Abstract
Recent research has uncovered complex transcription factor networks that control the processes of T-cell development and differentiation. RUNX (runt-related transcription factor) proteins are among the many factors that have crucial roles in these networks. In this Review, we examine the mechanisms by which RUNX complexes act together with other transcription factors, such as Th-POK (T-helper-inducing POZ/Kruppel-like factor) and GATA-binding protein 3 (GATA3) in determining the CD4/CD8 lineage choice of developing thymocytes. In addition, we discuss evidence indicating that RUNX complexes are also involved in the differentiation of effector T-cell subsets and that the molecular mechanisms by which RUNX proteins regulate T-cell fate decisions are conserved between the thymus and periphery.
The mechanism of T-cell lineage choice, by which progenitor double positive (DP) thymocytes give rise to MHC class I-specific CD8+ T cells and MHC class II- specific CD4+ T cells, has been a topic of long standing interest in the fields of immunology and developmental biology. Characterization of the transcriptional programmes that are required for the development of T cells into one lineage and not the other is crucial for understanding how the differentiation of each functional T-cell subset is achieved. More fundamentally, T-cell lineage choice is a paradigm for studying bipotential cell fate decisions at both phenotypical and genetic levels. Recent advances in the field have begun to reveal how a cross-regulatory network that involves many transcription factors regulates this developmental branch point.
After T-cell precursors enter the thymus, they progress through a series of developmental stages before they become either MHC class I-specific CD8+ T cells or MHC class II-specific CD4+ T cells. Initially, thymocytes do not express CD4 and CD8 (and are therefore designated as double negative (DN)) and begin rearranging the T-cell receptor (TCR)β-chain locus. Productive rearrangement allows the cells to pass the β-selection checkpoint and to upregulate the expression of both the CD4 and CD8 co-receptor genes to become DP cells. During the transition from the DN to the DP stage, a proliferative burst is followed by rearrangement of the TCRA locus and the expression of a mature αβTCR. Once the mature TCR is expressed, a thymocyte has one of three fates: apoptosis, negative selection or positive selection. More specifically, failure to produce a TCR that interacts with sufficient affinity with self peptide–MHC complexes causes apoptosis that is induced by a lack of TCR-mediated survival signals, or ‘death by neglect’. Cells with TCRs that bind too strongly to self peptide–MHC complexes are deleted in a process known as negative selection to rid the peripheral T-cell pool of potentially self-reactive lymphocytes. The few DP cells with TCRs of appropriate affinity undergo positive selection following interaction with self peptide–MHC complexes and develop into cells with a CD4+CD8low transitional phenotype. At some stage during or after positive selection, a CD4+CD8low thymocyte commits to either the CD4+ T-cell fate (if its TCR is MHC class II specific) or the CD8+ T-cell fate (if its TCR is MHC class I specific).
Several models have been proposed to explain how the MHC specificity of a developing T cell is linked to both co-receptor gene regulation and functional gene expression programmes. Initial models focused on whether co-receptor gene expression was stochastically determined or instructed by interactions between the co-receptor and the MHC molecule and, if instructed, whether the instructing signal was quantitative or qualitative1,2. Although it is now widely accepted that co-receptor gene regulation is not stochastic, the nature of the instructing signal has not been substantively clarified. It has been proposed that differences in the duration of the interactions between the TCR and the MHC molecule initiate distinct signals that direct cells to either the CD4+ or CD8+ T-cell fate3–5, which might be due to asymmetry in co-receptor expression at the CD4+CD8low stage. In addition, a distinction can clearly be made between positive selection and lineage commitment (the step at which the T-cell lineage fate is fixed), but it remains unclear precisely when lineage choice is established. The various models that have been proposed, and the data that support each of them, are the subject of a comprehensive recent review by Singer et al.6, and they will not be further discussed here. Instead, this Review focuses on recent findings that have led to a refinement of our current understanding of the transcription factor network that controls the CD4/CD8 lineage decision.
Recent studies (discussed below) have focused mainly on the transcription factors RUNX (runt-related transcription factor; specifically the family members RUNX1 and RUNX3) and Th-POK (T-helper-inducing POZ/Kruppel-like factor; encoded by ZBTB7B; also known as c-Krox) and have revealed that there is cross-regulation between these molecules, both in terms of their expression and their downstream function. It has also been observed that other transcription factors, notably GATA-binding protein 3 (GATA3) and thymocyte selection-associated high-mobility group box (TOX), are crucially involved in the CD4/CD8 lineage decision. Taken together, recent findings have emphasized that the initial specification of positively selected thymocytes to either the CD4+ or CD8+ T-cell lineage is reversible, whereas lineage commitment is a subsequent step at which T-cell fate is rendered irreversible. The current data indicate that RUNX proteins and Th-POK are lineage commitment factors that are required to repress the development of the alternative fate once a certain T-cell lineage has been specified, and that other transcription factors, including MYB, GATA3 and TOX, are lineage-specifying factors that cannot themselves enforce lineage choice. Recent studies have also revealed that RUNX complexes are involved in the differentiation of peripheral effector T-cell subsets and have shown that the regulatory mechanisms by which RUNX proteins enforce T-cell fate decisions, such as binding to silencer elements to repress alternative fates, are conserved between the thymus and the periphery.
RUNX proteins and expression of CD4 and CD8
RUNX proteins are a family of evolutionarily conserved heterodimeric transcription factors that have crucial roles during the development of many tissues7,8 and of the immune system (TABLE 1). RUNX complexes are composed of unique DNA-binding α-subunits (RUNX1, RUNX2 or RUNX3, which are homologues of the Drosophila runt gene) that pair with the non-DNA binding subunit core-binding factor β (CBFβ, which has been shown to stabilize the interaction of RUNX proteins with DNA). Targeted deletion of RUNX subunits has revealed distinct roles for these proteins in development, with RUNX1 being required for haematopoiesis9, RUNX2 for osteogenesis10,11 and RUNX3 for neurogenesis, thymopoiesis and the control of gastric epithelial-cell proliferation12–14. Because the CBFβ subunit acts as a binding partner for all RUNX proteins, targeted inactivation of its expression abrogates the activity of all RUNX complexes15,16.
Table 1.
RUNX complexes in the immune system
| Targeted gene | Phenotype of germline knockout | Refs | Phenotype of conditional knockout | Refs |
|---|---|---|---|---|
| Runx1 |
|
16,9 |
Mx-CRE:
|
64 |
Lck-CRE:
|
17,24 | |||
Cd4-CRE:
|
24,45 | |||
| Runx2 |
|
11,65 | ND | |
| Runx3 | 12,13 |
Cd4-CRE:
|
24,56 | |
| Cbfb | Identical to Runx1 germline knockout (see above) | 15, 16,66 |
Lck-CRE:
|
42 |
Cd4-CRE:
|
42 | |||
| Runx1 and Runx3 | Identical to Runx1 germline knockout (see above) |
Runx1fl/flRunx3fl/fl* and Cd4-CRE:
|
24 | |
Runx1Δ446/Δ446‡, Runx3fl/fl* and Cd4-CRE:
|
42 |
Runx1 alleles targeted with flanking loxP sites.
Runx1 alleles lack carboxy-terminal VWRPY (Groucho/TLE-binding) motif.
Cbfb, core-binding factor-β; DN, double negative; HSC, haematopoietic stem cell; ND, not determined; NKT, natural killer T; RUNX, runt-related transcription factor; SP, single positive.
The role of RUNX proteins in T-cell development was first suggested by the discovery that RUNX1 and RUNX3 sequentially interact with the Cd4 silencer17. This 434 base pair sequence is a cis-acting element in the Cd4 locus that restricts the expression of CD4 to appropriate thymocyte populations (FIG. 1) independently of its position and orientation18,19. Germline deletion of the Cd4 silencer resulted in de-repression of CD4 expression in both DN thymocytes and CD8+ T cells20,21; however, conditional deletion of the silencer in mature CD8+ T cells did not cause de-repression of CD4 expression21, which suggests that there are two different mechanisms of silencing22. At the DN stage, the Cd4 silencer may act to actively repress Cd4 transcription, but it is dispensable for keeping Cd4 silenced in peripheral CD8+ T cells; this suggests that Cd4 silencing becomes epigenetically fixed in developing CD8+ T cells23. Consistent with the expression profiles in thymocytes, RUNX1 was found to bind to the Cd4 silencer in DN thymocytes and RUNX3 to bind to the Cd4 silencer in CD8 single positive (SP) thymocytes. The finding that DN thymocytes from mice that were deficient for RUNX1 in the T-cell lineage showed de-repression of CD4 expression, as did CD8+ T cells from mice that were deficient for RUNX3, confirmed the requirement for RUNX complexes in Cd4 silencing17.
Figure 1. The structure of Cd4 and Cd8 loci, illustrating important cis-acting elements.
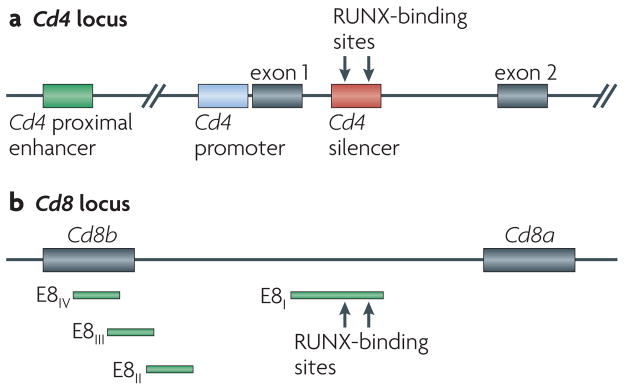
a | The expression of CD4 is driven by the Cd4 proximal enhancer in all T-cell populations and is restricted to the double-positive and CD4 single-positive thymocyte populations owing to the activity of the Cd4 silencer, which is found in intron 1. The binding of RUNX (runt-related transcription factor) proteins to the Cd4 silencer is necessary for silencer activity. b | The expression of Cd8ab is driven by a series of stage-specific enhancers (known as E8I – E8IV), which have overlapping activity.
Beyond their role in regulating stage-specific CD4 expression, RUNX proteins have been shown to have other functions in thymocyte development. RUNX1 was found to be required for effective β-selection and for the positive selection of both CD4+ and CD8+ thymocytes24. The differentiation of natural killer T cells that express invariant TCRs specific for CD1d was also found to depend on RUNX1 (REF. 25). It has been suggested that RUNX3 is involved in the re-expression of CD8 during progression towards the CD8+ T-cell lineage by transitional CD4+CD8low thymocytes that express MHC class I-specific TCRs26. The Cd8 locus is regulated by a combination of stage-specific enhancers27,28, most of which are located in the 35 kb region between the coding sequences of Cd8b and Cd8a (FIG. 1). DNase hypersensitivity mapping and transgenic analyses have shown that there are at least five enhancer elements, termed E8I, E8II, E8III, E8IV and E8V, which have overlapping roles in promoting the expression of CD8 (REFS 29,30). RUNX proteins have been shown to bind to some of these enhancers26, most prominently to E8I, which is capable of directing the expression of a reporter transgene specifically in mature CD8+ T cells, although whether the activity of the E8I enhancer depends on RUNX3 binding remains to be directly addressed31. The specific expression of RUNX3 in CD8 SP thymocytes24,26, along with the putative dual role of RUNX3 in co-receptor gene expression — that is, Cd4 silencing and Cd8 reactivation — suggests that the induction of RUNX3 expression might be central for directing CD8+ T-cell fate. However, ectopic expression of RUNX3 from the DP stage onwards failed to redirect many MHC class II-specific T cells to the CD8+ T-cell fate32,33. Thus, the physiological contribution of RUNX3 in determining the CD4/CD8 lineage choice remained uncertain until recently and awaited further studies, which are described below.
Th-POK and the CD4/CD8 lineage choice
Th-POK was identified as the product of a gene that, when mutated, was responsible for the phenotype of a mouse strain known as helper deficient34, which had been identified earlier as a strain that lacked CD4+ T helper (TH) cells owing to a spontaneous recessive mutation35. Unlike other mouse models in which loss of the CD4+ T-cell lineage can be traced to defects in cell survival, helper-deficient mice have MHC class II-specific T cells that have been redirected to the CD8+ T-cell lineage, as shown by the presence of CD8+ T cells in β2-microglobulin-deficient (and therefore MHC class I-deficient) helper-deficient mice36. This was the first demonstration that positive selection and CD4/CD8 lineage choice are distinct events. The mutation that is responsible for the helper-deficient phenotype was mapped to a single base pair substitution in the first coding exon of Zbtb7b, which is the gene that encodes Th-POK (as named by Kappes and colleagues)34.
Th-POK is a zinc-finger transcription factor of the C2H2-type family and contains a BTB/POZ domain37. Structural studies of other closely related C2H2-type zinc-finger proteins have revealed that each zinc finger comprises approximately 28 amino acids that are organized around a zinc atom that is coordinated by the C2H2 residues38. The mutation that is observed in helper-deficient mice (hd mutation) results in the substitution of a glycine for an arginine (Arg389Gly) in the putative second zinc-finger domain, which presumably abrogates the ability of Th-POK to bind to target DNA sequences and results in a loss of Th-POK function. This has been confirmed by comparative analysis of helper-deficient mice and Th-POK-deficient mice (in which the entire Th-POK coding sequence has been knocked out by homologous recombination), as these two mouse strains are phenotypically identical39. Further analyses showed that the ectopic expression of Th-POK (using transgenic Zbtb7b constructs) in MHC class I-specific thymocytes that would normally develop into CD8+ T cells caused these cells to be redirected to the CD4 lineage34,40. These initial studies identified Th-POK as a master regulator of CD4+ T-cell development, although, as discussed below, more recent studies suggest that the situation is more complicated.
Regulation of Th-POK expression
The breakthrough finding that Th-POK is required for the development of CD4+ T cells prompted investigation into the mechanism that regulates Th-POK expression to further elucidate how the lineage decision process is regulated. Using DNase hypersensitivity mapping and transgenic analyses, He et al.41 identified several cis-acting regulatory elements in the Zbtb7b locus. Of particular interest is the 500 base pair region known as the distal regulatory element (DRE), which is located upstream of exon 1a (FIG. 2). They showed that the DRE could confer CD4 lineage specificity to transgenic constructs regardless of whether the endogenous Zbtb7b promoter or an exogenous human CD2 promoter was used. These data identified that the DRE has both transcriptional silencer and enhancer activities. Following removal of the DRE from the Zbtb7b-promoter-driven construct, a reporter gene was expressed in CD8-lineage cells, which indicated that a silencer element in the DRE was responsible for repressing the expression of Th-POK in CD8-lineage cells and thereby for conferring lineage-specific expression of Th-POK in CD4-lineage cells. To identify the essential sequences and to find functional sites within the DRE, He et al. constructed a series of reporter transgenes with various truncations and mutations at the 500 base pair DRE. This led to the identification of a core 80 base pair sequence that was shown to be essential for repressing of the expression of the reporter transgene in CD8+ T cells. However, mutation of three E-box motifs (sequences to which transcription factors specifically bind and thereby regulate gene expression) within the 500 base pair DRE (including one in the core 80 base pair region), as well as two DRE truncations that excluded either a conserved GATA3-binding site or two RUNX-binding sites, did not significantly affect the downregulation of the reporter transgene in CD8+ cells41. The trans-acting factors that bind to the DRE silencer and the physiological relevance of the activity of this silencer in the expression of Th-POK remain to be elucidated. An independent study by Setoguchi et al.42 identified the same region as an essential transcriptional silencer and provided informative results on its mechanism of action. This is discussed below, in the context of the role of RUNX proteins in lineage decision.
Figure 2. The structure of the Zbtb7b locus, illustrating important cis-acting elements.
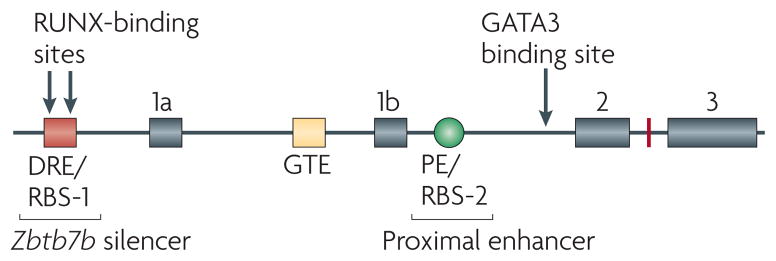
The Zbtb7b locus encodes Th-POK (T-helper-inducing POZ/Kruppel-like factor). The Zbtb7b silencer (designated the distal response element; DRE41 or RUNX (runt-related transcription factor)-binding site 1 (RBS-1)42) restricts the expression of Th-POK to MHC class II-specific thymocytes. The binding of RUNX to this region is necessary but not sufficient for silencer activity. The Zbtb7b silencer region also contains enhancer activity which, together with the general T-lymphoid element (GTE) may be involved in initiating the expression of Th-POK. The proximal enhancer (PE; also known as the proximal regulatory element (PRE)41 and/or RUNX- binding site 2 (RBS-2)42,44) is not required to initiate the expression of Th-POK following positive selection, but is required to maintain Th-POK expression in MHC class II-selected thymocytes that are destined to become CD4+ T cells. Loss of the PE in the Zbtb7b locus leads to gradual loss of Th-POK expression and thereby causes partial redirection of MHC class II-selected thymocytes to the CD8 lineage. GATA3, GATA-binding protein 3.
RUNX proteins and Th-POK repression
Initial studies on the role of RUNX proteins in the CD4/CD8 lineage choice were complicated by the finding that germline deletion of Runx genes is lethal9,12 (TABLE 1), by the redundancy and cross-regulation of RUNX1 and RUNX3 (REF. 43) and because incomplete deletion of conditional alleles leads to leaky RUNX protein expression. More recent work using mice that have a conditional allele of Cbfb (deletion of which results in the functional inactivation of all RUNX proteins)16 and mice with compound mutations in Runx1 and Runx3 has clarified these issues. Analyses of T-cell development in these mice showed that loss of RUNX complex function in DP thymocytes leads to loss of CD8+ T cells24,42 and, importantly, that MHC class I-specific T cells are redirected to the CD4+ T-cell lineage42, a crucial finding that had previously been obscured owing to the experimental limitations discussed above. Examination of thymocyte subsets in mice that lacked RUNX complex function revealed that there was increased expression of Th-POK at developmental stages as early as pre-positive-selection DP thymocytes. This increased expression of Th-POK presumably continued to the CD4+CD8low stage, which is when lineage choice is thought to occur42. Given that transgenic expression of Th-POK at the pre-positive-selection DP stage and at subsequent stages redirects MHC class I-specific thymocytes to the CD4+ T-cell lineage34,40, it is probable that the redirection of MHC class I-specific thymocytes to the CD4+ T-cell lineage in mice that lack functional RUNX proteins is due to an inappropriate upregulation of Th-POK expression. Whether RUNX proteins continue to repress Th-POK expression in CD8 SP cells has not been determined. Following inactivation of the expression of RUNX3, there is a compensatory upregulation of RUNX1, which may repress the expression of Th-POK; this may explain why MHC class I-selected T cells are not redirected to the CD4 lineage under these conditions.
To investigate whether RUNX proteins could directly repress Th-POK expression, Setoguchi et al.42 used a ChIP-on-chip approach to identify binding sites for RUNX complexes across the entire Zbtb7b locus. Using a CBFβ-specific antibody that binds to all RUNX complexes, they identified two RUNX-binding sequences (RBS-1 and RBS-2) that seemed to be bound by RUNX proteins in both Th-POK-expressing and non-expressing cells. The RBS-1 site that was identified in this study overlaps with the DRE site that was identified by He et al.41 (FIG. 2). Further analysis of the RBS-1 site using a reporter transgene expression assay identified that there was transcriptional silencer activity in this sequence42, as was observed in the study by He et al.41. Importantly, Setoguchi et al.42 confirmed the physiological relevance of the RBS-1 sequence by deleting it from the endogenous Zbtb7b allele. This resulted in the upregulation of Th-POK expression in CD69− DP cells, as occurred in RUNX-deficient DP thymocytes, and in the loss of CD8+ T cells (and presumed redirection of MHC class I-specific cells to the CD4 lineage), as had been observed in Zbtb7b transgenic animals34,40. In addition, the combined effect of deleting the RBS-1 silencer and the expression of a reporter transgene on the same Zbtb7b allele revealed that, in heterozygous mice, equal amounts of the reporter transgene were expressed in both CD4+ and CD8+ T cells, and the expression of the reporter transgene was upregulated in CD69− DP cells in a uniform manner42. These two independent studies clearly showed that the DRE/RBS-1 region contains a transcriptional silencer (named the Th-Pok silencer by Setoguchi et al.42 and hereafter described as the Zbtb7b silencer) that has an essential role in CD8+ T-cell development, as it prevents the inappropriate diversion of MHC class I-specific T cells to the CD4 lineage.
However, these two studies revealed a discrepancy in the requirement for RUNX recognition motifs in the DRE/RBS-1 region for Zbtb7b silencer function. In the study by Setoguchi et al.42, the activity of the Zbtb7b silencer was shown to largely depend on two RUNX-binding sites, whereas He et al.41 concluded that these RUNX sites were not necessary for the silencer to function. Therefore, the function of the RUNX-binding sites in physiological conditions will need to be addressed in the context of the endogenous Zbtb7b locus by gene targeting approaches. As RUNX complexes are bound to the Zbtb7b silencer even in Th-POK-expressing cells, it is clear that RUNX binding is not sufficient for Zbtb7b silencing. However, in light of the finding that Zbtb7b expression is de-repressed following the deletion of RUNX proteins, it is possible that RUNX proteins are recruited to the silencer by protein–protein interactions with factors that bind to the core 80 base pair region but have yet to be identified. Another aspect of RUNX-mediated Zbtb7b silencing that remains unexplored is the relative contribution of RUNX1, which is expressed predominantly in DN cells and at lower levels in DP cells, and RUNX3, the expression of which is detected only after positive selection in CD8-fated cells that have progressed beyond the CD4+CD8low stage. It is possible that differences in the DNA-binding affinities of RUNX1 and RUNX3, or in the different proteins with which RUNX1 or RUNX3 associate, contribute to distinct mechanisms of silencing that are mediated by each protein in pre- and post-selection thymocytes.
The finding that RUNX proteins bind to both the Cd4 silencer and the Zbtb7b silencer suggests that some aspects of the regulation of these two genes must be similar. However, there are clearly differences in the activities of the Cd4 and Zbtb7b silencers and, probably, in the mechanism of their regulation. This is most strongly supported by the observation that RUNX1 is sufficient to silence the expression of Th-POK but not CD4 in DP cells, whereas RUNX3 seems to silence the expression of both proteins in CD8 SP cells.
Th-POK in the commitment to the CD4+ T-cell lineage
The mechanism by which silenced Zbtb7b is activated following positive selection is still unclear, although recent work suggests that it requires both the activation of enhancers, including one that is regulated by the transcription factor GATA3 (discussed below), and the reversal of Zbtb7b silencing, which occurs at the DP stage. Evidence that both of these events occur was obtained by a study that examined CD69− DP thymocytes in mice in which the Zbtb7b silencer was deleted and the reporter gene encoding green fluorescent protein (GFP) was knocked into the Zbtb7b locus (Zbtb7bGFP knock-in mice). CD69− DP thymocytes from these mice had detectable and uniform levels of expression of GFP, which were lower than those of CD4 SP thymocytes, indicating that Zbtb7b is actively repressed in all pre-selection thymocytes and that Zbtb7b enhancers are weakly active in these cells. Appropriate induction of Th-POK expression in developing CD4+ T cells therefore requires both the reversal of Zbtb7b silencer activity and the activation of other enhancer elements.
The positive regulatory elements that control Th-POK expression following positive selection seem to be multiple and overlapping. In addition to acting as a silencer, the DRE (the region designated RBS-1 in REF. 42) was found to have enhancer activity (with CD4 lineage specificity presumably conferred by the silencer activity in the 80 base pair core region) when placed upstream of a heterologous promoter (minimal human CD2 promoter) that alone was insufficient to drive the expression of the transgene41. By crossing mice that expressed this transgenic construct with either MHC class I- or MHC class II-specific TCR-transgenic mice, He et al.41 found that reporter transgene expression was significantly higher in MHC class II-specific CD4+CD8low cells than in MHC class I-specific cells. As it is thought that some MHC class II-specific cells at the CD4+CD8low stage are not fully committed to the CD4 lineage5, this result suggests that the enhancer activity of the DRE is activated before full commitment to the CD4 lineage. In the context of the endogenous distal promoter of Zbtb7b, DRE enhancer activity was not sufficient to maintain the expression of the reporter transgene in peripheral CD4+ T cells41, which suggests that the enhancer in the DRE is specifically active in thymocytes and that additional enhancers in the Zbtb7b locus function in mature T cells. Indeed, He et al.41 identified another two enhancer elements, which were designated general T-lymphoid element (GTE) and proximal regulatory element (PRE) (FIG. 2). The GTE was shown to drive transgene expression in both CD4+ and CD8+ T cells independently of the DRE and the PRE, whereas only the PRE could drive the preferential expression of transgenes in mature CD4+ T cells. The PRE is of particular interest, as it is found in the same region of the Zbtb7b locus that contains the RBS-2 element described in the study by Setoguchi et al.42. This region was subsequently designated the proximal enhancer (PE)44. Setoguchi et al. also showed that this region contains enhancer activity that is necessary for maintaining reporter transgene expression in peripheral CD4+ T cells. Although the PE contains two RUNX recognition motifs, it remains unclear whether RUNX binding is necessary for its enhancer activity.
In a subsequent study, Muroi et al.44 examined the physiological role of the PE by removing it from either the Zbtb7b locus or the Zbtb7bGFP locus. Initial induction of GFP expression from the Zbtb7bGFP allele in the CD4+CD8low subset was not significantly affected by the removal of the PE, but upregulation of GFP expression at the transition to the CD4+CD8− stage was inefficient. The PE is therefore dispensable for the initial induction of Th-POK expression, presumably because this is controlled by the enhancer activity of the DRE, whereas it is essential for the maintenance of Th-POK expression in CD4-lineage thymocytes. Because of this stage-specific activity of the PE, homozygous deletion of the PE from the Zbtb7b locus resulted in a gradual loss of Th-POK expression in CD4-lineage-specified thymocytes and consequently a partial redirection of MHC class II-specific cells to the CD8 lineage44. A similar observation was reported independently in a study by Egawa and D.R.L.45, in which a hypomorphic mutant allele of Zbtb7b was used. Both studies demonstrated that CD4+ T cells that developed in the presence of a reduced amount of Th-POK (owing to either deletion of the PE or a hypomorphic mutation in Zbtb7b) showed de-repression of the expression of CD8+ T-cell-associated genes, such as Runx3 and Eomes, and displayed cytotoxic features, such as high levels of interferon-γ (IFNγ) production. Total ablation of Zbtb7b activity resulted in premature expression of a knocked-in reporter transgene under the control of the Runx3 distal promoter in CD4+CD8low transitional thymocytes45. In these mice, CD4+CD8− thymocytes expressed the reporter transgene and underwent progressive redirection to the CD8 lineage. Together, these findings indicate that Th-POK represses the Runx3 distal promoter, although the mechanism by which this occurs has not yet been elucidated. In addition, Th-POK has been shown to antagonize the ability of RUNX complexes to repress CD4 expression46, in part through direct binding to the Cd4 silencer44. These data suggest that antagonistic interplay between RUNX3 and Th-POK is an important component of the CD4/CD8 lineage choice.
Egawa and D.R.L.45 also showed that CD4+CD8− T cells could be generated in mice that were deficient for both RUNX proteins and Th-POK, indicating that the development of CD4-lineage cells might occur independently of Th-POK function if RUNX complexes are non-functional. The finding that CD4-lineage thymocytes and T cells are present in mice that lack Th-POK suggested that this transcription factor is not required for the initial specification of the CD4 T-cell fate during or after positive selection45. This conclusion was reinforced by the study of Wang et al.39 (discussed below), in which the expression of GATA3 was found to be upregulated in MHC class II-specific thymocytes and to be required for the expression of Th-POK. Therefore, Th-POK probably functions as a lineage commitment factor rather than a lineage-specifying factor by repressing the expression of RUNX3 and the activity of other components that are involved in promoting the CD8 lineage, such as Cd4 silencing.
Th-POK expression needs to be appropriately upregulated and maintained during the differentiation of MHC class II-specific cells into mature CD4 SP thymocytes. The results of Muroi et al.44 suggest a mechanism by which this upregulation of Th-POK expression occurs. More specifically, this group showed that Th-POK antagonizes the function of the Zbtb7b silencer by directly binding to it, and proposed that there is an auto-amplification loop in which a restricted amount of Th-POK, the expression of which is induced following receipt of lineage-specifying signals, amplifies its own expression by inhibiting the silencer in its own locus. In light of the observed transdifferentiation of CD4+ T cells into either CD4−CD8− or CD4−CD8+ cells owing to gradual elimination of the expression of Th-POK following the deletion of the PE, this auto-amplification loop would be essential to maintain and amplify the expression of Th-POK and seal the CD4 lineage fate in MHC class II-specific T cells. Further characterization of this auto-amplification loop will probably lead to advances in our understanding of the mechanism by which initial lineage-specifying signals are amplified and differences in TCR signalling are monitored and converted into genetic programmes.
CD4-lineage-specifying factors
Characterization of the interplay between RUNX complexes and Th-POK, and the effects of these proteins on the Zbtb7b, Runx3 and Cd4 loci, is central to our understanding of what solidifies T-cell lineage choice. However, it has become apparent that these are downstream events that reinforce earlier lineage specification events. Recent work has clearly shown that there are other transcription factors that act upstream of Th-POK but downstream of positive selection (and TCR signalling) and that have a role in T-cell lineage specification.
GATA3 has key functions at many stages of T-cell development, from early T-cell precursor specification to mature T-cell differentiation47,48, so analysis of its role in CD4/CD8 lineage choice has required the conditional inactivation of the Gata3 gene (which can be achieved with stage-specific CRE recombinase expression). Mice in which Gata3 was deleted in DP thymocytes had a marked reduction in the number of both CD4 SP cells and CD4+CD8low cells (which in this case were referred to as CD4+CD8int cells)39. Previous results with two different MHC class II-specific TCR-transgenic mouse strains (AND and DO.11.10 mice) did not indicate that GATA3-deficient MHC class II-specific cells were redirected to the CD8 lineage47,48. By contrast, using a third TCR-transgenic mouse strain (5CC7 mice), Wang et al.39 observed that GATA3-deficient thymocytes were redirected to the CD8 lineage, albeit inefficiently. To resolve these discrepant results and to avoid the complications that are associated with the use of TCR-transgenic mice (such as inappropriate early expression of the TCR and binding avidities and affinities that vary between the TCRs in different strains), Wang et al. carried out experiments using bone marrow chimeric mice. T-cell-depleted bone marrow from either wild-type or GATA3-deficient mice was adoptively transferred into lethally irradiated β2-microglobulin mutant mice that lacked cell surface MHC class I expression. A small number of mature CD8+ T cells was found in mice that received GATA3-deficient cells, conclusively showing that redirection could occur in the absence of GATA3 (REF. 39).
Because of the similarities between the phenotypes of Th-POK-deficient and GATA3-deficient mice, Wang et al.39 investigated whether GATA3 and Th-POK function upstream or downstream of one another, or whether they function independently of each other. Although GATA3 was expressed at wild-type levels in CD69+ thymocytes from Th-POK-deficient animals, Th-POK expression was not detected in CD69+ thymocytes from GATA3-deficient mice. Together with the finding that GATA3 binds to the Zbtb7b locus upstream of exon 2, this observation suggested that CD4+ T-cell differentiation is abrogated in GATA3-deficient animals because of a failure to induce Th-POK expression. To test this hypothesis, Wang et al.39 generated GATA3-deficient mice that expressed a Zbtb7b transgene that had previously been found to redirect all thymocytes to the CD4 lineage40. Interestingly, the Zbtb7b transgene failed to rescue CD4+ T-cell differentiation39, suggesting that the lack of Th-POK expression was not the sole cause of the absence of CD4+ T cells in the GATA3-deficient mice. By contrast, CD4−CD8+ T cells were absent from the GATA3-deficient Zbtb7b transgenic mice, although some CD4+CD8+ T cells developed39, suggesting that even though Th-POK cannot promote CD4 lineage differentiation in the absence of GATA3, its ability to repress the CD8 lineage, at least by antagonizing Cd4 silencer activity, is independent of GATA3.
MYB is another transcription factor that is involved in T-cell development. Conditional deletion of MYB in DP thymocytes resulted in a decrease in CD4 SP cell numbers and an increase in CD8 SP cell numbers, whereas the total number of thymocytes was not affected49. Deletion of MYB in MHC class II-specific TCR-transgenic animals, in which all thymocytes should be CD4+, resulted in a marked loss of CD4 SP cells but did not cause redirection of these MHC class II-specific cells to the CD8 lineage. Moreover, overexpression of the viral homologue vMYB in two MHC class I-specific TCR-transgenic models, in which all thymocytes normally develop into CD8 SP cells, blocked the differentiation of CD8 SP cells and caused an increase in the number of CD4+CD8low cells without redirecting them to the CD4 lineage. Interestingly, the expression of MYB and GATA3 increased and decreased in parallel, which suggests that MYB may positively regulate the expression of GATA3 following TCR activation, as supported by the finding that MYB directly binds to the Gata3 locus49.
TOX is a high-mobility group box transcription factor that was initially suggested to be involved in promoting CD8 lineage choice, based on the analysis of a Tox-transgenic mouse strain50. However, recent work examining a TOX-deficient mouse model has revealed that TOX is required for all CD4+ T-cell lineages. Similarly to Th-POK- and GATA3-deficient mice, TOX-deficient mice have a severe reduction in the number of CD4+ T cells51. In addition, TOX-deficient mice have a reduction in CD4+CD8low transitional cells and lack Th-POK expression in positively selected thymocytes, which is similar to the phenotype of GATA3-deficient mice but not to that of Th-POK-deficient mice. GATA3 expression, however, is unaffected by the loss of TOX expression, which suggests that TOX might function either between GATA3 and Th-POK or upstream of Th-POK and in parallel with GATA3, leading to CD4 T-cell lineage specification (FIG. 3). Indeed, MYB-deficient CD4+CD8low cells, in which the level of GATA3 expression is reduced, show only a slight reduction in the levels of TOX expression, which is consistent with the idea that TOX and GATA3 function in parallel. Nevertheless, it is possible that the reduced levels of GATA3 expression in MYB-deficient cells are sufficient to allow both TOX and Th-POK expression. Although the number of CD8 SP thymocytes was slightly decreased by the lack of TOX expression, it was increased in an MHC class I-deficient background. It remains unclear from these data whether there is any redirection of MHC class II-specific cells to the CD8 lineage in Tox−/− mice. It is also unclear whether the lack of CD4+ T cells in TOX-deficient mice is due to the loss of Th-POK expression. This was a plausible hypothesis, but the observation that a Zbtb7b transgene fails to rescue CD4 lineage development in GATA3-deficient mice begs the question of whether the same is true in TOX-deficient mice.
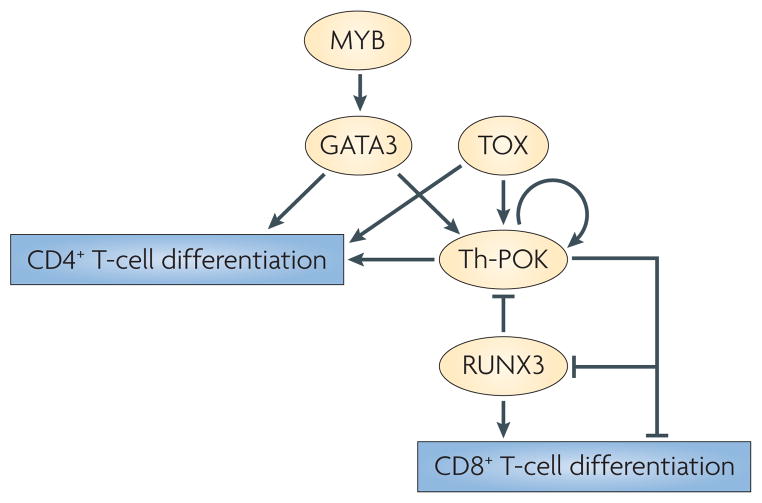
Figure 3. The transcription factor network that underlies T-cell lineage choice.
Th-POK (T-helper-inducing POZ/Kruppel-like factor) ‘seals’ CD4+ T-cell fate, which is specified by GATA-binding protein 3 (GATA3) and thymocyte selection-associated high-mobility group box (TOX), and represses CD8+ T-cell fate in part by repressing the expression of RUNX3 (runt-related transcription factor 3). In CD8+ T cells, RUNX3 maintains silencing of Th-POK expression, thereby repressing the CD4+ T-cell fate and irreversibly committing cells to the CD8 lineage. MYB is involved in promoting CD4 lineage choice, possibly by inducing the expression of GATA3 following T-cell-receptor activation.
A transcription factor network
The phenotypic analyses of thymocytes that are deficient for the transcription factors that are involved in lineage choice leads us to propose the following hierarchy of transcription factor networks (FIG. 3). The expression of Th-POK is initiated in MHC class II-specific thymocytes by the reversal of the activity of the Zbtb7b silencer and by the enhancer in the DRE/RBS-1 with a contribution from the GATA3-binding site. Initial Th-POK expression is increased by an auto-amplification loop that, together with continuous TCR signals triggered by MHC class II binding, keeps the Zbtb7b silencer in an inactive state until the Zbtb7b PE is activated and Th-POK expression reaches a level that is sufficient to antagonize both Cd4 and Zbtb7b silencers independently of TCR signals triggered by MHC class II binding. In parallel, Th-POK potentially activates other CD4 lineage genes and inactivates CD8 lineage genes, thereby sealing the CD4+ T-cell fate. Conversely, the TCR signal that is transduced by the CD8–MHC class I interaction is insufficient to reverse the activity of the Zbtb7b and Cd4 silencers and to maintain the initial expression of Th-POK. In the absence of Th-POK, activation of the distal promoter of Runx3 by mechanisms that have yet to be characterized induces RUNX3 expression. RUNX3 can then keep the expression of Cd4 and Zbtb7b silenced by directly binding to their silencers, in cooperation with other factors (at least in the case of the Zbtb7b silencer) and potentially by regulating the expression of other lineage-specific genes, thereby repressing the CD4 lineage and committing the cell to the CD8 lineage.
An important conclusion that has emerged from the most recent studies is that GATA3 — potentially by acting with other transcription factors — is required upstream of Th-POK to specify the CD4 lineage, and that Th-POK acts later by preventing the alternate lineage fate and thereby contributing to commitment of CD4+ thymocytes to the TH-cell programme.
RUNX regulation of effector T cells
As in many other developmental processes, the transcription factors discussed above have been found to have recurring roles throughout T-cell development and function. Similar to their role in repressing the transcription of Cd4 and Zbtb7b in developing SP thymocytes through binding to the silencers in these genes, RUNX1 and RUNX3 have recently been shown to have a role in repressing Il4 (interleukin-4) transcription in both naive CD4+ T cells and TH1 cells by binding to a previously identified Il4 silencer52. TH1- and TH2-cell subsets are characterized by the expression of the signature cytokines IFNγ and IL-4 and require T-bet (encoded by Tbx21) and GATA3 for their development, respectively53,54. The Il4 silencer was identified as a DNaseI hypersensitive site, the deletion of which resulted in increased IL-4 expression in both naive and TH1 cells52, in which IL-4 is not usually expressed. Two groups independently found that RUNX3 was specifically upregulated and bound to the Il4 silencer in TH1 cells. Djuretic et al.55 found that the upregulation of RUNX3 expression in TH1 cells was T-bet dependent and that both RUNX3 and T-bet were required for maximum IFNγ production. They further showed that T-bet and RUNX3 both associated with the Ifng promoter in a co-dependent manner. Forced retroviral expression of RUNX3 and T-bet in TH2 cells cooperatively repressed the expression of IL-4 and, similarly to their association with the Ifng promoter, RUNX3 and T-bet bound to the Il4 silencer cooperatively55. These results are consistent with the observation that RUNX3 overexpression could not repress the production of IL-4 by CD4+ T cells that lacked the Il4 silencer.
Another study observed that IL-4 expression was de-repressed in CBFβ-deficient CD4+ T cells that had been cultured in either neutral or TH1-cell-polarizing conditions, and that IL-4 production was significantly increased in TH2-cell-polarizing conditions56. Furthermore, the same study used chromatin immuno-precipitation to confirm that RUNX3 binds to the Il4 silencer56. Taken together, these results indicate that, similarly to its ability to inhibit Cd4 and Zbtb7b transcription in developing CD8+ T cells, RUNX3 can bind to the Il4 silencer to repress Il4 expression in differentiating TH1 cells while also cooperating with T-bet to induce maximum IFNγ production.
In addition to TH1 and TH2 cells, there are many other effector T-cell subsets that naive CD4+ T cells can differentiate into following activation, including TH17 cells and regulatory T (TReg) cells. A focus of many recent studies has been to understand the relationship between TH17 cells, which are characterized by the production of Il-17 and are thought to be involved in autoimmunity, and induced TReg cells, which differentiate in the periphery and are thought to be particularly important for controlling immune responses in the gut. Two recent studies have shown that RuNX1 can interact with the transcription factors retinoic-acid-receptor-related orphan receptor-γt (RORγt; encoded by Rorc) and forkhead box P3 (fOXP3), which are required for the differentiation of TH17 and TReg cells, respectively57,58. Zhang et al.59 found that RuNX1 is required for Il17 transcription in TH17 cells (by acting cooperatively with RORγt) and that both RuNX1 and RORγt bind to the Il17 locus. Consistent with these data, other work has shown that fOXP3 can inhibit the production of Il-17 by interacting with RORγt60–62. Similarly, Zhang et al.59 also found that the ability of fOXP3 to inhibit RORγt-dependent Il-17 production depended on its ability to bind to RuNX1 (REF. 59). Another study reported that a similar mechanism occurs at the Il2 locus, and showed that RuNX1 binds to the promoter of the Il2 gene and enhances the TCR-stimulated production of Il-2. In TReg cells, however, fOXP3 inhibits the production of Il-2 by physically interacting with RuNX1 (REF. 63). The study by Zhang et al.59 also showed that attenuation of RuNX1 function (either by gene knockdown or expression of a dominant-negative form of RuNX1) during the differentiation of TH17 cells in vitro decreased the expression of RORγt, suggesting another possible mechanism by which RuNX1 could promote TH17-cell differentiation. because these results were obtained in vitro, the physiological relevance of RuNX1-dependent regulation of TH17-cell differentiation requires clarification in vivo using mouse models. However, the evidence that RuNX1 interacts with T-bet, RORγt and fOXP3 (FIG. 4) indicates that RuNX transcription factors are important modifiers in the transcriptional cascade that underlies both the generation of CD4+ effector T-cell subsets and the lineage choice of DP thymocytes.
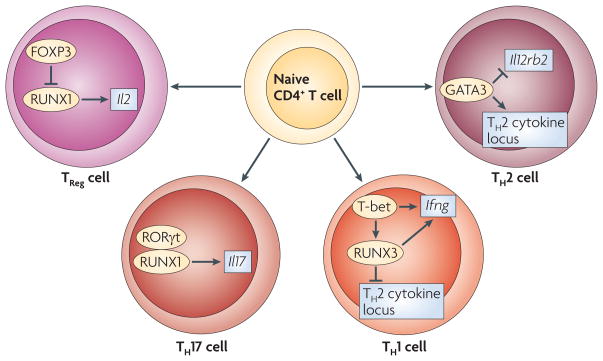
Figure 4. Transcription factor networks that underlie the differentiation of peripheral naive CD4+ T cells into T-helper- and regulatory T-cell subsets.
RUNX3 (runt-related transcription factor 3) represses the production of interleukin-4 (IL-4) by binding to the Il4 silencer in the T helper 2 (TH2) cytokine locus while cooperating with T-bet to promote the production of interferon-γ (IFNγ) in TH1 cells. GATA-binding protein 3 (GATA3), which is required for the expression of TH2-type cytokines (encoded by the TH2-cytokine locus), represses the expression of the TH1-cell-associated gene Il12rb2 in TH2 cells. In TH17 cells, RUNX1 cooperates with the transcription factor retinoic-acid-receptor-related orphan receptor-γt (RORγt) to induce the production of IL-17. In regulatory T (TReg) cells, forkhead box P3 (FOXP3) represses the RUNX1-mediated production of IL-2.
Concluding remarks
We are now beginning to understand the interplay of the transcription factors that control the CD4/CD8 lineage choice and the mechanisms by which they regulate their target genes. The finding that positive selection and lineage choice are distinct processes has been crucial for revising models of lineage choice and for guiding further investigations. The recent observation that some transcription factors are required to initiate lineage specification whereas others are required to repress the alternative cell fate also influences our understanding of this developmental decision. However, many questions remain unanswered. The regulation of the RUNX proteins is a surprisingly unexplored field, investigation of which will probably provide further insight into the role of these transcription factors in lineage choice. Aside from the regulation of Cd4 and Cd8, we have little information on the target genes that might be regulated by Th-POK and RUNX3 to ensure appropriate co-receptor expression for lineage choice and effector T-cell differentiation. Finally, linking the regulation of these transcription factors to the signalling events that are triggered by either CD4–TCR–MHC class II or CD8–TCR–MHC class I interactions will be essential for unravelling the instructing signals that ultimately determine T-cell fate.
Acknowledgments
We thank our many colleagues who have contributed to the study of T-cell lineage choice and apologize for not including all references in this Review owing to space limitations. We are grateful to the members of our laboratories for helpful discussion. The work of I.T. was supported by grants from Precursory Research for Embryonic Science and Technology (PREST) and the Japan Science and Technology Agency (JST). The work of D.R.L. was supported by a grant from the Howard Hughes Medical Institute.
Glossary
- β-selection
A process that, through a cell-autonomous signalling cascade, leads to the proliferation, differentiation and survival of thymocytes that have successfully recombined the β-chain of the T-cell receptor (TCR) locus to express a functional pre-TCR on their cell surface
- DNase hypersensitivity mapping
A technique that is used to investigate DNA regions that are likely to have regulatory roles in gene expression. It is based on the fact that the chromatin at regulatory regions is less condensed than at neighbouring regions, reflecting the need for increased accessibility to transcription factors, which results in higher sensitivity to digestion by endonucleases
- ChIP-on-chip
A technique that combines chromatin immunoprecipitation, which determines the binding of transcription factors to specific regions of genomic DNA, with microarray chips to determine the binding of transcription factors in a genome-wide, unbiased manner
- Hypomorphic mutant allele
A type of mutation in which either the altered gene product has a decreased level of activity or the wild-type gene product is expressed at a decreased level
- CRE recombinase
A site-specific recombination system. Two short DNA sequences (loxP sites) are engineered to flank the target DNA. Expression of the recombinase CRE leads to excision of the intervening sequence. Depending on the type of promoter that controls CRE expression, CRE can be expressed at specific times during development or by specific subsets of cells
- TH17 cell
A subset of CD4+ T helper (TH) cells that produce interleukin-17 (IL-17) and that are thought to be important in inflammatory and autoimmune diseases. Their generation involves IL-23 and IL-21, as well as the transcription factors retinoic-acid-receptor-related orphan receptor-γt and signal transducer and activator of transcription 3
- Regulatory T (TReg) cell
A specialized type of CD4+ T cell that can suppress the responses of other T cells. TReg cells provide a crucial mechanism for the maintenance of peripheral self tolerance and are characterized by the expression of CD25 (also known as the α-chain of the interleukin-2 receptor) and the transcription factor forkhead box P3
Footnotes
DATABASES
Entrez Gene: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene
FOXP3 | GATA3 | Il4 | MYB | RORγt | RUNX1 | RUNX3 | T-bet | TOX | ZBTB7B
FURTHER INFORMATION
Dan R. Littman’s homepage: http://www.med.nyu.edu/research/littmd01.html
Ichiro Taniuchi’s homepage: http://www.riken.jp/engn/r-world/research/lab/rcai/regu/index.html
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Robey EA, et al. Thymic selection in CD8 transgenic mice supports an instructive model for commitment to a CD4 or CD8 lineage. Cell. 1991;64:99–107. doi: 10.1016/0092-8674(91)90212-h. [DOI] [PubMed] [Google Scholar]
- 2.Davis CB, Killeen N, Crooks ME, Raulet D, Littman DR. Evidence for a stochastic mechanism in the differentiation of mature subsets of T lymphocytes. Cell. 1993;73:237–247. doi: 10.1016/0092-8674(93)90226-g. [DOI] [PubMed] [Google Scholar]
- 3.Yasutomo K, Doyle C, Miele L, Fuchs C, Germain RN. The duration of antigen receptor signalling determines CD4+ versus CD8+ T-cell lineage fate. Nature. 2000;404:506–510. doi: 10.1038/35006664. [DOI] [PubMed] [Google Scholar]
- 4.Singer A, Bosselut R. CD4/CD8 coreceptors in thymocyte development, selection, and lineage commitment: analysis of the CD4/CD8 lineage decision. Adv Immunol. 2004;83:91–131. doi: 10.1016/S0065-2776(04)83003-7. [DOI] [PubMed] [Google Scholar]
- 5.Brugnera E, et al. Coreceptor reversal in the thymus: signaled CD4+8+ thymocytes initially terminate CD8 transcription even when differentiating into CD8+ T cells. Immunity. 2000;13:59–71. doi: 10.1016/s1074-7613(00)00008-x. [DOI] [PubMed] [Google Scholar]
- 6.Singer A, Adoro S, Park JH. Lineage fate and intense debate: myths, models and mechanisms of CD4- versus CD8-lineage choice. Nature Rev Immunol. 2008;8:788–801. doi: 10.1038/nri2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito Y. RUNX genes in development and cancer: regulation of viral gene expression and the discovery of RUNX family genes. Adv Cancer Res. 2008;99:33–76. doi: 10.1016/S0065-230X(07)99002-8. [DOI] [PubMed] [Google Scholar]
- 8.Speck NA. Core binding factor and its role in normal hematopoietic development. Curr Opin Hematol. 2001;8:192–196. doi: 10.1097/00062752-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Okuda T, van Deursen J, Hiebert SW, Grosveld G, Downing JR. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell. 1996;84:321–330. doi: 10.1016/s0092-8674(00)80986-1. [DOI] [PubMed] [Google Scholar]
- 10.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfα1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 11.Komori T, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 12.Li QL, et al. Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell. 2002;109:113–124. doi: 10.1016/s0092-8674(02)00690-6. [DOI] [PubMed] [Google Scholar]
- 13.Levanon D, et al. The Runx3 transcription factor regulates development and survival of TrkC dorsal root ganglia neurons. EMBO J. 2002;21:3454–3463. doi: 10.1093/emboj/cdf370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inoue K, et al. Runx3 controls the axonal projection of proprioceptive dorsal root ganglion neurons. Nature Neurosci. 2002;5:946–954. doi: 10.1038/nn925. [DOI] [PubMed] [Google Scholar]
- 15.Sasaki K, et al. Absence of fetal liver hematopoiesis in mice deficient in transcriptional coactivator core binding factor beta. Proc Natl Acad Sci USA. 1996;93:12359–12363. doi: 10.1073/pnas.93.22.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Q, et al. The CBFβ subunit is essential for CBFα2 (AML1) function in vivo. Cell. 1996;87:697–708. doi: 10.1016/s0092-8674(00)81389-6. [DOI] [PubMed] [Google Scholar]
- 17.Taniuchi I, et al. Differential requirements for Runx proteins in CD4 repression and epigenetic silencing during T lymphocyte development. Cell. 2002;111:621–633. doi: 10.1016/s0092-8674(02)01111-x. This study shows that RUNX complexes bind to the Cd4 silencer and are required for the silencing of CD4 expression. [DOI] [PubMed] [Google Scholar]
- 18.Sawada S, Scarborough JD, Killeen N, Littman DR. A lineage-specific transcriptional silencer regulates CD4 gene expression during T lymphocyte development. Cell. 1994;77:917–929. doi: 10.1016/0092-8674(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 19.Siu G, Wurster AL, Duncan DD, Soliman TM, Hedrick SM. A transcriptional silencer controls the developmental expression of the CD4 gene. EMBO J. 1994;13:3570–3579. doi: 10.1002/j.1460-2075.1994.tb06664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leung RK, et al. Deletion of the CD4 silencer element supports a stochastic mechanism of thymocyte lineage commitment. Nature Immunol. 2001;2:1167–1173. doi: 10.1038/ni733. [DOI] [PubMed] [Google Scholar]
- 21.Zou YR, et al. Epigenetic silencing of CD4 in T cells committed to the cytotoxic lineage. Nature Genet. 2001;29:332–336. doi: 10.1038/ng750. [DOI] [PubMed] [Google Scholar]
- 22.Taniuchi I, Ellmeier W, Littman DR. The CD4/CD8 lineage choice: new insights into epigenetic regulation during T cell development. Adv Immunol. 2004;83:55–89. doi: 10.1016/S0065-2776(04)83002-5. [DOI] [PubMed] [Google Scholar]
- 23.Taniuchi I, Littman DR. Epigenetic gene silencing by Runx proteins. Oncogene. 2004;23:4341–4345. doi: 10.1038/sj.onc.1207671. [DOI] [PubMed] [Google Scholar]
- 24.Egawa T, Tillman RE, Naoe Y, Taniuchi I, Littman DR. The role of the Runx transcription factors in thymocyte differentiation and in homeostasis of naive T cells. J Exp Med. 2007;204:1945–1957. doi: 10.1084/jem.20070133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Egawa T, et al. Genetic evidence supporting selection of the Vα14i NKT cell lineage from double-positive thymocyte precursors. Immunity. 2005;22:705–716. doi: 10.1016/j.immuni.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 26.Sato T, et al. Dual functions of Runx proteins for reactivating CD8 and silencing CD4 at the commitment process into CD8 thymocytes. Immunity. 2005;22:317–328. doi: 10.1016/j.immuni.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 27.Ellmeier W, Sawada S, Littman DR. The regulation of CD4 and CD8 coreceptor gene expression during T cell development. Annu Rev Immunol. 1999;17:523–554. doi: 10.1146/annurev.immunol.17.1.523. [DOI] [PubMed] [Google Scholar]
- 28.Kioussis D, Ellmeier W. Chromatin and CD4, CD8A and CD8B gene expression during thymic differentiation. Nature Rev Immunol. 2002;2:909–919. doi: 10.1038/nri952. [DOI] [PubMed] [Google Scholar]
- 29.Ellmeier W, Sunshine MJ, Losos K, Littman DR. Multiple developmental stage-specific enhancers regulate CD8 expression in developing thymocytes and in thymus-independent T cells. Immunity. 1998;9:485–496. doi: 10.1016/s1074-7613(00)80632-9. [DOI] [PubMed] [Google Scholar]
- 30.Hostert A, et al. Hierarchical interactions of control elements determine CD8α gene expression in subsets of thymocytes and peripheral T cells. Immunity. 1998;9:497–508. doi: 10.1016/s1074-7613(00)80633-0. [DOI] [PubMed] [Google Scholar]
- 31.Ellmeier W, Sunshine MJ, Losos K, Hatam F, Littman DR. An enhancer that directs lineage-specific expression of CD8 in positively selected thymocytes and mature T cells. Immunity. 1997;7:537–547. doi: 10.1016/s1074-7613(00)80375-1. [DOI] [PubMed] [Google Scholar]
- 32.Kohu K, et al. Overexpression of the Runx3 transcription factor increases the proportion of mature thymocytes of the CD8 single-positive lineage. J Immunol. 2005;174:2627–2636. doi: 10.4049/jimmunol.174.5.2627. [DOI] [PubMed] [Google Scholar]
- 33.Grueter B, et al. Runx3 regulates integrin αE/CD103 and CD4 expression during development of CD4−/CD8+ T cells. J Immunol. 2005;175:1694–1705. doi: 10.4049/jimmunol.175.3.1694. [DOI] [PubMed] [Google Scholar]
- 34.He X, et al. The zinc finger transcription factor Th-POK regulates CD4 versus CD8 T-cell lineage commitment. Nature. 2005;433:826–833. doi: 10.1038/nature03338. [DOI] [PubMed] [Google Scholar]
- 35.Dave VP, Allman D, Keefe R, Hardy RR, Kappes DJ. HD mice: a novel mouse mutant with a specific defect in the generation of CD4+ T cells. Proc Natl Acad Sci USA. 1998;95:8187–8192. doi: 10.1073/pnas.95.14.8187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keefe R, Dave V, Allman D, Wiest D, Kappes DJ. Regulation of lineage commitment distinct from positive selection. Science. 1999;286:1149–1153. doi: 10.1126/science.286.5442.1149. [DOI] [PubMed] [Google Scholar]
- 37.Bilic I, Ellmeier W. The role of BTB domain-containing zinc finger proteins in T cell development and function. Immunol Lett. 2007;108:1–9. doi: 10.1016/j.imlet.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 38.Klevit RE. Recognition of DNA by Cys2, His2 zinc fingers. Science. 1991;253:1367, 1393. doi: 10.1126/science.1896847. [DOI] [PubMed] [Google Scholar]
- 39.Wang L, et al. Distinct functions for the transcription factors GATA-3 and ThPOK during intrathymic differentiation of CD4+ T cells. Nature Immunol. 2008;9:1122–1130. doi: 10.1038/ni.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun G, et al. The zinc finger protein cKrox directs CD4 lineage differentiation during intrathymic T cell positive selection. Nature Immunol. 2005;6:373–381. doi: 10.1038/ni1183. Along with reference 34, this study establishes the involvement of Th-POK in the differentiation of CD4+ T cells. [DOI] [PubMed] [Google Scholar]
- 41.He X, et al. CD4–CD8 lineage commitment is regulated by a silencer element at the ThPOK transcription-factor locus. Immunity. 2008;28:346–358. doi: 10.1016/j.immuni.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 42.Setoguchi R, et al. Repression of the transcription factor Th-POK by Runx complexes in cytotoxic T cell development. Science. 2008;319:822–825. doi: 10.1126/science.1151844. References 41 and 42 identify a silencer element in the Zbtb7b locus that restricts full Th-POK expression to CD4-fated thymocytes. [DOI] [PubMed] [Google Scholar]
- 43.Woolf E, et al. Runx3 and Runx1 are required for CD8 T cell development during thymopoiesis. Proc Natl Acad Sci USA. 2003;100:7731–7736. doi: 10.1073/pnas.1232420100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muroi S, et al. Cascading suppression of transcriptional silencers by ThPOK seals helper T cell fate. Nature Immunol. 2008;9:1113–1121. doi: 10.1038/ni.1650. [DOI] [PubMed] [Google Scholar]
- 45.Egawa T, Littman DR. ThPOK acts late in specification of the helper T cell lineage and suppresses Runx-mediated commitment to the cytotoxic T cell lineage. Nature Immunol. 2008;9:1131–1139. doi: 10.1038/ni.1652. Together with references 39 and 44, this study helped to define the hierarchy of transcription factors that are involved in T-cell lineage choice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wildt KF, et al. The transcription factor Zbtb7b promotes CD4 expression by antagonizing Runx-mediated activation of the CD4 silencer. J Immunol. 2007;179:4405–4414. doi: 10.4049/jimmunol.179.7.4405. [DOI] [PubMed] [Google Scholar]
- 47.Hernandez-Hoyos G, Anderson MK, Wang C, Rothenberg EV, Alberola-Ila J. GATA-3 expression is controlled by TCR signals and regulates CD4/CD8 differentiation. Immunity. 2003;19:83–94. doi: 10.1016/s1074-7613(03)00176-6. [DOI] [PubMed] [Google Scholar]
- 48.Pai SY, et al. Critical roles for transcription factor GATA-3 in thymocyte development. Immunity. 2003;19:863–875. doi: 10.1016/s1074-7613(03)00328-5. [DOI] [PubMed] [Google Scholar]
- 49.Maurice D, Hooper J, Lang G, Weston K. c-Myb regulates lineage choice in developing thymocytes via its target gene Gata3. EMBO J. 2007;26:3629–3640. doi: 10.1038/sj.emboj.7601801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilkinson B, et al. TOX: an HMG box protein implicated in the regulation of thymocyte selection. Nature Immunol. 2002;3:272–280. doi: 10.1038/ni767. [DOI] [PubMed] [Google Scholar]
- 51.Aliahmad P, Kaye J. Development of all CD4 T lineages requires nuclear factor TOX. J Exp Med. 2008;205:245–256. doi: 10.1084/jem.20071944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ansel KM, et al. Deletion of a conserved Il4 silencer impairs T helper type 1-mediated immunity. Nature Immunol. 2004;5:1251–1259. doi: 10.1038/ni1135. [DOI] [PubMed] [Google Scholar]
- 53.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 54.Szabo SJ, et al. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 55.Djuretic IM, et al. Transcription factors T-bet and Runx3 cooperate to activate Ifng and silence Il4 in T helper type 1 cells. Nature Immunol. 2007;8:145–153. doi: 10.1038/ni1424. [DOI] [PubMed] [Google Scholar]
- 56.Naoe Y, et al. Repression of interleukin-4 in T helper type 1 cells by Runx/Cbfβ binding to the Il4 silencer. J Exp Med. 2007;204:1749–1755. doi: 10.1084/jem.20062456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ivanov I, et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 58.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nature Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 59.Zhang F, Meng G, Strober W. Interactions among the transcription factors Runx1, RORγt and Foxp3 regulate the differentiation of interleukin 17-producing T cells. Nature Immunol. 2008;9:1297–1306. doi: 10.1038/ni.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou L, et al. TGF-β-induced Foxp3 inhibits TH17 cell differentiation by antagonizing RORγt function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ichiyama K, et al. Foxp3 inhibits RORγt-mediated IL-17A mRNA transcription through direct interaction with RORγt. J Biol Chem. 2008;283:17003–17008. doi: 10.1074/jbc.M801286200. [DOI] [PubMed] [Google Scholar]
- 62.Yang XO, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ono M, et al. Foxp3 controls regulatory T-cell function by interacting with AML1/Runx1. Nature. 2007;446:685–689. doi: 10.1038/nature05673. [DOI] [PubMed] [Google Scholar]
- 64.Ichikawa M, et al. AML-1 is required for megakaryocytic maturation and lymphocytic differentiation, but not for maintenance of hematopoietic stem cells in adult hematopoiesis. Nature Med. 2004;10:299–304. doi: 10.1038/nm997. [DOI] [PubMed] [Google Scholar]
- 65.Otto F, et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 66.Niki M, et al. Hematopoiesis in the fetal liver is impaired by targeted mutagenesis of a gene encoding a non-DNA binding subunit of the transcription factor, polyomavirus enhancer binding protein 2/core binding factor. Proc Natl Acad Sci USA. 1997;94:5697–5702. doi: 10.1073/pnas.94.11.5697. [DOI] [PMC free article] [PubMed] [Google Scholar]