favorable and within the ranges expected of a healthy female population. The patch was well tolerated and treatment compliance was high.
Keywords: transdermal, female contraception, gestodene, ethinyl estradiol, pearl index
Introduction
The systemic delivery of steroid hormones by transdermal technology using patches containing an estrogen, or an estrogen plus a progestin, is well established in postmenopausal women.1 Transdermal hormone delivery has also been successfully used for the purposes of contraception, with a transdermal contraceptive patch approved in 2002 in Europe that releases ethinyl estradiol (EE) and norelgestromin (NGMN) over a 7-day application period. This patch is associated with the same systemic exposure to EE and NGMN that is recorded after daily administration of a combined oral contraceptive pill (COC) containing 33.9 µg EE and 203 µg NGMN.2
Recently, a novel transparent transdermal patch that delivers low doses of EE and gestodene (GSD) has been developed. The 7-day application period of this patch results in the same systemic hormone exposure as observed with daily administration of a COC containing 0.02 mg EE and 0.06 mg GSD.3
Daily administration of COCs represents the most common contraceptive choice for women in the developed world4 and is highly effective in preventing pregnancy. However, poor compliance with this treatment is a common problem and can result in greatly impaired efficacy.5 There are additional problems with COCs, such as the low bioavailability of EE (38%-48%) after oral administration,6 rapid and large fluctuations in serum hormone concentrations,7 and large intra- and interindividual pharmacokinetic variations.8 Transdermal delivery of contraceptive hormones, in contrast, offers several advantages over oral administration, including effective absorption and the provision of relatively constant serum concentrations.7,9 In addition to the convenience offered by weekly patch application, transdermal delivery of contraception provides a further choice for women, which may increase compliance and, thus, efficacy.
Both EE and GSD are a good choice for transdermal delivery since each is well absorbed through the skin.7,10 Indeed, EE is the most potent estrogen agonist available and has long been used in COCs,11 while GSD is a well-researched progestin that has an established efficacy and safety profile and has been widely used in Europe for contraceptive purposes for more than 20 years.12–14 Together with its good skin absorption properties,10 the low absolute dose of GSD needed for contraception15 allows for a small patch size.
The success of new nondaily hormonal contraceptive products, such as the once-a-week transdermal patch, EVRA® (Janssen-Cilag International N.V., Belgium), has highlighted the need for additional nondaily and/or nonoral hormonal contraceptive choices. A recent study showed that although many women discuss COCs with their health care provider, few discuss non-COC alternatives.16 Furthermore, previous studies investigating women’s views of contraception have reported that women admit to risk-taking behavior in terms of their compliance with contraceptive methods. Some women continue with the use of oral COCs that are seen as a familiar and acceptable contraceptive option, despite concerns such as skipping a pill dose. They report disliking methods of contraception that are invasive or involve a vaginal examination and have a low opinion of health care professionals’ advice.17 Negative views on methods of long-acting reversible contraception cannot be completely dispelled by providing women with accurate information on their efficacy and tolerability alone.17
The principal aim of the present study was to investigate the efficacy and safety of the EE/GSD patch. As one of the major reasons women discontinue use of hormonal contraceptives is abnormal uterine bleeding,18 it is essential that any new hormonal contraceptive entering the market is evaluated for its effect on both cycle control and bleeding pattern. Therefore, an additional aim of the study was to assess both the bleeding pattern and the compliance associated with the EE/GSD patch, since each may plausibly be improved due to the convenience of weekly patch application.
Participants and Methods
Objectives and Study Design
The primary objective of the study was to investigate the contraceptive efficacy of a transdermal contraceptive patch containing 0.55 mg EE and 2.1 mg GSD, which results in the same systemic hormone exposure as that observed with daily administration of a COC containing 0.02 mg EE and 0.06 mg GSD. Secondary objectives included the assessment of bleeding pattern, cycle control, compliance, and safety. This was a Phase III, open-label study conducted at 60 study centers in 7 countries (Germany, Italy, France, Spain, Chile, Mexico, and Australia). The study comprised 13 cycles, each consisting of 21 days of treatment (1 patch per week for 3 weeks) followed by a 7-day, patch-free interval.
Participants
Eligible participants were healthy women, aged 18 to 35 years (smokers: 18-30 years), with a normal cervical smear not requiring further follow up and a history of regular cyclic menstrual periods. As such, the study did not recruit women having hormonal imbalance with reduced fecundity. Written informed consent was obtained from all participants. Key exclusion criteria included pregnancy or lactation (fewer than 3 menstrual cycles since delivery, abortion, or lactation before start of treatment); obesity (body mass index [BMI] > 30 kg/m2); conditions or medications that could alter the pharmacokinetics of the study drugs, for example, St. John’s wort, antiepileptics, anticoagulants, hypnotics or sedatives, tuberculostatics, virostatic agents, oral antimycotics, additional sex steroids and other drugs impairing ovarian function, and phenylbutazone within 28 days before start of treatment; and use of antibiotics during treatment and within 7 days before start of treatment. Other exclusion criteria included diseases that could worsen during hormonal treatment; undiagnosed abnormal genital bleeding; abuse of alcohol, drugs, or medications; and use of other contraceptive methods (eg, sterilization, oral, vaginal, or transdermal hormonal contraception, intrauterine devices, implants, and long-acting preparations).
Assessments
The primary efficacy variable was the occurrence of unintended pregnancies during the study period, up to 7 days after removal of the last patch, as assessed by life table analysis and the Pearl Index. Pregnancy tests were conducted during the first and last visit, immediately before the first patch application and, if required, at each study visit (cycles 3, 7, and 10). Furthermore, participants were instructed to perform a home pregnancy test in case of absence of withdrawal bleeding. Secondary efficacy variables were the number of days with bleeding within 4 reference periods of 90 days each, and bleeding pattern and cycle control during 1 treatment year (13 treatment cycles, each of 28 days). Each volunteer kept a cycle diary in which occurrence/severity of bleeding and spotting was recorded daily. Participants were instructed that bleeding was defined as vaginal blood loss requiring the use of sanitary protection, whereas spotting was bleeding not requiring sanitary protection. Vaginal bleeding episodes were categorized as withdrawal bleeding when the bleeding started either after the last treatment day of the cycle (day 21) or not more than 4 days before treatment withdrawal in the case of a bleeding episode ongoing on the last day of treatment (day 21) and on the following day. All other bleeding episodes were considered as intracyclic bleeding, apart from application-deviation bleeding which was defined as bleeding resulting from complete/partial patch detachment for more than 24 hours or a patch not being replaced within 48 hours after the due time.
Safety variables were adverse events, results of physical examination, including vital signs, weight, gynecologic examination, cytologic cervical smear test, pregnancy test, and prior and concomitant medication. Adverse events were continuously monitored and recorded throughout the study. Compliance was assessed using diary cards. Efficacy and safety were analyzed in a full analysis set (FAS), defined as all participants who applied at least 1 study patch and for whom at least 1 observation after dosing was recorded.
Ethical Approval
The study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use Guideline E6: Good Clinical Practice. The study was approved by the individual Ethics Committees of the participating sites.
Statistical Analyses
A sample size of at least 950 women (for 13 cycles each) was considered necessary to obtain a 2-sided 95% confidence interval (CI) for the Pearl Index with a probability of at least 90% so that the upper limit of the CI did not exceed 2.0 when the Pearl Index was 1.0. Statistical evaluations were performed using the SAS software package (release 9.1 or higher; SAS Institute Inc, Cary, North Carolina). All variables were analyzed by descriptive statistical methods, with frequency tables generated for categorical data.
Results
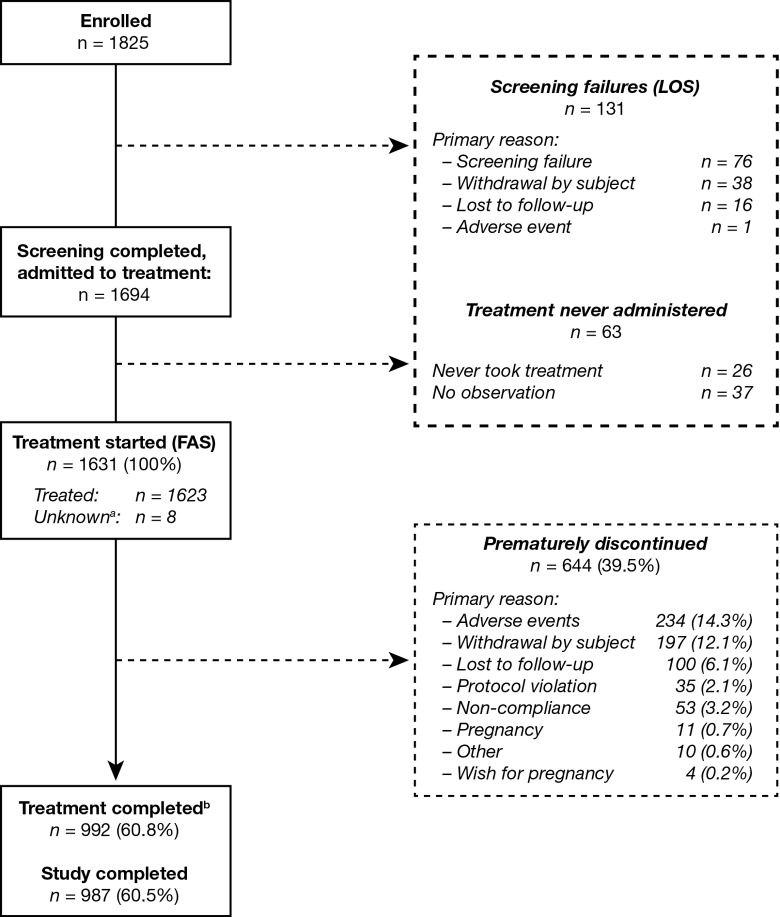
In total, 1631 women were included in the FAS, including 8 women who had received study treatment but were subsequently lost to follow up and who were conservatively assumed to have applied at least 1 patch. The baseline demographic characteristics of the participants in the FAS are listed in Table 1. In total, 987 participants completed the study. The most common reasons for withdrawal were adverse events (14.3%) and personal reasons (12.1%; Figure 1). Participants excluded from the FAS were not included in any statistical analysis and were classified as the “listing-only set.” The listing-only set also included 26 participants who never took study medication and 37 participants who had no observation after admission to treatment.
Table 1.
Baseline Demographic Characteristics, Full Analysis Set.a
| Age, height, body weight, BMI, mean ± SD (range) | |
| Age, years | 25.3 ± 4.4 (18-36) |
| Height, cm | 164.1 ± 6.7 (143-188) |
| Weight, kg | 61.3 ± 9.0 (39.8-94.0) |
| BMI, kg/m2 | 22.8 ± 3.0 (15.0-30.0) |
| Race, n (%) | |
| White | 1570 (96.3) |
| Black | 9 (0.6) |
| Asian | 15 (0.9) |
| Other | 37 (2.3) |
| Ethnicity,b n (%) | |
| Not Hispanic/Latino | 1135 (69.6) |
| Hispanic/Latino | 486 (29.8) |
| Not reported | 10 (0.6) |
| Educational level, n (%) | |
| Missing | 1 (<0.1) |
| Elementary | 92 (5.6) |
| Secondary | 697 (42.7) |
| College/university | 841 (51.6) |
| Smoking history, n (%) | |
| Never | 954 (58.5) |
| Former | 145 (8.9) |
| Current | 532 (32.6) |
| Alcohol consumption, n (%) | |
| Abstinent | 542 (33.2) |
| Light | 1061 (65.1) |
| Moderate | 28 (1.7) |
| At least 1 birth or abortion, n (%) | |
| Yes | 594 (36.4) |
| No | 1037 (63.6) |
| Time since last birth or abortion, mean ± SD (range) | |
| Days | 1360.8 ± 1162.8 (52-6590) |
| Blood pressurec, mean ± SD (range) | |
| Diastolic, mm Hg | 69.0 ± 8.3 (40-95) |
| Systolic, mm Hg | 113.0 ± 11.5 (68-163) |
| Contraceptive method used within 28 days before screening, n (%) | |
| None | 178 (10.9) |
| Barrier methods | 426 (26.1) |
| Hormonal methods | 1007 (61.7) |
| Intra-uterine device | 13 (0.8) |
| Other | 7 (0.4) |
Abbreviations: BMI, body mass index; SD, standard deviation.
a n = 1631.
b Ethnicity was not reported by 10 participants. The difference between race and ethnicity is not universally recognized, and an absence in reporting one or the other may result from the assumption that “race” is the same as “ethnicity.” Moreover, some defined ethnic groups (eg, Hispanics) do not identify with racial categories (eg, white) or find the race question confusing (http://www.census.gov/population/www/socdemo/race/racefactcb.html).
c n = 1629.
Figure 1.
Disposition of the study population. aFor 8 participants, it is not known whether treatment was started; these participants were included in the FAS. The LOS comprises those participants who failed to satisfy the selection criteria for the study and never received any treatment. These data were not included in the subsequent statistical analysis. bTreatment was completed if a subject applied at least 1 patch after day 14 of cycle 13 according to the diary, or if the completion date was available and was at least 350 days after start of study treatment. FAS indicates full analysis set; LOS, listing-only set.
Efficacy
Based on 14 pregnancies and 428 521 days of exposure, the unadjusted Pearl Index until 7 days after removal of the last patch was 1.19 (upper limit of 2-sided 95% CI: 2.00). Of the 14 pregnancies, 5 were considered to be the result of noncompliance. Based on the remaining 9 pregnancies and 403 361 days of relevant exposure, the adjusted Pearl Index until 7 days after removal of the last patch was 0.81 (upper limit of 2-sided 95% CI: 1.55). The Kaplan-Meier probability of contraceptive protection after 364 days of treatment was 98.8% (Figure 2). The separate calculation of the Pearl Index in the European population (women recruited in France, Germany, Italy, and Spain), based on six pregnancies of which three were considered a result of non-compliance and 287,861 days of exposure, resulted in an unadjusted Pearl Index of 0.76 (upper limit of 95% CI: 1.66). Based on the remaining three pregnancies and 272,242 days of exposure, the adjusted Pearl Index for method failure was 0.40 (upper limit of 95% CI: 1.18).
Figure 2.
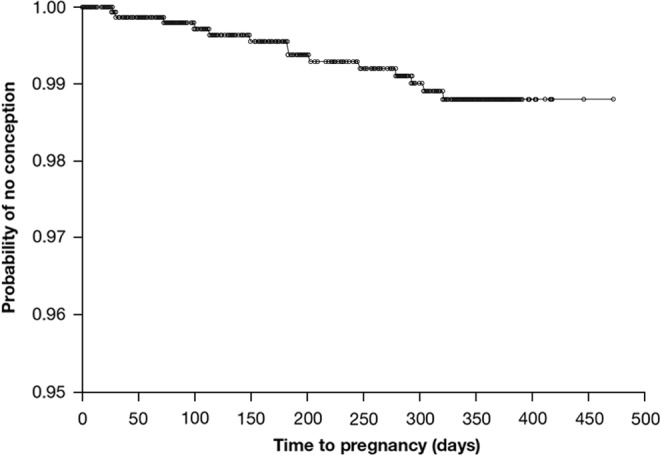
Probability of contraceptive protection with the EE/GSD patch. EE indicates ethinyl estradiol; GSD, gestodene.
Bleeding Pattern
As expected, the mean number of bleeding/spotting days per 90-day reference period was higher in period 1 than in subsequent periods, since the first administration of the patch in period 1 started on the first day of bleeding in those participants who had not used hormonal contraception in the cycle immediately before entering the study; the mean number of bleeding/spotting days decreased from 19.8 ± 8.1 in period 1 to 15.7 ± 4.9 in period 4 (Figure 3A). The number of bleeding/spotting episodes per 90-day reference period did not change during the study (Figure 3B).
Figure 3.
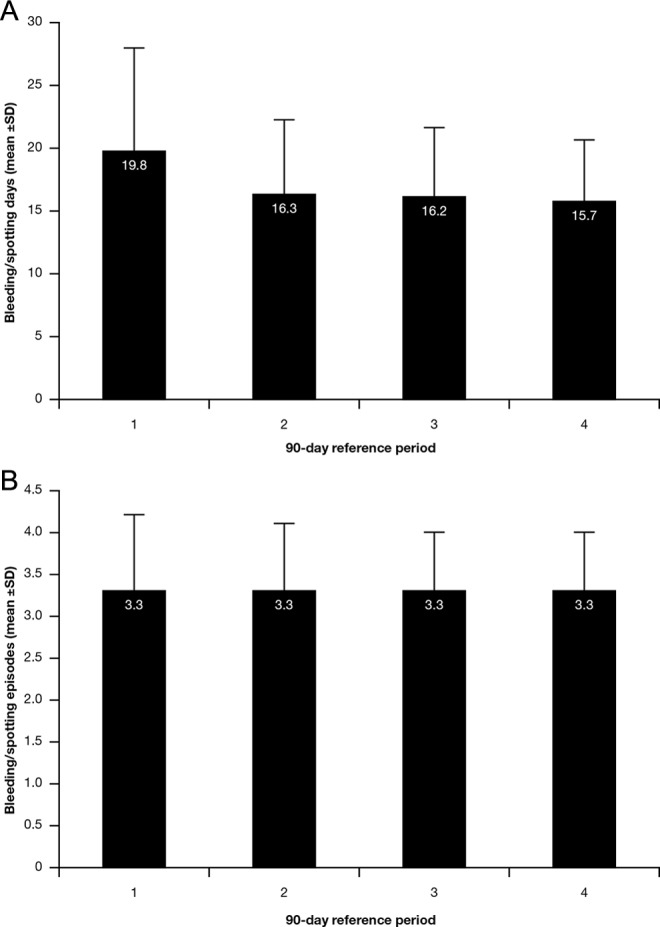
A, Bleeding pattern in terms of mean number of bleeding/spotting days per 90-day reference period. B, Bleeding pattern in terms of mean number of bleeding/spotting episodes per 90-day reference period. SD indicates standard deviation.
Results from cycle 13 are not included in this analysis as diary entries for a given cycle were only valid when the withdrawal bleeding (or its absence) was documented as well as at least 2 days without bleeding after that event. Most participants, however, ended diary entries once they removed the last study patch. During cycles 1 to 12, almost all participants in the FAS experienced withdrawal bleeding, ranging from 90.8% of participants in cycle 1 to 97.6% of those in cycle 11. The intensity of withdrawal bleeding episodes was stable throughout the study (Figure 4). In addition, the onset of withdrawal bleeding after patch removal was stable over time (range: 2.7-3.0 days). The length of withdrawal bleeding episodes was stable throughout the study and ranged from 4.9 ± 1.8 days (cycle 2) to 5.1 ± 2.1 days (cycle 1).
Figure 4.
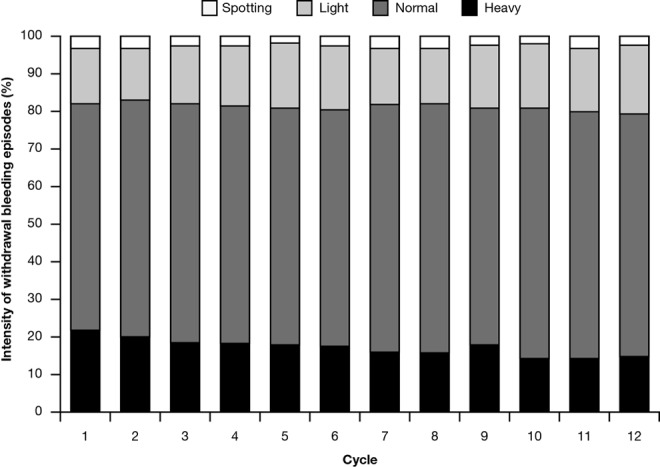
Intensity of withdrawal bleeding episodes.
The percentage of participants with intracyclic bleeding/spotting episodes decreased over time from 11.4% to 6.8% in cycles 1 and 12, respectively (Figure 5A). Approximately half of these participants (between 43.0% in cycle 8 and 54.4% in cycle 2) reported intracyclic spotting only, which likewise decreased with time (cycle 1: 5.6% of participants; cycle 2: 6.5% of participants; cycle 11: 3.0% of participants; and cycle 12: 4.0% of participants). The intensity of bleeding/spotting remained stable over time (Figure 5B).
Figure 5.
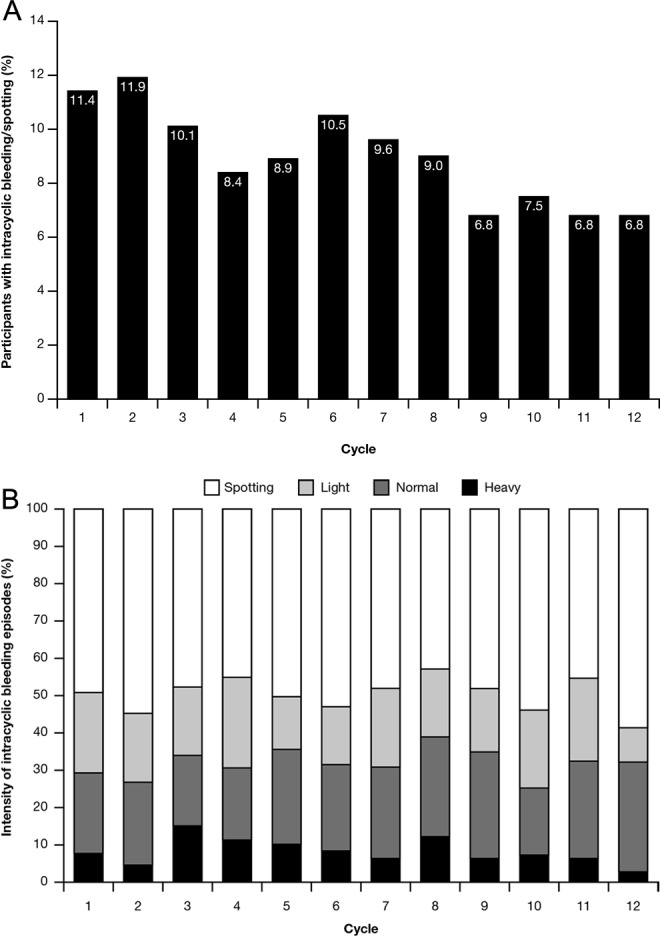
A, Proportion of participants reporting intracyclic bleeding/spotting episodes by cycle. B, Intensity of intracyclic bleeding/spotting episodes by cycle.
Compliance with the patch was very high, with a mean of 97.9% and a median of 100% (range, 3.0%-124.1%). The patch adhesion rate was also high, with complete and complete/partial patch detachments occurring in 5.7% and 15.3% of the study population, respectively. Considerable deviation from scheduled patch application (ie, detached patches were not replaced within 24 hours or patches were not changed after the 7-day wearing period plus an additional 48 hours) was recorded for 64 participants in cycle 1 and 21 participants in cycle 12, with a corresponding bleeding event occurring in 8 and 3 of these participants, respectively.
Safety
In total, 1007 (61.7%) of 1631 participants included in the FAS experienced at least 1 adverse event during treatment. The most frequently occurring adverse events were headache (9.5%) and application-site reaction (8.5%; Table 2). Discontinuation due to adverse events occurred in 234 (14.3%) participants (Figure 1). No clinically significant trends or safety concerns were observed in the evaluation of other safety parameters, and no deaths were reported during the study.
Table 2.
Adverse Events Occurring in ≥2% of Patients (Full Analysis Set, n = 1631) During Treatment.a
| MedDRA Preferred Term | n (%) |
|---|---|
| At least 1 adverse event | 1007 (61.7) |
| Headache | 155 (9.5) |
| Application-site reaction | 139 (8.5) |
| Nasopharyngitis | 114 (7.0) |
| Cervical dysplasiab | 101 (6.2) |
| Application-site erythema | 80 (4.9) |
| Metrorrhagia | 73 (4.5) |
| Application-site pruritus | 64 (3.9) |
| Application-site hypersensitivity | 59 (3.6) |
| Application-site rash | 56 (3.4) |
| Vulvovaginal mycotic infection | 46 (2.8) |
| Cystitis | 43 (2.6) |
| Breast pain | 43 (2.6) |
| Dysmenorrhea | 41 (2.5) |
| Application-site irritation | 39 (2.4) |
| Bronchitis | 38 (2.3) |
| Tonsillitis | 37 (2.3) |
| Nausea | 36 (2.2) |
| Influenza | 34 (2.1) |
| Urinary tract infection | 33 (2.0) |
Abbreviations: MedDRA, Medical Dictionary for Regulatory Activities.
a Participants may have experienced more than one adverse event.
b Abnormal cervical smears had to be reported by the investigators as adverse events. These events included the category “atypical squamous cells of undetermined significance,” for which there is no immediate risk of cancer.
Overall, 35 (2.1%) participants experienced at least 1 serious adverse event, 4 participants experienced events related to pregnancies with abnormal outcome (2 cesarean sections, 1 double loop of the umbilical cord around the neck, and a case of equinovarus foot in the child), and 3 (0.2%) participants reported serious adverse events considered by the investigator to be related to study treatment: 1 case of reactive depression and suicidal ideation and 2 cases of pulmonary embolism. One of these pulmonary embolism cases occurred in an 18-year-old nonsmoker who also experienced deep vein thrombosis. This woman had no history of pregnancy and had used a subcutaneous hormonal contraceptive implant (68 mg etonogestrel, slow release over 3 years) for approximately 9 months before enrollment. She was overweight (BMI, 29.7 kg/m2) and had received study treatment for more than 9 months prior to the event. Diagnostic evaluations revealed a heterozygous factor V Leiden mutation (associated with an increased risk of developing venous thrombosis) and a suspicion of iliac vein compression syndrome (May-Thurner syndrome). Both the deep vein thrombosis and pulmonary embolism were regarded as drug related; however, the woman’s genetic profile was suggestive of thrombophilia, and both vascular events were possibly complicated by the external compression of the left common iliac vein. The second case of pulmonary embolism occurred in a 21-year-old light smoker approximately 4 months after study medication was initiated; this study participant also had a pulmonary infarction. The woman had no history of pregnancy and had previously used barrier contraception. Diagnostic investigations detected no deep vein thrombosis, and markers of thrombophilia were negative. Both these serious adverse events were considered to be drug related.
Discussion
The results of this study show that contraceptive efficacy with the EE/GSD patch was good, with unadjusted and adjusted Pearl Indices of 1.19 and 0.81, respectively. In addition, withdrawal bleeding pattern was favorable over the course of the study and the average number of bleeding/spotting days tended to decrease over time. Cycle control parameters showed an equally favorable pattern and were within the ranges expected of a healthy female population (an average withdrawal bleed of around 5 days with normal bleeding intensity). The number of participants with intracyclic bleeding/spotting also decreased during the study to a value in cycle 12 that was approximately half that of cycle 1. Together, these data suggest that the novel transdermal patch, which provides the same systemic exposure as seen after oral administration of a COC containing 0.02 mg EE/0.06 mg GSD, constitutes an effective and well-tolerated contraceptive option for women.
High levels of compliance were reported in this study, which is in line with previous findings. A recent study using diary cards to measure compliance with a transdermal contraceptive patch containing 0.6 mg EE/6 mg NGMN versus other forms of contraception (oral and barrier) found compliance with oral contraception to be poor, with 77.8% reporting missed doses. In contrast, 90.5% of recorded cycles in the patch group were completed with perfect compliance.19
Of the 1631 women who received treatment, 2 were diagnosed with pulmonary embolism. However, it should be noted that these were the only cases that have occurred in a development program that encompassed >3700 women, thus this number of thromboembolic events was expected. Moreover, although both cases were considered by the investigators to be related to study treatment, the first woman was overweight, heterozygous for a factor V Leiden mutation, and the investigator suspected the presence of May-Thurner syndrome, which may have predisposed the participant to venous thrombosis. The second case of pulmonary embolism was diagnosed after 4 months of study treatment, with no obvious predisposing factors other than the woman being a light smoker.
It should be noted that all combined hormonal contraceptives carry a small increased risk of venous thromboembolisms associated with the estrogen component.20 Best evidence indicates that venous thromboembolism rates in nonusers of reproductive age approximate 4 to 5 of 10 000 women per year; rates in oral contraceptive users are in the range of 9 to 10 of 10 000 women per year.21 For comparison, venous thromboembolism rates in pregnancy approach 29 of 10000 overall and may reach 300 to 400 of 10 000 in the immediate postpartum period.20,21 The risk of venous thromboembolism associated with oral contraceptive use is also increased in women with genetic thrombophilias such as Leiden V mutations22 and in women who smoke or have a raised BMI.20,22 However, there is an ongoing unresolved debate, based on data from oral contraceptive studies, about whether contraceptives containing third- and fourth-generation progestogens (such as GSD) carry an increased risk of venous thromboembolisms compared with levonorgestrel-containing COCs. The risk of death from venous thromboembolism is low.20 Based on a relative risk of 2, the excess risk of death for a woman taking modern combined hormonal contraceptives is 1 in 100 000, which is much lower than the risk of everyday activities such as cycling.23 There is also conflicting evidence regarding whether the transdermal route of administration is associated with a higher incidence of venous thromboembolism. Several studies have indicated a 2-fold increase among patch users24 while others have not confirmed this.25 This highlights the need to develop transdermal hormonal contraception that delivers lower levels of EE.24 Although the risk of venous thromboembolism associated with COC use is known to decrease with decreasing estrogen dose, the role of different progestogens in affecting risk remains controversial.26
Although this study was open label in design and consisted of a single treatment arm, the results presented here suggest that the novel transdermal contraceptive patch is a promising option for women who currently consider their contraceptive choice to be limited or unacceptable in terms of effects on bleeding pattern and cycle control. However, it should be noted that this study was limited by the fact that it only included healthy women with regular cycles and excluded obese women; this impacts on the generalizability of these study findings to the general population.
In conclusion, our study found this novel, transdermal contraceptive patch to be highly effective in terms of preventing pregnancy, with an adjusted Pearl Index of 0.81. Menstrual bleeding pattern was also favorable, and the patch was well tolerated with no unexpected findings related to use of a transdermal hormonal contraceptive.
Acknowledgments
The authors would like to thank Ogilvy 4D, Oxford, United Kingdom, for providing editorial assistance.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: IW reports receiving lecture fees and travel reimbursements from Bayer HealthCare, Germany; Jenapharm GmbH & Co. KG, Germany; MSD Sharp & Dohme GmbH, Germany; Rottapharm Madaus, Germany; and Dr Kade/Besins Pharma GmbH, Germany. She has participated on advisory panels for Bayer HealthCare, Germany; MSD Sharp & Dohme GmbH, Germany; and Rottapharm Madaus, Germany. No other potential conflict of interest relevant to this article was reported. SB has received research funding from Bayer Pharma AG, Germany. No other potential conflict of interest relevant to this article was reported. EW is an occasional speaker for Bayer HealthCare, Germany and MSD Sharp & Dohme GmbH, Germany, and has been on advisory panels for both. She has received funding support from NIH, the Australian Research Council, The Population Council, Bayer HealthCare, Germany, and MSD Sharp & Dohme GmbH, Germany. UM and MM are salaried employees of Bayer Pharma AG, Germany.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Bayer Pharma AG, Germany.
References
- 1. Nelson HD. Commonly used types of postmenopausal estrogen for treatment of hot flashes: scientific review. JAMA. 2004;291(13):1610–1620. [DOI] [PubMed] [Google Scholar]
- 2. Janssen-Cilag. Evra® transdermal patch. Summary of Product Characteristics; 2012. http://www.medicines.org.uk/emc/medicine/12124/spc Accessed April 17, 2014.
- 3. Bayer Apleek®. Summary of Product Characteristics. http://www.mhra.gov.uk/home/groups/spcpil/documents/spcpil/con1394776320327.pdf Accessed April 17, 2014.
- 4. UN Department of Economic and Social Affairs Population Division. World contraceptive use. http://www.un.org/esa/population/publications/contraceptive2011/contraceptive2011.htm Accessed April 17, 2014.
- 5. Aubeny E, Buhler M, Colau JC, Vicaut E, Zadikian M, Childs M. The Coraliance study: non-compliant behavior. Results after a 6-month follow-up of patients on oral contraceptives. Eur J Contracept Reprod Health Care. 2004;9(4):267–277. [DOI] [PubMed] [Google Scholar]
- 6. Goldzieher JW, Brody SA. Pharmacokinetics of ethinyl estradiol and mestranol. Am J Obstet Gynecol. 1990;163(6 pt 2):2114–2119. [DOI] [PubMed] [Google Scholar]
- 7. Kuhl H. Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric. 2005;(8 suppl 1):3–63. [DOI] [PubMed] [Google Scholar]
- 8. Jung-Hoffmann C, Kuhl H. Intra- and interindividual variations in contraceptive steroid levels during 12 treatment cycles: no relation to irregular bleedings. Contraception. 1990;42(4):423–438. [DOI] [PubMed] [Google Scholar]
- 9. Burkman RT. Transdermal hormonal contraception: benefits and risks. Am J Obstet Gynecol. 2007;197(2):134 e131–e136. [DOI] [PubMed] [Google Scholar]
- 10. Heger-Mahn D, Warlimont C, Faustmann T, Gerlinger C, Klipping C. Combined ethinylestradiol/gestodene contraceptive patch: two-center, open-label study of ovulation inhibition, acceptability and safety over two cycles in female volunteers. Eur J Contracept Reprod Health Care. 2004;9(3):173–181. [DOI] [PubMed] [Google Scholar]
- 11. Coelingh Bennink HJ. Are all estrogens the same? Maturitas. 2004;47(4):269–275. [DOI] [PubMed] [Google Scholar]
- 12. Barbosa IC, Filho CI, Faggion D, Jr, , Baracat EC. Prospective, open-label, noncomparative study to assess cycle control, safety and acceptability of a new oral contraceptive containing gestodene 60 microg and ethinylestradiol 15 microg (Minesse). Contraception. 2006;73(1):30–33. [DOI] [PubMed] [Google Scholar]
- 13. Kaplan B. Desogestrel, norgestimate, and gestodene: the newer progestins. Ann Pharmacother. 1995;29(7-8):736–742. [DOI] [PubMed] [Google Scholar]
- 14. Wilde MI, Balfour JA. Gestodene. A review of its pharmacology, efficacy and tolerability in combined contraceptive preparations. Drugs. 1995;50(2):364–395. [DOI] [PubMed] [Google Scholar]
- 15. Benagiano G, Primiero FM, Farris M. Clinical profile of contraceptive progestins. Eur J Contracept Reprod Health Care. 2004;9(3):182–193. [DOI] [PubMed] [Google Scholar]
- 16. Beligotti F, Gordon K. Findings from the International ‘I plan on…survey’: women’s awareness, misconceptions, and preferences regarding their contraceptive options. Eur J Contracept Reprod Health Care. 2012;17(S1):S98. [Google Scholar]
- 17. Glasier A, Scorer J, Bigrigg A. Attitudes of women in Scotland to contraception: a qualitative study to explore the acceptability of long-acting methods. J Fam Plann Reprod Health Care. 2008;34(4):213–217. [DOI] [PubMed] [Google Scholar]
- 18. Schrager S. Abnormal uterine bleeding associated with hormonal contraception. Am Fam Physician. 2002;65(10):2073–2080. [PubMed] [Google Scholar]
- 19. Jakimiuk AJ, Crosignani PG, Chernev T, et al. High levels of women’s satisfaction and compliance with transdermal contraception: results from a European multinational, 6-month study. Gynecol Endocrinol. 2011;27(10):849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weisberg E. Developments in contraception. Expert Opin Pharmacother. 2014;15(2):203–210. [DOI] [PubMed] [Google Scholar]
- 21. Reid R, Leyland N, Wolfman W, et al. SOGC clinical practice guidelines: oral contraceptives and the risk of venous thromboembolism: an update. J Obstet Gynaecol Can. 2010;32(12):1192–1204. [DOI] [PubMed] [Google Scholar]
- 22. Vandenbroucke JP, Koster T, Briet E, Reitsma PH, Bertina RM, Rosendaal FR. Increased risk of venous thrombosis in oral-contraceptive users who are carriers of factor V Leiden mutation. Lancet. 1994;344(8935):1453–1457. [DOI] [PubMed] [Google Scholar]
- 23. Bitzer J, Amy JJ, Beerthuizen R, et al. Statement on combined hormonal contraceptives containing third- or fourth-generation progestogens or cyproterone acetate, and the associated risk of thromboembolism. J Fam Plann Reprod Health Care. 2013;39(3):156–159. [DOI] [PubMed] [Google Scholar]
- 24. Cole JA, Norman H, Doherty M, Walker AM. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol. 2007;109(2 pt 1):339–346. [DOI] [PubMed] [Google Scholar]
- 25. Jick SS, Kaye JA, Russmann S, Jick H. Risk of nonfatal venous thromboembolism in women using a contraceptive transdermal patch and oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception. 2006;73(3):223–228. [DOI] [PubMed] [Google Scholar]
- 26. Lidegaard O, Nielsen LH, Skovlund CW, Lokkegaard E. Venous thrombosis in users of non-oral hormonal contraception: follow-up study, Denmark 2001-10. BMJ . 2012;344:e2990. [DOI] [PMC free article] [PubMed] [Google Scholar]