Abstract
Purified Clostridium histolyticum collagenase (CHC), an Food and Drug Administration-approved drug that does not affect nerves or blood vessels, was assessed as a potential treatment for fibroids in this proof-of-principle study. Fibroids (1-4 cm, capsules intact) and myometrial specimens from 5 patients were injected posthysterectomy with CHC or vehicle containing methylene blue and incubated for 24 hours. Percentage of collagen-stained area was estimated using Masson-Trichrome-stained slides. Collagen fibers were observed with picrosirius staining. Tissue stiffness was objectively measured by rheometry (complex shear modulus [Pa]). Injected materials spread within and beyond fibroids as visualized by methylene blue. Of the 8 treated fibroids, 7 were softened and some contained liquefied centers. Relative percentage of collagen-stained area (mean ± standard deviation) in treated fibroids (38 ± 12%; n = 7) was less than that in control fibroids (66 ± 17%; n = 5). Treated myometrium (40 ± 30% collagen; n = 3) was similar to control myometrium (53 ± 8%; n = 2). Picrosirius staining demonstrated loss of collagen fibers in treated fibroids. Treated fibroids were less stiff (3630 ± 2410 Pa; n = 4) than controls (5930 ± 830 Pa; n = 4). Treated and control myometrium had similar stiffness (2149 ± 927 Pa; n = 3 and 3314 ± 494 Pa; n = 2, respectively) and were never liquefied. In conclusion, injections of CHC into encapsulated fibroids are feasible and effective. Heterogeneity of collagen types and quantities within individual fibroids may contribute to varied responses and need additional investigation. Further study of collateral effects on myometrium is indicated. Injected CHC has potential for treatment of fibroids.
Keywords: collagenase, fibroid, leiomyoma, clostridium histolyticum, clostridium
Introduction
Uterine leiomyomas (fibroids) are one of the most common tumors in reproductive-age women in the United States, with an incidence of >80% in African American women and 70% in white women detectable with ultrasonography by age 50.1 They cause symptoms of menorrhagia, dysmenorrhea, pelvic pain and pressure, and infertility and are the reason for over 200 000 hysterectomies annually.2 The estimated annual direct costs for medical and surgical management of fibroids is substantial, ranging from approximately 4 to 9 billion US dollars.2
For women with contraindications to hysterectomy, who desire less invasive treatment or who wish to preserve their fertility, there are multiple less invasive medical and surgical treatment options. These include oral and intramuscular contraceptives, estrogen and progesterone receptor modulators, gonadotropin-releasing hormone agonists, myomectomy, uterine artery embolization (UAE), high-intensity focused ultrasound (HIFU), and laparoscopic ultrasound-guided radiofrequency volumetric thermal ablation.3–7 Targeted destruction methods induce necrosis, and hormonal treatments can affect fertility and are contraindicated in patients with certain comorbidities.3–7 There would be an advantage to a minimally invasive fibroid treatment that does not induce necrosis and is nonhormonal.
Fibroids contain abundant and disorganized extracellular matrix (ECM). The main components of this ECM are abundant, disorganized collagens.8 As one of the main ECM components, collagens may serve as a vulnerable nonhormonal target for treatment of fibroids.9
Purified Clostridium histolyticum collagenase (CHC) was Food and Drug Administration (FDA) approved in 2010 for the treatment of Dupuytren contracture by local injection.10,11 The CHC is being studied as a potential treatment of other multiple fibrotic diseases, including keloid scars, and was approved in 2013 for the treatment of Peyronie disease.12 The CHC consists of class I and class II collagenases with potent binding affinity to interstitial collagens, particularly collagens I and III. Class I CHC has particularly high affinity to mature triple helical interstitial collagen with a preferred cleavage site at the N and C termini. Class II CHC preferentially cleaves the internal peptides and its preferred substrate is small denatured peptides.13–19 The CHC mostly attacks collagen types I and III, which are the most abundant collagen types found in fibroids. The CHC cleaves multiple bonds in the collagen molecule and thus is capable of completely degrading the collagen molecules in the treated tissue.
This collagenase is readily inhibited by serum proteins and thus is not active in the presence of serum.11,20,21 The CHC does not degrade type IV collagen, which is found in nerve and blood vessel basement membranes.10,11 Clinical studies using this CHC showed no evidence of systemic side effects nor blood vessel or nerve destruction in adjacent tissues, including a recent study assessing CHC for the treatment of Peyronie plaques, which immunohistochemically showed that CHC had no detectable effect on blood vessels or nerves at multiple doses and varying incubation periods.22 Reported adverse events with CHC injection for Dupuytren contracture most commonly included local reactions, including peripheral edema, contusion, injection site pain or hemorrhage, extremity pain, injection site swelling, tenderness, ecchymosis, lymphadenopathy, pruritus, and skin laceration.11 Possible indirect nerve damage or resulting paresthesias from the injection process could be attributed to edema, placing pressure on the nerve itself, or directly hitting the nerve with the injection needle; however, there is no evidence for nerve degeneration as a result of this drug. Antibody formation to CHC I and II has been detected in serum after treatment; however, no adverse effects have been noted from these antibodies.10,11 All of this suggest that CHC would be a safe candidate for direct injection into fibroids.
Our previous proof-of-principle studies showed the efficacy of CHC injected into small pieces of fibroids. Tissues were softened and partially liquefied within 24 hours.23 The CHC specifically affected the ECM with no observed changes in cellular architecture and nuclear structures. Therefore, pathological evaluation of treated tissues was still possible.
This study was designed as a feasibility study and sought to establish whether injection of CHC into intact encapsulated fibroids is possible and will effectively degrade the fibroid’s interstitial collagens and result in softening of the treated fibroids. We used a fixed amount of collagenase and examined injection volumes and distribution within and throughout the injected tissue and monitored reduction in collagen and tissue stiffness. This study provides necessary data to better design future clinical studies.
Materials and Methods
Five women undergoing hysterectomy for benign indications between August 2012 and March 2013 were selected for this Duke University Medical Center institutional review board (IRB)-approved study. English-speaking adult patients with fibroids 1 to 4 cm in diameter were included. Patients with suspicion of malignancy, minors, and non-English speakers were excluded. Since the goal of this study was limited to the effect of CHC on fibroid collagen as well on the ability to inject encapsulated tumors, the IRB approved the utilization of only completely deidentified tissue. Demographic information such as age or race, past medical or surgical history, and past hormonal treatments of the patients were unknown; however, these variables should not affect collagen degradation in this experiment. After hysterectomy, uteri were examined under the supervision of surgical pathology. Up to 3 intact fibroids 1 to 4 cm in diameter with up to 2 cm of surrounding myometrium were removed per patient. Additional myometrial samples were also obtained, up to 2 samples per patient. All solutions used in these experiments contained penicillin, streptomycin, and amphotericin B to prevent bacterial or fungal growth during incubation times.
All samples were washed 3 times in phosphate-buffered saline (PBS). Tissues were photographed, measured, and palpated for firmness. Between 2 and 4 hours after hysterectomy, fibroids were injected using a 20G needle into the fibroid center with 0 to 4 mg/mL of purified collagenase (BioSpecifics Technologies Corp, Lynbrook, New York). The collagenase was added to a vehicle of 0.01% methylene blue in 0.3% CaCl2 in normal saline. Methylene blue was selected as a gross marker for spread of injection.24 Injection volume was determined by calculating total fibroid volume. Initially, tissue was injected with 100 µL/0.53 cm3 of vehicle (0.01% methylene blue in 0.3% CaCl2 in normal saline) with or without collagenase at 1 mg/mL. After observing easy penetration of injected fluid throughout and beyond the injected fibroids in the first two patients, collagenase concentrations were increased to 4 mg/mL and volumes decreased to 25 µL/0.53 cm3. One control sample did not receive any injection. All samples were incubated in Dulbecco Modified Eagle Medium/F12 culture media in a humidified CO2 incubator at 37°C for 24 hours.
After incubation, the samples were again grossly palpated, photographed, and measured. All samples were bivalved at the injection site. Specimens were frozen at −80°C or formalin-fixed and paraffin-embedded for histology. Slides were stained with hematoxylin and eosin (H&E), Masson Trichrome (MT), or Picrosirius Red (PR). Slides were imaged using a Zeiss Axio Imager (Zeiss, Peabody, Massachusetts) microscope. The PR-stained slides were imaged under polarized light.
Tissue stiffness was ascertained by determining the complex shear modulus on thawed specimens on an AR-G2 rheometer.25 Tissue for the studies of tissue stiffness were frozen at minus 80°C until tested. Rheometry is a time-consuming process, and consistent handling of samples was ensured by freezing all samples at the time of collection and only thawing them shortly before measurements were taken. In addition, many CHC-treated samples were extremely soft, and obtaining punch biopsies of equal size was only possible from frozen tissue. The freezing and thawing process does not affect the stability of collagen. We had previously measured very similar stiffness (complex shear moduli) values in samples before and after refreezing. Briefly, frozen tissue samples were partially thawed, and depending on the size of the tissue samples, 1 or 2 punch biopsies were cored (5-mm diameter by 2- to 5-mm high) from each sample. Cored samples were placed in a PBS bath between 2 plates, and a compressive offset strain of 20% was applied. Dynamic frequency sweeps were performed at an increasing angular frequency from 1 to 50 rad/s. The magnitude of the complex shear modulus was then calculated at 10 rad/sec. Results from multiple punches of the same tissue (up to 2) were averaged.
Cellular architecture was evaluated by observation of the H&E sections. Percentage of collagen was assessed on MT slides, with collagen defined by the blue–green spectrum, 5 to 10 nonoverlapping areas (depending on the specimen size) at 10× magnification were imaged as high-resolution .tiff files, which then underwent semiquantitative analysis using Adobe Photoshop CS6. All clear areas, including any vessel lumens, were excluded from the total pixel count. The number of the blue–green pixels as a percentage of total pixels was determined for each image and averaged with all images from that slide. Differences in ECM organization were subjectively assessed using PR staining, viewed under polarized light. Our study design included descriptive statistics but did not include analysis of statistical significance between groups since this was an exploratory study to determine injection feasibility. Given the small number of samples, we could not assess for any possible dose–response relationship between the varying concentrations and amounts of collagenase used. However, determination of dose response was not our goal in this proof-of-concept study.
Results
Five consented patients met all selection criteria. Thirteen fibroids, 1 to 4 cm in diameter, were used. All fibroids were stiff, intact, encapsulated, and had 1 to 2 cm of attached myometrium. In all, 4 fibroids were used for vehicle injections, 1 fibroid was not injected, and 8 received collagenase injections. Five separate myometrial samples were used for injection of vehicle (n = 2) or collagenase (n = 3).
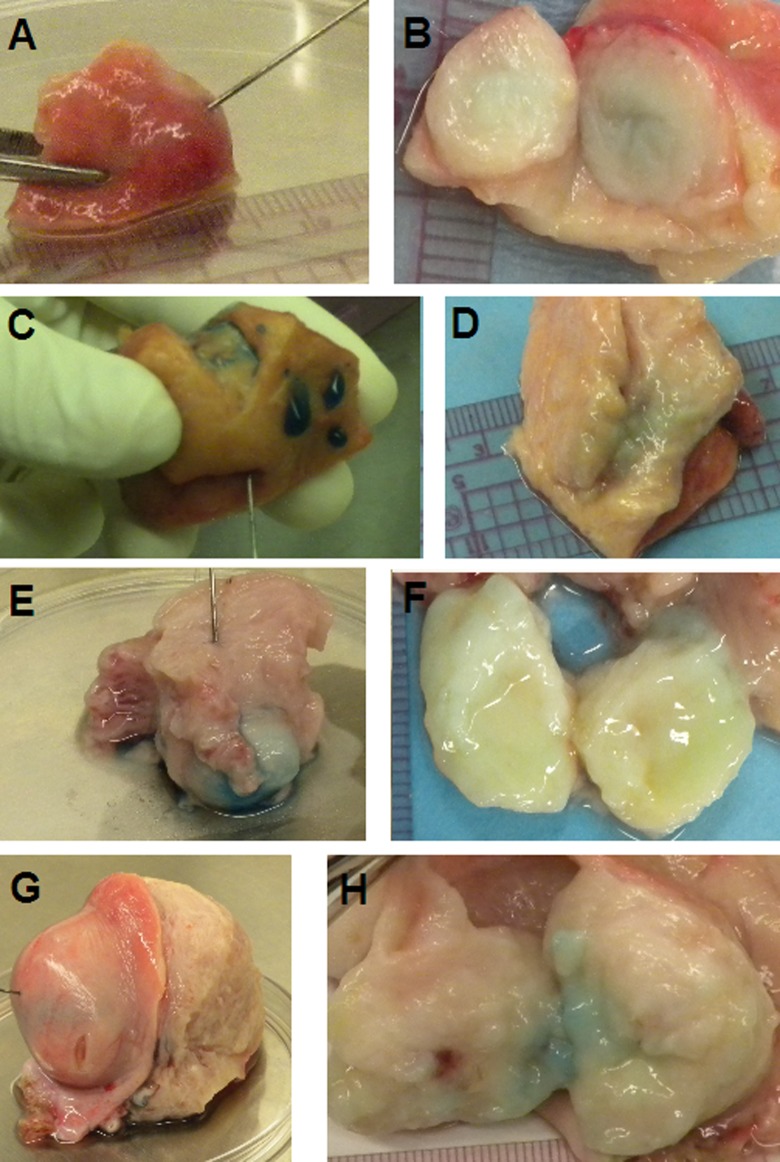
Vehicle-injected fibroids before and after incubation were not visibly changed (Figure 1A and B). Collagenase-injected fibroids were less stiff by palpation after incubation in 7 of 8 samples. Of 8 collagenase-injected fibroids, 4 were partially liquefied (Figure 1 C through H). Collagenase-injected myometrium was less stiff by palpation than vehicle-injected myometrium. For all samples, measurement of fibroid diameter was not different pre- and postincubation. However, many of the collagenase-injected fibroids did have distortion of their shapes, most notably in a fibroid 4 cm in diameter (Figure 1G and H).
Figure 1.
Fibroids during injection (left column A, C, E, and G) and post-24-hour incubation and bisection (right column B, D, F, H). Row 1: (A, B) 1-cm vehicle-injected fibroid (26 μL of 0 mg/mL); note that the center stayed firm. Row 2: (C, D) 1.8-cm collagenase-injected fibroid (350 μL of 1 mg/mL). Row 3: (E, F) 2-cm collagenase-injected fibroid (400 μL of 1 mg/mL); note liquefaction in the center. Row 4: (G, H) 4-cm collagenase-injected fibroid (840 μL of 2 mg/mL). In some specimens, methylene blue dye is still visible upon dissection.
In 7 of the 12 injected fibroids, penetration of methylene blue dye into the surrounding myometrium was observed. This was most noticeable in patient 1, where 350 µL of 1 mg/mL collagenase was injected into the 1.8-cm fibroid and penetration was noted 2 cm distal to the fibroid outer edge (Figure 1C and D). After observing penetration of the dye into surrounding myometrium in patients 1 and 2, the collagenase concentration was then progressively increased and injection volume decreased; however, the initially planned dosage of CHC per total fibroid volume remained constant. This decreased the penetration of dye into the surrounding tissues. Three 1-cm fibroids from patients 4 and 5 were injected with less than 50 µL of 4 mg/mL, with minimal (up to 0.5 cm) or no collateral spread of blue-stained injection material. The maximum collagenase concentration used in this study was 4 mg/mL, and injection of 25 µl of the 4 mg/mL preparation into a 1-cm fibroid was feasible and preferred over the initial preparation, given the lack of gross collateral spread.
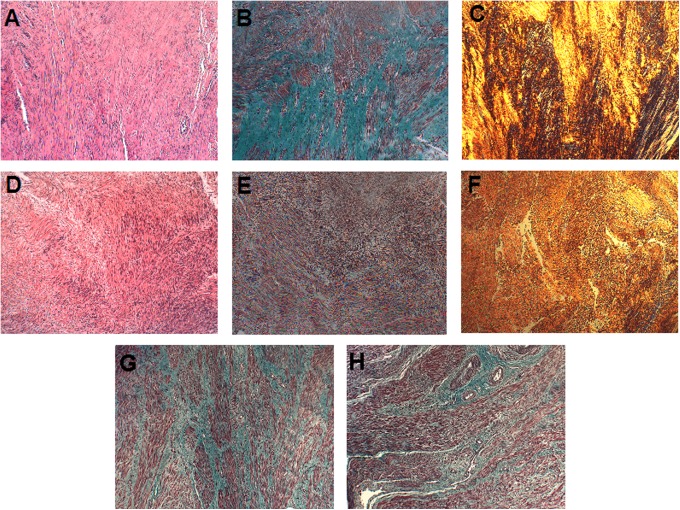
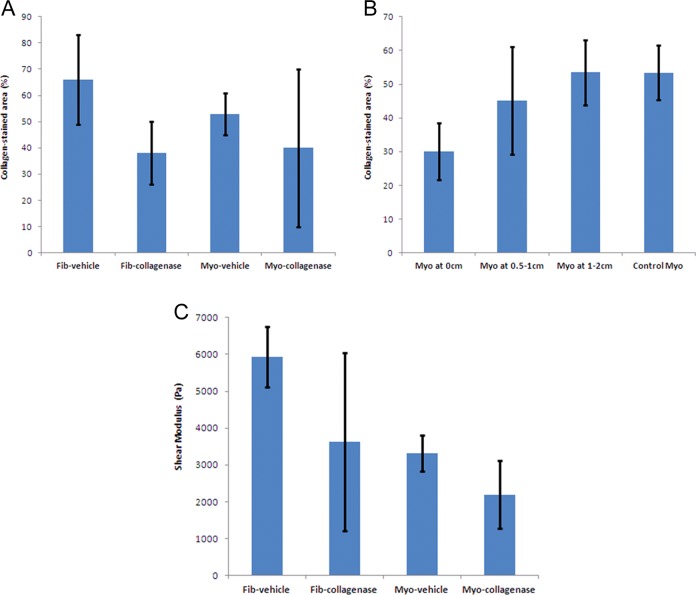
Hematoxylin and eosin-stained slides showed that cellular architecture was maintained in collagenase-injected samples (Figure 2A and D). The MT and PR slides showed a decrease in the collagen-stained areas in treated samples (Figure 2 B, C, E, and F). Myometrium adjacent to the injected fibroids’ capsules showed less difference in percentage of collagen-stained area between control and treated specimens, when compared to the fibroids themselves (Figure 2G and H). The collagen-stained area was measured by digitized pixel counts in MT-stained slides. Values in collagenase-injected fibroids ranged from 21% to 95%, with median of 41% (n = 8). The fibroid with 95% collagen-stained area was the only one with densely hyalinized collagen and was very stiff even after collagenase injection and 24-hour incubation. In vehicle-injected fibroids, collagen-stained area ranged from 38% to 83% with median of 66%. Collagen-stained area (mean ± standard deviation [SD]) was less in nonhyalinized collagenase-injected fibroids (38% ± 12%; n = 7) than in vehicle-injected fibroids (66 ± 17%; n = 5; Figure 3A). Collagenase-injected myometrium values ranged from 14% to73%, median 31% (n = 3). Vehicle-injected myometrium ranged from 48% to 59% collagen (n = 2). We collected surrounding myometrium from 4 of the collagenase-treated fibroids at 0 cm (n = 3), 0.5 to 1 cm (n = 3), and 1.5 to 2 cm (n = 4) away from the fibroid capsule. There was less collagen-stained area in the attached myometrium proximal to the fibroid capsule than distal, suggesting that the effect of collagenase decreases rapidly with increasing distance from the fibroid. Samples 1.5 to 2 cm from the fibroid capsule had 53% ± 10% collagen-stained area, similar to vehicle-injected myometrium (Figure 3B). The PR slides were viewed under polarized light to visualize birefringence of collagen fibers and the content was subjectively judged. We observed a loss of collagen fibers in all treated fibroids except for 1 fibroid from patient 5, which contained abundant amounts of hyalinized collagen.
Figure 2.
Histology slides using Hematoxylin & Eosin (H&E; A, D), Masson Trichrome (MT; B, E, G, H), and Picrosirius Red (PR; C, F) staining, at 10× magnification. Collagen appears blue–green on MT slides and yellow–orange on PR slides. Row 1: (A, B, C) a 1-cm control fibroid injected with 100 μL of vehicle. Row 2: (D, E, F) a 1-cm collagenase-injected (D, E, F) fibroid that was treated with 100 μL of 1 mg/mL Clostridium histolyticum collagenase (CHC) in vehicle. G, Myometrium proximal to a control 1-cm fibroid injected with 25 μL of vehicle. H, Myometrium proximal to a 1 cm treated fibroid injected with 25 μL of 4 mg/mL CHC in vehicle. (The color version of this figure is available at rs.sagepub.com.)
Figure 3.
A, Percentage of collagen-stained area ± standard deviation (SD) on Masson Trichrome slides for vehicle-injected and collagenase-injected fibroid and myometrium groups. B, Percentage of collagen-stained area ± SD on Masson Trichrome slides for uninjected myometrium at 0, 0.5 to 1, and 1.5 to 2 cm from the capsule of collagenase-injected fibroids as well as for vehicle-injected myometrium that serves as control. C, Tissue stiffness measured as complex Shear moduli (average ± SD) for vehicle-injected and collagenase-injected fibroid and myometrium.
Objective measurements of tissue stiffness were obtained by determining the complex shear modulus using a rheometer. Values for collagenase-injected fibroids ranged from 1,382 to 16,065 Pa, median 3,677 Pa (n = 5), with the previously described hyalinized fibroid measuring 16,065 Pa. Vehicle-injected fibroids ranged from 4,832 to 6,829 Pa, median 6,031 Pa (n = 4). Shear modulus (mean ± SD) was less in nonhyalinized collagenase-injected fibroids (3630 ± 2410 Pa; n = 4) than vehicle-injected fibroids (5930 ± 830 Pa; n = 4). Collagenase-injected myometrial samples ranged 1,432 to 3,226 Pa with median of 1,924 Pa (n = 3), similar to the range for vehicle-injected myometrial tissues (2965-3664 Pa; n = 2; Figure 3C).
Discussion
Our data indicate that CHC injected into intact fibroids degraded collagen and reduced stiffness in those fibroids, as evidenced by decreased collagen staining and decreased complex shear modulus. Reduction in collagen in myometrium adjacent to collagenase-injected fibroids may be due to the penetration of injected materials as visualized by methylene blue. It is understood that the distribution of the methylene blue and diluents may have been different from the distribution of CHC. However, methylene blue was still a useful tool for determining the full extent of spread of the injection materials. It is also likely that CHC penetrated through the samples and into the culture media, as blue dye was seen seeping out of the tissue edges and then was able to diffuse through the myometrial tissue. Observation of myometrial collagen content at varying distances from the fibroid capsule suggests that the effect of collagenase may decrease rapidly with increasing distance from the fibroid, an important point in assessing the safety of this injection technique. Our observations led us to reduce injection volumes in patients 3, 4, and 5 and reduced the penetration of dye into surrounding myometrium. The important finding was that increasing the concentration of the CHC in a smaller volume of vehicle (diluent) limited the diffusion of the enzyme into the surrounding myometrium. Drug concentration can be further adjusted in future clinical trials.
We can speculate why the 1 hyalinized fibroid from patient 5 was the only fibroid unaffected by the CHC. The appearance, size, and subjective firmness of this fibroid was similar to those from other patients and did not show any evidence of increased calcium deposits on gross inspection or H&E staining. The collagen content and shear modulus were much higher than the other studied fibroids, including control fibroids from the same patient. It is possible that the dosage used in our study or the incubation time was not sufficient to degrade the collagen in this specific tissue. It is also possible that serum inhibitors of collagenase may have rendered the injections less effective, as there appeared to be more residual blood in this fibroid following the first wash. Serum inhibition of collagenase implies that a higher dose of collagenase may be necessary to achieve an in vivo therapeutic effect, a phenomenon that was noted in studies conducted for treatment of Dupuytren contractions.11 It has been shown that fibroids, even within the same uterus, have varied growth rates, and some will grow while others regress.26,27 This suggests differences in molecular composition of individual fibroids may exist, in fact, unique ratios of collagen types have been observed in different fibroids.28
Fibrosis in tissues is felt to be due to the continued secretion of ECM, especially interstitial collagens leading to increased extracellular pressure and the induction of the mechanotransduction pathways promoting myofibroblast formation and inhibiton of apoptosis. By degrading interstitial collagens, CHC may reduce tissue stiffness and arrest the mechanotransduction signaling that allows for continued buildup of matrix in the treated tissue29 and may even induce apoptosis of myofibroblasts.30 This theory, tested in other tissues, will be evaluated in future clinical studies. Our current study was not designed to determine whether ECM–cell mechanical dynamics would in fact cause apoptosis of fibroid cells. This particular evaluation is to be accomplished in planned future studies. It was not feasible to evaluate this in our current study since the effect of the reduction in mechanical tension takes place over a longer period of time than the 24-hour time point evaluated in these experiments.
In situ destruction of fibroids by HIFU, radiofrequency ablation, and UAE, all cause necrosis of the target tissues, making analysis by pathologists difficult should the tissues need to be removed later. The CHC-treated tissues show no change in cellular and nuclear architecture, which poses a distinct advantage over the other necrosis-inducing minimally invasive options, as it allows for pathological diagnosis. Since we only assessed cellular architecture on slides and did not perform tests on cellular function, we cannot know at this time whether the fibroid cells remain functional after collagenase treatment, and this will need to be assessed in further studies.
Before clinical studies can be initiated, volumes and concentrations of injected CHC relative to the size of the fibroid have to be determined. Volumes that are too large and concentrations that are too high could adversely affect the surrounding myometrium. Cells were visualized to remain intact, despite digestion of some proximal myometrial ECM in this study. Future clinical studies are needed to determine the dose and time required to soften CHC-injected fibroids in vivo in the presence of intact circulation and serum. Our current data inform the design of those clinical studies as we have determined that CHC at the dose of 0.1 mg/0.53 cm3 with a concentration of 4 mg/mL can be injected into intact, encapsulated fibroids and lead to the softening of the treated tissue. We have also determined that an injection volume of 25 μL/0.53 cm3 will minimize penetration into surrounding tissues while allowing for digestion within the fibroid. At this volume, there is a very limited diffusion of CHC into the surrounding myometrium.
In summary, we have shown that CHC can be injected into whole encapsulated fibroids and we have demonstrated that in 4 of 5 patients, CHC degraded fibroid interstitial collagen sufficiently to change the tissue stiffness and collagen content. This study is part of ongoing studies investigating the use of this FDA-approved drug in the treatment of fibroids by local injection either during hysteroscopy or during laparoscopy, or perhaps by ultrasound-guided direct injection, for the treatment of uterine fibroids in an outpatient setting. The 2 current FDA-approved indications for CHC include Peyronie disease and Dupuytren contracture, both of which are extraperitoneal, superficially treatable conditions. Fibroid intratumoral injection in situ has the added challenges of accessibility to the tissue, and the possibility of adverse reactions of surrounding intraperitoneal structures. Safely accessing the fibroids could be done by transabdominal or transvaginal injection with ultrasound guidance or by hysteroscopic or laparoscopic injection. Reproductive endocrinologists are experienced in transvaginal egg retrieval done under ultrasound guidance, using current high-resolution ultrasound that allows them to identify and avoid blood vessels. Fibroid vasculature is most dense at the capsule, and since we intend to inject fibroids at their core, where there are fewer vessels,31 there would be a lower risk of intravascular injection. We already know that CHC is inactivated by serum proteins, and it is likely that intraperitoneal fluid may also inactivate CHC, so a higher dose of CHC than was used in these experiments may be required. We do not know whether digested collagen that may leak into the peritoneal cavity could trigger an inflammatory reaction in nearby organs and tissues. However, degraded collagen is known to be eliminated in tissues by the active scavenger response of activated macrophages under physiological conditions. Thus, a local inflammatory response would be anticipated. This would not of itself be harmful unless it became chronic. Further studies will be crucial in answering this question. As not all fibroids are alike in terms of collagen content or type ratios, not all fibroids may respond in the same way to treatment with CHC, or they may require higher doses or multiple treatments to achieve a therapeutic effect. It is possible that collateral digestion might be prevented by serum inhibition as occurs with the injection of Dupuytren cords. The CHC injection does not cause tissue necrosis and this may be beneficial over other uterus-preserving, in situ destructive treatments for fibroids that induce tissue necrosis.
Acknowledgments
We wish to thank BioSpecifics Technologies Corp for providing the purified collagenase and performing enzyme activity testing. We also wish to thank Dr Farshid Guilak, Franklin T. Moutos, and Elisabeth M. Flannery in the Department of Orthopedic Surgery at DUMC, Dr Rex Bentley of the Department of Pathology at DUMC, and the Duke Women’s Health Associates of Durham, North Carolina, for their generous assistance with this project.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Grant support: Charles B. Hammond Fund, Department of Obstetrics and Gynecology, Duke University Medical Center, Durham, NC. This article won first prize at the Charles B. Hammond Resident Research Day, Department of Obstetrics and Gynecology, Duke University Medical Center, Durham, NC on May 31, 2013.
References
- 1. Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188 (1):100–107. [DOI] [PubMed] [Google Scholar]
- 2. Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol. 2012;206 (3):211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Munro MG. Uterine leiomyomas, current concepts: pathogenesis, impact on reproductive health, and medical, procedural, and surgical management. Obstet Gynecol Clin North Am. 2011;38 (4):703–731. [DOI] [PubMed] [Google Scholar]
- 4. Taylor DK, Leppert PC. Treatment for uterine fibroids: searching for effective drug therapies. Drug Discov Today Ther Strateg. 2012;9 (1):e41–e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Levy G, Hill MJ, Beall S, Zarek SM, Segars JH, Catherino WH. Leiomyoma: genetics, assisted reproduction, pregnancy, and therapeutic advances. J Assist Reprod Genet. 2012;29 (8):703–712 doi: 10.1007/s10815-012-9784-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sabry M, Al-Hendy A. Medical treatment of uterine leiomyoma. Reprod Sci. 2012;19 (4):339–353 doi: 10.1177/1933719111432867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chudnoff SG, Berman JM, Levine DJ, Harris M, Guido RS, Banks E. Outpatient procedure for the treatment and relief of symptomatic uterine myomas. Obstet Gynecol. 2013 May;121 (5):1075–82. [DOI] [PubMed] [Google Scholar]
- 8. Leppert PC, Baginski T, Prupas C, Catherino WH, Pletcher S, Segars JH. Comparative ultrastructure of collagen fibrils in uterine leiomyomas and normal myometrium. Fertil Steril. 2004;82 (suppl 3):1182–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Malik M, Norian J, McCarthy-Keith D, Britten J, Catherino WH. Why leiomyomas are called fibroids: the central role of extracellular matrix in symptomatic women. Semin Reprod Med. 2010;28 (3):169–179. [DOI] [PubMed] [Google Scholar]
- 10. Thomas A, Bayat A. The emerging role of Clostridium histolyticum collagenase in the treatment of Dupuytren disease. Ther Clin Risk Manag. 2010;6:557–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Badalamente MA, Hurst LC. Efficacy and safety of injectable mixed collagenase subtypes in the treatment of Dupuytren’s contracture. J Hand Surg Am. 2007;32 (6):767–774. [DOI] [PubMed] [Google Scholar]
- 12. Gelbard M, Goldstein I, Hellstrom WJ, et al. Clinical efficacy, safety, and tolerability of collagenase clostridium histolyticum for the treatment of peyronie disease in 2 large double-blind, randomized, placebo controlled phase 3 studies. J Urol. 2013;190 (1):199–207 doi: 10.1016/j.juro.2013.01.087. [DOI] [PubMed] [Google Scholar]
- 13. Toyoshima T, Matsushita O, Minami J, Nisen N, Okobe a, Itano T. Collagen-binding domain of clostridium histolyticum exhibits a broad substrate spectrum both in vitro and in vivo. Connect Tissue Res. 2001;42 (2):281–290. [DOI] [PubMed] [Google Scholar]
- 14. Han S, Makareeva E, Kuznetsova NV, et al. Molecular mechanism of type I collagen homotrimer resistance to mammalian collagenases. J Biol Chem. 2010;285 (29):22276–22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roberts-Pilgrim AM, Makareeva E, Myles MH, et al. Deficient degradation of homotrimeric type I collagen, α1(I)3 glomerulopathy in oim mice. Mol Genet Metab. 2011;104 (3):373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bromley JW, Osman M, Steinlauf P, Gennace T, Stern H. Collagenase: an experimental study of intervertebral disc dissolution. Spine. 1980;5 (2):126–136. [PubMed] [Google Scholar]
- 17. Friedman K, Poliak SV, Manning T, Pennell SR. Degradation of porcine dermal connective tissue by collagenase and by hyaluronidase. Br J Dermatol. 1986;115 (4):403–408. [DOI] [PubMed] [Google Scholar]
- 18. Miyabashi T, Lord PF, Dubiolzig PR, Biller DS, Manley PA. Chemonucleolysis with collagenase: a radiographic and pathologic study in dogs. Vet Surg. 1992;21 (3):189–194. [DOI] [PubMed] [Google Scholar]
- 19. Mallya SK, Mookhtiar KA, van Wart HE. Kinetics of hydrolysis of type I, II, and III collagens by the class I and II Clostridium histolyticum collagenases. J Protein Chem. 1992;11 (1):99–107. [DOI] [PubMed] [Google Scholar]
- 20. Borth W, Menzel EJ, Salzer M, Steffen C. Human serum inhibitors of collagenase as revealed by preparative isoelectric focusing. Clin Chim Acta. 1981;117 (2):219–225. [DOI] [PubMed] [Google Scholar]
- 21. Nagase H, Itoh Y, Binner S. Interaction of alpha 2-macroglobulin with matrix metalloproteinases and its use for identification of their active forms. Ann N Y Acad Sci. 1994;732:294–302. [DOI] [PubMed] [Google Scholar]
- 22. Levine LA, Schmid TM, Emeigh-Hart SG, Tittlebach T, McLane MP, Tursi JB. Collagenase clostiridium histolyticum degrades type I and II collagen while sparing type IV collagen in vitro in Peyronie’s plaque explants. PD 22-03 [published online May 19, 2014] Am Urol Assoc. 2014. [Google Scholar]
- 23. Jayes FL, Ma X, Flannery EM, Moutos FT, Guilak F, Leppert PC. Biomechanical evaluation of human uterine fibroids after exposure to purified clostridial collagenase. In: Supplement to Biology of Reproduction for the 46th Annual Meeting of the Society for the Study of Reproduction, 22–26 July 2013, Montreal, Quebec, Canada A743; 2013: 334. [Google Scholar]
- 24. Peter C, Hongwan D, Küpfer A, Lauterburg BH. Pharmacokinetics and organ distribution of intravenous and oral methylene blue. Eur J Clin Pharmacol. 2000;56 (3):247–250. [DOI] [PubMed] [Google Scholar]
- 25. Moutos FT, Estes BT, Guilak F. Multifunctional hybrid three-dimensionally, woven scaffolds for cartilage tissue engineering. Macromol Biosci. 2010:10 (11):1355–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Laughlin SK, Hartmann KE, Baird DD. Postpartum factors and natural fibroid regression. Am J Obstet Gynecol. 2011;204(6):496.e1–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peddada SD, Laughlin SK, Miner K, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci USA. 2008;105 (50):19887–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Feng L, Aviles N, Leikin S, Leppert P. Pattern of collagen types in uterine fibroids. Reprod Sci. 2010;17:270A. [Google Scholar]
- 29. Rogers R, Norian J, Malik M, et al. Mechanical homeostasis is altered in uterine leiomyoma. Am J Obstet Gynecol. 2008;198(4):474.e1–e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hinz B, Gabbiani G. Fibrosis: recent advances in myofibroblast biology and new therapeutic perspectives. F1000 Biol Rep. 2010;2:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Walocha JA, Litwin JA, Miodoński AJ. Vascular system of intramural leiomyomata revealed by corrosion casting and scanning electron microscopy. Hum Reprod. 2003;18 (5):1088–1093. [DOI] [PubMed] [Google Scholar]