Abstract
The clinical presentation of autonomic failure is orthostatic hypotension. Severely affected patients require pharmacologic treatment to prevent pre-syncopal symptoms or frank syncope. We previously reported in a proof of concept study that pediatric doses of the norepinephrine transporter blockade, atomoxetine, increases blood pressure in autonomic failure patients with residual sympathetic activity compared with placebo. Given that the sympathetic nervous system is maximally activated in the upright position, we hypothesized that atomoxetine would be superior to midodrine, a direct vasoconstrictor, in improving upright blood pressure and orthostatic hypotension-related symptoms. To test this hypothesis, we compared the effect of acute atomoxetine versus midodrine on upright systolic blood pressure and orthostatic symptom scores in 65 patients with severe autonomic failure. There were no differences in seated systolic blood pressure (95% CI= −7.3 to 7.9, P=0.94) or seated diastolic blood pressure (95% CI= −3.4 to 4.2, P=0.83) between atomoxetine and midodrine. In contrast, atomoxetine produced a greater pressor response in upright systolic blood pressure (95% CI= 0.6 to 15, P=0.03) and upright diastolic blood pressure (95% CI=-0.05 to 8.3, P=0.05), compared with midodrine. Furthermore, atomoxetine (95% CI=0.1 to 0.8, P=0.02), but not midodrine (95% CI=-0.1 to 1.0, P=0.08), improved orthostatic hypotension-related symptoms as compared with placebo. The results of our study suggest that atomoxetine could be a superior therapeutic option than midodrine for the treatment of orthostatic hypotension in autonomic failure.
Keywords: autonomic failure, orthostatic hypotension, treatment, atomoxetine, midodrine
INTRODUCTION
The autonomic nervous system is responsible for maintaining blood pressure in the upright position. When this system fails, as in autonomic failure, orthostatic hypotension (OH) occurs. Severely affected patients with autonomic failure can stand only for a few minutes before developing disabling pre-syncopal symptoms or frank syncope. Although non-pharmacologic measures, such as physical counter-maneuvers and the use of compression garments, are the first step in the treatment of OH,1 these interventions may not suffice to improve standing blood pressure and control OH-related symptoms. Thus, the use of pharmacological agents often becomes necessary.2
The α-1 adrenergic agonist midodrine has become the mainstay treatment in patients with autonomic failure and neurogenic OH. This drug induces direct vasoconstriction and improves upright systolic blood pressure (SBP) following acute and chronic administration.3-5 The Food and Drug Administration (FDA), however, recently proposed the withdrawal of midodrine because of the lack of post-marketing studies supporting its clinical efficacy in reducing symptoms.6,7 More recently, droxidopa was approved by the FDA for the treatment of OH as a synthetic pro-drug to norepinephrine and epinephrine. This drug, however, is still not commercially available and there is no information about its long-term efficacy and safety.
We and others have reported that even in patients with severe autonomic failure there is some degree of residual sympathetic activity. For instance, patients with multiple systems atrophy (MSA) have intact peripheral noradrenergic fibers8 despite impairment in central autonomic pathways. In patients with pure autonomic failure (PAF), the peripheral noradrenergic denervation9 is heterogeneous and does not occur in all vascular beds.10 This raises the possibility that blockade of the norepinephrine transporter (NET), which increases the availability of norepinephrine in the synaptic gap, may be an effective therapy in autonomic failure. Indeed, our proof of concept study showed that acute administration of atomoxetine, a selective NET blocker, is an effective pressor agent in autonomic failure.11 Considering that the sympathetic nervous system is maximally activated in the upright position, the purpose of this study was to test the hypothesis that atomoxetine is better than midodrine in improving upright SBP in patients with neurogenic OH.
METHODS
Subjects
Patients with severe autonomic failure (PAF, MSA, and Parkinson’s disease12) were recruited from referrals to the Paden Autonomic Dysfunction Center at Vanderbilt University. The diagnosis of autonomic failure was confirmed using standardized autonomic function testing13,14 (Table 1). OH was defined as a decrease in SBP of at least 20 mm Hg or diastolic blood pressure (DBP) of at least 10 mm Hg within 3 minutes of standing or 60° head-up tilt.15 Patients were excluded if they had autonomic failure secondary to diabetes mellitus, amyloidosis, or paraneoplastic syndrome. All studies adhered to the principles of the Declaration of Helsinki and Title 45, U.S. Code of Federal Regulations, Part 46, Protection of Human Subjects. The study was approved by the Vanderbilt Institutional Review Board, and studies were conducted in accordance with institutional guidelines. All subjects provided written informed consent. The study was registered at ClinicalTrials.gov under “Treatment of Orthostatic Hypotension in Autonomic Failure” (NCT00223691).
Table 1.
Patient characteristics.
| Parameters | PAF (n=26) |
PD (n=12) |
MSA (n=21) |
Undetermined (n=10) |
All Patients (n=69) |
|---|---|---|---|---|---|
| Sex, male/female | 17/11 | 6/5 | 9/11 | 6/4 | 38/31 |
| Age, years | 68±10 | 69±7 | 59±7 | 67±7 | 65±9 |
| BMI, kg/m2 | 27±4 | 24±3 | 27±4 | 27±2 | 26±4 |
| Systolic BP, mm Hg | |||||
| Supine | 144±31 | 142±24 | 140±20 | 133±32 | 141±26 |
| Upright | 77±21 | 79±20 | 84±15 | 76±26 | 79±20 |
| Diastolic BP, mm | |||||
| Hg | 81±13 | 78±12 | 85±14 | 74±6 | 81±12 |
| Supine | 49±12 | 51±12 | 58±10 | 52±19 | 53±12 |
| Upright | |||||
| Heart rate, bpm | |||||
| Supine | 69±11 | 69±14 | 74±10 | 64±9 | 68±11 |
| Upright | 81±15 | 82±13 | 87±14 | 83±12 | 86±12 |
| Norepinephrine, pg/mL |
|||||
| Supine | 90±89 | 115±62 | 181±86 | 116±65 | 126±89 |
| Upright | 145±128 | 256±282 | 384±299 | 277±192 | 257±249 |
All values are expressed in mean ± standard deviation.
Procedures
All subjects were admitted to the Vanderbilt Clinical Research Center. Patients were fed a low-monoamine, caffeine-free diet containing 150 mEq/day sodium and 60-80 mEq/day potassium for at least 3 days before evaluation. Medications affecting the autonomic nervous system, blood pressure, and blood volume were discontinued for at least 5 half-lives before admission.
Medication trials
All studies were conducted in the morning, in a post-void state, at least 2.5 hours after breakfast to avoid the post-prandial hemodynamic effects. On separate days, patients were given atomoxetine (18 mg, Eli Lilly Pharmaceuticals, Indianapolis, IN), midodrine (5-10 mg, Shire Pharmaceuticals Inc., Wayne, PA), or placebo in a randomized, single-blind, crossover fashion. Studies were conducted with the patients seated in a chair, with their feet on the floor.
During baseline, SBP, DBP and heart rate (HR) were measured every 5 minutes for 30 minutes. Orthostatic vital signs were obtained at 1, 3, 5 and 10 minutes or until tolerated. The patients were asked to rate their OH-related symptoms using the Orthostatic Hypotension Questionnaire (OHQ).16 The study drug was then administered and the SBP, DBP, and HR were assessed every 5 minutes for 60 minutes. We repeated the orthostatic vital signs assessment and symptom evaluation at the end of this period, as described previously. Blood pressure and HR were measured using an automated brachial sphygmomanometer (Dinamap, GE Medical Systems Information Technologies, Milwaukee, WI). Data were digitally transferred into a custom-designed database (Access, Microsoft Corporation, Bellevue, WA).
The hemodynamic parameters and symptom questionnaires were evaluated at baseline and 60 minutes after drug administration, consistent with the time for active metabolites of midodrine and atomoxetine to reach their peak plasma levels.5,17,18
Assessment of orthostatic hypotension-related symptoms
The OHQ was previously validated as a tool to assess OH-related symptoms in clinical trials.16 The questionnaire consists of 6 questions (Q), each rating the intensity of the following symptoms: Q1 = dizziness, lightheadedness, feeling faint, or feeling like you might black out, Q2 = problems with vision (blurring, seeing spots, tunnel vision), Q3 = generalized weakness, Q4 = fatigue, Q5 = trouble concentrating and Q6 = head/neck discomfort. This questionnaire addresses the severity of OH-related symptoms, with the absence of a symptom being rated as 0 and a maximal severity of symptoms as 10. In the present study, we evaluate the effect of our interventions on OH-related symptoms in two different ways. We reported changes in the total OHQ score and in Q1 only. The latter was done because the FDA approved the drug droxidopa for the treatment of OH based on an improvement in 1 point in Q1.19
Statistics
The primary endpoint was the post-treatment upright SBP at 1 minute. For comparison, previous studies have used the upright SBP as a surrogate biomarker for OH improvement.5 Secondary endpoints included post-treatment seated SBP and DBP, upright DBP and HR, and OHQ and Q1 symptom scores. The pre- and post-treatment OHQ and Q1 scores were square-rooted transformed (SQRT) to reduce skewness of its distribution.
Data are expressed as mean ± standard deviation (SD). The analyses were performed using a random-effects model that takes into account correlation due to repeated measurements within the same subject. Post-treatment standing SBP, DBP, and HR were adjusted for each pre-treatment baseline measurement as well as age and gender.
We also performed subgroup analyses according to the seated pressor response to both midodrine and atomoxetine. Seated pressor response was defined a priori as an increase of at least 15 mm Hg in seated SBP at 60 minutes after drug administration. We determined differences in upright SBP and OH-related symptoms in the subjects who had a seated pressor response to both midodrine and atomoxetine versus those who did not. All analyses were performed using STATA 11.0 (StataCorp, College Station, TX).
Sample size calculations were performed using paired t-test analysis in PS software (Version 3.0.34).20 A blinded analysis was performed on 20 random patients enrolled in this study to obtain an estimate of variance, and showed an approximate 22 mm Hg standard deviation of difference in upright SBP among study interventions (midodrine, atomoxetine). An increase in upright SBP of 8 mm Hg with atomoxetine vs. midodrine would be a clinically meaningful difference, representing the magnitude of response achieved with other drugs that primarily increase upright blood pressure.21 Based on these data, we estimated that 65 patients would have 80% power to detect a difference in means among treatment groups.
RESULTS
Subject Characteristics and Autonomic Evaluation Data
Figure S1 shows the study flow diagram according to CONSORT.22 A total of 69 patients with autonomic failure and neurogenic OH participated in this trial. The clinical characteristics of these patients are shown in Table 1. All patients had a profound decrease in SBP and DBP from the supine to the upright position (−63±29 and −29±16 mm Hg, respectively) without adequate increase in heart rate (12±15 bpm). Norepinephrine levels were low in supine posture (126±89 pg/mL) and did not increase appropriately during upright posture (257±249 pg/mL).
The results of the autonomic function assessment are presented in Table S1. All patients had an exaggerated decrease in SBP during phase II and absence of SBP overshoot during phase IV of the Valsalva maneuver. Pressor responses to isometric handgrip and cold pressor test were also impaired suggesting sympathetic failure. The sinus arrhythmia ratio was decreased in these patients indicating impaired parasympathetic function.
Pressor Response to Drugs
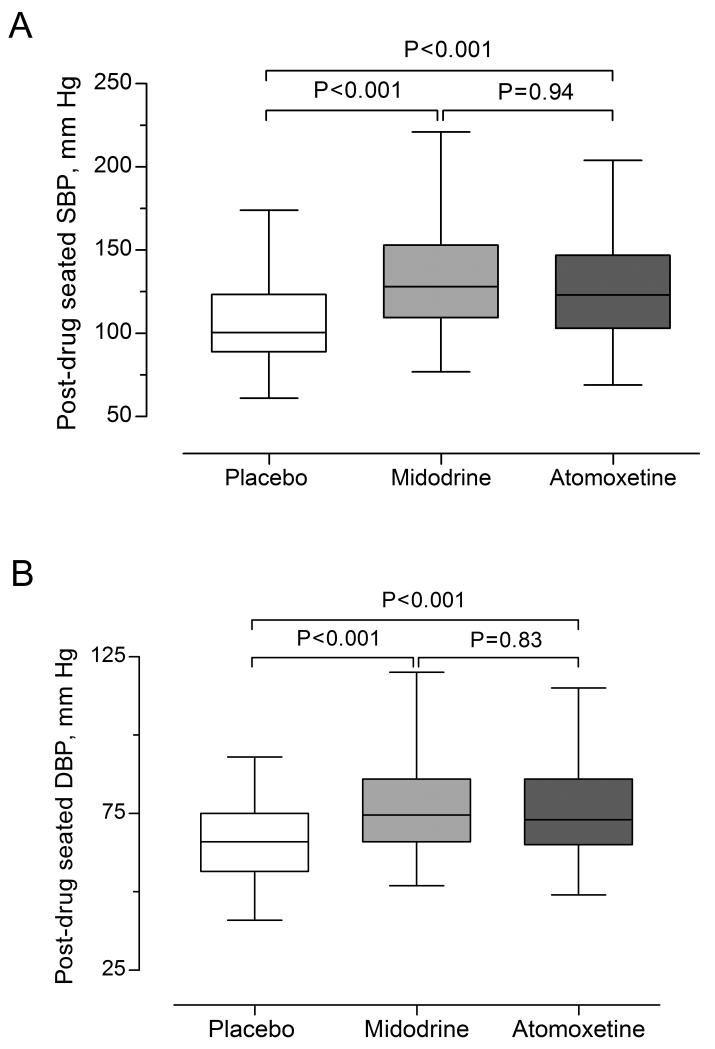
Atomoxetine increased seated SBP by 20 mm Hg (95% CI = 14 to 27, P<0.001) and seated DBP by 10 mm Hg (95% CI = 7 to 13, P<0.001), compared with placebo. Similarly, midodrine increased seated SBP by 20 mm Hg (95% CI = 13 to 28, P<0.001) and seated DBP by 10 mm Hg (95% CI= 7 to 14, P<0.001), compared with placebo, Figure 1. There was no difference between atomoxetine and midodrine in seated SBP (means difference= 0.3 mm Hg, 95% CI= −7.3 to 7.9, P=0.94) or seated DBP (means difference = 0.4 mm Hg, 95% CI= −3.4 to 4.2, P = 0.83).
Figure 1.
Post-drug seated SBP [A] and DBP [B]. Boxes and whiskers plot displays unadjusted data. The P-values were generated by comparing post-drug seated SBP [A] and DBP [B] using random-effects model. The model was adjusted for baseline seated SBP or DBP, age, and gender.
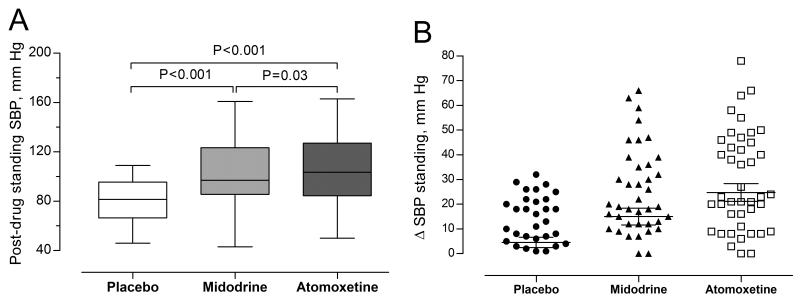
Atomoxetine significantly increased upright SBP by 20 mm Hg (95% CI = 13 to 27, P<0.001) and upright DBP by 11 mm Hg (95% CI = 7 to 14, P<0.001), compared with placebo. Likewise, midodrine increased upright SBP by 12 mm Hg (95% CI = 6 to 19, P<0.001) and upright DBP by 7 mm Hg (95% CI = 3 to 11, P=0.001), compared with placebo. Atomoxetine, however, improved upright SBP to a greater extent than midodrine (means difference = 7.5 mm Hg, 95% CI = 0.6 to 14.5, P=0.03). Upright SBP responses to atomoxetine was variable as shown in Figure 2.
Figure 2.
[A] Post-drug standing SBP. Boxes and whiskers plot displays unadjusted post-drug standing SBP. P-values were generated by comparing post-drug standing SBP using random-effects model. The model was adjusted for pre-drug standing SBP, age, and gender. [B] Scatter plot showing individual values for the changes in standing SBP from baseline values.
Heart Rate Response to Drugs
The effects of atomoxetine and midodrine on HR were also assessed after 1 minute of upright posture. Post-drug upright HR after atomoxetine administration was greater than after midodrine by 4 bpm (95% CI = 1.4 to 7.3, P=0.003). The post-drug upright HR after midodrine was lower than placebo (3 bpm, 95% CI = −4.9 to −1.1, P=0.002). Conversely, the post-drug upright HR after atomoxetine was similar to placebo (1.4 bpm, 95% CI = −1.5 to 4.2, P = 0.34).
Orthostatic Hypotension-Related Symptoms
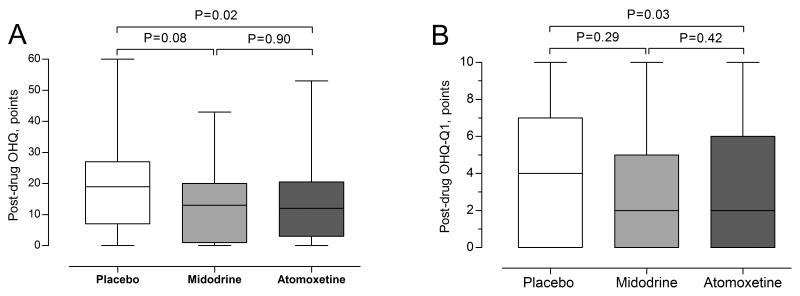
Atomoxetine significantly improved OH-related symptoms compared with placebo in the total OHQ score (0.4 SQRT, 95% CI = −0.1 to −0.8, P = 0.02) and Q1 (0.6 SQRT points, 95% CI = −0.1 to 1.7, P = 0.03). There was a tendency for an improvement in OH-related symptoms with midodrine versus placebo in the total OHQ score (0.5 SQRT point, 95% CI = −0.1 to 1.0, P = 0.08). However, there was no improvement in Q1 (0.6 SQRT point, 95% CI = −1.6 to 0.5, P = 0.29). No differences in total OHQ score or Q1 were found between atomoxetine and midodrine (P = 0.9 and P = 0.42, respectively), Figure 3.
Figure 3.
Total OHQ [A] and Q1 [B]. Boxes and whiskers plots displays unadjusted scores. The P-values were generated by comparing the squared root of the post-drug OHQ or Q1 using random-effects model. The model was adjusted for baseline squared rooted OHQ or Q1, age, and gender.
Subgroup analyses: Effect of study drug on upright SBP, OHQ and Q1 in subjects with and without a seated pressor response to atomoxetine and midodrine
We performed subgroup analyses in patients who had a seated pressor response to both midodrine and atomoxetine defined a priori as an increase of at least 15 mm Hg in seated SBP, post-drug administration (N = 36 patients, 52%). In these patients, atomoxetine increased upright SBP by 26 mm Hg (95% CI = 16 to 37, P<0.001) compared with placebo. Similarly, midodrine increased standing SBP by 19 mm Hg (95% CI = 9 to 29, P<0.001) compared with placebo. Compared with midodrine, atomoxetine increased upright SBP by 8 mm Hg (95% CI = −3 to 18, P = 0.16). After atomoxetine administration, the OHQ score was lower than placebo by 0.7 SQRT points (95% CI = −0.2 to −1.3, P = 0.013). Similarly, the OHQ score after midodrine administration was lower than placebo by 1.0 SQRT point (95% CI= − 0.3 to −1.7, P = 0.004). After atomoxetine administration, Q1 was lower than placebo by 1.4 SQRT points (95% CI = 0.4 to 2.5, P = 0.008). Similarly, after midodrine administration Q1 was lower than placebo by 1.8 SQRT points (95% CI = 0.6 to 2.9, P = 0.004). No differences in total OHQ or Q1 were found between atomoxetine or midodrine.
In patients who failed to increase their seated SBP more than 15 mm Hg, atomoxetine significantly increased upright SBP by 11 mm Hg (95% CI= 2.8 to 18.7, P=0.009) compared with placebo. There was no difference in upright SBP between midodrine and placebo (4 mm Hg, 95% CI = −3.7 to 11.8, P = 0.3). Similarly, there was no difference between atomoxetine and midodrine on upright SBP (7 mm Hg, 95% CI = −1.2 to 14.7, P = 0.095). Furthermore, no improvement in symptoms was found with any of these interventions.
DISCUSSION
The main finding of this study is that the NET blocker atomoxetine preferentially improves upright blood pressure to a greater extent than midodrine, the current standard of care, in patients with severe autonomic failure. In addition, only atomoxetine improved OH-related symptoms as shown by a decrease in the total OHQ and Q1 scores compared with placebo. These findings suggest that atomoxetine, when given at a pediatric dose, may be an alternative therapy for patients who do not experience symptom relief with midodrine. NET blockers, and atomoxetine in particular, are approved for the treatment of attention deficit/hyperactivity disorder, and are prescribed chronically in seven million patients with this condition.23 Repurposing atomoxetine for the treatment of autonomic failure would be of major advantage because this medication is already commercially available, and therefore its cardiovascular safety has been assessed in post-marketing studies, as well as in a large pharmacoepidemiology study.24
The treatment of neurogenic OH is challenging. A stepwise approach is recommended based on the severity of the symptoms.9 The first step includes the use of non-pharmacological treatment such as custom-fitted compression stocking, abdominal binders and physiological counter maneuvers, these measures are aimed at reducing venous pooling in the lower body and thereby improving cardiac output on standing. The first step includes the use of nonpharmacological approaches to decrease venous pooling, avoid volume depletion or increase plasma volume. In the majority of patients, however, non-pharmacological measures are unable to control symptoms or reduce syncope and falls. Therefore, the use of pharmacological agents such as short acting pressor agents is often needed. pressor agents will increase blood pressure for 2 to 3 hours at a time and are best given on a PRN basis, to be taken 30 to 45 minutes before upright activities.25 Evening doses should be avoided because of increased risk of supine hypertension.
Since its approval by the FDA in 1995, the α1 adrenergic agonist, midodrine has been the current standard of care for the treatment of OH. The efficacy of midodrine in improving 1-minute upright SBP has been demonstrated in double-blind, placebo-controlled trials.3,4,26 The use of midodrine, however, is limited in some patients by adverse effects such as pilomotor reactions, pruritus of the scalp, urinary urgency or retention, and supine hypertension.4,27 Furthermore, previous reports have described that midodrine may not work in a sub-group of patients with autonomic failure. In the present study, approximately 36% of patients did not experience an increase in seated blood pressure or improvement in OH-related symptoms despite receiving therapeutic doses of midodrine. Of note, we recently reported poor treatment adherence with midodrine in elderly patients with neurogenic OH enrolled in the Tennessee Medicare Program (TENNCARE).28 In this context, our findings suggest that atomoxetine could be an alternative therapeutic agent for treatment of patients who do not respond or develop side effects to midodrine.
In patients with autonomic failure, the decline in blood pressure on standing induces a reduction in cerebral blood flow,29 which in turn translates into pre-syncopal symptoms and even syncope. Cerebral blood flow could be preserved with the use of systemic pressor agents, such as midodrine. 30 Indeed, midodrine did not improved OH-related symptoms when the entire cohort was analyzed, however, in our sub-group analysis midodrine improved symptoms in patients who had an increase in seated SBP of at least 15 mm Hg. Atomoxetine, on the other hand, may improve OH-related symptoms not only through its effect on systemic blood pressure but also locally by directly increasing cerebral blood flow. Atomoxetine crosses the blood-brain barrier, and has been shown to increase cerebral blood flow in areas of high norepinephrine-transporter density in healthy individuals.31
The effect of atomoxetine on upright heart rate is worth discussing. A normal baroreflex mediated response to blood pressure changes leads to a compensatory increase or decrease in HR. This response is impaired in patients with autonomic failure because of failure of baroreflex-mediated efferents.32 When a pressor agent is administered, however, the increase in blood pressure attenuates the increase in HR otherwise seen during OH, thus suggesting that baroreflex failure is not complete in patients with autonomic failure. Indeed, in our study, midodrine significantly decreased upright HR by 4 bpm compared to placebo. In contrast, atomoxetine did not decrease upright HR compared with placebo. This finding suggests that atomoxetine has a different mechanism of action that might be explained by an indirect stimulation of β1 adrenergic receptors in the heart by increasing plasma norepinephrine in the synaptic junction, where the synaptic cleft width is narrow.33
We previously reported that atomoxetine preferentially increases seated SBP in patients with central autonomic failure, but not in those with peripheral autonomic failure.11 In this study, we did not perform a sub-group analysis based on patient diagnosis because our cohort included a large number of patients, in whom the precise subtype of primary autonomic failure was undetermined. We observed heterogeneity in the upright pressor response to atomoxetine, which suggests that residual sympathetic tone differs among severe autonomic failure patients. Previous observations by our group showed that residual sympathetic tone can be regulated by α2 receptors. For instance, clonidine lowers blood pressure in autonomic failure patients.34 On the other hand, we have not observed a clonidine-like effect of atomoxetine in our proof of concept study.11 We believe that the best explanation for this apparent discrepancy could be that even though postsynaptic α2 receptors are intact and able to regulate residual sympathetic tone, it is likely that presynaptic norepinephrine fibers are depleted and, therefore, NET blockade is devoid of a clonidine-like effect that would lower blood pressure in these patients. In support of this, previous studies have shown that patients with MSA and other primary autonomic disorders such as Parkinson’ disease and PAF have marked central norepinephrine deficiency as shown by a decrease in dihydroxyphenylglycol (DPHG), the main neuronal metabolite of norepinephrine in their cerebral spinal fluid,35 and by the depletion of catecholamine neurons in the rostral ventrolateral medulla.36 Therefore, the histological and neuro-hormonal evidence of norepinephrine depletion in the brain of patients with severe autonomic failure may prevent a hypotensive response secondary to NET blockade. Nevertheless, a clonidine-like reaction with paradoxical hypotension cannot be excluded in patients with mild autonomic failure. Thus, clinicians should consider testing the pressor response to atomoxetine when considering its use for the treatment of OH.
Our study has some potential limitations. First, autonomic failure patients were enrolled at a tertiary care center for autonomic disorders, which may not reflect the broader and less severe disease population. Second, we did not assess supine blood pressure during the study because of the risk of supine hypertension; we designed our study to comply with standard of care in patients with autonomic failure in whom we recommend as a routine clinical practice not to lay down after receiving pressor agents. We did not monitor blood pressure beyond 1 hour after drug administration. It is possible that some patients may have prolonged pressor responses, particularly those who are poor metabolizers of cytochrome P450 CYP2D6 which metabolizes atomoxetine.37,38 Finally, our study only addressed the effect of acute administration of atomoxetine on blood pressure and OH-related symptoms, it would be important to assess in future studies the long-term efficacy of this drug for the treatment of OH.
PERSPECTIVES
Atomoxetine, a selective norepinephrine transporter blocker increases upright blood pressure and improves OH-related symptoms to a greater extent than midodrine, the current standard of care. Atomoxetine could be a new therapeutic alternative for the treatment of orthostatic hypotension in patients with autonomic failure. Further studies are required to address its long-term efficacy given that atomoxetine is not FDA-approved for the treatment of OH.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is new?
We show that pediatric doses of the ADHD-approved drug atomoxetine, improve upright systolic blood pressure and pre-syncopal symptoms in patients with primary forms of autonomic failure to a greater extent than the standard of care, midodrine.
What is relevant?
Patients with autonomic failure often develop disabling orthostatic hypotension. Midodrine is the current standard of care for this condition. However, some patients may not respond to this medication or develop intolerable side effects. Our study provides an alternative therapeutic option for these challenging patients.
Summary
The NET blocker atomoxetine improves upright blood pressure to a greater extent than midodrine, the current standard of care, in patients with severe autonomic failure. Only atomoxetine improved orthostatic hypotension-related symptoms compared with placebo. These findings suggest that atomoxetine, when given at a pediatric dose, could be an alternative therapy for patients who do not experience symptom relief with midodrine. Atomoxetine is not an FDA-approved for the treatment of OH and further studies are required to address its long-term efficacy.
Acknowledgments
SOURCES OF FUNDING This work was supported in part by grant P01 HL056693, Autonomic Rare Diseases Clinical Research Consortium Grant U54 NS065736, the Vanderbilt Clinical and Translational Science Award grant UL1 RR024975 from the National Center for Research Resources, and the National Institutes of Health. Cyndya Shibao is supported by grant K23 HL103976 from the National Institutes of Health, PhRMA Foundation Career Development Award and grant 10CRP4310026 from the American Heart Association Clinical Research Program.
Footnotes
DISCLOSURES None.
REFERENCES
- (1).Lahrmann H, Cortelli P, Hilz M, Mathias CJ, Struhal W, Tassinari M. EFNS guidelines on the diagnosis and management of orthostatic hypotension. Eur J Neurol. 2006;13:930–936. doi: 10.1111/j.1468-1331.2006.01512.x. [DOI] [PubMed] [Google Scholar]
- (2).Shibao C, Lipsitz LA, Biaggioni I. Evaluation and treatment of orthostatic hypotension. J Am Soc Hypertens. 2013;7:317–324. doi: 10.1016/j.jash.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Jankovic J, Gilden JL, Hiner BC, Kaufmann H, Brown DC, Coghlan CH, Rubin M, Fouad-Tarazi FM. Neurogenic orthostatic hypotension: a double-blind, placebo-controlled study with midodrine. Am J Med. 1993;95:38–48. doi: 10.1016/0002-9343(93)90230-m. [DOI] [PubMed] [Google Scholar]
- (4).Low PA, Gilden JL, Freeman R, Sheng KN, McElligott MA. Efficacy of midodrine vs placebo in neurogenic orthostatic hypotension. A randomized, double-blind multicenter study. Midodrine Study Group. JAMA. 1997;277:1046–1051. [PubMed] [Google Scholar]
- (5).Wright RA, Kaufmann HC, Perera R, Opfer-Gehrking TL, McElligott MA, Sheng KN, Low PA. A double-blind, dose-response study of midodrine in neurogenic orthostatic hypotension. Neurology. 1998;51:120–124. doi: 10.1212/wnl.51.1.120. [DOI] [PubMed] [Google Scholar]
- (6).Dhruva SS, Redberg RF. Accelerated approval and possible withdrawal of midodrine. JAMA. 2010;304:2172–2173. doi: 10.1001/jama.2010.1695. [DOI] [PubMed] [Google Scholar]
- (7).Walsh S. [Accesed April 22];FDA Proposes Withdrawal of Low Blood Pressure Drug. U.S. Food and Drug Administration. 2013 Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm222580.htm.
- (8).Diedrich A, Jordan J, Tank J, Shannon JR, Robertson R, Luft FC, Robertson D, Biaggioni I. The sympathetic nervous system in hypertension: assessment by blood pressure variability and ganglionic blockade. J Hypertens. 2003;21:1677–1686. doi: 10.1097/00004872-200309000-00017. [DOI] [PubMed] [Google Scholar]
- (9).Shibao C, Okamoto L, Biaggioni I. Pharmacotherapy of autonomic failure. Pharmacol Ther. 2012;134:279–286. doi: 10.1016/j.pharmthera.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Meredith IT, Esler MD, Cox HS, Lambert GW, Jennings GL, Eisenhofer G. Biochemical evidence of sympathetic denervation of the heart in pure autonomic failure. Clin Auton Res. 1991;1:187–194. doi: 10.1007/BF01824986. [DOI] [PubMed] [Google Scholar]
- (11).Shibao C, Raj SR, Gamboa A, Diedrich A, Choi L, Black BK, Robertson D, Biaggioni I. Norepinephrine transporter blockade with atomoxetine induces hypertension in patients with impaired autonomic function. Hypertension. 2007;50:47–53. doi: 10.1161/HYPERTENSIONAHA.107.089961. [DOI] [PubMed] [Google Scholar]
- (12).Goldenberg MM. Pharmaceutical approval update. Pharmacy and Therapeutics. 2014;39:337–344. [PMC free article] [PubMed] [Google Scholar]
- (13).Mosqueda-Garcia R. Evaluation of the autonomic nervous system. In: Robertson D, Biaggioni I, editors. Disorders of the Autonomic Nervous System. Harwood Academy Press; London: 1995. pp. 25–29. [Google Scholar]
- (14).Robertson D. Clinical pharmacology: assessment of autonomic function. In: Baughman K, Greene B, editors. Clinical Diagnostic Manual for the House Officer. William and Wilkins; Baltimore, Maryland: 1981. pp. 86–101. [Google Scholar]
- (15).Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Auton Neurosci. 2011;161:46–48. doi: 10.1016/j.autneu.2011.02.004. [DOI] [PubMed] [Google Scholar]
- (16).Kaufmann H, Malamut R, Norcliffe-Kaufmann L, Rosa K, Freeman R. The Orthostatic Hypotension Questionnaire (OHQ): validation of a novel symptom assessment scale. Clin Auton Res. 2012;22:79–90. doi: 10.1007/s10286-011-0146-2. [DOI] [PubMed] [Google Scholar]
- (17).Witcher JW, Long A, Smith B, Sauer JM, Heilgenstein J, Wilens T, Spencer T, Biederman J. Atomoxetine pharmacokinetics in children and adolescents with attention deficit hyperactivity disorder. J Child Adolesc Psychopharmacol. 2003;13:53–63. doi: 10.1089/104454603321666199. [DOI] [PubMed] [Google Scholar]
- (18).Zachariah PK, Bloedow DC, Moyer TP, Sheps SG, Schirger A, Fealey RD. Pharmacodynamics of midodrine, an antihypotensive agent. Clin Pharmacol Ther. 1986;39:586–591. doi: 10.1038/clpt.1986.101. [DOI] [PubMed] [Google Scholar]
- (19).Chelsea Therapeutics, Inc. [Accessed March 11];NortheraTM (Droxidopa) Advisory Committee Briefing Document. U.S. Food and Drug Administration. 2014 Available at: http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/cardiovascularandrenaldrugsadvisorycommittee/ucm381155.pdf.
- (20).Dupont WD, Plummer WD., Jr. Power and sample size calculations. Control Clin Trials. 1990;11:116–128. doi: 10.1016/0197-2456(90)90005-m. [DOI] [PubMed] [Google Scholar]
- (21).Singer W, Sandroni P, Opfer-Gehrking TL, Suarez GA, Klein CM, Hines S, O’Brien PC, Slezak J, Low PA. Pyridostigmine treatment trial in neurogenic orthostatic hypotension. Arch Neurol. 2006;63:513–518. doi: 10.1001/archneur.63.4.noc50340. [DOI] [PubMed] [Google Scholar]
- (22).Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Mao AR, Babcock T, Brams M. ADHD in adults: current treatment trends with consideration of abuse potential of medications. J Psychiatr Pract. 2011;17:241–250. doi: 10.1097/01.pra.0000400261.45290.bd. [DOI] [PubMed] [Google Scholar]
- (24).Cooper WO, Habel LA, Sox CM, et al. ADHD drugs and serious cardiovascular events in children and young adults. N Engl J Med. 2011;365:1896–1904. doi: 10.1056/NEJMoa1110212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Shibao C, Lipsitz LA, Biaggioni I. ASH position paper: evaluation and treatment of orthostatic hypotension. J Clin Hypertens (Greenwich ) 2013;15:147–153. doi: 10.1111/jch.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Kaufmann H, Brannan T, Krakoff L, Yahr MD, Mandeli J. Treatment of orthostatic hypotension due to autonomic failure with a peripheral alpha-adrenergic agonist (midodrine) Neurology. 1988;38:951–956. doi: 10.1212/wnl.38.6.951. [DOI] [PubMed] [Google Scholar]
- (27).Fouad-Tarazi FM, Okabe M, Goren H. Alpha sympathomimetic treatment of autonomic insufficiency with orthostatic hypotension. Am J Med. 1995;99:604–610. doi: 10.1016/s0002-9343(99)80246-0. [DOI] [PubMed] [Google Scholar]
- (28).Shibao C, Grijalva CG, Lipsitz LA, Biaggioni I, Griffin MR. Early discontinuation of treatment in patients with orthostatic hypotension. Auton Neurosci. 2013;177:291–296. doi: 10.1016/j.autneu.2013.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Novak V, Novak P, Spies JM, Low PA. Autoregulation of cerebral blood flow in orthostatic hypotension. Stroke. 1998;29:104–111. doi: 10.1161/01.str.29.1.104. [DOI] [PubMed] [Google Scholar]
- (30).Matsubara S, Sawa Y, Yokoji H, Takamori M. Shy-Drager syndrome. J Neurol Neurosurg Psychiatry. 1990;53:994–997. doi: 10.1136/jnnp.53.11.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Marquand AF, O’Daly OG, De SS, Alsop DC, Maguire RP, Williams SC, Zelaya FO, Mehta MA. Dissociable effects of methylphenidate, atomoxetine and placebo on regional cerebral blood flow in healthy volunteers at rest: a multi-class pattern recognition approach. Neuroimage. 2012;60:1015–1024. doi: 10.1016/j.neuroimage.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Robertson D, Hollister AS, Carey EL, Tung CS, Goldberg MR, Robertson RM. Increased vascular beta2-adrenoceptor responsiveness in autonomic dysfunction. J Am Coll Cardiol. 1984;3:850–856. doi: 10.1016/s0735-1097(84)80264-8. [DOI] [PubMed] [Google Scholar]
- (33).Osswald W. Transmitter disposition mechanisms. In: Bevan J, Johansson B, Nedergaard O, editors. Vascular Neuroeffector Mechanisms. Karger; Basel: 1976. pp. 123–130. [Google Scholar]
- (34).Shibao C, Gamboa A, Abraham R, Raj SR, Diedrich A, Black B, Robertson D, Biaggioni I. Clonidine for the treatment of supine hypertension and pressure natriuresis in autonomic failure. Hypertension. 2006;47:522–526. doi: 10.1161/01.HYP.0000199982.71858.11. [DOI] [PubMed] [Google Scholar]
- (35).Goldstein DS, Holmes C, Sharabi Y. Cerebrospinal fluid biomarkers of central catecholamine deficiency in Parkinson’s disease and other synucleinopathies. Brain. 2012;135:1900–1913. doi: 10.1093/brain/aws055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Benarroch EE, Smithson IL, Low PA, Parisi JE. Depletion of catecholaminergic neurons of the rostral ventrolateral medulla in multiple systems atrophy with autonomic failure. Ann Neurol. 1998;43:156–163. doi: 10.1002/ana.410430205. [DOI] [PubMed] [Google Scholar]
- (37).Kupfer A, Preisig R. Inherited defects of hepatic drug metabolism. Semin Liver Dis. 1983;3:341–354. doi: 10.1055/s-2008-1040786. [DOI] [PubMed] [Google Scholar]
- (38).Price-Evans D. Therapy. In: Kalow W, Goedde H, Agarwal D, editors. Ethnic differences in reaction to drug and xenobiotics. Liss; New York: 1986. pp. 491–526. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.