Abstract
Chemo- and radiation therapies used to treat cancer can have the unintended effect of making patients infertile. Clinically established fertility preservation methods, such as egg and embryo cryopreservation, are not applicable to all patients, which has motivated the development of strategies that involve ovarian tissue removal and cryopreservation before the first sterilizing treatment. To restore fertility at a later date, the early-stage follicles present in the tissue must be matured to produce functional oocytes, a process that is not possible using existing cell culture technologies. This review describes the application of tissue engineering principles to promote ovarian follicle maturation and produce mature oocytes through either in vitro culture or transplantation. The design principles for these engineered systems are presented, along with identification of emerging opportunities in reproductive biology.
Keywords: oncofertility, biomaterial, transplantation, oocyte, infertility, tissue engineering
1. INTRODUCTION
Strategies for facilitating fertility have revolutionized multiple aspects of medicine, a fact that was recognized with the awarding of the Nobel Prize in Medicine for 2010 to Dr. Robert Edwards “for the development of in vitro fertilization.” Since the birth of Louise Brown in 1978, the number of babies conceived by in vitro fertilization (IVF) has exceeded 5 million (1). Although IVF has enabled infertile couples to have or expand their families, IVF is not applicable to all cases of infertility (2, 3). Infertility can result from disorders such as polycystic ovary syndrome (PCOS) or premature ovarian failure (POF). The treatment of cancer with alkylating agents and platinum-based drugs is also associated with infertility, as these agents damage growing follicles and their enclosed oocytes (4, 5). Cancer treatments may also cause uterine problems requiring a gestational surrogate. Compared with that of their age-matched peers, the five-year relative survival rate of cancer patients is now 68%, an increase from 50% 30 years ago (6). At least 1 in 250 women of reproductive age are survivors of childhood cancer. Today, 90% of these young women will be cured of their cancer. The increased survival rates that have resulted from advances in chemo-and radiation therapies have shifted the discussion of cancer care to issues related to the quality of life after cancer, including the ability to have a family.
Most young women with cancer are highly interested in preserving their fertility so they might have children in the future (7). For many cancer patients, the decision to protect their fertility from the damaging effects of radiation and chemotherapy is complicated by their age, marital status, whether they can delay treatment, and sometimes, the uncertainty of survival. Young cancer patients, facing the devastating news that their college plans, early careers, or childhood playtime will be forever changed by the fight against this ruthless disease, are sometimes unaware that the life-preserving treatments they will undergo can also threaten their future fertility. Fertility preservation may be equally important for pediatric patients, who may not understand the full implications of cancer treatment for future parenthood and for whom the currently available technologies may not be applicable. Ten years ago, the phrase “families after cancer” for women would have been an oxymoron, if it were considered at all. Today, owing to the impressive increase in the number of cancer survivors, this phrase not only is well used but is also an issue that women increasingly want addressed.
For more than 20 years, males diagnosed with cancer have had the option of banking sperm to preserve fertility; however, the options for females have been slower to develop. At the time of a cancer diagnosis, plans for fertility preservation must consider the individual patient’s priorities in conjunction with the cancer treatment strategy. Several options are available to women with cancer who wish to preserve their germ lines. Patients may elect to delay cancer treatment (though this may not be an option for some patients with aggressive cancers) in order to undergo one cycle of hormone stimulation to induce follicle growth and ovulation, followed by cryopreservation of either a mature oocyte or an embryo (8, 9). Cryopreservation of mature oocytes is now considered standard of care, based on the most recent American Society for Reproductive Medicine (ASRM) guidelines (10). Embryo and oocyte cryopreservation dominate fertility interventions used today; however, the hormonal stimulation needed to harvest mature eggs for use in IVF or for oocyte banking is time consuming and may be contraindicated in women with hormone-responsive cancers. Moreover, breast cancer patients with the high-risk BRCA1 and BRCA2 mutations may have lower baseline fertility, resulting in fewer eggs retrieved after hormonal stimulation (11). Patients with less prognostically favorable, hormone receptor–negative tumors may be better candidates for fertility measures that involve exposure to elevated hormone levels; however, recent data also suggest that estrogens may have an indirect mitogenic effect on hormone receptor–negative cancers (12). Treatment with antiestrogens, including letrozole, has been used to minimize the short-term hormone effects on cancer growth during an induced ovulation cycle (13).
Investigational fertility preservation techniques that do not require hormonal stimulation are available and involve cryopreservation of ovarian tissue, rather than mature eggs or embryos. Ovarian tissue can be obtained at the time of diagnosis without additional hormonal stimulation, and this technique has the added benefit of only minimally interfering with the patient’s cancer treatment plan. Depending on the day of the menstrual cycle, oocytes may be aspirated from the ovary and then matured in vitro and cryopreserved for later use (14). In addition, individual follicles or strips of ovarian cortical tissue can be cryopreserved for future use in either in vitro follicle maturation or tissue transplantation. Thus far, 24 live births have been reported in women with cancer who underwent autologous transplantation of cryopreserved ovarian tissue (15, 16). In patients without cancer, orthotopic ovarian tissue transplantation between monozygotic twins has resulted in 7 live births (17).
Fertility preservation techniques hold promise for young cancer patients who want a family in the future; however, some of these technologies require more time than is available to a patient or are not feasible depending on a patient’s age or relationship status, among other variables (3). If one evaluates the continuum of technologies, one finds that likelihood of success is inversely related to the time the biological materials (oocytes, follicles, and ovarian tissues) are handled ex vivo and the reliance on freezing for storage of gametes (Figure 1). Standard of care, with the highest success rate, includes natural conception, IVF using intracytoplasmic sperm injection (ICSI) followed by embryo transfer, and natural cycle IVF/ICSI. IVF using cryopreserved eggs or in vitro–matured immature oocytes are also now standard of care, but these approaches have a lower live-birth rate than IVF using fresh tissues does. As we move toward the more investigational technologies, fertoprotection is an important emerging area of clinical intervention [using gonadotropin-releasing hormone (GnRH) analogs] and investigation (18, 19), and in vitro follicle growth and follicle transplantation are the focus of a great deal of preclinical research. Possible fertility preservation methods for the future include the development of inducible pluripotent stem cells (iPSC), production of stem cell–derived gametes, and use of germ-line stem cells isolated from the ovary (20, 21). The primary focus of this review is to describe how the application of biomaterials science to reproductive biology has led to development and advances in in vitro follicle growth and ovarian tissue transplantation, though emerging opportunities in fertility preservation are also addressed.
Figure 1.
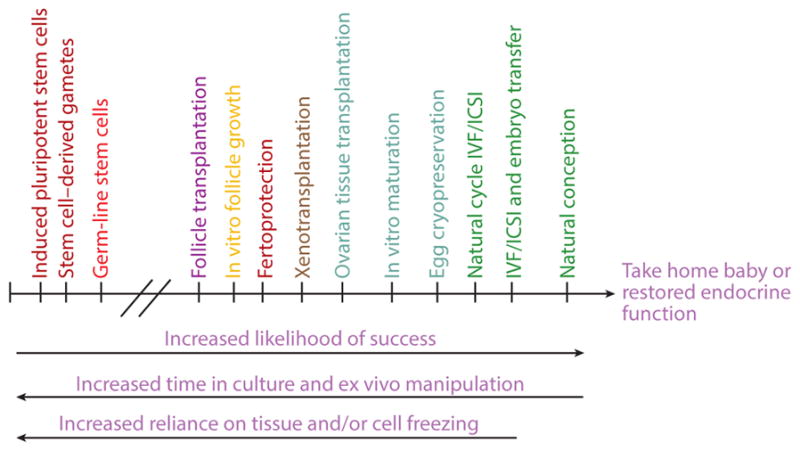
Continuum of fertility preservation options based on likelihood of success in terms of successful pregnancy or restoration of endocrine function. Success rates decrease for options that involve tissue or cell cryopreservation or require longer times in culture or ex vivo manipulation. Natural conception has the highest rate of success, followed by IVF and oocyte cryopreservation, whereas more investigational options such as ovarian tissue transplantation and in vitro follicle growth are less successful. Abbreviations: ICSI, intracytoplasmic sperm injection; IVF, in vitro fertilization.
2. BIOLOGY OF FOLLICLE DEVELOPMENT
The follicle is the functional unit of the ovary and is composed of a germ cell (the oocyte) and layers of somatic cells (granulosa and theca cells). At puberty, a human female will typically have on the order of 105 follicles in each ovary, whereas at menopause there are less than 1,000. Primordial follicles are the most immature class and are formed during embryonic (human) or immediately postembryonic (murine) life (22). The ovary contains numerous immature follicles—constituting the ovarian reserve—that have the potential to develop and produce mature oocytes capable of fertilization in a highly regulated process called folliculogenesis. The oocytes within primordial follicles are arrested in the first meiotic prophase. Follicle activation from the nonreplenishable ovarian reserve is a process that is not well understood. Nevertheless, selected primordial follicles are activated and grow to form primary follicles, which have larger oocytes and a layer of cuboidal granulosa cells enclosed by a basement membrane. Subsequently, the granulosa cells proliferate and form several layers around the oocyte. Theca cells begin to surround the basement membrane of the follicle. These more mature follicles are called secondary and multilayer follicles. Under the influence of follicle-stimulating hormone (FSH), the granulosa cells proliferate and differentiate into cumulus (surrounding the oocyte) and mural (inside the basement membrane) granulosa cells. Likewise, the theca cells differentiate into theca interna (androgen-secreting cells) and externa (connective and supportive tissue). The follicle continues to increase in size and develops a fluid-filled cavity called an antrum. The oocyte and the surrounding somatic cells must communicate throughout development and do so through transzonal projections (TZPs), which extend through the glycoprotein-rich zona pellucida and connect via gap junctions on the oocyte membrane. Under the influence of luteinizing hormone (LH), the oocytes in antral follicles complete the first meiotic cell division and then pause in the second meiotic metaphase (MII). These MII oocytes are capable of fertilization upon ovulation. Folliculogenesis within the ovary is highly regulated, with only a few follicles per cycle (or only one in humans) selected for maturation while other growing follicles undergo atresia.
Ovarian stromal cells may have significant roles in folliculogenesis, particularly in the development of early-stage (primordial and primary) follicles. The ovarian stroma contains connective tissue, neurons, blood vessels, and macrophages. Stromal cells, which are similar in morphology to fibroblasts (i.e., spindle shaped), compose the connective tissue throughout the ovary and surround follicles. The stroma expresses collagen, fibronectin, and laminin extracellular matrix (ECM) proteins. The morphology of the stromal cells varies between the cortex and medulla of the ovary. In the cortex, the stromal cells are organized parallel to the surface and have a rounded structure. In the medulla, the cells exhibit random organization and have an elongated structure. The stromal cells in the medulla have greater steroidogenic capacity than the stromal cells in the cortex, a fact that has supported the hypothesis that the stromal cells are recruited by the follicle and differentiate into theca cells. Stromal cells provide structural support to the follicle and have complex bidirectional paracrine signaling that influences folliculogenesis.
3. TECHNOLOGIES FOR IN VITRO FOLLICLE CULTURE
The major challenge for these technologies lies in the development of systems that support maturation for all follicle stages in an in vitro setting. The most mature technology, which is routinely practiced in IVF labs worldwide, involves the in vitro culture of fully mature (MII) oocytes and embryos. Following hormonal stimulation of oocyte maturation, retrieved MII oocytes are fertilized and cultured to support development of multicellular embryos for implantation. For fertility preservation applications, oocytes or embryos can be cryopreserved prior to initiation of cancer treatment. For a patient who delays cancer treatment to undergo a cycle of hormonal stimulation, the retrieved oocytes represent her future reproductive potential using these traditional IVF techniques.
Technologies to culture follicles rather than mature oocytes or embryos may enable the development of alternative fertility preservation strategies for those patients who either cannot delay cancer treatment or are unable to undergo hormonal stimulation (e.g., prepubertal patients). Pioneering work with the culture of ovarian tissue and ovarian follicles demonstrated the potential to support folliculogenesis in vitro. The first successful attempt to grow ovarian tissue in vitro was performed by Martinovitch in 1937 (23). Whole mouse and rat ovaries were cultured on watch glasses in a primitive culture medium, and growth and differentiation of germ cells were observed in all cultures.
Following this initial work, techniques were developed to culture individual follicles, with the goal of recapitulating each stage of follicle development in vitro. Isolating follicles for in vitro culture removes ovarian regulation of follicle growth, and various culture environments have been tested to enable growth of isolated follicles. In vitro culture of individual follicles was first demonstrated by Eppig in 1977 (24). Initial systems were based on group cultures of secondary follicles (initial diameter of ~120 μm) on polystyrene, though adaptations have since been made for the culture of individual follicles in wells. In such systems, isolated follicles are placed on a two-dimensional surface of either tissue culture plastic or surfaces coated with molecules such as collagen or polylysine. The follicles attach to the surface, and the proliferating granulosa cells break through the basement membrane and migrate onto the culture surface, resulting in a diffuse morphology (25, 26). These studies determined that only oocytes that remained within the follicle structure underwent significant growth, demonstrating that oocytes must remain in contact with granulosa cells to grow and mature. A fraction of the oocytes retrieved at the end of culture could be fertilized, with implantation of the embryos leading to live births (25). A number of culture systems have evolved from this initial system (27, 28), some of which are detailed below.
These two-dimensional culture systems applied to isolated secondary follicles have contributed to our ability to analyze the biology of individual follicles (26), raised the possibility of personalized follicle culture, and become a key technology enabling the study of reproductive toxicology (29). Yet although these systems have proven successful in producing high-quality oocytes and live (murine) offspring from secondary follicles cultured in vitro (30, 31), they have not been as successful for the culture of early-stage (primordial and primary) follicles or follicles from large animal species [e.g., bovine (32), ovine (33), and human (34)]. This limitation is likely due to the fact that earlier-stage follicles demonstrate a greater propensity than secondary follicles to lose the critical physical association between the oocyte and granulosa cells. The inability to culture primordial and primary follicles has limited the investigation of early follicle biology and factors that promote and/or restrict the initial steps in folliculogenesis. For large animal species, maintenance of the oocyte–somatic cell interactions is particularly difficult because the follicles reach a much larger size and require a longer time in culture (35). In our hands, human follicles rapidly lose the connections between oocyte and somatic cells after a few days in culture on either a transwell or an adhesive substrate (M. Xu, unpublished observations; see Figure 2a). The development of culture systems that maintain follicle architecture (Figure 2b), thereby retaining the cell–cell and cell–matrix interactions that are critical in follicle and oocyte development (36), would not only permit studies on the earliest stages of follicle development but also enable development of new technological approaches to fertility preservation.
Figure 2.
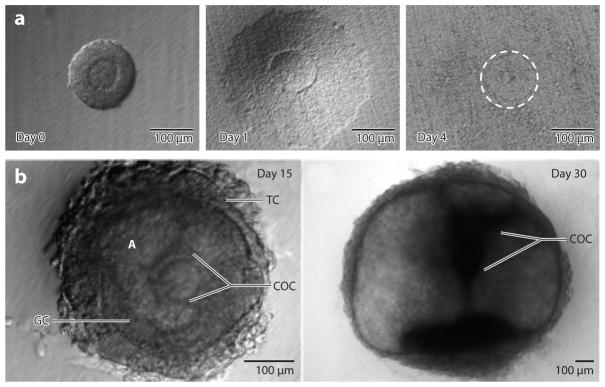
Human follicles cultured on tissue culture polystyrene and within alginate hydrogels. (a) Human secondary follicles cultured on polystyrene lose their normal follicular architecture after a few days in culture, and the follicles are nonviable. (b) Culture of human follicles in alginate hydrogels retains the architecture, and follicles increase in size and develop morphological features (e.g., antrum) consistent with in vivo follicle growth. Abbreviations: A, antrum; COC, cumulus oocyte complex; GC, granulosa cell; TC, theca cell.
3.1. Designer Environments for Follicle Culture
Our laboratories have been developing designer microenvironments that maintain the architecture of cultured follicles and deliver factors to promote follicle growth. In primary follicles cultured on flat surfaces, physical manipulation has been used to prevent cell attachment and migration of somatic cells in an attempt to maintain follicle structure (37). However, we have focused on the design of three-dimensional culture systems that use hydrogels to encapsulate follicles, providing physical support that preserves oocyte–somatic cell connections and promotes survival of early-stage follicles, without the need for manipulation during culture. Several design criteria for these three-dimensional hydrogel-based follicle culture systems have been identified. First, the encapsulation and culture conditions must be mild, as years of experience with IVF have established that oocyte quality is significantly impacted by the culture medium and conditions. Second, the growth of the follicle within the hydrogel must maintain the cell–cell connectivity and overall architecture of the follicle, yet enable the follicle to expand. Murine secondary follicles with an initial diameter of 120 μm will grow to a final diameter of approximately 400 μm, which represents an approximate 37-fold increase in volume. Human secondary follicles, with an initial diameter of 120 μm, undergo a 4.7 × 106–fold increase in volume to their final diameter of 20 mm. The mechanical properties of the hydrogel and/or matrix degradation must accommodate this exponential increase in size during culture. Third, the follicle and the oocyte must be easily retrieved from the hydrogel upon termination of culture in order to study biological endpoints or for use of the recovered oocyte for IVF. Finally, the culture system environment must be easily modulated to contain biological signals such as growth factors, hormones, or ECM peptides or proteins.
A guiding principle in the design of these three-dimensional culture environments is the accurate presentation of the various signals (growth factors, hormones, ECM, mechanics, etc.) that will allow for coordinated growth of the multiple cellular compartments of the follicle—the oocyte, granulosa cells, and theca cells—in vitro. The ovarian environment is dynamic, governed by cyclic changes in endocrine factors in the systemic circulation and local signals produced by the ovary. To mimic the ovarian environment in vitro, these factors must be added to the cell culture medium at the appropriate times and concentrations and transported to the follicle by diffusion through the hydrogel to recapitulate the changes that occur during folliculogenesis. The signals presented to the follicle must promote the growth of the somatic cells in conjunction with, and not at the expense of, the oocyte. The hydrogel interacts with the follicle based on its mechanical properties (38), and adhesive proteins or peptides incorporated into the matrix can also influence the function of the somatic cells on the follicle exterior (38, 39). Importantly, the hydrogel matrix and the formation of the multicellular structure of the growing follicle can impose limitations on the transport of hormones and factors to the follicle.
In vitro follicle culture has become a powerful bioassay (40, 41) to investigate the mechanisms by which factors (or environmental toxins) present in the systemic circulation impact follicle growth and development. Both two-dimensional and three-dimensional culture systems are being employed to identify signals that maximally promote follicle growth and oocyte quality, as well as to investigate how disruption of this environment—from exposure to environmental toxins, disease, or disease therapies—may underlie infertility.
3.2. Alginate Hydrogel Culture Systems
We have pioneered the use of alginate as a hydrogel for the in vitro culture of ovarian follicles (42). Alginate is a linear polysaccharide derived from algae and composed of repeating units of β-D-mannuronic acid and α-L-guluronic acid (43) that gels by ionic cross-linking of the guluronic residues (44). This mild gelation process has led to the use of alginate in a number of applications (45). The hydrogel can also be dissolved through the addition of calcium chelators (46); however, these agents may affect the follicle gap junctions required for oocyte–somatic cell communication. Alternatively, the alginate hydrogel can be degraded with alginate lyase, a bacterially derived enzyme that has had no observable effects on the follicle. Alginate lyase has been employed to retrieve intact follicles from the matrix for subsequent in vitro maturation of the oocyte. Granulosa and theca cells do not have adhesion receptors for alginate and thus do not interact with the hydrogel; however, ECM peptides or proteins can be immobilized to or entrapped within the hydrogel matrix to interact with the follicle (39). Small molecules with molecular weights less than 20 kDa diffuse through alginate gels with the same diffusivity as in water (47). Larger molecules diffuse through the alginate matrix pores, which have diameters ranging from 5 nm to 200 nm, in a molecular weight–dependent manner (44). The high porosity of the alginate gels allows FSH added to the culture medium to diffuse into the matrix within 15 min and reach a steady level within 4 h (P. Kreeger, L.D. Shea & T.K. Woodruff, unpublished observations). The alginate matrix also helps to maintain paracrine signaling by factors produced by the follicle and can be modified to mimic the ovarian stroma (39).
Follicles cultured within alginate maintain both an in vivo–like morphology, with a centrally located oocyte and surrounding layers of granulosa and theca cells, and cell–cell connections between the somatic cells and also between the granulosa cells and oocyte (42, 48). Two-layered secondary follicles cultured in alginate–collagen I gels are FSH responsive and show follicle growth and lactate production, whereas growth of multilayered secondary follicles cultured in alginate matrices is FSH dependent (49); this phenocopies the in vivo acquisition of gonadotropin responsiveness during folliculogenesis. Follicle growth and hormone secretion patterns of secondary follicles cultured in vitro in the alginate system mimic those of follicles in vivo (50). Oocytes retrieved following the culture of secondary follicles in alginate hydrogels develop the capacity for fertilization similar to that of in vivo–matured oocytes. Embryos derived from cultured oocytes fertilized in vitro and transferred to pseudopregnant female mice are viable, and both male and female offspring are fertile (51). Viability rates are significantly greater than those reported in other culture systems.
The physical properties of the alginate hydrogel determine whether the environment is permissive for follicle growth and development (52, 53). These properties include factors such as mesh size, which determines hormone and growth factor transport from the culture medium through the hydrogel, and mechanical properties, which cells translate to a biochemical signal in a process known as mechanotransduction. We varied the concentration of alginate, which influences both transport and mechanical properties, and then determined the impact on follicle growth in vitro (52). A 0.25% alginate hydrogel, which creates relatively soft beads, was more permissive for follicle growth than the other concentrations tested (0.5%, 1%, and 1.5%). The 0.25% alginate hydrogel improved growth, increased steroidogenesis (estradiol and androstenedione), and produced a greater yield of MII oocytes.
The contribution of the mechanical properties and transport capacity of the alginate hydrogel to follicle growth was further investigated through chemical modification of the alginate. Irradiation or oxidation of alginate breaks individual polymer chains to reduce the average molecular weight, which in turn decreases the rigidity of the cross-linked hydrogel at a fixed-solids concentration (53). For hydrogels with a fixed-solids content, decreasing the elastic modulus led to improved follicle growth. Furthermore, for hydrogels with a similar elastic modulus, follicles encapsulated in materials with low solids content had higher rates of antrum formation than did follicles encapsulated in materials with high solids content. Alginate hydrogels formed with a lower solids content have a larger pore size, suggesting that transport of diffusible factors is significant in follicle culture even at low concentrations of alginate. Changing the physical properties of the alginate hydrogel influenced other aspects of follicle growth. Under certain conditions, follicle growth in vitro was similar to that seen in PCOS; these findings are discussed below in the models of infertility.
3.3. Cell-Responsive Culture Systems
We have also investigated degradable matrices applied to follicle culture that allow for matrix remodeling in response to follicle growth. As a follicle grows in a three-dimensional environment in vitro, its diameter increases, and an encapsulating hydrogel such as alginate, which is not readily degradable, will exert a compressive force on the follicle in response to the expansion. The compressive force is dependent upon the elastic strength of the hydrogel, as well as the change in size of the follicle. The volume of the hydrogel that is displaced by the developing follicle increases as r3, where r is the radius of the follicle, but the surface area that is acted on by the compressive force increases only as r2. Though nondegradable alginate hydrogel systems support the culture of mouse secondary follicles, translation of these systems to either earlier-stage follicles or follicles of large animal species may be challenging owing to the significantly greater volumetric increase that must occur during culture. The stress profile in a human follicle may significantly differ from that in a murine follicle and may partially contribute to the challenge of culturing human follicles in vitro.
Naturally derived polymers such as collagen and fibrin have been used extensively in regenerative medicine applications and can degrade in response to growing follicles to allow for expansion of the follicle, yet the success of these materials for in vitro follicle culture has been modest. Collagen was one of the first biomaterials used for three-dimensional in vitro follicle culture (54). Follicles cultured in collagen gels survived in vitro for 2 weeks and multilayered follicles were formed, but they were unable to proceed to the antral stage (54). More recently, however, buffalo preantral follicles encapsulated in collagen were shown to develop an antrum (55). Fibrin has also been employed for follicle culture; this protein is responsible for blood clotting and is formed via enzymatic cross-linking. Follicles encapsulated and cultured in fibrin hydrogels secreted enzymes that rapidly degraded the matrix, and the integrity of the follicle architecture was lost once the follicle fell from the degraded gel onto the culture surface (56). Thus, fibrin alone cannot support three-dimensional in vitro culture of follicles.
A hydrogel consisting of degradable and nondegradable components was developed based on a combination of fibrin and alginate to create a fibrin–alginate interpenetrating network (FA-IPN) (56, 57). An interpenetrating network (IPN) is a combination of polymers in network form, in which at least one polymer is synthesized and/or cross-linked in the presence of the other, either simultaneously or sequentially (58). Chains of the individual polymers are completely entangled, and there may or may not be chemical bonds between the combined networks. The overall IPN structure behavior reflects the characteristics of each individual polymer (59). In the FA-IPN hydrogel, the fibrin component is bioactive and can be degraded by the follicle to create space for outward expansion, whereas the alginate component provides long-term stability to maintain the follicle architecture. Also, the alginate concentration is significantly reduced relative to the alginate-only hydrogels described earlier (56). Importantly, the FA-IPN material has dynamic mechanical properties consistent with the needs of the growing follicle. Initially, the combination of fibrin and alginate is relatively rigid, which supports follicular structure. As the follicle grows, the fibrin component is gradually degraded, thereby decreasing the elastic modulus and providing less resistance to follicle expansion. Fibrin and alginate can be gelled simultaneously under mild conditions to facilitate cell encapsulation, and the initial mechanics of the hydrogel can be modified depending on the properties of each component. The FA-IPNs promoted follicle growth and increased the number of meiotically competent oocytes relative to follicles cultured in either fibrin or alginate alone (56).
Encapsulating matrices were subsequently developed that provided even greater control over the degradation rate of the hydrogel, allowing it to be matched more closely to the growth rate of the follicle. Naturally occurring biomaterials, such as collagen and fibrin, have the advantage of intrinsic biological activity; however, these materials are difficult to modify for desired physical properties, such as degradation. Hydrogels were developed that were based on peptide cross-linking of a nondegradable polymer with peptide cross-linkers, and the peptide sequences could be tuned to modulate the degradation rate of the hydrogel. Poly(ethylene glycol) (PEG) was modified with vinyl sulfone groups to allow cross-linking through peptides that contain multiple cysteine residues. Initial studies of follicle culture with four-arm PEG and bifunctional peptides, which have been successful for the culture of many cell types (60), were unsuccessful for the culture of ovarian follicles. The bifunctional peptides required extended gelation times that led to dehydration of the oocyte, and the buffer conditions were harmful to the follicles. A trifunctional peptide, which avoids elastically inactive loops and minimizes defects in the network, reduced gelation time to prevent oocyte dehydration and enabled the use of more cell-compatible buffer conditions (61). Tunable degradation of the plasmin-sensitive PEG gels was achieved using peptide sequences with varying plasmin sensitivity. Sequences that were highly sensitive to plasmin (YKNR) resulted in loss of the follicle from the gel. Conversely, sequences that were relatively insensitive to plasmin (YKND) did not allow follicle growth. However, peptide sequences with moderate sensitivity (YKNS) limited hydrogel degradation to the area immediately around the follicles, forming a soft pocket inside an otherwise rigid matrix, suggesting that the cell-mediated proteolysis was localized to the follicle–hydrogel interface. Tuning the peptide sequences to achieve the appropriate degradation rate of the hydrogel allowed for the coordinated growth of the multiple cellular compartments of the encapsulated follicle.
4. FOLLICLE CULTURE SYSTEM APPLICATIONS
4.1. Early-Stage Follicles
Fertility preservation approaches based on the use of cryopreserved ovarian tissue rely on the ability to culture the small, early-stage follicles that are enriched in the ovarian cortex. The culture of early-stage follicles has been an underlying motivation for the application of biomaterials to ovarian follicle culture. Follicle culture systems with flat surfaces have supported the growth of mouse primary follicles (80–90 μm) (37) by using repeated physical manipulations to prevent cell attachment and migration away from the oocyte. Alginate-based hydrogels have been successful in maintaining the follicle architecture and survival of early-stage follicles; however, the follicles did not readily grow in vitro. The growth of primordial and primary follicles in vitro has been successful using in situ organ culture (25, 62). In this approach, ovarian stromal cells provide structural support and participate in bidirectional paracrine signaling with the primordial follicles (63), leading to theca cell differentiation and primary follicle development. Secondary follicles can be isolated from the organ culture and cultured individually to produce mature oocytes. Although organ culture has been successful in supporting the growth of early-stage follicles, these systems do not lend themselves to controlled studies to investigate the biology of individual follicles in culture.
Cocultures of early-stage follicles with stromal cells or granulosa cells, which act as feeder cells, have been employed to provide the factors to promote follicle growth. Coculture studies with follicles and surfaces employing stromal and granulosa cells demonstrated enhanced follicle survival and growth (64). Theca–interstitial cells (TICs) were isolated from the ovary and plated on a tissue culture surface, and primary follicles (90 μm) entrapped within alginate hydrogels were subsequently added to the culture. The presence of the TICs improved the survival and growth of follicles to approximately 240 μm in diameter (65). A challenge presented by the TIC coculture is that the cells represent the relatively heterogeneous cell population that may change during culture. Mouse embryonic fibroblasts (MEFs), which have been used for the coculture of stem cells (66), have been used in a manner similar to the TIC cocultures while providing a more uniform and consistent cell population (67). Follicles experience increased growth, granulosa cell proliferation, and survival when cultured in the presence of MEFs. MEFs enabled the culture of early secondary and primary mouse ovarian follicles within alginate hydrogels. Primary follicles with an initial diameter of 70 μm were cultured to the antral stage and matured to produce MII eggs. Although the mechanisms remain unclear, the MEFs secreted paracrine factors that stimulated mouse follicle growth, allowing the follicle to survive, transition to the secondary stage, and produce oocytes responsive to in vitro maturation.
The development of a feeder-free culture medium for early-stage follicles has begun based on the recent development of stem cell media, which have the potential to accelerate basic science discovery and clinical application of in vitro follicle culture for fertility preservation. Although the feeder cell populations are effective in enabling the survival, growth, and development of early stage follicles, which can produce mature eggs, these coculture methods promote growth by undefined mechanisms and have batch-to-batch variability, and clinical translation would risk transmission of pathogens. Studies employing embryonic stem cell (ESC) media formulations (mTeSR1 and E8), which contain factors such as FGF2, TGF-β1, and ascorbic acid that are associated with ovarian follicle development, were successful in supporting the culture of early-stage follicles in the absence of feeder cells (67). The methodical removal of factors from the stem cell medium ultimately revealed that the formulation that maximized survival and growth of early-stage follicles (80 μm) in alginate hydrogels was αMEM/F12 supplemented with fetuin, insulin, transferrin, selenium, and FSH. Although fetuin remains an incompletely defined component, this formulation provides a foundation for more systematic analysis of factors that promote the growth of early-stage follicles in vitro.
4.2. Follicles from Large Animal Species
The ability to culture follicles from large animal species will open tremendous opportunities that extend beyond fertility preservation for women and girls to include preservation of endangered species (e.g., cats and pandas) and valuable livestock (e.g., bovine, sheep, and horses). However, the follicle culture systems that have worked in mice have not translated well to cow, goat, sheep, pig, cat, buffalo, or human follicle growth and development (68–70). In vitro cultures of follicles from large animal species require longer times in culture and expand to greater diameters during folliculogenesis. For culture on transwells or adhesive surfaces, follicles attach to the surface and the proliferating granulosa cells break through the basement membrane and migrate onto the culture surface, resulting in a diffuse morphology. Maintaining the associations between the oocyte and granulosa cells has been particularly challenging in follicles from large animal species. This disruption of follicle architecture, resulting from disruption of cell–cell and cell–matrix interactions that play a role in follicle and oocyte development, must be overcome to create an effective in vitro culture system.
Alginate-based encapsulation has enabled the in vitro culture of follicles from several species, including dogs, macaques, baboons, and humans. The alginate hydrogel culture system sustained growth and steroidogenesis of pre- and early-antral dog follicles for 10 days (71). Further development of this culture system may contribute to the conservation of a growing list of wild canid species threatened in nature. Baboon preantral follicles were encapsulated in fibrin-alginate-matrigel matrices and cultured to the small antral stage in an FSH-independent manner (72). FSH negatively impacted follicle health by disrupting the integrity of the contacts between the oocyte and cumulus cells, whereas follicles grown in the absence of FSH produced MII oocytes with normal spindle structure. Baboon luteal-phase cumulus–oocyte complexes (COCs) and oocytes from cultured preantral follicles could be matured in vitro, and oocyte meiotic competence correlated positively with the number of cumulus cell layers. For rhesus macaque secondary follicles, alginate hydrogel cultures maintained the three-dimensional structure, permitted follicle growth, and supported steroidogenesis (73). Interestingly, secondary follicles cultured in alginate alone produced an MII oocyte, which cleaved and reached the morula stage after in vitro fertilization (32). In the same study, a single macaque primary follicle cultured in fibrin-alginate yielded an MII oocyte that arrested without cell division after fertilization (74). Human ovarian follicles cultured in alginate hydrogels became steroidogenically active and developed from the early secondary to antral stage in vitro (Figure 2b; 75). The follicles contained healthy, growing oocytes that were connected by transzonal projections between the somatic cells and oocyte (73). The performance of in vitro follicle culture systems varies significantly by species. For example, nonhuman primate primary follicles do not require coculture (32), whereas mouse primary follicles responded best when additional factors were present.
4.3. Infertility Models
In vitro follicle culture provides a tool for new discoveries regarding the physiology and mechanisms governing follicle development and oocyte maturation; however, these systems are also being employed to model various disease states to identify the deregulated processes and the mechanisms by which these processes influence normal follicle growth.
4.3.1. Polycystic ovary syndrome
PCOS is a complex disease that includes endocrine, reproductive, and metabolic features. Diagnostic criteria include hyperandrogenism (biochemical and/or clinical), chronic oligo- or anovulation, and polycystic ovaries (76). With a prevalence of 4–10% (77, 78), it is the most common endocrinopathy among reproductive-age women and a leading cause of anovulatory infertility. PCOS is frequently associated with metabolic abnormalities including insulin resistance. As such, it is also associated with significant sequelae such as type 2 diabetes, endometrial hyperplasia/carcinoma, and cardiovascular disease (76). PCOS is likely a multifactorial disorder and its etiology remains unclear.
The culture of follicles in alginate hydrogels of varying physical properties impacts follicular growth and hormone production (38, 50, 52). Interestingly, follicles grown in more rigid conditions synthesize a greater ratio of androgen to estradiol, mimicking the principal endocrinological defect in PCOS (38, 53). Additionally, immature follicles cultured in a more dense, rigid three-dimensional environment were less able to proceed through folliculogenesis to the antral stage (52, 53), suggesting that more dense, rigid hydrogels phenocopy the stroma-rich PCOS ovary and that the physical state of the ovary may play an important role in PCOS pathogenesis (79). Disruption of the physical environment in tissues has been associated with several disease phenotypes (e.g., arthrosclerosis); however, this hypothesis was novel within the context of PCOS. A pathway candidate approach was applied to identify a candidate list of mechanosensitive genes in a microarray performed on mRNA from murine follicles grown in two hydrogel concentrations mimicking normal and PCOS stromal properties. Starting from this list, a single-nucleotide polymorphism (SNP) analysis of samples from PCOS and control cohorts identified SNPs in 13 mechanosensitive genes associated with PCOS. Thus, the analysis of in vitro cultured murine follicles enabled the discovery of mechanosensitive pathways in PCOS.
4.3.2. Obesity
Obesity impacts at least 30% of reproductive-age women (80) and is associated with an increased risk of early loss of pregnancies achieved from either natural conception or assisted reproductive technologies (ART) (80). Excluding PCOS patients and correcting for anovulation, studies indicate that higher body mass index (BMI) is associated with worse IVF pregnancy outcomes (81, 82). The mechanisms by which obesity impacts pregnancy outcomes are unclear; it may negatively affect oocyte and embryo quality or uterine implantation (83). In parallel to findings in the human, diet-induced obese mouse studies have shown a wide range of negative reproductive phenotypes. Obesity has been linked to an increase in free oxygen radicals that may negatively impact oocyte health (84). Diet-induced obese mice have greater numbers of apoptotic follicles, impaired oocyte developmental competence, and smaller and fewer eggs compared with lean control animals (83). In addition, poor reproductive outcomes have been observed, as diet-induced obese mice produce zygotes with compromised mitochondrial metabolism, smaller fetuses, and smaller pups with a predisposition for metabolic abnormalities (82).
Follicles isolated from obese mice and cultured in alginate hydrogels in vitro were of distinctly poorer quality compared with those isolated from leaner control animals (85). Fewer follicles were isolated from obese mice compared with controls, and of those follicles that were isolated, fewer survived in culture. Interestingly, follicles from obese and lean mice had similar growth trajectories; however, follicles from obese mice secreted larger amounts of androgen and estrogen with a significant shift to androgen relative to estrogen compared with those of controls. This high androgen environment may negatively impact oocyte quality (86). Oocytes from follicles isolated from obese animals differed in their ability to resume meiosis relative to those from controls. In addition, the meiotic spindle length in MII-arrested eggs derived from obese follicles was larger compared with that of eggs from controls. Taken together, the altered global metabolic and hormonal environment of the animal may translate to poor follicle function and poor gamete quality when follicles from obese animals are used for in vitro follicle growth.
4.3.3. Aging
Fertility and fecundity sharply decrease in women 35 years of age and older (84). The capacity of ART to overcome the maternal age factor is limited in patients older than 40 years (87); even in cases of successful conception with ART, there is an increase in spontaneous miscarriage among older women (84, 88). The observed age-related loss of fertility has been largely attributed to decreased oocyte quality and increased incidence of aneuploidy (88). This phenomenon has also been shown to occur in mouse models where the mechanisms of aneuploidy are currently being investigated (89–91). Relative to those of young mice, follicles from old mice cultured in alginate hydrogels secreted abnormal levels of hormones, contained oocytes with decreased meiotic competence, and ultimately produced MII-arrested eggs with chromosome alignment defects (85). Interestingly, the spindle length in eggs derived from old follicles was shorter compared with that in eggs from controls, which contrasted with the observations in obese mice. These findings suggest that the mechanisms that reduce follicle quality may be different between aged and obese animals. For instance, prolonged meiotic arrest and deterioration of oocyte factors may contribute to the age-associated decline in oocyte and egg quality (88, 92).
The impact of age on follicle growth was also modeled using alginate hydrogels for the culture of multiple follicles. As females age, the follicle number and quality steadily decline; thus follicle density within the ovary would be expected to differ between young and old females. Isolated primary ovarian follicles survived and grew when cultured in the presence of other follicles in a size-and number-dependent manner. Follicles grown in groups had more transzonal projections than those grown individually. This increase in cellular connections likely prevented the dissociation of the oocyte and granulosa cells, a phenotype commonly observed when primary follicles are grown individually in vitro. Follicles grown together secrete a factor or combination of factors that, through paracrine signaling, promotes the growth and survival of neighboring follicles (93). Thus, in the in vitro system, we observe that follicle quality—based on the ability to survive, grow, and produce mature gametes—is diminished with decreasing follicle number, potentially mimicking what occurs in the ovary during natural aging.
5. TECHNOLOGIES FOR OVARIAN TISSUE OR FOLLICLE TRANSPLANTATION
Investigational options for fertility preservation revolve around the use of ovarian tissue that has been removed and cryopreserved prior to the initiation of cancer treatment. Individual follicles may be isolated from the cryopreserved ovarian tissue and cultured in vitro in the three-dimensional systems described above to produce MII oocytes for in vitro maturation and IVF. Alternatively, the thawed tissue may be transplanted back into the patient, where the follicles will develop in vivo (either with resumption of natural cycles or with hormonal stimulation) to produce mature oocytes capable of fertilization. Successful fresh human ovary transplantation was first reported between monozygotic twins discordant for premature ovarian failure (POF) using a cortical grafting technique (94). Normal menstrual cycles resumed after 4 months, and spontaneous pregnancy leading to a healthy child occurred 1 month later. Transplantation of cryopreserved ovarian tissue has since resulted in at least 24 live births (16, 95). This number of live births demonstrates the feasibility of the approach, but it also indicates that advances are needed to make the procedure more widespread.
The ovary at any age contains mainly primordial follicles (>70%), the most immature stage of follicle development, along with smaller subsets of follicles at multiple stages of development (primary, preantral, and antral). Primordial follicles have the greatest potential to survive cryo-preservation and transplantation because of their small size (30 μm) relative to follicles that have been activated into the growing pool (80–500 μm in mice). The ovarian tissue cryopreservation process preserves a large number of immature follicles, compared with the small number of mature oocytes that are recovered from hormonal stimulation in traditional IVF cycles. Harnessing the potential of these earliest-stage follicles to develop and produce mature eggs may provide greater opportunities for fertility preservation. Also, transplantation of tissue with a large number of immature follicles could restore both endocrine function and produce a large source of follicles for recruitment into the growing pool over multiple cycles. The cryopreservation and autotrans-plantation of ovarian tissue is a powerful approach for preserving fertility for patients who are at risk of losing ovarian function, either owing to diseases such as PCOS or autoimmune disorders or as a consequence of exposure to radiation or chemotherapy.
The most common approach to ovarian tissue transplantation involves cortical grafting rather than whole-ovary microsurgical transplantation. Whole-ovary microsurgical transplantation may provide the opportunity to avoid or significantly limit ischemic damage, but this approach is more complicated owing to the challenge of cryopreserving an intact ovary (96). For cortical grafting, cryopreservation systems based on vitrification of ovarian pieces have been highly successful at preserving multiple follicle classes (97, 98). However, transplantation of cortical grafts has had only sporadic reported success, with the primary obstacle attributed to the survival and engraftment of transplanted tissue, which is particularly critical for patients with a low follicular count in the graft. The combination of biomaterial and drug delivery technologies is aimed at enhancing integration of the transplanted cortical tissue with the host by providing factors during the days and weeks after transplantation, the time frame associated with the greatest risk of graft loss. Shikanov et al. (99) demonstrated the efficiency of this approach in the orthotopic mouse model of infertility (Figure 3a). The ovarian cortical tissue was vitrified, thawed, encapsulated in fibrin hydrogels loaded with vascular endothelial growth factor (VEGF), and placed in the bursal sac. The combination of fibrin to improve implant–host connectivity and the delivery of angiogenic VEGF resulted in a greater degree of graft revascularization (Figure 3b), facilitated the engraftment of early-stage follicles (Figure 3c), and enabled earlier onset of pregnancy compared with controls.
Figure 3.
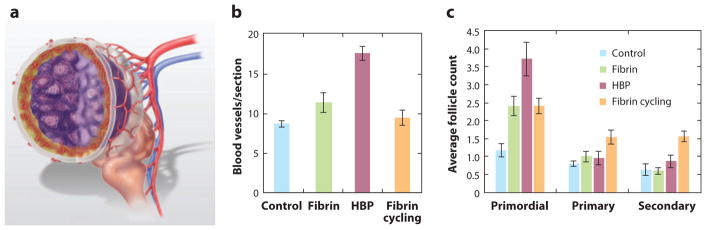
Ovarian tissue transplantation. (a) Schematic of ovarian tissue transplant into the bursa, in which the biomaterial surrounds the ovarian tissue. In the mouse, the ovary resides within the bursa. For transplantation, the bursa was opened, the ovary removed, and the biomaterial encapsulated ovarian tissue graft inserted. The biomaterial supports cell infiltration and thus promotes integration of the graft with the host. Localized delivery of angiogenic factors promotes revascularization of the transplanted tissue, with vessel growth primarily from the bursa. Quantification of (b) blood vessels and (c) follicle numbers within encapsulated ovarian tissue transplanted into noncycling mice with no material (control), fibrin gel alone, or HBP (fibrin gel modified with heparin-binding peptides that deliver angiogenic factors), or within fibrin-encapsulated ovarian tissue transplanted into cycling mice (fibrin cycling).
A critical concern with using ovarian tissue transplantation to restore fertility in cancer survivors is whether cancer cells may be present within the cryopreserved ovarian tissue and inadvertently reintroduced to the patient upon transplantation (100–102). Hodgkin lymphoma is considered to be the safest setting for transplanting ovarian tissue back to the patient (103). However, leukemia survivors may have cancer cells within the vasculature of the ovary. Non-Hodgkin lymphomas and colon, melanoma, pancreas, gastric, and breast cancers can metastasize to the ovary to form what are called Krukenberg tumors (100). For these patients, the isolation and transplantation of individual ovarian follicles, rather than intact cortical tissue, has the potential to significantly reduce or eliminate the risk of reseeding disease. The transplantation of individual follicles has not been widely reported in preclinical models, but the potential of the approach has been demonstrated (104). Gosden (105) pioneered this approach by transplanting enzymatically disintegrated infant mouse ovaries to the ovarian bursal sac of sterile adults. This experiment resulted in a live birth, though the litter size was small and the graft longevity was relatively short. A similar approach was investigated by encapsulating approximately 400 freshly isolated primordial follicles in a fibrin clot (105a). The transplanted follicles restored ovarian function in an infertile mouse, which was demonstrated by a substantial decline in the serum FSH levels and morphologically distinguishable ovarian tissue. In addition to avoiding risk of seeding cancer cells, the transplantation of individually isolated follicles delivers a known number and density of follicles, and standardization of this procedure may improve transplant outcomes. However, this approach is challenging owing to the initial lack of stromal support in the graft and requires a smart design of the newly constructed ovarian matrix. Ultimately, the delivery of isolated purified follicles in an ovarian-specific matrix has the potential to overcome the currently low and inconsistent follicular counts and eliminate the risk of reseeding cancer.
6. EMERGING OPPORTUNITIES
6.1. Artificial Ovary
Deconstructing ovarian tissue into individual components, such as follicles, granulosa cells, and theca cells, with subsequent reconstruction into an artificial ovary can help uncover the contribution of each component to overall ovarian function and may also be useful clinically to restore one or more aspects of ovarian function. However, the presence of multiple differentiated cell types within each follicle, the need for cyclic exposure to endocrine hormones and local factors, and the presence of follicles at different developmental stages make engineering a functional artificial ovary a formidable challenge. Several approaches to develop modular ovarian components are under way. Theca and granulosa cells encapsulated in multilayer alginate capsules respond to gonadotropins and secrete sex hormones, which is similar to what occurs in an intact follicle (106). This multilayered engineered ovarian tissue could be applied to treat the loss of ovarian function by providing physiological serum levels of hormones in response to gonadotropins. Another method has been proposed for self-assembly of three-dimensional microtissues from granulosa and theca cells isolated from adult human ovaries. The cells are seeded into the recesses of micromolded agarose gels (107) and more closely recreate the three-dimensional interaction between the granulosa cells, theca cells, and oocyte that is critical to follicular maturation. These artificial ovaries could be used to mature early antral oocytes, and serve as tools for testing follicular physiology and toxicology in preclinical drug development. The integration of multiple cellular, molecular, and physicochemical properties within a structural design will result in powerful models to investigate fundamental questions about primordial follicle assembly or activation, follicle–stroma cell interactions, and ovarian tissue regeneration.
6.2. Transplantation of Oogonial Stem Cells
Newly discovered oogonial stem cells (OSC) and iPSC represent possible sources of ovarian follicles for fertility preservation and may be an alternative to the removal and cryopreservation of ovarian tissue. Although the number of OSC in the ovary is low, representing ~0.014% of the total ovarian population, once isolated the cells stably proliferate in vitro for months and spontaneously generate what seem to be immature oocytes, as determined by morphology, gene expression, and haploid status (20). After intraovarian transplantation, mouse OSC generated functional eggs, which were fertilized and resulted in two-cell embryos that progressed to a hatching blastocyst stage. Alternatively, Hayashi et al. (21) demonstrated that mouse ESC and iPSC could be reprogrammed to primordial germ cell–like cells that can be developed to produce fertile offspring after in vitro maturation and fertilization. Female (XX)-derived ESC bearing Prdm1-mVenus and Dppa3-ECFP transgenes were first induced into epiblast-like cells (EpiLC) and then reprogrammed to primordial germ cell–like cells (PGCLC). To obtain mature eggs and offspring from ESC and iPSC, the induced cells were reconstituted with embryonic gonadal somatic cells (d3, d6, and E12.5), which were then transplanted orthotopically or under the kidney capsule. Similar to outcomes achieved with OSC, the efficiency of obtaining PGCLC-derived pups using this procedure was low (~3.9%), possibly because of the large number of triploid zygotes with two maternal chromosomes. Therapeutic translation of OSC and PGCLC will require the development of strategies to more efficiently direct their differentiation, such as the application of tissue engineering principles. Biomaterials designed to support the development and expansion of OSC coimplanted with developed follicles could improve follicle survival. Approaches using OSC and ESC/iPSC for ovarian tissue reconstruction open up new fertility restoration options for patients who have low ovarian follicle counts or did not preserve their tissue before undergoing ovotoxic treatments.
6.3. Systems Biology Analysis
Computational techniques applied to reproductive biology have enabled a greater understanding of biological processes, but the opportunity exists for greater integration of molecular and cellular processes. Mathematical models of follicle progression have been developed, such as predicting the menstrual cycle (108), signaling pathways (109), and oxygen demand during folliculogenesis (110), or the dynamics of follicle progression (111–113). Many of these models do not explicitly include the molecular mechanism behind the processes (endocrine, paracrine, and autocrine regulation), though there are exceptions. These examples are based on mechanistic considerations (i.e., bottom-up approach). However, the proposed mechanisms are not derived from the data or from proposing mechanisms that are consistent with the data. This alternative top-down approach employs data from high-throughput techniques (114) to develop mechanistic models and underlies the field of systems biology.
Systems biology aims to merge computational techniques and experimental data to uncover the mechanisms that control biological systems. In the past 15 years, there has been an explosion in the “omics” world, including transcriptomics, proteomics (115, 116), metabolomics (117), and epigenomics (118). Microarrays have been used extensively to study folliculogenesis in isolated ovaries (119), follicles (120), oocytes (121), and granulosa or theca cells (122) and in untreated (123, 124) or treated cell lines (125–127). Unfortunately, most of these studies have limited themselves to identifying the most important upregulated or downregulated genes. Some have reported the most likely signaling and metabolic pathways based on over- or underexpressed genes, with the exception of understanding the primordial to primary transition (63). Through a combination of analytical approaches, such as transcriptomics and proteomics, key molecules involved in paracrine and autocrine signaling in the follicle can be identified to inform the development of novel media formulations to better support in vitro follicle culture or organ culture. Metabolomics may help identify the metabolites that are required at the different stages during folliculogenesis. Systems biology has the potential to construct dynamic models that may predict the impact of different environmental insults or gene mutations on follicle phenotype. Finally, the application of evolution theories could enable the translation of results from mouse models to humans to inform the development of fertility preservation techniques.
6.4. Reproductive Tract Engineering
The female reproductive tract is required for the production of ova, the secretion of sex hormones, and the gestation and delivery of a fetus. The reproductive tract tissues and the hormones they produce are essential for entry and advancement through puberty, establishment of normal menstrual cycles, support of a potential intervening pregnancy, and endocrine support of peripheral tissues such as the bone, brain, and heart. Each reproductive tract organ has major responsibilities that are autonomous (e.g., oocyte maturation, gestation), but the organs are physically connected to form a structure through which gametes travel or the embryo implants, and rely on each other for hormonal support. Moreover, each organ has cellular contributions from multiple lineages (e.g., myometrium and endometrium), which provide local feedback.
The development of systems that contain all the components of the reproductive tract could enhance drug testing by recapitulating ex vivo the intimate relationship between cells in each organ and between individual organs in the tract. Early testing of candidate drugs or existing pharmaceuticals on reproductive function is difficult owing to ethical considerations and the real and perceived difficulties of studying biomarkers that change during a menstrual cycle. Moreover, few drugs have been brought to market for pregnant women because of the difficulties of informed consent, tragic outcomes due to untested drug treatments (e.g., thalidomide) where no good in vivo or in vitro model was available, and the inability to do controlled clinical trials in the pregnant population. As a result, there is a significant gender imbalance in drug development and testing (most developmental research is conducted in males, and most drug studies are conducted in postmenopausal women or males) and a lack of data on effects of drugs during pregnancy. This inequity could be mitigated by the development of a robust three-dimensional ex vivo reproductive tract that is a physiologic mimic of the in vivo biology and provides multimodal readout over long-term culture. A recent example where an in vitro system might provide earlier or more definitive data for evaluation of efficacy is the development of a vaccine against the H1N1 virus, which is most lethal to pregnant women. A vaccine was eventually developed and tested in pregnant women. Any potential toxic effects on the reproductive tract of the initial vaccine candidates or the final version could have been discovered rapidly in an ex vivo reproductive tract model, perhaps bringing a final vaccine to the population more quickly. As another example, the active agent in Plan B emergency contraceptive is a propregnancy progestin (levonorgestrel). The mechanism of action for this drug is thought to be the inhibition of ovulation and alternation of fallopian tube transport. These effects could be tested in a suitable in vitro system with strong, definitive endpoints, providing additional data for review by the FDA and other agencies charged with drug approval. Finally, vaccines against human papillomavirus (HPV) are now available, but an in vitro system that represents an intact cervix and is amenable to high-content screening for efficacy and toxicology would have facilitated vaccine development and perhaps reduced the number of young women who will develop cervical cancer in the next 20 years. Clearly, an urgent unmet need is to develop suitable models of individual organs, alone and combined within an ex vivo female reproductive tract, which would have an immediate impact on drug toxicity testing, expand the number of preclinical drugs that can be screened, and hasten the approval of drugs (e.g., vaccines) to meet emerging health crises.
7. SUMMARY
Bioengineering principles have numerous applications in follicle development and in the field of reproductive biology more broadly. Novel solutions to ongoing clinical issues may emerge from research into biomaterials, drug delivery technologies, and engineering of the follicle microenvironment. The need for fertility preservation options for young women facing toxic cancer therapies has revealed an opportunity for combining biomaterials technology and the field of reproductive biology. These technologies may enable patients to bank a portion of an ovary for later use in ovarian tissue transplantation or in vitro culture to preserve patients’ fertility. Furthermore, in vitro culture systems can be designed to model disease processes and serve as tools for evaluation of drug toxicity or drug screening. Opportunities abound for the application of bioengineering principles to key challenges within reproductive biology.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Lonnie D. Shea, Email: l-shea@northwestern.edu.
Teresa K. Woodruff, Email: tkw@northwestern.edu.
Ariella Shikanov, Email: shikanov@umich.edu.
LITERATURE CITED
- 1.Adamson GD, Tabangin M, Macaluso M, de Mouzon J. The number of babies born globally after treatment with the assisted reproductive technologies (ART) Fertil Steril. 2013;100(Suppl):S42. [Google Scholar]
- 2.Nieman CL, Kazer R, Brannigan RE, Zoloth LS, Chase-Lansdale PL, et al. Cancer survivors and infertility: a review of a new problem and novel answers. J Support Oncol. 2006;4:171–78. [PubMed] [Google Scholar]
- 3.Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. N Engl J Med. 2009;360:902–11. doi: 10.1056/NEJMra0801454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson RA, Themmen AP, Al-Qahtani A, Groome NP, Cameron DA. The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve in premenopausal women with breast cancer. Hum Reprod. 2006;21:2583–92. doi: 10.1093/humrep/del201. [DOI] [PubMed] [Google Scholar]
- 5.Meirow D, Nugent D. The effects of radiotherapy and chemotherapy on female reproduction. Hum Reprod Update. 2001;7:535–43. doi: 10.1093/humupd/7.6.535. [DOI] [PubMed] [Google Scholar]
- 6.Natl. Cancer Inst. SEER data, 1973–2010. Surveillance, Epidemiology, and End Results (SEER) Program. 2012 http://seer.cancer.gov/data.
- 7.Schover LR, Rybicki LA, Martin BA, Bringelsen KA. Having children after cancer. A pilot survey of survivors’ attitudes and experiences. Cancer. 1999;86:697–709. doi: 10.1002/(sici)1097-0142(19990815)86:4<697::aid-cncr20>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 8.Gosden RG. Prospects for oocyte banking and in vitro maturation. J Natl Cancer Inst Monogr. 2005;2005:60–63. doi: 10.1093/jncimonographs/lgi007. [DOI] [PubMed] [Google Scholar]
- 9.Ata B, Chian RC, Tan SL. Cryopreservation of oocytes and embryos for fertility preservation for female cancer patients. Best Pract Res Clin Obstet Gynaecol. 2010;24:101–12. doi: 10.1016/j.bpobgyn.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Loren AW, Mangu PB, Beck LN, Brennan L, Magdalinski AJ, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:2500–10. doi: 10.1200/JCO.2013.49.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oktay K. Fertility preservation: We are in this for a long haul. Am J Obstet Gynecol. 2013;209:77–79. doi: 10.1016/j.ajog.2013.05.036. [DOI] [PubMed] [Google Scholar]
- 12.Siegelmann-Danieli N, Tamir A, Zohar H, Papa MZ, Chetver LL, et al. Breast cancer in women with recent exposure to fertility medications is associated with poor prognostic features. Ann Surg Oncol. 2003;10:1031–38. doi: 10.1245/aso.2003.03.068. [DOI] [PubMed] [Google Scholar]
- 13.Malloch L, Rhoton-Vlasak A. An assessment of current clinical attitudes toward letrozole use in reproductive endocrinology practices. Fertil Steril. 2013;100:1740–44. doi: 10.1016/j.fertnstert.2013.08.040. [DOI] [PubMed] [Google Scholar]
- 14.Davis VJ. Female gamete preservation. Cancer. 2006;107:1690–94. doi: 10.1002/cncr.22105. [DOI] [PubMed] [Google Scholar]
- 15.Silber S, Kagawa N, Kuwayama M, Gosden RG. Duration of fertility after fresh and frozen ovary transplantation. Fertil Steril. 2010;94:2191–96. doi: 10.1016/j.fertnstert.2009.12.073. [DOI] [PubMed] [Google Scholar]
- 16.Donnez J, Dolmans MM, Pellicer A, Diaz-Garcia C, Sanchez Serrano M, et al. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil Steril. 2013;99:1503–13. doi: 10.1016/j.fertnstert.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 17.Silber SJ, DeRosa M, Pineda J, Lenahan K, Grenia D, et al. A series of monozygotic twins discordant for ovarian failure: ovary transplantation (cortical versus microvascular) and cryopreservation. Hum Reprod. 2008;23:1531–37. doi: 10.1093/humrep/den032. [DOI] [PubMed] [Google Scholar]
- 18.Ahn RW, Barrett SL, Raja MR, Jozefik JK, Spaho L, et al. Nano-encapsulation of arsenic trioxide enhances efficacy against murine lymphoma model while minimizing its impact on ovarian reserve in vitro and in vivo. PLoS ONE. 2013;8:e58491. doi: 10.1371/journal.pone.0058491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Constine LS, Woolf PD, Cann D, Mick G, McCormick K, et al. Hypothalamic-pituitary dysfunction after radiation for brain tumors. N Engl J Med. 1993;328:87–94. doi: 10.1056/NEJM199301143280203. [DOI] [PubMed] [Google Scholar]
- 20.Woods DC, Tilly JL. Isolation, characterization and propagation of mitotically active germ cells from adult mouse and human ovaries. Nat Protoc. 2013;8:966–88. doi: 10.1038/nprot.2013.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayashi K, Ohta H, Kurimoto K, Aramaki S, Saitou M. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell. 2011;146:519–32. doi: 10.1016/j.cell.2011.06.052. [DOI] [PubMed] [Google Scholar]
- 22.Tingen C, Kim A, Woodruff TK. The primordial pool of follicles and nest breakdown in mammalian ovaries. Mol Hum Reprod. 2009;15:795–803. doi: 10.1093/molehr/gap073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinovitch PN. The development in vitro of the mammalian gonad: ovary and ovogenesis. Proc R Soc Ser B. 1938;125:232–49. [Google Scholar]
- 24.Eppig JJ. Mouse oocyte development in vitro with various culture systems. Dev Biol. 1977;60:371–88. doi: 10.1016/0012-1606(77)90135-x. [DOI] [PubMed] [Google Scholar]
- 25.Eppig JJ, Schroeder AC. Capacity of mouse oocytes from preantral follicles to undergo embryogenesis and development to live young after growth, maturation, and fertilization in vitro. Biol Reprod. 1989;41:268–76. doi: 10.1095/biolreprod41.2.268. [DOI] [PubMed] [Google Scholar]
- 26.Cortvrindt R, Smitz J, Van Steirteghem AC. In-vitro maturation, fertilization and embryo development of immature oocytes from early preantral follicles from prepuberal mice in a simplified culture system. Hum Reprod. 1996;11:2656–66. doi: 10.1093/oxfordjournals.humrep.a019188. [DOI] [PubMed] [Google Scholar]
- 27.Telfer EE, Zelinski MB. Ovarian follicle culture: advances and challenges for human and non-human primates. Fertil Steril. 2013;99:1523–33. doi: 10.1016/j.fertnstert.2013.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smitz J, Dolmans MM, Donnez J, Fortune JE, Hovatta O, et al. Current achievements and future research directions in ovarian tissue culture, in vitro follicle development and transplantation: implications for fertility preservation. Hum Reprod Update. 2010;16:395–414. doi: 10.1093/humupd/dmp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhattacharya P, Keating AF. Impact of environmental exposures on ovarian function and role of xenobiotic metabolism during ovotoxicity. Toxicol Appl Pharmacol. 2012;261:227–35. doi: 10.1016/j.taap.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eppig JJ, O’Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biol Reprod. 1996;54:197–207. doi: 10.1095/biolreprod54.1.197. [DOI] [PubMed] [Google Scholar]
- 31.O’Brien MJ, Pendola JK, Eppig JJ. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod. 2003;68:1682–86. doi: 10.1095/biolreprod.102.013029. [DOI] [PubMed] [Google Scholar]
- 32.Xu J, Lawson MS, Yeoman RR, Molskness TA, Ting AY, et al. Fibrin promotes development and function of macaque primary follicles during encapsulated three-dimensional culture. Hum Reprod. 2013;28:2187–200. doi: 10.1093/humrep/det093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tambe SS, Nandedkar TD. Steroidogenesis in sheep ovarian antral follicles in culture: time course study and supplementation with a precursor. Steroids. 1993;58:379–83. doi: 10.1016/0039-128x(93)90041-k. [DOI] [PubMed] [Google Scholar]
- 34.Roy SK, Treacy BJ. Isolation and long-term culture of human preantral follicles. Fertil Steril. 1993;59:783–90. [PubMed] [Google Scholar]
- 35.Ksiazkiewicz LK. Recent achievements in in vitro culture and preservation of ovarian follicles in mammals. Reprod Biol. 2006;6:3–16. [PubMed] [Google Scholar]
- 36.Woodruff TK, Shea LD. The role of the extracellular matrix in ovarian follicle development. Reprod Sci. 2007;14:6–10. doi: 10.1177/1933719107309818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lenie S, Cortvrindt R, Adriaenssens T, Smitz J. A reproducible two-step culture system for isolated primary mouse ovarian follicles as single functional units. Biol Reprod. 2004;71:1730–38. doi: 10.1095/biolreprod.104.028415. [DOI] [PubMed] [Google Scholar]
- 38.West-Farrell ER, Xu M, Gomberg MA, Chow YH, Woodruff TK, Shea LD. The mouse follicle microenvironment regulates antrum formation and steroid production: alterations in gene expression profiles. Biol Reprod. 2009;80:432–39. doi: 10.1095/biolreprod.108.071142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kreeger PK, Woodruff TK, Shea LD. Murine granulosa cell morphology and function are regulated by a synthetic Arg-Gly-Asp matrix. Mol Cell Endocrinol. 2003;205:1–10. doi: 10.1016/s0303-7207(03)00209-0. [DOI] [PubMed] [Google Scholar]
- 40.Lenie S, Cortvrindt R, Eichenlaub-Ritter U, Smitz J. Continuous exposure to bisphenol A during in vitro follicular development induces meiotic abnormalities. Mutat Res. 2008;651:71–81. doi: 10.1016/j.mrgentox.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 41.Paulose T, Tannenbaum LV, Borgeest C, Flaws JA. Methoxychlor-induced ovarian follicle toxicity in mice: dose and exposure duration-dependent effects. Birth Defects Res B Dev Reprod Toxicol. 2012;95:219–24. doi: 10.1002/bdrb.21007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pangas SA, Saudye H, Shea LD, Woodruff TK. Novel approach for the three-dimensional culture of granulosa cell-oocyte complexes. Tissue Eng. 2003;9:1013–21. doi: 10.1089/107632703322495655. [DOI] [PubMed] [Google Scholar]
- 43.Amsden B, Turner N. Diffusion characteristics of calcium alginate gels. Biotechnol Bioeng. 1999;65:605–10. doi: 10.1002/(sici)1097-0290(19991205)65:5<605::aid-bit14>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 44.Wee S, Gombotz WR. Protein release from alginate matrices. Adv Drug Deliv Rev. 1998;31:267–85. doi: 10.1016/s0169-409x(97)00124-5. [DOI] [PubMed] [Google Scholar]
- 45.Machluf M, Carroll RS. Cell-based delivery systems for antiangiogenic therapy. Biotechnol Genet Eng Rev. 2003;20:183–95. doi: 10.1080/02648725.2003.10648043. [DOI] [PubMed] [Google Scholar]
- 46.Wang L, Shansky J, Borselli C, Mooney D, Vandenburgh H. Design and fabrication of a biodegradable, covalently crosslinked shape-memory alginate scaffold for cell and growth factor delivery. Tissue Eng Part A. 2012;18:2000–7. doi: 10.1089/ten.tea.2011.0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanaka H, Matsumura M, Veliky IA. Diffusion characteristics of substrates in Ca-alginate gel beads. Biotechnol Bioeng. 1984;26:53–58. doi: 10.1002/bit.260260111. [DOI] [PubMed] [Google Scholar]
- 48.Barrett SL, Albertini DF. Allocation of γ-tubulin between oocyte cortex and meiotic spindle influences asymmetric cytokinesis in the mouse oocyte. Biol Reprod. 2007;76:949–57. doi: 10.1095/biolreprod.106.057141. [DOI] [PubMed] [Google Scholar]
- 49.Kreeger PK, Deck JW, Woodruff TK, Shea LD. The in vitro regulation of ovarian follicle development using alginate-extracellular matrix gels. Biomaterials. 2006;27:714–23. doi: 10.1016/j.biomaterials.2005.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parrish EM, Siletz A, Xu M, Woodruff TK, Shea LD. Gene expression in mouse ovarian follicle development in vivo versus an ex vivo alginate culture system. Reproduction. 2011;142:309–18. doi: 10.1530/REP-10-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu M, Kreeger PK, Shea LD, Woodruff TK. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006;12:2739–46. doi: 10.1089/ten.2006.12.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu M, West E, Shea LD, Woodruff TK. Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol Reprod. 2006;75:916–23. doi: 10.1095/biolreprod.106.054833. [DOI] [PubMed] [Google Scholar]
- 53.West ER, Xu M, Woodruff TK, Shea LD. Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials. 2007;28:4439–48. doi: 10.1016/j.biomaterials.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Torrance C, Telfer E, Gosden RG. Quantitative study of the development of isolated mouse pre-antral follicles in collagen gel culture. J Reprod Fertil. 1989;87:367–74. doi: 10.1530/jrf.0.0870367. [DOI] [PubMed] [Google Scholar]
- 55.Sharma GT, Dubey PK, Meur SK. Survival and developmental competence of buffalo preantral follicles using three-dimensional collagen gel culture system. Anim Reprod Sci. 2009;114:115–24. doi: 10.1016/j.anireprosci.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 56.Shikanov A, Xu M, Woodruff T, Shea L. Interpenetrating fibrin–alginate matrices for in vitro ovarian follicle development. Biomaterials. 2009;30:5476–85. doi: 10.1016/j.biomaterials.2009.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shikanov A, Xu M, Woodruff TK, Shea LD. A method for ovarian follicle encapsulation and culture in a proteolytically degradable 3 dimensional system. J Vis Exp. 2011;2011(49):2695. doi: 10.3791/2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sperling LH, Mishra V. The current status of interpenetrating polymer networks. Polym Adv Technol. 1996;7:197–208. [Google Scholar]
- 59.Rowe SL, Stegemann JP. Interpenetrating collagen-fibrin composite matrices with varying protein contents and ratios. Biomacromolecules. 2006;7:2942–48. doi: 10.1021/bm0602233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kraehenbuehl TP, Zammaretti P, Van der Vlies AJ, Schoenmakers RG, Lutolf MP, et al. Three-dimensional extracellular matrix-directed cardioprogenitor differentiation: systematic modulation of a synthetic cell-responsive PEG-hydrogel. Biomaterials. 2008;29:2757–66. doi: 10.1016/j.biomaterials.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 61.Shikanov A, Smith RM, Xu M, Woodruff TK, Shea LD. Hydrogel network design using multi-functional macromers to coordinate tissue maturation in ovarian follicle culture. Biomaterials. 2011;32:2524–31. doi: 10.1016/j.biomaterials.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jin SY, Lei L, Shikanov A, Shea LD, Woodruff TK. A novel two-step strategy for in vitro culture of early-stage ovarian follicles in the mouse. Fertil Steril. 2010;93:2633–39. doi: 10.1016/j.fertnstert.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nilsson EE, Savenkova MI, Schindler R, Zhang B, Schadt EE, Skinner MK. Gene bionetwork analysis of ovarian primordial follicle development. PLoS ONE. 2010;5:e11637. doi: 10.1371/journal.pone.0011637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Osborn SM, Gook DA, Stern C, Speirs AL. The isolation and culture of human primordial follicles from fresh ovarian tissue. Hum Reprod. 1997;12(Suppl 1):153. [Google Scholar]
- 65.Tingen CM, Kiesewetter SE, Jozefik J, Thomas C, Tagler D, et al. A macrophage and theca cell-enriched stromal cell population influences growth and survival of immature murine follicles in vitro. Reproduction. 2011;141:809–20. doi: 10.1530/REP-10-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tagler D, Tu T, Smith RM, Anderson NR, Tingen CM, et al. Embryonic fibroblasts enable the culture of primary ovarian follicles within alginate hydrogels. Tissue Eng Part A. 2012;18:1229–38. doi: 10.1089/ten.tea.2011.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tagler D, Makanji Y, Anderson NR, Woodruff TK, Shea LD. Supplemented αMEM/F12-based medium enables the survival and growth of primary ovarian follicles encapsulated in alginate hydrogels. Biotechnol Bioeng. 2013;110:3258–68. doi: 10.1002/bit.24986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gutierrez CG, Ralph JH, Telfer EE, Wilmut I, Webb R. Growth and antrum formation of bovine preantral follicles in long-term culture in vitro. Biol Reprod. 2000;62:1322–28. doi: 10.1095/biolreprod62.5.1322. [DOI] [PubMed] [Google Scholar]
- 69.McLaughlin M, Bromfield JJ, Albertini DF, Telfer EE. Activin promotes follicular integrity and oogenesis in cultured pre-antral bovine follicles. Mol Hum Reprod. 2010;16:644–53. doi: 10.1093/molehr/gaq021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jewgenow K, Paris MC. Preservation of female germ cells from ovaries of cat species. Theriogenology. 2006;66:93–100. doi: 10.1016/j.theriogenology.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 71.Songsasen N, Woodruff TK, Wildt DE. In vitro growth and steroidogenesis of dog follicles are influenced by the physical and hormonal microenvironment. Reproduction. 2011;142:113–22. doi: 10.1530/REP-10-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu M, Fazleabas AT, Shikanov A, Jackson E, Barrett SL, et al. In vitro oocyte maturation and preantral follicle culture from the luteal-phase baboon ovary produce mature oocytes. Biol Reprod. 2011;84:689–97. doi: 10.1095/biolreprod.110.088674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu M, West-Farrell ER, Stouffer RL, Shea LD, Woodruff TK, Zelinski MB. Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biol Reprod. 2009;81:587–94. doi: 10.1095/biolreprod.108.074732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hornick JE, Duncan FE, Shea LD, Woodruff TK. Isolated primate primordial follicles require a rigid physical environment to survive and grow in vitro. Hum Reprod. 2012;27:1801–10. doi: 10.1093/humrep/der468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu M, Barrett SL, West-Farrell E, Kondapalli LA, Kiesewetter SE, et al. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum Reprod. 2009;24:2531–40. doi: 10.1093/humrep/dep228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456–88. doi: 10.1016/j.fertnstert.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 77.Michelmore KF, Balen AH, Dunger DB, Vessey MP. Polycystic ovaries and associated clinical and biochemical features in young women. Clin Endocrinol (Oxf) 1999;51:779–86. doi: 10.1046/j.1365-2265.1999.00886.x. [DOI] [PubMed] [Google Scholar]
- 78.Michelmore KF. Polycystic ovary syndrome in adolescence and early adulthood. Hum Fertil (Camb) 2000;3:96–100. doi: 10.1080/1464727002000198771. [DOI] [PubMed] [Google Scholar]
- 79.Woodruff TK, Shea LD. A new hypothesis regarding ovarian follicle development: ovarian rigidity as a regulator of selection and health. J Assist Reprod Genet. 2011;28:3–6. doi: 10.1007/s10815-010-9478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nelson SM, Fleming R. Obesity and reproduction: impact and interventions. Curr Opin Obstet Gynecol. 2007;19:384–89. doi: 10.1097/GCO.0b013e32825e1d70. [DOI] [PubMed] [Google Scholar]
- 81.Lintsen AM, Pasker–de Jong PC, de Boer EJ, Burger CW, Jansen CA, et al. Effects of subfertility cause, smoking and body weight on the success rate of IVF. Hum Reprod. 2005;20:1867–75. doi: 10.1093/humrep/deh898. [DOI] [PubMed] [Google Scholar]
- 82.Robker RL. Evidence that obesity alters the quality of oocytes and embryos. Pathophysiology. 2008;15:115–21. doi: 10.1016/j.pathophys.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 83.Purcell SH, Moley KH. The impact of obesity on egg quality. J Assist Reprod Genet. 2011;28:517–24. doi: 10.1007/s10815-011-9592-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev. 2009;30:465–93. doi: 10.1210/er.2009-0006. [DOI] [PubMed] [Google Scholar]
- 85.Hirshfeld-Cytron JE, Duncan FE, Xu M, Jozefik JK, Shea LD, Woodruff TK. Animal age, weight and estrus cycle stage impact the quality of in vitro grown follicles. Hum Reprod. 2011;26:2473–85. doi: 10.1093/humrep/der183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Teissier MP, Chable H, Paulhac S, Aubard Y. Comparison of follicle steroidogenesis from normal and polycystic ovaries in women undergoing IVF: relationship between steroid concentrations, follicle size, oocyte quality and fecundability. Hum Reprod. 2000;15:2471–77. doi: 10.1093/humrep/15.12.2471. [DOI] [PubMed] [Google Scholar]
- 87.Malizia BA, Hacker MR, Penzias AS. Cumulative live-birth rates after in vitro fertilization. N Engl J Med. 2009;360:236–43. doi: 10.1056/NEJMoa0803072. [DOI] [PubMed] [Google Scholar]
- 88.Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet. 2001;2:280–91. doi: 10.1038/35066065. [DOI] [PubMed] [Google Scholar]
- 89.Duncan FE, Chiang T, Schultz RM, Lampson MA. Evidence that a defective spindle assembly checkpoint is not the primary cause of maternal age-associated aneuploidy in mouse eggs. Biol Reprod. 2009;81:768–76. doi: 10.1095/biolreprod.109.077909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pan H, Ma P, Zhu W, Schultz RM. Age-associated increase in aneuploidy and changes in gene expression in mouse eggs. Dev Biol. 2008;316:397–407. doi: 10.1016/j.ydbio.2008.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Duncan FE, Hornick JE, Lampson MA, Schultz RM, Shea LD, Woodruff TK. Chromosome cohesion decreases in human eggs with advanced maternal age. Aging Cell. 2012;11:1121–24. doi: 10.1111/j.1474-9726.2012.00866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hunt P, Hassold T. Female meiosis: coming unglued with age. Curr Biol. 2010;20:R699–702. doi: 10.1016/j.cub.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 93.Hornick JE, Duncan FE, Shea LD, Woodruff TK. Multiple follicle culture supports primary follicle growth through paracrine-acting signals. Reproduction. 2013;145:19–32. doi: 10.1530/REP-12-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Silber SJ, Lenahan KM, Levine DJ, Pineda JA, Gorman KS, et al. Ovarian transplantation between monozygotic twins discordant for premature ovarian failure. N Engl J Med. 2005;353:58–63. doi: 10.1056/NEJMoa043157. [DOI] [PubMed] [Google Scholar]
- 95.Donnez J. Introduction. Fertility preservation, from cancer to benign disease to social reasons: the challenge of the present decade. Fertil Steril. 2013;99:1467–68. doi: 10.1016/j.fertnstert.2013.03.040. [DOI] [PubMed] [Google Scholar]
- 96.Gosden RG. Ovary and uterus transplantation. Reproduction. 2008;136:671–80. doi: 10.1530/REP-08-0099. [DOI] [PubMed] [Google Scholar]
- 97.Ting AY, Yeoman RR, Lawson MS, Zelinski MB. In vitro development of secondary follicles from cryopreserved rhesus macaque ovarian tissue after slow-rate freeze or vitrification. Hum Reprod. 2011;26:2461–72. doi: 10.1093/humrep/der196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nisolle M, Casanas-Roux F, Qu J, Motta P, Donnez J. Histologic and ultrastructural evaluation of fresh and frozen-thawed human ovarian xenografts in nude mice. Fertil Steril. 2000;74:122–29. doi: 10.1016/s0015-0282(00)00548-3. [DOI] [PubMed] [Google Scholar]
- 99.Shikanov A, Zhang Z, Xu M, Smith RM, Rajan A, et al. Fibrin encapsulation and vascular endothelial growth factor delivery promotes ovarian graft survival in mice. Tissue Eng Part A. 2011;17:3095–104. doi: 10.1089/ten.tea.2011.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dolmans MM, Luyckx V, Donnez J, Andersen CY, Greve T. Risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue. Fertil Steril. 2013;99:1514–22. doi: 10.1016/j.fertnstert.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 101.Dolmans MM, Marinescu C, Saussoy P, Van Langendonckt A, Amorim C, Donnez J. Reimplantation of cryopreserved ovarian tissue from patients with acute lymphoblastic leukemia is potentially unsafe. Blood. 2010;116:2908–14. doi: 10.1182/blood-2010-01-265751. [DOI] [PubMed] [Google Scholar]
- 102.Poirot CJ, Martelli H, Genestie C, Golmard JL, Valteau-Couanet D, et al. Feasibility of ovarian tissue cryopreservation for prepubertal females with cancer. Pediatr Blood Cancer. 2007;49:74–78. doi: 10.1002/pbc.21027. [DOI] [PubMed] [Google Scholar]
- 103.Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Fertility preservation: successful transplantation of cryopreserved ovarian tissue in a young patient previously treated for Hodgkin’s disease. Oncologist. 2007;12:1437–42. doi: 10.1634/theoncologist.12-12-1437. [DOI] [PubMed] [Google Scholar]
- 104.Vanacker J, Luyckx V, Dolmans MM, Des Rieux A, Jaeger J, et al. Transplantation of an alginate-matrigel matrix containing isolated ovarian cells: first step in developing a biodegradable scaffold to transplant isolated preantral follicles and ovarian cells. Biomaterials. 2012;33:6079–85. doi: 10.1016/j.biomaterials.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 105.Gosden RG. Restitution of fertility in sterilized mice by transferring primordial ovarian follicles. Hum Reprod. 1990;5:499–504. doi: 10.1093/oxfordjournals.humrep.a137132. [DOI] [PubMed] [Google Scholar]
- 105a.Smith RM, Shikanov A, Kniazeva E, Ramadurai D, Woodruff TK, Shea LD. Fibrin-mediated delivery of an ovarian follicle pool in a mouse model of infertility. Tissue Eng Part A. 2014 doi: 10.1089/ten.tea.2013.0675. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sittadjody S, Saul JM, Joo S, Yoo JJ, Atala A, Opara EC. Engineered multilayer ovarian tissue that secretes sex steroids and peptide hormones in response to gonadotropins. Biomaterials. 2013;34:2412–20. doi: 10.1016/j.biomaterials.2012.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Krotz SP, Robins JC, Ferruccio TM, Moore R, Steinhoff MM, et al. In vitro maturation of oocytes via the pre-fabricated self-assembled artificial human ovary. J Assist Reprod Genet. 2010;27:743–50. doi: 10.1007/s10815-010-9468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Reineeke I, Deuflhard PA. A complex mathematical model of the human menstrual cycle. J Theor Biol. 2007;247:303–30. doi: 10.1016/j.jtbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 109.Clement F, Monniaux D, Stark J, Hardy K, Thalabard JC, et al. Mathematical model of FSH-induced cAMP production in ovarian follicles. Am J Physiol Endocrinol Metab. 2001;281:E35–53. doi: 10.1152/ajpendo.2001.281.1.E35. [DOI] [PubMed] [Google Scholar]
- 110.Redding GP, Bronlund JE, Hart AL. Mathematical modelling of oxygen transport-limited follicle growth. Reproduction. 2007;133:1095–106. doi: 10.1530/REP-06-0171. [DOI] [PubMed] [Google Scholar]
- 111.Wallace WH, Kelsey TW. Human ovarian reserve from conception to the menopause. PLoS ONE. 2010;5:e8772. doi: 10.1371/journal.pone.0008772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Echenim N, Monniaux D, Sorine M, Clement F. Multi-scale modeling of the follicle selection process in the ovary. Math Biosci. 2005;198:57–79. doi: 10.1016/j.mbs.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 113.Bristol-Gould SK, Kreeger PK, Selkirk CG, Kilen SM, Cook RW, et al. Postnatal regulation of germ cells by activin: the establishment of the initial follicle pool. Dev Biol. 2006;298:132–48. doi: 10.1016/j.ydbio.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 114.Lawrence ND, Girolami M, Rattray M, Sanguinetti G, editors. Learning and Inference in Computational Systems Biology. Cambridge, MA: MIT Press; 2010. [Google Scholar]
- 115.Memili E, Peddinti D, Shack LA, Nanduri B, McCarthy F, et al. Bovine germinal vesicle oocyte and cumulus cell proteomics. Reproduction. 2007;133:1107–20. doi: 10.1530/REP-06-0149. [DOI] [PubMed] [Google Scholar]
- 116.Peddinti D, Memili E, Burgess SC. Proteomics-based systems biology modeling of bovine germinal vesicle stage oocyte and cumulus cell interaction. PLoS ONE. 2010;5:e11240. doi: 10.1371/journal.pone.0011240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Revelli A, Delle Piane L, Casano S, Molinari E, Massobrio M, Rinaudo P. Follicular fluid content and oocyte quality: from single biochemical markers to metabolomics. Reprod Biol Endocrinol. 2009;7:40. doi: 10.1186/1477-7827-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vanselow J, Spitschak M, Nimz M, Furbass R. DNA methylation is not involved in preovulatory down-regulation of CYP11A1, HSD3B1, and CYP19A1 in bovine follicles but may have a role in permanent silencing of CYP19A1 in large granulosa lutein cells. Biol Reprod. 2010;82:289–98. doi: 10.1095/biolreprod.109.079251. [DOI] [PubMed] [Google Scholar]
- 119.Hasegawa A, Kumamoto K, Mochida N, Komori S, Koyama K. Gene expression profile during ovarian folliculogenesis. J Reprod Immunol. 2009;83:40–44. doi: 10.1016/j.jri.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 120.Yoon SJ, Kim KH, Chung HM, Choi DH, Lee WS, et al. Gene expression profiling of early follicular development in primordial, primary, and secondary follicles. Fertil Steril. 2006;85:193–203. doi: 10.1016/j.fertnstert.2005.07.1296. [DOI] [PubMed] [Google Scholar]
- 121.Pan H, O’Brien MJ, Wigglesworth K, Eppig JJ, Schultz RM. Transcript profiling during mouse oocyte development and the effect of gonadotropin priming and development in vitro. Dev Biol. 2005;286:493–506. doi: 10.1016/j.ydbio.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 122.Skinner MK, Schmidt M, Savenkova MI, Sadler-Riggleman I, Nilsson EE. Regulation of granulosa and theca cell transcriptomes during ovarian antral follicle development. Mol Reprod Dev. 2008;75:1457–72. doi: 10.1002/mrd.20883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sasson R, Dantes A, Tajima K, Amsterdam A. Novel genes modulated by FSH in normal and immortalized FSH-responsive cells: new insights into the mechanism of FSH action. FASEB J. 2003;17:1256–66. doi: 10.1096/fj.02-0740com. [DOI] [PubMed] [Google Scholar]
- 124.Sriraman V, Rudd MD, Lohmann SM, Mulders SM, Richards JS. Cyclic guanosine 5′-monophosphate-dependent protein kinase II is induced by luteinizing hormone and progesterone receptor-dependent mechanisms in granulosa cells and cumulus oocyte complexes of ovulating follicles. Mol Endocrinol. 2006;20:348–61. doi: 10.1210/me.2005-0317. [DOI] [PubMed] [Google Scholar]
- 125.Chin KV, Seifer DB, Feng B, Lin Y, Shih WC. DNA microarray analysis of the expression profiles of luteinized granulosa cells as a function of ovarian reserve. Fertil Steril. 2002;77:1214–18. doi: 10.1016/s0015-0282(02)03114-x. [DOI] [PubMed] [Google Scholar]
- 126.Hernandez-Gonzalez I, Gonzalez-Robayna I, Shimada M, Wayne CM, Ochsner SA, et al. Gene expression profiles of cumulus cell oocyte complexes during ovulation reveal cumulus cells express neuronal and immune-related genes: Does this expand their role in the ovulation process? Mol Endocrinol. 2006;20:1300–21. doi: 10.1210/me.2005-0420. [DOI] [PubMed] [Google Scholar]
- 127.Wood JR, Nelson VL, Ho C, Jansen E, Wang CY, et al. The molecular phenotype of polycystic ovary syndrome (PCOS) theca cells and new candidate PCOS genes defined by microarray analysis. 2003 doi: 10.1074/jbc.M300688200. [DOI] [PubMed] [Google Scholar]


