Abstract
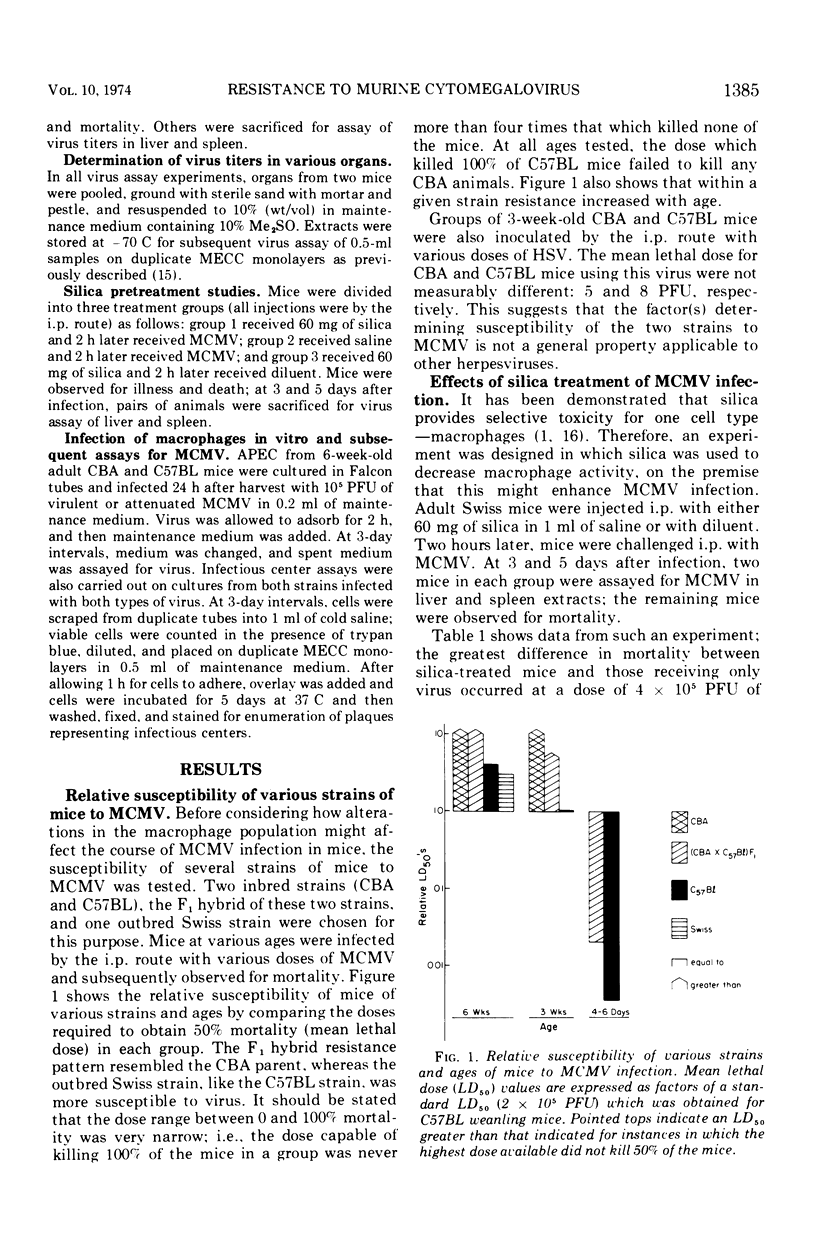
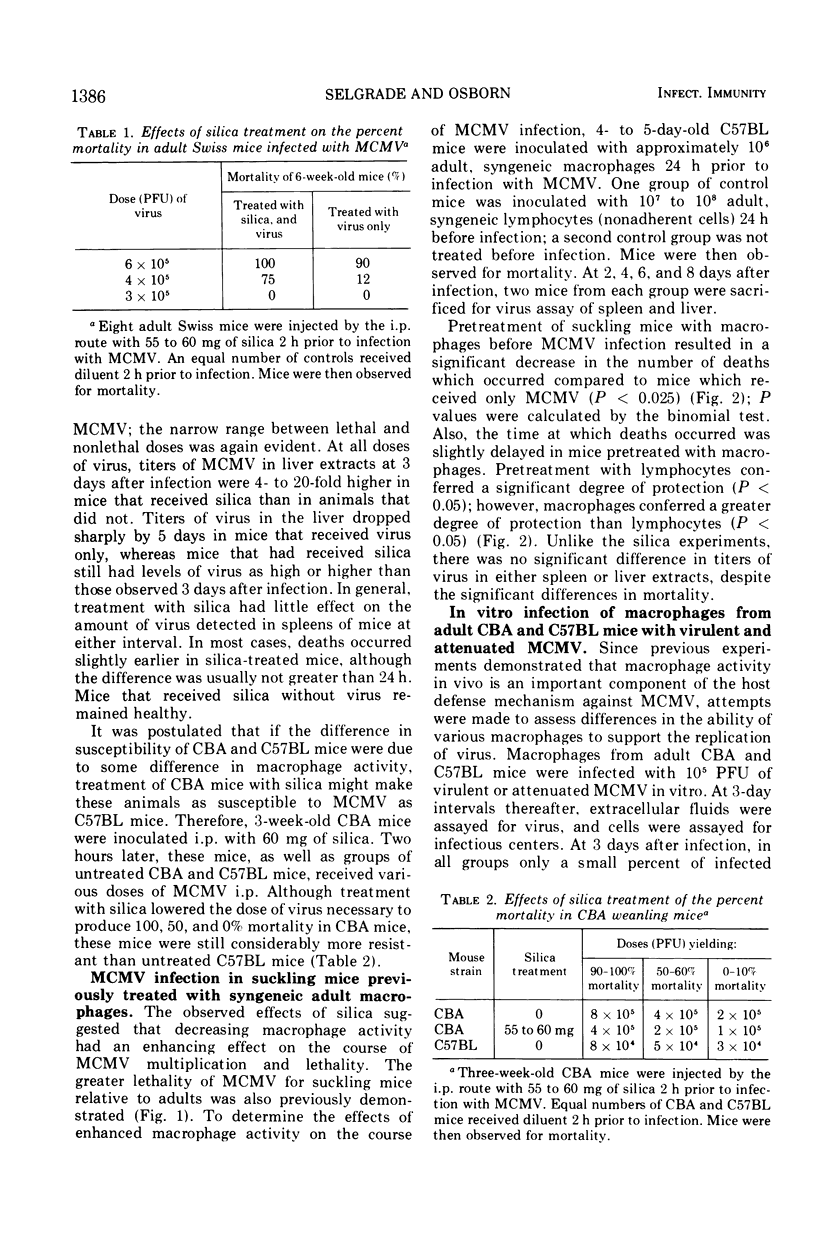
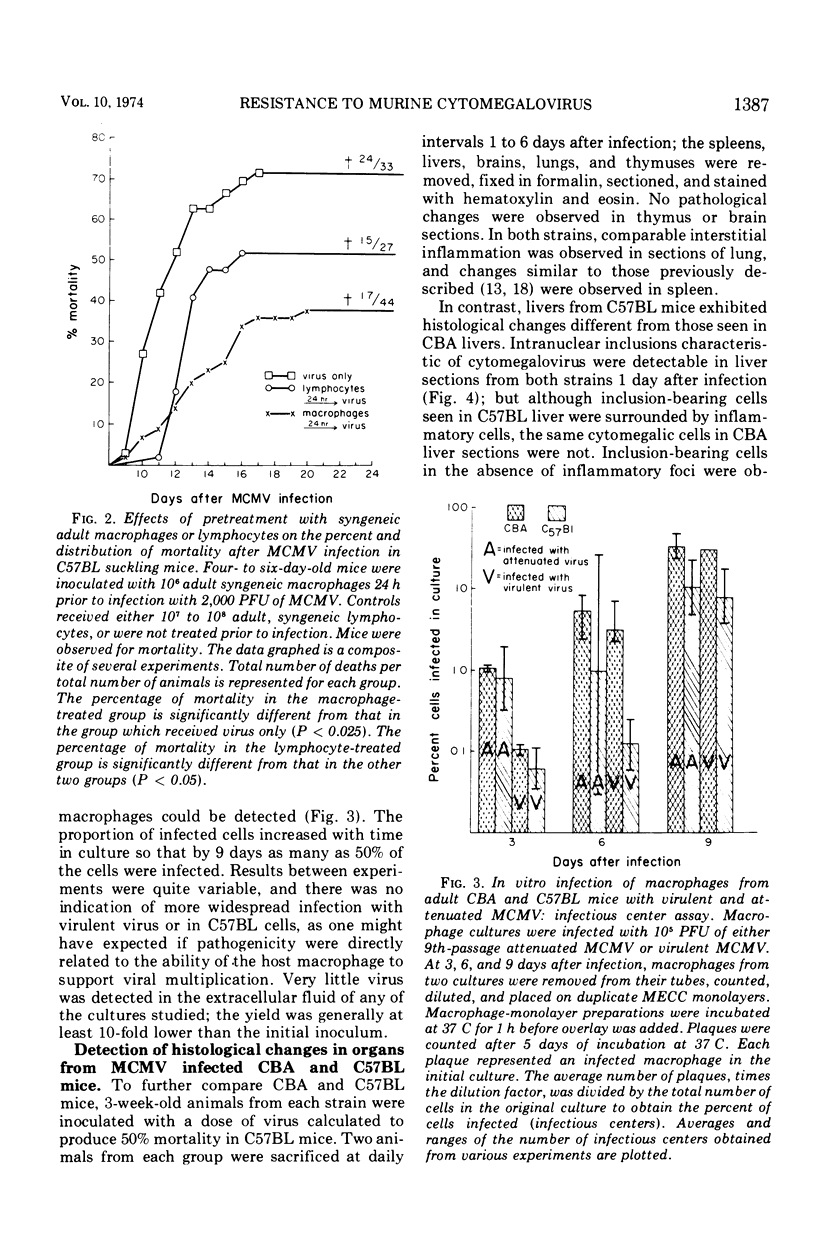
The role of macrophages in protecting mice from murine cytomegalovirus (MCMV) was studied in Swiss, CBA/J, and C57BL/6J mice. CBA/J mice were more resistant to virus than were C57BL/6J mice at all ages tested. Prior treatment of adult Swiss mice with 60 mg of silica, a dose selectively toxic to macrophages, increased mortality due to MCMV infection. Transfer of syngeneic adult macrophages to suckling mice significantly increased their resistance to subsequent MCMV infection. Transfer of syngeneic, nonimmune adult lymphocytes to suckling mice also had a lesser but significant protective effect against subsequent MCMV challenge. In vitro infection of adult CBA/J and C57BL/6J macrophages with virulent and attenuated MCMV resulted in productive infection in only a small percentage of cells and recovery of very little virus from the extracellular fluid. Infection of CBA macrophages was no less productive than C57BL/6J nor was infection with virulent virus more productive than with attenuated virus. Histological examination of the livers of MCMV-infected CBA/J and C57BL/6J mice suggested that divergent cellular immune responses to infection might account for differences in susceptibility. It is postulated that the macrophage may facilitate the inductive phase of cellular immunity, one possible explanation for its demonstrated importance in host defenses against MCMV.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Harington J. S., Birbeck M. An examination of the cytotoxic effects of silica on macrophages. J Exp Med. 1966 Aug 1;124(2):141–154. doi: 10.1084/jem.124.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyris B. F. Role of macrophages in immunological maturation. J Exp Med. 1968 Sep 1;128(3):459–467. doi: 10.1084/jem.128.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang F. B., Warwick A. MOUSE MACROPHAGES AS HOST CELLS FOR THE MOUSE HEPATITIS VIRUS AND THE GENETIC BASIS OF THEIR SUSCEPTIBILITY. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1065–1075. doi: 10.1073/pnas.46.8.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diosi P., Arcan P., Plavoşin L. Genetic control of resistance to mouse cytomegalovirus infection. Arch Gesamte Virusforsch. 1974;44(1):23–27. doi: 10.1007/BF01242177. [DOI] [PubMed] [Google Scholar]
- GOODMAN G. T., KOPROWSKI H. Study of the mechanism of innate resistance to virus infection. J Cell Comp Physiol. 1962 Jun;59:333–373. doi: 10.1002/jcp.1030590313. [DOI] [PubMed] [Google Scholar]
- Hirsch M. S., Zisman B., Allison A. C. Macrophages and age-dependent resistance to Herpes simplex virus in mice. J Immunol. 1970 May;104(5):1160–1165. [PubMed] [Google Scholar]
- JOHNSON R. T. THE PATHOGENESIS OF HERPES VIRUS ENCEPHALITIS. II. A CELLULAR BASIS FOR THE DEVELOPMENT OF RESISTANCE WITH AGE. J Exp Med. 1964 Sep 1;120:359–374. doi: 10.1084/jem.120.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANTOCH M., WARWICK A., BANG F. B. The cellular nature of genetic susceptibility to a virus. J Exp Med. 1963 May 1;117:781–798. doi: 10.1084/jem.117.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANNINI A., MEDEARIS D. N., Jr Mouse salivary gland virus infections. Am J Hyg. 1961 May;73:329–343. doi: 10.1093/oxfordjournals.aje.a120192. [DOI] [PubMed] [Google Scholar]
- Mirchamsy H., Rapp F. A new overlay for plaquing animal viruses. Proc Soc Exp Biol Med. 1968 Oct;129(1):13–17. doi: 10.3181/00379727-129-33237. [DOI] [PubMed] [Google Scholar]
- Nachkov D., Dumanova L., Dimitrova A., Christophorov L. Phagocytic activity of macrophages from C57-Bl and CBA mice. Boll Ist Sieroter Milan. 1973 Sep-Dec;52(5):402–404. [PubMed] [Google Scholar]
- Osborn J. E., Medearis D. N., Jr Suppression of interferon and antibody and multiplication of Newcastle disease virus in cytomegalovirus infected mice. Proc Soc Exp Biol Med. 1967 Feb;124(2):347–353. doi: 10.3181/00379727-124-31740. [DOI] [PubMed] [Google Scholar]
- Osborn J. E., Shahidi N. T. Thrombocytopenia in murine cytomegalovirus infection. J Lab Clin Med. 1973 Jan;81(1):53–63. [PubMed] [Google Scholar]
- Osborn J. E., Walker D. L. Enhancement of infectivity of murine cytomegalovirus in vitro by centrifugal inoculation. J Virol. 1968 Sep;2(9):853–858. doi: 10.1128/jvi.2.9.853-858.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn J. E., Walker D. L. Virulence and attenuation of murine cytomegalovirus. Infect Immun. 1971 Feb;3(2):228–236. doi: 10.1128/iai.3.2.228-236.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearsall N. N., Weiser R. S. The macrophage in allograft immunity. I. Effects of silica as a specific macrophage toxin. J Reticuloendothel Soc. 1968 Apr;5(2):107–120. [PubMed] [Google Scholar]
- ROBERTS J. A. GROWTH OF VIRULENT AND ATTENUATED ECTROMELIA VIRUS IN CULTURED MACROPHAGES FROM NORMAL AND ECTROMELIAIMMUNE MICE. J Immunol. 1964 Jun;92:837–842. [PubMed] [Google Scholar]
- Ruebner B. H., Hirano T., Slusser R., Osborn J., Medearis D. N., Jr Cytomegalovirus infection. Viral ultrastructure with particular reference to the relationship of lysosomes to cytoplasmic inclusions. Am J Pathol. 1966 Jun;48(6):971–989. [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. J., Craighead J. E. Infection of adult mouse macrophages in vitro with cytomegalovirus. Proc Soc Exp Biol Med. 1968 Dec;129(3):690–694. doi: 10.3181/00379727-129-33399. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. The regulatory role of macrophages in antigenic stimulation. Adv Immunol. 1972;15:95–165. doi: 10.1016/s0065-2776(08)60684-7. [DOI] [PubMed] [Google Scholar]
- Waldron J. A., Jr, Horn R. G., Rosenthal A. S. Antigen-induced proliferation of guinea pig lymphocytes in vitro: obligatory role of macrophages in the recognition of antigen by immune T-lymphocytes. J Immunol. 1973 Jul;111(1):58–64. [PubMed] [Google Scholar]
- Zisman B., Hirsch M. S., Allison A. C. Selective effects of anti-macrophage serum, silica and anti-lymphocyte serum on pathogenesis of herpes virus infection of young adult mice. J Immunol. 1970 May;104(5):1155–1159. [PubMed] [Google Scholar]