Abstract
Purpose
We evaluated the utility of 10-, 12-, and 16-core prostate biopsies for detecting prostate cancer (PCa) and correlated the results with prostate-specific antigen (PSA) levels, prostate volumes, Gleason scores, and detection rates of high-grade prostatic intraepithelial neoplasia (HGPIN) and atypical small acinar proliferation (ASAP).
Materials and Methods
A prospective controlled study was conducted in 354 consecutive patients with various indications for prostate biopsy. Sixteen-core biopsy specimens were obtained from 351 patients. The first 10-core biopsy specimens were obtained bilaterally from the base, middle third, apex, medial, and latero-lateral regions. Afterward, six additional punctures were performed bilaterally in the areas more lateral to the base, middle third, and apex regions, yielding a total of 16-core biopsy specimens. The detection rate of carcinoma in the initial 10-core specimens was compared with that in the 12- and 16-core specimens.
Results
No significant differences in the cancer detection rate were found between the three biopsy protocols. PCa was found in 102 patients (29.06%) using the 10-core protocol, in 99 patients (28.21%) using the 12-core protocol, and in 107 patients (30.48%) using the 16-core protocol (p=0.798). The 10-, 12-, and 16-core protocols were compared with stratified PSA levels, stratified prostate volumes, Gleason scores, and detection rates of HGPIN and ASAP; no significant differences were found.
Conclusions
Cancer positivity with the 10-core protocol was not significantly different from that with the 12- and 16-core protocols, which indicates that the 10-core protocol is acceptable for performing a first biopsy.
Keywords: Needle biopsy, Prostate, Prostatic neoplasms
INTRODUCTION
Other than skin cancers, prostate cancer (PCa) is the most common cancer in men and the second cause of death after lung cancer. The estimated numbers of new cases of PCa and deaths in the United States in 2014 are 233,000 and 29,480, respectively [1]. In Brazil, the number of deaths in 2011 was 13,129, and the estimated number of new cases for 2014 is 68,800 [2].
Screening for PCa is accomplished by digital rectal examination (DRE) and by measuring serum prostate-specific antigen (PSA) levels. A DRE can be uncomfortable and is not welcomed by patients; however, this type of examination is an important screening and staging tool despite the disadvantages of subjectivity and interpersonal variability among examiners. The examination can aid in the detection of tumors in men with low levels of PSA [3].
Transrectal ultrasound (TRUS)-guided biopsy is the most accepted method for diagnosing PCa, which is detected in 30% to 40% of biopsy specimens [4]. This method did not gain popularity until the mid-1980s, when understanding of the anatomy of the prostate for radical prostatectomy and PSA measurement stimulated enthusiasm for early detection of PCa [5]. With the advent of TRUS, non-palpable nodules began to be visualized and biopsied.
Hodge et al. [6] proposed the sextant technique for PCa detection, which consists of the collection of six-core biopsy specimens targeted at the base, middle third, and apex regions of the prostate in sagittal line bilaterally. Subsequent studies have shown that sextant biopsies yield false-negative results in 30% of cases [7].
The sextant method was modified by the inclusion of more lateral biopsies (the method of five regions); four fragments (two on each side) of the most lateral regions and three from the median line were added, totaling 13 fragments. With this technique the number of false-negative results decreased by 35% [8]. In a subsequent study, Presti Jr et al. [9] showed the advantages of prostate biopsy techniques involving a larger number of core fragments and including the latero-lateral regions.
In our Department of Urology at the Botucatu Medical School, the 10-core protocol is standard; the sextant biopsy protocol, extended to obtain 12 cores during the first biopsy, is used at the Brazilian Society of Urology.
The aim of this study was to compare PCa detection in prostate biopsy specimens between the 10-core protocol (protocol of Botucatu Medical School) and the 12-core (used at the Brazilian Society of Urology) and 16-core (overall total) protocols. The number of cores collected during prostate biopsy was also compared with stratified PSA levels, stratified prostate volumes, Gleason scores, and detection rates of high-grade prostatic intraepithelial neoplasia (HGPIN) and atypical small acinar proliferation (ASAP).
MATERIALS AND METHODS
The current prospective controlled study was conducted from January 2011 to February 2012 at the Department of Urology, Botucatu Medical School, Sao Paulo State University, after approval by the Research Ethics Committee.
The criteria for inclusion in the study were as follows: DRE results suggestive of neoplasia, elevated PSA (>4.0 ng/mL in men older than 55 years and >2.5 ng/mL in men younger than 55 years), a PSA density >0.15 ng/mL, and an annual increase in the rate of PSA levels >0.75 ng/mL. Carriers of coagulopathies, individuals with urinary tract infections (whether diagnosed at the time of biopsy or during treatment), and individuals who refused to provide informed written consent were excluded from the study.
Consecutive patients (n=354) were recruited for the study; however, three of these patients were excluded: two for not consenting to participate in the study (did not sign the consent form) and another because they underwent sextant biopsy as a result of an unfavorable medical condition. The patient's medical records were reviewed, and variables such as age, race, serum total PSA (current and previous), free PSA, free PSA/total PSA, and biopsy indication were analyzed.
The biopsy was performed on an outpatient basis in a room equipped with all material necessary for emergency intervention. Sedation and anesthesia were achieved by using 50-mcg fentanyl citrate and 5-mg midazolam. The biopsies were performed by two experienced urologists.
On the morning of the procedure, a rectal enema (250 mL) was performed, and antibiotic prophylaxis was achieved with the oral administration of 500-mg ciprofloxacin 2 hours prior to the procedure and again 8 hours afterward. The procedure was performed while the patient was in the left lateral position with the thighs flexed. The procedure was performed by using Dornier TRUS equipment with a 6.5-MHz multiplanar probe, an auto-fire gun, and an 18-gauge needle.
Initially 10 punctures were performed, yielding core specimens from the following regions of the prostate bilaterally: base, middle third, apex, medial (transitional zone), and latero-lateral. After these specimens were collected, six additional punctures were performed bilaterally in the same specimens in the more lateral regions of the base, middle third, and apex (Fig. 1).
FIG. 1.
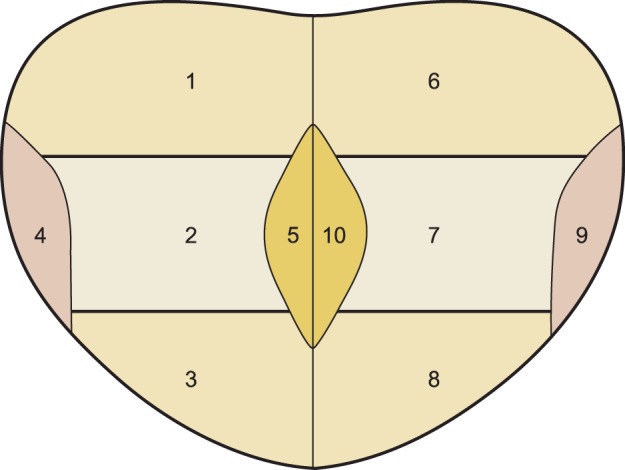
Regions where punctures were made to collect prostate cores: 1, right base; 2, right middle third; 3, right apex; 4, latero-lateral right; 5, right medial; 6, left base; 7, left middle third; 8, left apex; 9, latero-lateral left; 10, left medial.
A positive diagnosis of PCa was compared between the 10-core protocol (base, middle third, apex, medial [transitional zone] and latero-lateral, bilaterally) and the 12-core (base, middle third, apex, and more lateral regions of the base, middle third, and apex, bilaterally) and 16-core (overall total) protocols (Fig. 2). Tumor detection with the 10-, 12-, and 16-core protocols was correlated with PSA levels, prostate volumes, Gleason scores, and detection rates of HGPIN and ASAP.
FIG. 2.
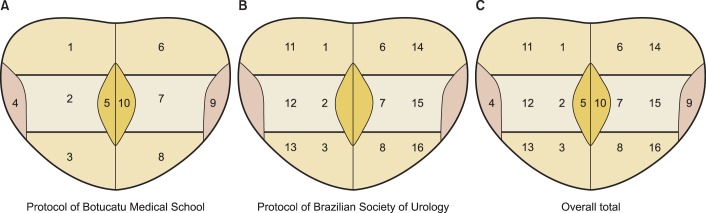
Biopsy protocols: Protocol of Botucatu Medical School with 10 cores (A) - Protocol of Brazilian Society of Urology with 12 cores (B) - Overall total with 16 cores (C). 1, right base; 2, right middle third; 3, right apex; 4, latero-lateral right; 5, right medial; 6, left base; 7, left middle third; 8, left apex; 9, latero-lateral left; 10, left medial: 11, right base; 12, right middle third; 13, right apex; 14, left base; 15, left middle third; and 16, left apex.
Data were collected and recorded on an Excel spreadsheet and analyzed by using SAS 9.2 (SAS Institute Inc., Cary, NC, USA). Results for age, PSA level, and prostate volume are expressed as means and standard deviations. Qualitative variables are expressed as frequencies and percentages. Chi-square and Fisher exact tests were used to evaluate differences between the variables; the significance level was set at 5%. The prevalence of PCa diagnosis, with 90% power, was determined by using a one-sided hypothesis test and a significance level of 5%.
RESULTS
A total of 351 consecutive patients underwent TRUS-guided prostate biopsy with 16-core fragments. Mean age, total PSA level, prostate volume, and race are shown in Table 1. Four patients had PSA levels >100 ng/mL (137, 198, 2,000, and 2,585 ng/mL)-greater than the mean and standard deviation.
TABLE 1.
Characteristics of patients undergoing prostate biopsy according to age, prostate-specific antigen level, prostate volume, and race
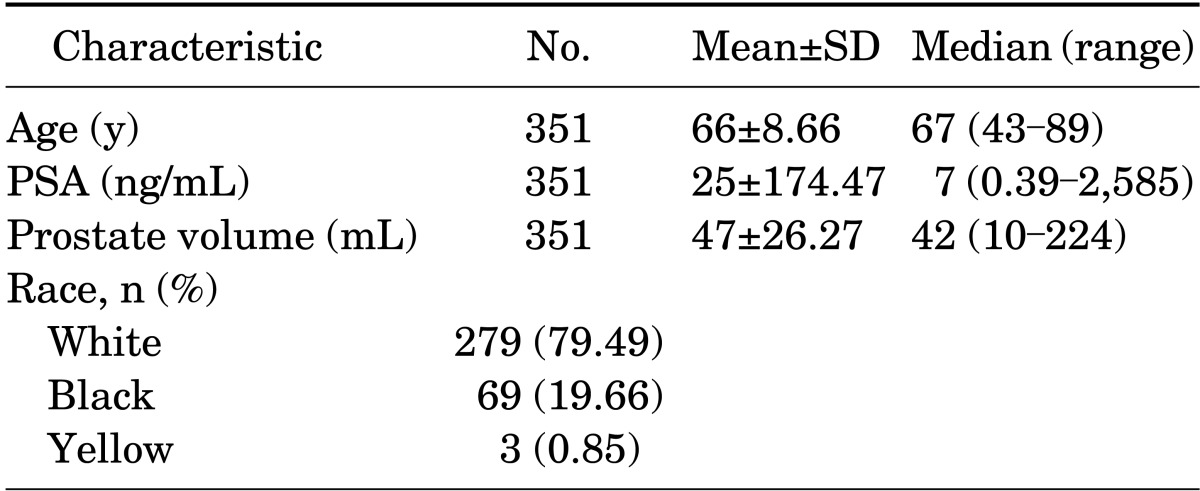
SD, standard deviation; PSA, prostate-specific antigen.
Most patients (68.81%) had a high PSA level, 13.11% had an abnormal DRE result, 12.25% had an association between a high PSA and the presence of a nodule or an abnormal DRE result, and 5.83% had an increased PSA velocity. Results of the DRE performed before prostate biopsy were abnormal in 93 patients (26.50%) and were normal in 258 patients (73.50%) (T1c). Examination of the prostate by TRUS, before the prostate biopsy, detected hypoechoic nodules in 98 of the patients (27.92%).
PCa positivity in prostate biopsy specimens, by the number and location of the biopsy cores, is shown in Fig. 3. The cancer detection rate was not significantly different between the three biopsy protocols. PCa was detected in 102 patients (29.06%) with the 10-core protocol, in 99 patients (28.21%) with the 12-core protocol, and in 107 patients (30.48%) with the 16-core protocol (p=0.79).
FIG. 3.
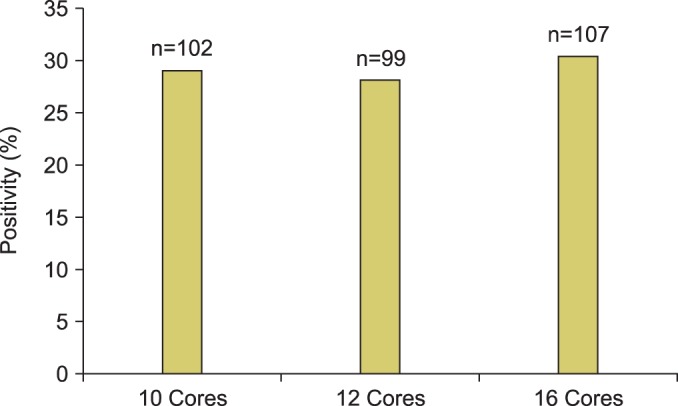
Percentage of cancer positivity by the number of core biopsy specimens collected.
The PSA level was stratified as 0 to 2.5, 2.6 to 4.0, 4.1 to 10.0, and >10.0 ng/mL, and prostate volume was stratified as ≤20, 20 to 50, and >50 cm3. PCa positivity in the prostate biopsy specimens in relation to stratified PSA levels (ng/mL), stratified prostate volumes (cm3), and number of core biopsy specimens is shown in Table 2. Elevated PSA levels were associated with greater PCa positivity, especially when levels were >10.0 ng/mL. In two patients with a PSA level <2.0 ng/mL, PCa was detected on biopsy. A comparison of PSA levels with the number of core biopsy specimens showed no significant differences, nor did a comparison of prostate volumes with the number of core biopsy specimens. PCa detection rates were greatest at prostate volumes between 20 and 50 cm3 and were lower at prostate volumes >50 cm3.
TABLE 2.
Cancer detection rates with prostate biopsies in relation to stratified prostate-specific antigen level, stratified prostate volume, and number of core biopsies
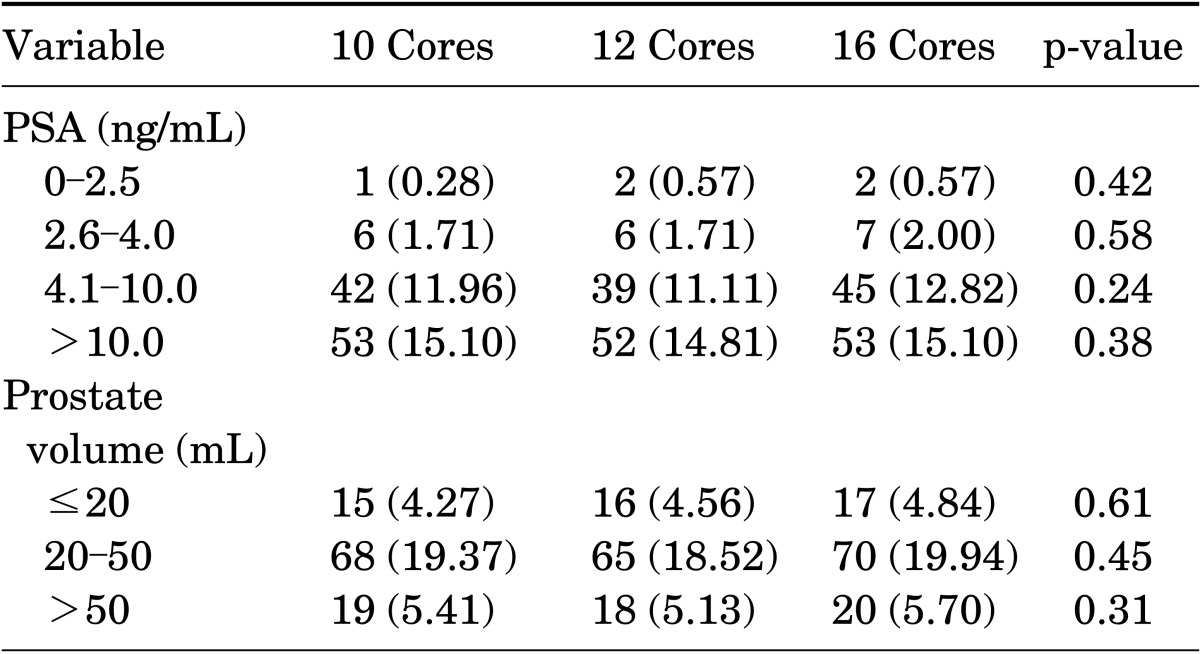
Values are presented as number (%).
Correlations between tumor Gleason scores, number of prostate biopsy specimens, and PSA values are shown in Table 3. Most patients had tumors with a Gleason score of 7 followed by a Gleason score of 6, and few patients had a Gleason score of 8 or 9. No significant differences in cancer detection rates with the three biopsy protocols were found in relation to Gleason scores and PSA values. The detection rates of ASAP and HGPIN in patients with negative biopsy results were stratified according to the three biopsy protocols and PSA values (Table 4). No statistically significant differences were found in the detection of ASAP and HGPIN in relation to the number (10, 12, or 16) of cores collected.
TABLE 3.
Cancer detection rates with different biopsy protocols in relation to Gleason scores and prostate-specific antigen levels
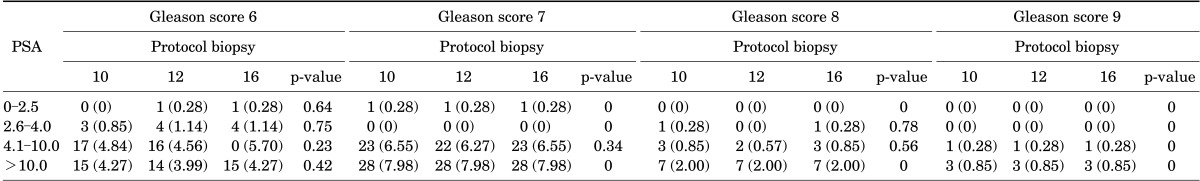
TABLE 4.
Detection rates of high-grade prostatic intraepithelial neoplasia and ASAP in patients with negative biopsy results in relation to stratified prostate-specific levels and the number of core biopsies
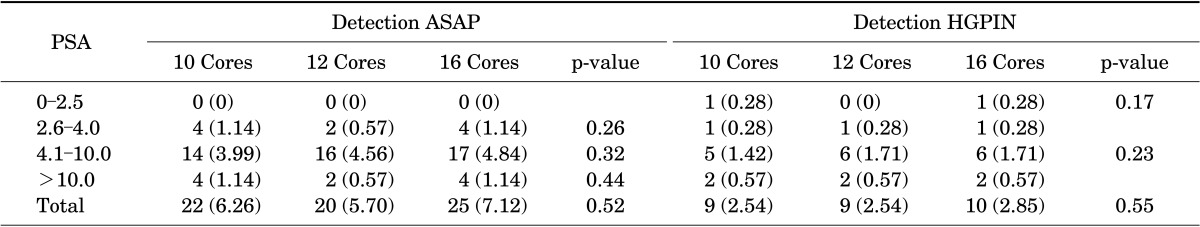
Values are presented as number (%).
ASAP, atypical small acinar proliferation; HGPIN, high-grade prostatic intraepithelial neoplasia.
DISCUSSION
PCa is an insidious neoplasm, and, as with any other malignancy, early detection is important. The introduction of serum PSA screening for PCa was a major breakthrough in the early diagnosis of the disease, which allows for the detection of subclinical malignancies. The development of treatments such as radical prostatectomy has led to permanent cure in a large number of patients or to an improved life expectancy.
TRUS-guided prostate biopsy has been the standard method for diagnosing PCa, but there is no consensus about the exact number of fragments to be collected. Several studies have attempted to define this number. Initially, Hodge et al. [6] proposed the sextant technique; however, subsequent studies have shown that sextant biopsies yield false-negative results in 30% of cases. Eskew et al. [8], in a study of 119 patients, added five more core specimens to the sextant biopsy, which improved the cancer detection rate by 35%. Levine et al. [7] added six more core specimens to the sextant biopsy, which resulted in the detection of an additional 30% of cancer cases. However, Naughton et al. [10], in a prospective randomized study of 244 patients, found that the 6- and 12-core protocols yielded similar cancer positivity: 26% and 27%, respectively (p=0.9). Presti Jr et al. [9] reported that sextant biopsies failed to detect PCa in 20% of 483 patients as compared with the 8- and 10-core protocols collected from lateral regions.
Presti Jr [11] reviewed available studies that analyzed various biopsy protocols and suggested that an initial biopsy should include a minimum of 12 cores (extended biopsy), with special attention to the lateral regions of the prostate. In our study, PCa detection rates with the 10-, 12-, and 16-core protocols were 29.06%, 28.21%, and 30.48%, respectively (p=0.79). No statistically significant differences in PCa detection rates were found between these three protocols.
The saturation biopsies with more than 20 cores were proposed for patients with multiple previous negative biopsy results. However, it is worth noting that the false-negative rate of saturation biopsy was reported to be similar to that found in patients who underwent traditional initial extended biopsy [12]. Jones et al. [13], in a study of 226 patients, reported that PCa positivity rates were 44.6% and 51.7%, respectively (p>0.9), with a saturation biopsy consisting of 24 cores compared with 10 cores. Pepe and Aragona [14] compared saturation biopsies (24-37 cores) with 18- and 12-core protocols; the detection rates of PCa on initial biopsy were 46.9%, 49% (p=0.6), and 39.8% (p=0 3), respectively. However, on second and third rebiopsies, the saturation biopsy had a higher detection rate than did the 18-core biopsy: 22% versus 10.9% (p=0.003) and 6.2% versus 0%, respectively. Nomikos et al. [15], in a retrospective study, compared 24-core and 10-core protocols; PCa detection rates were 34.55% and 39.09%, respectively (p=0.43). However, the study by Ceylan et al. [16] showed a benefit of saturation biopsy. In a group of 1,120 patients, 8-, 10-, 12-, 16-, and 20-core protocols yielded cancer detection rates of 18.3%, 14.8%, 24%, 22.1%, and 30.3%, respectively (p=0.008).
According to the literature reviewed, the sextant biopsy yields false-negative results in 30% of cases and should not be used. Saturation biopsy as initial biopsy does not appear to be more effective at diagnosing PCa than does extended biopsy. In summary, extended biopsy is indicated for the first biopsy and saturation biopsy may be indicated for rebiopsies.
Establishing a cutoff value for PSA aims to ensure greater diagnostic accuracy. Published data show that high-grade tumors may be found in patients with a PSA level as low as <4.0 ng/mL, which led to a reduction in the cutoff value to 2.5 ng/mL in some guidelines, especially for younger men, i.e., <60 years [17]. Physicians should evaluate PSA cutoff values for each patient individually to allow the diagnosis of aggressive tumors without increasing the diagnosis of indolent tumors.
The higher the PSA level, the greater the PCa positivity rate (especially when >10 ng/mL), which was also evidenced in other studies [15,18]. In two patients with a PSA level <2.0 ng/mL, PCa was detected on biopsy. The indication for biopsy was the presence of prostatic nodules detected on DRE, which indicated that this type of examination may reveal tumors in men with a low PSA level [3]. In other studies also, no statistically significant differences were found between PSA levels by the number of cores [15,16,18].
The number of cores required to diagnose PCa in relation to the size of the prostate is not yet defined. Many studies have shown that the greater the number of cores collected in larger prostates, the greater the PCa detection rate [16,18,19]. The Vienna nomogram was developed to define an appropriate number of prostate biopsy cores to improve the detection of PCa based on the age of the patient and the prostate volume. Remzi et al. [20] showed that the detection rate of PCa with the Vienna nomogram was 36.7% compared with 22% on first biopsy in the control group of eight cores. However, Lecuona and Heyns [21], in a prospective controlled clinical trial, suggested that there was no significant advantage to using the Vienna nomogram to determine the number of prostate biopsies to be performed compared with the control group of eight fragments. In our study, we found no statistically significant differences in cancer detection rates when we compared prostate volumes with the number of cores collected (10, 12, and 16). PCa positivity in prostates >50 cm3 was lower when compared with prostates between 20 and 50 cm3. It remains doubtful whether more than 16 core fragments could increase the cancer positivity rate in prostates >50 cm3.
The correlation between Gleason scores and the biopsy protocol and PSA values in other studies also showed no significant differences in tumor detection rates [15,22]. Mian et al. [23] studied 426 patients, 221 of whom had undergone sextant biopsy and 205 saturation biopsy before radical prostatectomy. The sextant biopsy had a lower concordance rate with Gleason scores than did the saturation biopsy when compared with Gleason scores with radical prostatectomy. Other studies have also shown that the increase in the number of cores collected improves the concordance rate between Gleason scores with prostate biopsy and radical prostatectomy [24]. In our study, the correlation of prostate biopsy Gleason scores with radical prostatectomy Gleason scores was not evaluated.
Few studies have addressed the influence of increasing the number of fragments in prostate biopsy on the detection rates of HGPIN and ASAP. Ploussard et al. [25] published a study in which HGPIN and ASAP were detected in 35.7% of cases by sextant biopsy, in 28.6% of cases with 6 additional cores (total of 12 cores), and in 35.7% of cases with 21 cores. Epstein and Potter [26] also showed no relationship between the number of cores collected during prostate biopsy and the incidence of HGPIN and ASAP. Nomikos et al. [15], in a retrospective study involving patients with a PSA level <10 ng/mL, reported that a 24-core prostate biopsy protocol increased the detection rate of HGPIN by 20% (p=0.0008) compared with a 10-core protocol. In our study, no statistically significant difference was found in the detection rates of ASAP and HGPIN in relation to the number of core fragments (10, 12, or 16) collected. The detection rates of ASAP and HGPIN were approximately 6% and 2.5%, respectively, consistent with the literature [27].
In our study, we collected cores bilaterally in the medial, transitional zone. The detection rate was low (1.8%). Corroborating the data of Pelzer et al. [28], we found that it did not improve the cancer detection rate, and there is currently no requirement to collect cores from the transitional zone. In our sample of 351 patients, only 1 patient (0.28%) would have remained undiagnosed with PCa if cores from the transitional zone were not collected.
Considering the data presented, we concluded that the protocol with 10 cores on first biopsy is sufficient to obtain a high positivity rate (29.06%) as compared with protocols with more cores. The most important fact is that additional biopsies should capture more lateral regions of the prostate. In the current study, extended biopsies of additional lateral tissue increased the PCa detection rate by 20% compared with the sextant biopsy. Thus, we observed that the number of lateral biopsies collected is more important than the total number of biopsies collected. Indeed, biopsies of the transitional zone did not increase the PCa detection rate. However, it is clear from the literature that a greater number of biopsies is required to yield higher detection rates with rebiopsies (extended or saturation biopsies). We also propose a modification to the biopsy protocol used at the Botucatu Medical School, i.e., collect 10 cores, do not collect the 2 medial cores, and collect more lateral cores (base, middle third, apex and two latero-lateral, bilaterally).
Furthermore, we must consider that increasing the number of biopsy specimens collected increases the duration of the procedure and, consequently, the discomfort of patients, especially when analgesia is induced with local anesthetics, which is the most commonly used method. However, the number of biopsy specimens collected is not as important when using intravenous sedation and analgesia, as is performed when it is necessary to obtain a large number of fragments [29]. However, collection of a greater number of fragments could be a risk factor for complications after biopsy. de Jesus et al. [30] reported that the collection of more than eight fragments increases the likelihood of infectious complications.
CONCLUSIONS
Cancer positivity with the 10-core protocol was not significantly different from that with the 12- and 16-core protocols, which indicates that the 10-core protocol is acceptable for performing a first biopsy.
Footnotes
The authors have nothing to disclose.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Estimativa 2014 - Incidência de câncer no Brasil [Internet] Rio de Janeiro: Instituto Nacional do Câncer; 2014. [cited 2011 Jun 1]. Available from: http://www2.inca.gov.br/wps/wcm/connect/tiposdecancer/site/home/prostata. [Google Scholar]
- 3.Faria EF, Carvalhal GF, Vieira RA, Silva TB, Mauad EC, Carvalho AL. Program for prostate cancer screening using a mobile unit: results from Brazil. Urology. 2010;76:1052–1057. doi: 10.1016/j.urology.2010.02.044. [DOI] [PubMed] [Google Scholar]
- 4.Escudero Bregante JF, Lopez Cubillana P, Cao Avellaneda E, Lopez Lopez AI, Maluff Torres A, Lopez Gonzalez PA, et al. Clinical efficacy of prostatic biopsy. Experience in our center from 1990 to 2002. Actas Urol Esp. 2008;32:713–716. doi: 10.1016/s0210-4806(08)73919-6. [DOI] [PubMed] [Google Scholar]
- 5.Walsh PC, Lepor H, Eggleston JC. Radical prostatectomy with preservation of sexual function: anatomical and pathological considerations. Prostate. 1983;4:473–485. doi: 10.1002/pros.2990040506. [DOI] [PubMed] [Google Scholar]
- 6.Hodge KK, McNeal JE, Terris MK, Stamey TA. Random systematic versus directed ultrasound guided transrectal core biopsies of the prostate. J Urol. 1989;142:71–74. doi: 10.1016/s0022-5347(17)38664-0. [DOI] [PubMed] [Google Scholar]
- 7.Levine MA, Ittman M, Melamed J, Lepor H. Two consecutive sets of transrectal ultrasound guided sextant biopsies of the prostate for the detection of prostate cancer. J Urol. 1998;159:471–475. doi: 10.1016/s0022-5347(01)63951-x. [DOI] [PubMed] [Google Scholar]
- 8.Eskew LA, Bare RL, McCullough DL. Systematic 5 region prostate biopsy is superior to sextant method for diagnosing carcinoma of the prostate. J Urol. 1997;157:199–202. [PubMed] [Google Scholar]
- 9.Presti JC, Jr, Chang JJ, Bhargava V, Shinohara K. The optimal systematic prostate biopsy scheme should include 8 rather than 6 biopsies: results of a prospective clinical trial. J Urol. 2000;163:163–166. [PubMed] [Google Scholar]
- 10.Naughton CK, Miller DC, Mager DE, Ornstein DK, Catalona WJ. A prospective randomized trial comparing 6 versus 12 prostate biopsy cores: impact on cancer detection. J Urol. 2000;164:388–392. [PubMed] [Google Scholar]
- 11.Presti JC., Jr Prostate biopsy strategies. Nat Clin Pract Urol. 2007;4:505–511. doi: 10.1038/ncpuro0887. [DOI] [PubMed] [Google Scholar]
- 12.Lane BR, Zippe CD, Abouassaly R, Schoenfield L, Magi-Galluzzi C, Jones JS. Saturation technique does not decrease cancer detection during followup after initial prostate biopsy. J Urol. 2008;179:1746–1750. doi: 10.1016/j.juro.2008.01.049. [DOI] [PubMed] [Google Scholar]
- 13.Jones JS, Patel A, Schoenfield L, Rabets JC, Zippe CD, Magi-Galluzzi C. Saturation technique does not improve cancer detection as an initial prostate biopsy strategy. J Urol. 2006;175:485–488. doi: 10.1016/S0022-5347(05)00211-9. [DOI] [PubMed] [Google Scholar]
- 14.Pepe P, Aragona F. Saturation prostate needle biopsy and prostate cancer detection at initial and repeat evaluation. Urology. 2007;70:1131–1135. doi: 10.1016/j.urology.2007.07.068. [DOI] [PubMed] [Google Scholar]
- 15.Nomikos M, Karyotis I, Phillipou P, Constadinides C, Delakas D. The implication of initial 24-core transrectal prostate biopsy protocol on the detection of significant prostate cancer and high grade prostatic intraepithelial neoplasia. Int Braz J Urol. 2011;37:87–93. doi: 10.1590/s1677-55382011000100011. [DOI] [PubMed] [Google Scholar]
- 16.Ceylan C, Doluoglu OG, Aglamis E, Baytok O. Comparison of 8, 10, 12, 16, 20 cores prostate biopsies in the determination of prostate cancer and the importance of prostate volume. Can Urol Assoc J. 2014;8:E81–E85. doi: 10.5489/cuaj.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohler J, Bahnson RR, Boston B, Busby JE, D'Amico A, Eastham JA, et al. NCCN clinical practice guidelines in oncology: prostate cancer. J Natl Compr Canc Netw. 2010;8:162–200. doi: 10.6004/jnccn.2010.0012. [DOI] [PubMed] [Google Scholar]
- 18.Yoon BI, Shin TS, Cho HJ, Hong SH, Lee JY, Hwang TK, et al. Is it effective to perform two more prostate biopsies according to prostate-specific antigen level and prostate volume in detecting prostate cancer? Prospective study of 10-core and 12-core prostate biopsy. Urol J. 2012;9:491–497. [PubMed] [Google Scholar]
- 19.Mariappan P, Chong WL, Sundram M, Mohamed SR. Increasing prostate biopsy cores based on volume vs the sextant biopsy: a prospective randomized controlled clinical study on cancer detection rates and morbidity. BJU Int. 2004;94:307–310. doi: 10.1111/j.1464-410X.2004.04928.x. [DOI] [PubMed] [Google Scholar]
- 20.Remzi M, Fong YK, Dobrovits M, Anagnostou T, Seitz C, Waldert M, et al. The Vienna nomogram: validation of a novel biopsy strategy defining the optimal number of cores based on patient age and total prostate volume. J Urol. 2005;174(4 Pt 1):1256–1260. doi: 10.1097/01.ju.0000173924.83392.cc. [DOI] [PubMed] [Google Scholar]
- 21.Lecuona A, Heyns CF. A prospective, randomized trial comparing the Vienna nomogram to an eight-core prostate biopsy protocol. BJU Int. 2011;108:204–208. doi: 10.1111/j.1464-410X.2010.09887.x. [DOI] [PubMed] [Google Scholar]
- 22.Naya Y, Ochiai A, Troncoso P, Babaian RJ. A comparison of extended biopsy and sextant biopsy schemes for predicting the pathological stage of prostate cancer. J Urol. 2004;171(6 Pt 1):2203–2208. doi: 10.1097/01.ju.0000127729.71350.7f. [DOI] [PubMed] [Google Scholar]
- 23.Mian BM, Lehr DJ, Moore CK, Fisher HA, Kaufman RP, Jr, Ross JS, et al. Role of prostate biopsy schemes in accurate prediction of Gleason scores. Urology. 2006;67:379–383. doi: 10.1016/j.urology.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 24.Leite KR, Srougi M, Dall'Oglio MF, Sanudo A, Camara-Lopes LH. Histopathological findings in extended prostate biopsy with PSA < or = 4 ng/mL. Int Braz J Urol. 2008;34:283–290. doi: 10.1590/s1677-55382008000300005. [DOI] [PubMed] [Google Scholar]
- 25.Ploussard G, Plennevaux G, Allory Y, Salomon L, Azoulay S, Vordos D, et al. High-grade prostatic intraepithelial neoplasia and atypical small acinar proliferation on initial 21-core extended biopsy scheme: incidence and implications for patient care and surveillance. World J Urol. 2009;27:587–592. doi: 10.1007/s00345-009-0413-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Epstein JI, Potter SR. The pathological interpretation and significance of prostate needle biopsy findings: implications and current controversies. J Urol. 2001;166:402–410. [PubMed] [Google Scholar]
- 27.Iczkowski KA, Chen HM, Yang XJ, Beach RA. Prostate cancer diagnosed after initial biopsy with atypical small acinar proliferation suspicious for malignancy is similar to cancer found on initial biopsy. Urology. 2002;60:851–854. doi: 10.1016/s0090-4295(02)01981-7. [DOI] [PubMed] [Google Scholar]
- 28.Pelzer AE, Bektic J, Berger AP, Halpern EJ, Koppelstatter F, Klauser A, et al. Are transition zone biopsies still necessary to improve prostate cancer detection? Results from the tyrol screening project. Eur Urol. 2005;48:916–921. doi: 10.1016/j.eururo.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 29.Tsuji FH, Chambo RC, Agostinho AD, Trindade Filho JC, de Jesus CM. Sedoanalgesia with midazolam and fentanyl citrate controls probe pain during prostate biopsy by transrectal ultrasound. Korean J Urol. 2014;55:106–111. doi: 10.4111/kju.2014.55.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Jesus CM, Correa LA, Padovani CR. Complications and risk factors in transrectal ultrasound-guided prostate biopsies. Sao Paulo Med J. 2006;124:198–202. doi: 10.1590/S1516-31802006000400005. [DOI] [PMC free article] [PubMed] [Google Scholar]


