Abstract
BACKGROUND
Using phase 3 trial data for sunitinib versus interferon (IFN)-α in treatment-naive patients with metastatic renal cell carcinoma, retrospective analyses characterized sunitinib-associated fatigue and its impact on patient-reported health-related quality of life (HRQoL).
METHODS
Patients received sunitinib at a dose of 50 mg/day on a schedule of 4 weeks on/2 weeks off (375 patients) or IFN-α at a dose of 9 MU subcutaneously 3 times per week (360 patients). HRQoL was self-assessed using the Functional Assessment of Cancer Therapy-Kidney Symptom Index–15-item (FKSI-15) questionnaire, with fatigue assessed using its Disease-Related Symptoms subscale. Fatigue was also assessed by providers using Common Terminology Criteria for Adverse Events (CTCAE). A repeated-measures model (M1) and random intercept-slope model (M2) characterized sunitinib-associated fatigue over time. Another repeated-measures model examined the relationship between HRQoL scores and CTCAE fatigue grade.
RESULTS
M1 demonstrated that the initial increase in patient-reported fatigue with sunitinib was worst during cycle 1, with mean values numerically better at subsequent cycles; most pairwise comparisons of consecutive CTCAE fatigue cycle means were not found to be statistically significant. M2 demonstrated that the overall trend (slope) for patient-reported and CTCAE fatigue with sunitinib was not statistically different from 0. The relationship between most HRQoL scores and CTCAE fatigue was close to linear regardless of treatment, with lower scores (worse HRQoL) corresponding to higher fatigue grade. The majority of HRQoL scores were better with sunitinib versus IFN-α for the same CTCAE fatigue grade.
CONCLUSIONS
Patients reported worse fatigue during the first sunitinib cycle. However, in subsequent consecutive cycles, less fatigue was reported with no statistically significant worsening. CTCAE fatigue assessment may not fully capture patient treatment experience. Cancer 2014;120:1871–1880. © 2014 American Cancer Society.
Using phase 3 trial data for sunitinib versus interferon-α in treatment-naive patients with metastatic renal cell carcinoma, retrospective analyses characterized sunitinib-associated fatigue and its impact on patient-reported health-related quality of life. Patients reported worse fatigue during the first sunitinib cycle, but in subsequent consecutive cycles less fatigue was reported with no statistically significant worsening; provider-assessed fatigue did not appear to fully capture patient treatment experience.
Keywords: sunitinib, metastatic renal cell carcinoma, fatigue, health-related quality of life, phase 3
INTRODUCTION
In patients with advanced cancer, fatigue is frequently a priority symptom1 and may adversely impact quality of life because of its effects on physical functioning, social functioning, activity level, and emotional well-being. Fatigue is also a common adverse event (AE) associated with treatments of advanced cancer, including interferon (IFN) and targeted therapies (such as those approved for advanced renal cell carcinoma [RCC]), as well as tyrosine kinase and mammalian target of rapamycin inhibitors.2–4 For example, fatigue has been reported as an AE in approximately 40% to 70% of patients with advanced RCC treated in clinical trials with sunitinib malate.5–8 Sunitinib is an orally administered multitargeted inhibitor of vascular endothelial growth factor receptors, platelet-derived growth factor receptors, and other receptor tyrosine kinases, and is approved globally for the treatment of patients with advanced RCC; imatinib-resistant/imatinib-intolerant gastrointestinal stromal tumors; and progressive, well-differentiated pancreatic neuroendocrine tumors.
In oncology trials, the severity of AEs is usually assessed by the treating physician using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE).9 Because this assessment is performed by an outside observer and not the patient, it has generally been considered an “objective” measure of treatment impact. However, provider-reported ratings may not accurately or consistently depict the full effect of an AE on a patient, particularly when the AE is subjective, such as with fatigue, nausea, or pain.10–13 Furthermore, reporting of AEs from clinical trials is typically based on overall frequency, neglecting the time course (ie, when AEs develop during treatment, whether they persist or resolve, etc). In contrast, relative to CTCAE assessment, patient-reported outcomes may provide a more detailed understanding of symptom data and their effects on functioning and well-being, including their development and impact over time.13
Using data from a pivotal phase 3 trial of sunitinib versus IFN-α in treatment-naive patients with metastatic RCC,6,7 2 independent but complementary retrospective analyses were conducted to characterize the patient fatigue experience while undergoing sunitinib treatment and to investigate the subsequent impact of fatigue on patient-reported health-related quality of life (HRQoL). The latter analysis was conducted because patients may not experience fatigue uniformly across treatments and HRQoL assessment has the potential to provide a more holistic picture. In brief, a better understanding of these issues may allow for more timely and effective intervention to help manage fatigue and thus optimize clinical benefit with sunitinib in patients with advanced RCC.
MATERIALS AND METHODS
Study Population
The current study enrolled patients aged ≥ 18 years with histologically confirmed metastatic RCC with clear cell histology. Key eligibility criteria included6,7: no previous systemic therapy for RCC; measurable disease; an Eastern Cooperative Oncology Group performance status of 0 or 1; no known brain metastases; and adequate hepatic, renal, and cardiac function. All patients provided written informed consent.
Study Design and Treatment
A total of 750 patients with treatment-naive metastatic RCC were randomized 1:1 to receive sunitinib at a dose of 50 mg/day for 4 weeks, followed by 2 weeks off treatment (schedule 4/2), in repeated 6-week cycles, or IFN-α as a subcutaneous injection on 3 nonconsecutive days per week at a dose of 3 million units (MU) for the first week, 6 MU for the second week, and 9 MU thereafter. In both treatment arms, dose modifications were allowed to manage toxicity. Treatment continued until disease progression, unacceptable toxicity, or withdrawal of consent.
HRQoL Outcomes
As previously reported,6,14 HRQoL was assessed during the study using 3 psychometrically tested patient self-reported questionnaires: the Functional Assessment of Cancer Therapy-Kidney Symptom Index–15-item (FKSI-15) (Fig. 1),15 the Functional Assessment of Cancer Therapy-General (FACT-G),16 and the EuroQoL Group's 5-dimension (EQ-5D) questionnaire.17 Patients completed the questionnaires on days 1 and 28 of each treatment cycle (reflecting sunitinib's intermittent treatment schedule) to measure HRQoL across the full course of therapy, both during active treatment and after a treatment break, and at the end of treatment or study withdrawal. HRQoL scores were averaged for each patient by cycle.
Figure 1.
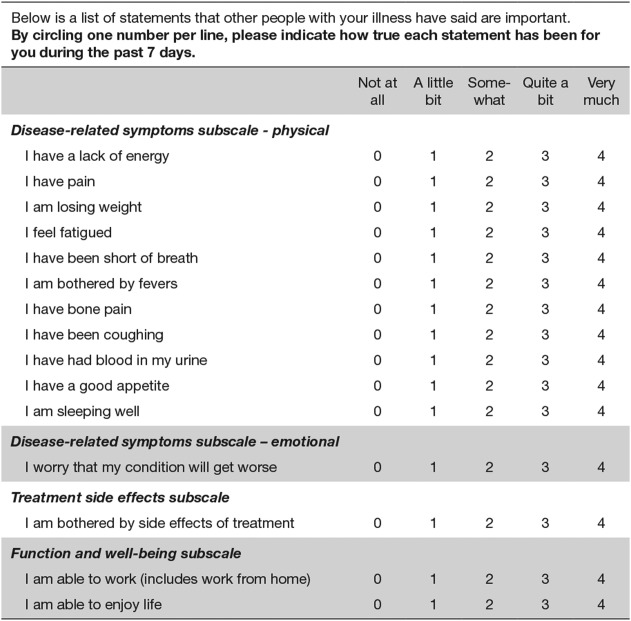
Functional Assessment of Cancer Therapy-Kidney Symptom Index-15 long-form questionnaire is shown.
HRQoL outcomes evaluated in the current analysis were scored as follows. The FKSI-15 total score ranged from 0 (most severe symptoms and concerns) to 60 (no symptoms and concerns). The 9-item FKSI–Disease-Related Symptoms (FKSI-DRS) subscale had a potential total score of 0 (all most severe symptoms) to 36 (no symptoms), with the 15 individual items of the FKSI each scored on a range from 0 to 4. The total FACT-G score ranged from 0 (worst cancer-related QoL) to 108 (best cancer-related QoL), in which each question of the FACT-G Physical Well-Being, Social/Family Well-Being, Emotional Well-Being, and Functional Well-Being subscores ranged from 0 to 4. The EQ-5D–weighted health index score included scores of 1, 0, or < 0, denoting that the corresponding health state is valued by the population as equivalent to full health, death, or worse than death, respectively. The EQ-visual analog scale or health state thermometer score ranged from 0 (worst imaginable health state) to 100 (best imaginable health state).
Fatigue Assessment
Fatigue was self-assessed by patients as part of the FKSI-DRS subscale, scoring the item “I feel fatigued” from 0 (very much) to 4 (not at all). As with other AEs (when applicable), fatigue was also provider-assessed, monitored throughout the study, and graded on a scale from 1 (lowest) to 4 (highest) by treating physicians using NCI CTCAE (version 3.0),18 in which grade 1 is defined as mild fatigue over baseline, grade 2 as moderate or causing difficulty performing some activities of daily living, grade 3 as severe fatigue interfering with activities of daily living, and grade 4 as disabling fatigue. Fatigue could be spontaneously reported as an AE at different time points because it occurred within and across cycles (with no baseline value, unlike HRQoL assessment) and at different grades. In this retrospective assessment, reported severity grades for AE fatigue were averaged by cycle for each patient. A value of 0 was assigned if a patient did not report fatigue as an AE during a given cycle.
Statistical Analysis
Two types of models were used to investigate the time course of patient-reported fatigue and provider-assessed AE fatigue across cycles with sunitinib treatment: a repeated-measures model and a random intercept-slope model.19,20 They included all available longitudinal data on the outcome variable for every patient from the sunitinib treatment arm and considered the FKSI-DRS item “I feel fatigued” and the AE fatigue as outcome (dependent) variables in separate models with time as a predictor. In the models with the FKSI-DRS fatigue item as the outcome, its baseline score was also included as a covariate. In the repeated-measures model, time was represented by a cycle and taken as a categorical predictor, indicating that no functional relationship was imposed between outcome and time. To account for repeated measurements on an individual, the covariance of the residual (error) terms over time was based on a first-order autoregressive structure. In this model, FKSI scores were averaged for each patient by cycle. Because of the small number of observations at later cycles, data from cycles 22 to 30 were collapsed and represented as 1 (mean) value per patient, which is consistent with the analytical approach used for previous analyses of HRQoL data from this trial.21 In the random intercept-slope model, time was used as a continuous predictor, with a linear relationship imposed between outcome and time. The covariance structure of the random effects (intercepts and slopes) was unstructured.
In a complementary analysis, another repeated-measures mixed-effects model was used to examine the relationship between provider-assessed AE fatigue and patient-reported HRQoL to determine whether patients with the same grade of fatigue severity have the same HRQoL while receiving 2 different treatments (sunitinib and IFN-α). All available longitudinal data regarding each HRQoL outcome variable for every patient from both treatment arms were used. Averaged fatigue grades for each patient by cycle were rounded into original metric values (eg, 0, 1, or 2). The AE-fatigue-grade-by-treatment interaction term was used as the predictor. Because AE fatigue grade was used as a categorical variable, no functional relationship between HRQoL scores and fatigue grade was imposed. Because there were no reports of CTCAE grade 5 fatigue and only very few cases of grade 4 fatigue reported, data for grade 4 fatigue were collapsed together with those for grade 3. Therefore, the AE fatigue was represented only by grades 0, 1, 2, and 3 in the analyses. Again, because of the small number of observations, data from cycles 22 to 30 were averaged together. To account for repeated measurements on the same individual, the covariance of the residual (error) terms over time was based on a first-order autoregressive structure. Because the analysis itself did acknowledge and account for the longitudinal structure of data, estimated relationships between the AE fatigue and HRQoL scores can be applied at any time point.
Finally, for each modeled outcome, we used residual diagnostics to assess the adequacy of the fitted model. Residual plots were studied (scaled residuals vs predicted means and scaled residuals vs theoretical normal quantiles).
RESULTS
Patients
As previously reported,6,14 there were no significant differences noted with regard to the baseline characteristics of the patients randomized to sunitinib or IFN-α, including baseline QoL scores, which were in the moderate range (eg, baseline FKSI-DRS scores [mean ± standard deviation] were 29.74 ± 5.24 and 29.55 ± 5.03, respectively, for patients on the sunitinib and IFN-α treatment arms).
Fit of Models
For each modeled outcome, scaled residuals versus theoretical normal quantiles formed a straight line for the vast majority of the observations; scaled residuals versus predicted means did not demonstrate any systematic patterns and centered randomly around 0. This set of results suggests that the models adequately fit the data and that the normal distribution assumption for the residuals is tenable.
Modeling the Time Course of Patient-Reported Fatigue
Results from the repeated-measures model with the FKSI-DRS item “I feel fatigued” (Fig. 2) demonstrated that the initial increase in patient-reported fatigue was worst during the first cycle of sunitinib (from a mean of 2.91 at baseline to a least-squares mean of 2.29 at cycle 1, corresponding to an estimated effect size of 0.58 for the change in fatigue). In addition, mean fatigue values for subsequent cycles were numerically better than (and for most comparisons, also statistically different from) the value in the first cycle (Table1).
Figure 2.
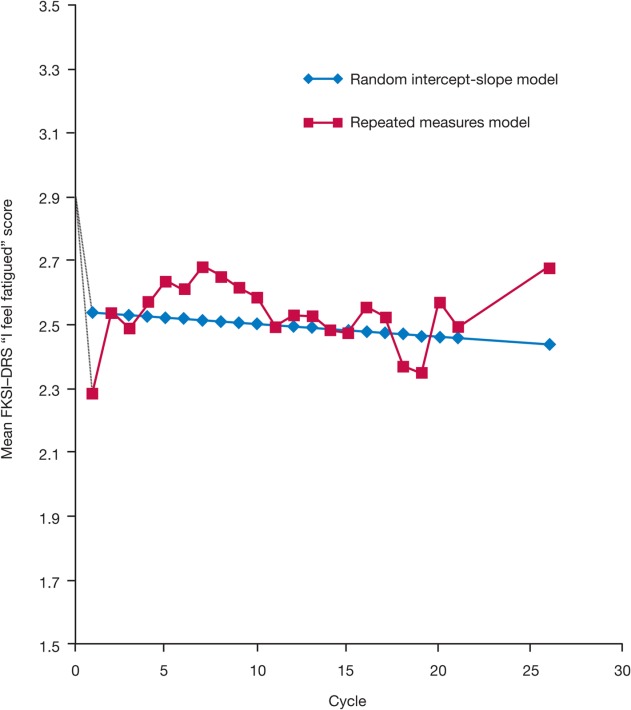
Modeling of the time course of the Functional Assessment of Cancer Therapy-Kidney Symptom Index–Disease-Related Symptoms (subscale) (FKSI-DRS) item “I feel fatigued” score with sunitinib treatment using the repeated-measures and random intercept-slope models is shown. Higher scores indicate better outcome (less fatigue). Random intercept-slope model: slope = −0.00416 per cycle (or per 6 weeks); P = .3488.
Table 1.
Difference of the Least-Squares Means of the FKSI-DRS Item “I Feel Fatigued” Compared With Cycle 1 During Sunitinib Treatment, Using the Repeated-Measures Modela
| Cycle | Least-Squares Meanb | Difference (95% CI; Cycle n–Cycle 1) | Difference P |
|---|---|---|---|
| 1 | 2.29 | — | — |
| 2 | 2.54 | 0.25 (0.18-0.33) | <.0001 |
| 3 | 2.49 | 0.20 (0.11-0.30) | <.0001 |
| 4 | 2.58 | 0.29 (0.18-0.40) | <.0001 |
| 5 | 2.64 | 0.35 (0.23-0.47) | <.0001 |
| 6 | 2.62 | 0.33 (0.20-0.46) | <.0001 |
| 7 | 2.68 | 0.40 (0.26-0.53) | <.0001 |
| 8 | 2.65 | 0.36 (0.22-0.50) | <.0001 |
| 9 | 2.62 | 0.33 (0.18-0.48) | <.0001 |
| 10 | 2.59 | 0.30 (0.15-0.45) | .0001 |
| 11 | 2.50 | 0.21 (0.05-0.37) | .0096 |
| 12 | 2.53 | 0.24 (0.08-0.41) | .0036 |
| 13 | 2.53 | 0.24 (0.07-0.41) | .0054 |
| 14 | 2.49 | 0.20 (0.03-0.38) | .0247 |
| 15 | 2.48 | 0.19 (0.01-0.37) | .0398 |
| 16 | 2.56 | 0.27 (0.08-0.46) | .0047 |
| 17 | 2.53 | 0.24 (0.05-0.43) | .0156 |
| 18 | 2.37 | 0.09 (−0.11 to 0.29) | .3965 |
| 19 | 2.35 | 0.06 (−0.15 to 0.28) | .5643 |
| 20 | 2.57 | 0.29 (0.05-0.52) | .0155 |
| 21 | 2.49 | 0.21 (−0.06 to 0.47) | .1285 |
| 22-30 | 2.68 | 0.39 (0.09-0.70) | .0118 |
Abbreviations: 95% CI, 95% confidence interval; FKSI, Functional Assessment of Cancer Therapy-Kidney Symptom Index; DRS, Disease-Related Symptoms (subscale).
Higher scores indicate better outcome (less fatigue).
All least-squares mean values were significantly differed from 0 (P <.0001).
Results from the random intercept-slope model also demonstrated that fatigue was stable over time (after the initial increase in fatigue during the first cycle to a least-squares mean of 2.55, corresponding to an estimated effect size of 0.34 for the change in fatigue). The overall trend (slope) for the FKSI-DRS item “I feel fatigued” was not statistically different from 0 (Fig. 2).
Modeling the Time Course of Provider-Assessed AE Fatigue
Results from the repeated-measures model demonstrated some fluctuations in AE fatigue over time with sunitinib treatment (Fig. 3), but the differences were small and most pairwise comparisons of the cycle mean values (with the exception of one value) were not statistically significant, indicating that the mean values at every cycle were actually not different from one another.
Figure 3.
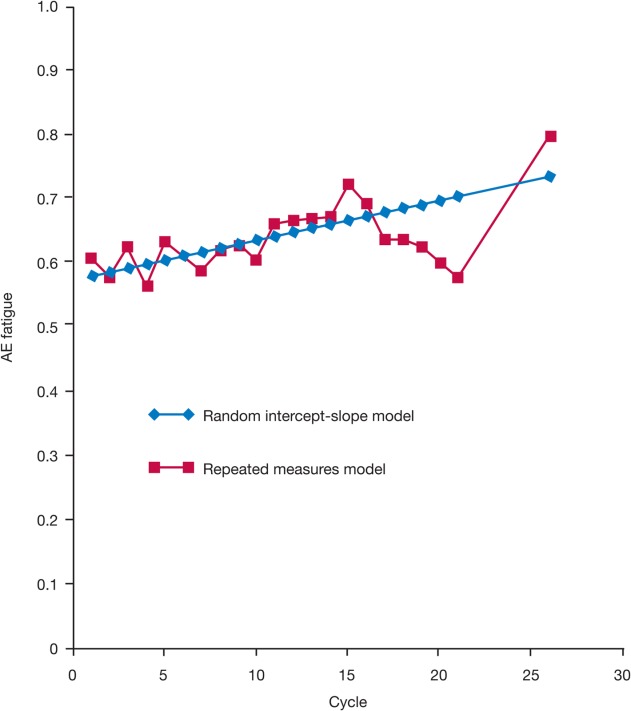
Modeling the time course of the adverse event (AE) of fatigue with sunitinib treatment using the repeated-measures and random intercept-slope models. Random intercept-slope model: slope = 0.006309 per cycle (or per 6 weeks); P = .0927.
Results from the random intercept-slope model demonstrated that the overall trend (or slope) for AE fatigue was not statistically different from 0, indicating no significant change in AE fatigue over time with sunitinib treatment (Fig. 3).
Comparison of HRQoL in Patients Reporting the Same AE Fatigue
An independent but complementary analysis demonstrated that the relationship between most HRQoL outcomes and CTCAE fatigue was close to linear in both treatment groups, with lower (worse) HRQoL scores on most measures corresponding to a higher mean fatigue grade (Figs. 4 and 5), except for HRQoL measures not directly linked with fatigue (ie, the FACT-G Social/Family Well-Being subscore and the FKSI items “I have bone pain,” “I have been coughing,” “I am sleeping well,” “I worry that my condition will get worse,” and “I am bothered by fevers”). For nearly all outcomes (FKSI, FKSI-DRS, FACT-G, and EQ-5D [data not shown]), regardless of whether they were aggregated or single items, patients treated with sunitinib had noticeably better HRQoL scores than patients in the IFN-α group for the same CTCAE severity of fatigue. For example, the FKSI-DRS subscale and FKSI items “I have a lack of energy” and “I feel fatigued” scores were both higher with sunitinib than with IFN-α at each CTCAE grade of fatigue (Figs. 4A-4C). The same pattern was observed for the relationship between the total FACT-G score and the FACT-G Physical Well-Being and Social/Family Well-Being subscores (Figs. 5A-5C).
Figure 4.
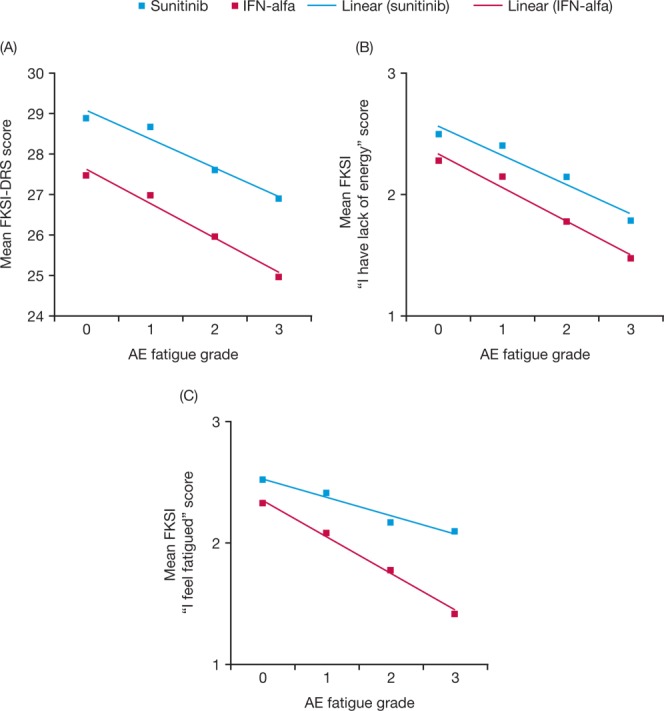
Mean (A) Functional Assessment of Cancer Therapy-Kidney Symptom Index–Disease-Related Symptoms (subscale) (FKSI-DRS) score, (B) FKSI item “I have lack of energy” score, and (C) FKSI item “I feel fatigued” score are shown by adverse event (AE) fatigue grade with sunitinib and interferon-α (IFN-α). Higher scores indicate better outcome (better health-related quality of life or fewer symptoms).
Figure 5.
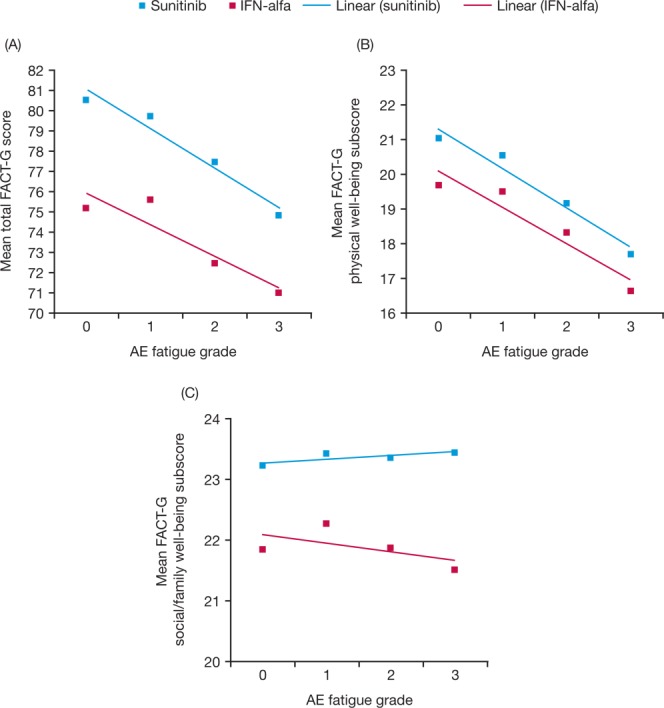
Mean (A) Functional Assessment of Cancer Therapy-General (FACT-G) score, (B) FACT-G physical Well-Being subscore, and (C) FACT-G Social/Family Well-Being subscore are shown by adverse event (AE) fatigue grade with sunitinib and interferon-α (IFN-alfa). Higher scores indicate better outcome (better health-related quality of life or fewer symptoms).
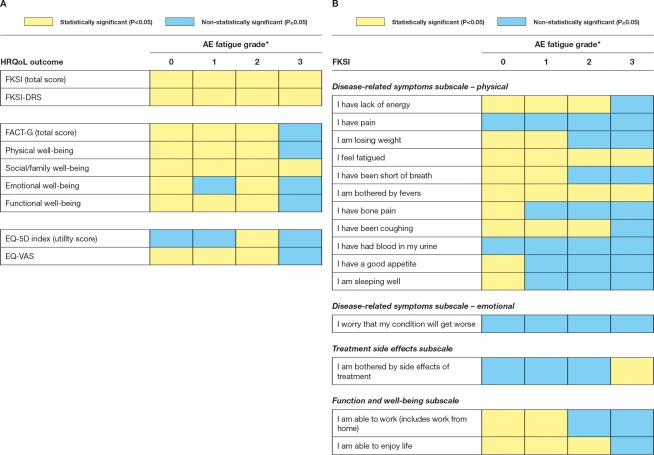
The statistical significance of each between-treatment difference in HRQoL outcome by CTCAE fatigue grade (0-3) is shown in Figure 6. In the absence of fatigue (ie, grade 0), scores for all outcomes were numerically superior with sunitinib compared with IFN-α. In most instances, these differences were also statistically significant (P < .05). For fatigue grades 1 and 2, differences in all outcomes but one (the FKSI item “I have pain”) numerically favored sunitinib and the majority of differences were statistically significant. A similar pattern for grade 3 fatigue also favored sunitinib, but only a few results were found to be statistically significant, potentially due to the smaller sample size.
Figure 6.
Statistical significance of between-treatment differences are shown in (A) health-related quality of life (HRQoL) outcomes and (B) Functional Assessment of Cancer Therapy-Kidney Symptom Index–Disease-Related Symptoms (subscale) (FKSI-DRS) subscales by adverse event (AE) fatigue grade. *The number of AE fatigue observations for grade 0 ranged, depending on the outcome modeled, from 1949 to 1967 for sunitinib and from 1007 to 1019 for interferon-α (IFN-α); for grade 1, the observations ranged from 1101 to 1106 for sunitinib and from 504 to 508 for IFN-α; for grade 2, the observations ranged from 487 to 490 for sunitinib and from 256 to 262 for IFN-α; and for grade 3, the observations ranged from 29 to 31 for sunitinib and from 38 to 40 for IFN-α. Note that all statistically significant between-treatment differences favored sunitinib over IFN-α. FACT-G indicates Functional Assessment of Cancer Therapy-General; EQ-5D, EuroQoL Group's 5-dimension questionnaire; EQ-VAS, EuroQoL visual analog scale questionnaire.
DISCUSSION
The purpose of these unique retrospective analyses was to characterize the patient fatigue experience while receiving sunitinib treatment and to investigate its expected adverse impact on patient-reported HRQoL. The results demonstrated that patients with metastatic RCC reported worse fatigue during the first cycle of sunitinib treatment compared with baseline; however, no significant worsening of fatigue was reported in all consecutive treatment cycles. The overall trend was stable, demonstrating no significant increase in reported fatigue after the first follow-up assessment in cycle 2. More specifically, based on the estimated effect size for the random intercept-slope model, the magnitude of change in fatigue during the first cycle can be characterized as a small-to-medium change22,23 that continued during subsequent cycles, whereas according to the repeated-measures model, the magnitude of change in fatigue during the first cycle can be described as a medium change22,23 that recovered to the effect levels of the random intercept-slope model at subsequent cycles. Similarly, provider-assessed fatigue, using the NCI CTCAE grading system, suggested no significant change in the AE fatigue over time for patients treated with sunitinib, even though subjective symptoms such as fatigue are often not uniformly reported between clinicians and patients.24 Findings similar to those of the current study may have been reported anecdotally by some clinicians, given their experience with sunitinib, but to the best of our knowledge the current study is the first formal analysis to confirm and quantify this observation.
It is interesting to note that patients treated with sunitinib who experienced the same CTCAE grade of fatigue as patients treated with IFN-α generally had more favorable HRQoL scores, as measured by the FKSI-15, FKSI-DRS, FACT-G, and EQ-5D questionnaires. As expected, fatigue had a negative impact on HRQoL outcomes, except when assessed by HRQoL measures not directly linked with fatigue (such as the FKSI item “I have been coughing”). The relationship between most HRQoL outcomes and fatigue was close to linear in both treatment groups, with lower HRQoL scores (representing worsening HRQoL) generally corresponding to a higher mean fatigue grade. These findings are consistent with previous studies across a range of cancer diagnoses in which the degree of patient-reported fatigue was inversely correlated with physical functioning, performance of everyday activities, and HRQoL.25–27
The finding that HRQoL scores were superior with sunitinib compared with IFN-α is also consistent with previous reports of HRQoL data from this trial, in which within-treatment decreases for FKSI-15 and FKSI-DRS scores after the first cycle of treatment were also reported (although more pronounced for IFN-α).14,21 HRQoL scores may have been influenced by other AEs and disease-relieving symptoms that favored sunitinib over IFN-α (eg, its superior efficacy as measured by progression-free survival).6 For example, it was previously reported that patients receiving sunitinib had less experience with lack of energy, breathlessness, weight loss, and fever as well as less fatigue than patients treated with IFN-α in this trial.21 Patients treated with sunitinib were also significantly more likely to report being able to sleep well, enjoy life, and have a good appetite, all of which would impact on their overall QoL.21 Other factors that may have affected HRQoL scores were the timing and frequency of fatigue, the dosing schemes used (schedule 4/2 for sunitinib vs continuous treatment for IFN-α), and/or the timing of HRQoL assessment; further research on this topic is warranted. It is interesting to note that the incidence of treatment-related CTCAE (version 3) fatigue of any grade reported in the current trial was similar for the sunitinib and IFN-α treatment groups (54% and 52%, respectively) and the incidence of grade 3/4 fatigue did not significantly differ between treatments (11% and 13%, respectively).7 Hence, relying on frequency estimates of AEs from clinical trials may provide an inaccurate and/or incomplete understanding of differences in tolerability between treatments (eg, lack of information regarding onset of AEs), which is an important concern in comparative efficacy trials in which provider-rated CTCAE assessment is relied on to determine differences in patient experience.
Although the results of the current analyses are consistent with those of previous studies, some limitations are recognized. First, because clinician reporting of AEs depends on what the patient conveys, the severity of AE reporting can be influenced by patient and provider characteristics that influence communication, such as assertiveness, stoicism, and clinician bias. In addition, there was the potential for variability in how physicians chose to manage fatigue, from “doing nothing” to dose modification to possible active treatment (eg, methylphenidate). There may also be more specific or “endorsed” tools for assessing fatigue than those used here (eg, the Brief Fatigue Inventory or the Functional Assessment of Chronic Illness Therapy Fatigue Scale).28,29 The possible influences of dose reduction and/or patient dropout after the first cycle were also not examined, although the latter was investigated previously for between-treatment HRQoL results in this trial, using a pattern mixture model as a sensitivity analysis.21 According to the results of the pattern mixture model, patient dropout did not notably influence the results. In addition, the possible role of a “response shift” effect,30 in which health changes lead to shifts in internal standards, values, and conceptualization of HRQoL by patients, was not investigated or taken into account. Finally, in exploratory studies such as this, in which data are collected with an objective but not with a prespecified hypothesis, multiplicity of data, hypotheses, and analyses can result in errors of inference, requiring the use of multiplicity corrections.31 However, no adjustment was made for multiple testing in the current study. Therefore, “significant” results (P < .05) based on these retrospective analyses must be qualified as having resulted from retrospective data, and any corresponding hypotheses must be tested in confirmatory studies.
In conclusion, the finding that sunitinib-related fatigue occurs early can be used to enhance education and preparedness among patients who have been prescribed sunitinib for the treatment of advanced RCC. It also highlights the importance of setting upfront expectations regarding the fatigue experience so that patients do not prematurely withdraw from treatment, thereby failing to take advantage of the potential clinical benefit of sunitinib.32,33 For example, retrospective analyses of pooled clinical trial data for sunitinib in patients with metastatic RCC have preliminarily identified treatment-associated fatigue as a potential biomarker of sunitinib efficacy.34 In addition, the results of the current study may lead to improved implementation of fatigue-specific coping strategies35 by both the patient and those involved with his/her care, thereby helping to motivate the patient to remain on therapy and adapt to this potentially disabling AE over the course of treatment. That CTCAE assessment of fatigue does not fully capture the patient's treatment experience indicates that a more comprehensive measure is needed, one that includes the patient's perspective in addition to the physician's assessment (which neither version 3 or the most recent update, version 4, of the NCI CTCAE do)36; however, the contribution of insufficient grade level options to the inadequacy of CTCAE assessment cannot be excluded as a possible factor (in addition to inappropriate physician reporting). In addition, the impact of fatigue severity must be understood in the context of the treatment being received, including other toxicities. Use of HRQoL measures can be an important tool for the treating physician in this regard, complementing CTCAE assessment. Such measures can provide a better understanding of more subjective AEs such as fatigue, which are reported sporadically, and take into account the entire treatment experience over time, ultimately leading to improved patient management.
FUNDING SUPPORT
This study was sponsored by Pfizer Inc. Medical writing support was provided by Andy Gannon at Acumed (New York, NY) with funding from Pfizer.
CONFLICT OF INTEREST DISCLOSURES
Dr. Cella reports receiving grants and personal fees from Pfizer during the conduct of the current study and grants and personal fees for work as a consultant from Pfizer, Novartis, Aveo, GlaxoSmithKline, and Bayer for work performed outside of the current study. Dr. Davis has received a research grant from Pfizer. Dr. Negrier reports receiving honoraria from Pfizer and Novartis and research grants from Pfizer, Roche, and GlaxoSmithKline for work performed outside of the current study. Mr. Bushmakin, Dr. Cappelleri, Dr. Sandin, Ms. Korytowsky, Dr. Charbonneau, and Dr. Matczak are employees of Pfizer and own Pfizer stock. Dr. Motzer reports receiving research funding and consultant fees from Pfizer for expert testimony, as well as grants from GlaxoSmithKline and Novartis and personal fees from Genentech for work performed outside of the current study.
REFERENCES
- Butt Z, Rosenbloom SK, Abernethy AP, et al. Fatigue is the most important symptom for advanced cancer patients who have had chemotherapy. J Natl Compr Canc Netw. 2008;6:448–455. doi: 10.6004/jnccn.2008.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson TE, Figlin RA, Kuhn JG, Motzer RJ. Targeted therapies for metastatic renal cell carcinoma: an overview of toxicity and dosing strategies. Oncologist. 2008;13:1084–1096. doi: 10.1634/theoncologist.2008-0120. [DOI] [PubMed] [Google Scholar]
- Larkin JM, Pyle LM, Gore ME. Fatigue in renal cell carcinoma: the hidden burden of current targeted therapies. Oncologist. 2010;15:1135–1146. doi: 10.1634/theoncologist.2010-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik UR, Makower DF, Wadler S. Interferon-mediated fatigue. Cancer. 2001;92:1664–1668. doi: 10.1002/1097-0142(20010915)92:6+<1664::aid-cncr1494>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Gore ME, Szczylik C, Porta C, et al. Safety and efficacy of sunitinib for metastatic renal-cell carcinoma: an expanded-access trial. Lancet Oncol. 2009;10:757–763. doi: 10.1016/S1470-2045(09)70162-7. [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584–3590. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzer RJ, Hutson TE, Olsen MR, et al. Randomized phase II trial of sunitinib on an intermittent versus continuous dosing schedule as first-line therapy for advanced renal cell carcinoma. J Clin Oncol. 2012;30:1371–1377. doi: 10.1200/JCO.2011.36.4133. [DOI] [PubMed] [Google Scholar]
- Trotti A, Colevas AD, Setser A, Basch E. Patient-reported outcomes and the evolution of adverse event reporting in oncology. J Clin Oncol. 2007;25:5121–5127. doi: 10.1200/JCO.2007.12.4784. [DOI] [PubMed] [Google Scholar]
- Brundage MD, Pater JL, Zee B. Assessing the reliability of 2 toxicity scales: implications for interpreting toxicity data. J Natl Cancer Inst. 1993;85:1138–1148. doi: 10.1093/jnci/85.14.1138. [DOI] [PubMed] [Google Scholar]
- Jensen MP. The validity and reliability of pain measures in adults with cancer. J Pain. 2003;4:2–21. doi: 10.1054/jpai.2003.1. [DOI] [PubMed] [Google Scholar]
- Kaba H, Fukuda H, Yamamoto S, Ohashi Y. Reliability at the National Cancer Institute-Common Toxicity Criteria version 2.0 [in Japanese] Gan To Kagaku Ryoho. 2004;31:1187–1192. [PubMed] [Google Scholar]
- Bruner DW. Should patient-reported outcomes be mandatory for toxicity reporting in cancer clinical trials&quest. J Clin Oncol. 2007;25:5345–5347. doi: 10.1200/JCO.2007.13.3330. [DOI] [PubMed] [Google Scholar]
- Cella D, Li JZ, Cappelleri JC, et al. Quality of life in patients with metastatic renal cell carcinoma treated with sunitinib or interferon alfa: results from a phase III randomized trial. J Clin Oncol. 2008;26:3763–3769. doi: 10.1200/JCO.2007.13.5145. [DOI] [PubMed] [Google Scholar]
- Cella D, Yount S, Du H, et al. Development and validation of the Functional Assessment of Cancer Therapy-Kidney Symptom Index (FKSI) J Support Oncol. 2006;4:191–199. [PubMed] [Google Scholar]
- Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33:337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- Cancer Therapy Evaluation Program. 2013. Common Terminology Criteria for Adverse Events. Version 3.0. Bethesda, MD: Division of Cancer Treatment and Diagnosis, National Cancer Institute, National Institutes of Health, Department of Health and Human Services; 2006. ctep.cancer.gov . Accessed May 29,
- Fairclough D. Design and Analysis of Quality of Life Studies in Clinical Trials. 2nd ed. Boca Raton, FL: Chapman & Hall/CRC; 2010. [Google Scholar]
- Fitzmaurice G, Laird N, Ware J. Applied Longitudinal Analysis. 2nd ed. Hoboken, NJ: John Wiley & Sons; 2011. [Google Scholar]
- Cella D, Michaelson MD, Bushmakin AG, et al. Health-related quality of life in patients with metastatic renal cell carcinoma treated with sunitinib vs interferon-alpha in a phase III trial: final results and geographical analysis. Br J Cancer. 2010;102:658–664. doi: 10.1038/sj.bjc.6605552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappelleri JC, Bushmakin AG. Interpretation of patient-reported outcomes [published online ahead of print February 19, 2013] Stat Methods Med Res. doi: 10.1177/0962280213476377. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- Basch E, Iasonos A, McDonough T, et al. Patient versus clinician symptom reporting using the National Cancer Institute Common Terminology Criteria for Adverse Events: results of a questionnaire-based study. Lancet Oncol. 2006;7:903–909. doi: 10.1016/S1470-2045(06)70910-X. [DOI] [PubMed] [Google Scholar]
- Brown DJ, McMillan DC, Milroy R. The correlation between fatigue, physical function, the systemic inflammatory response, and psychological distress in patients with advanced lung cancer. Cancer. 2005;103:377–382. doi: 10.1002/cncr.20777. [DOI] [PubMed] [Google Scholar]
- Mallinson T, Cella D, Cashy J, Holzner B. Giving meaning to measure: linking self-reported fatigue and function to performance of everyday activities. J Pain Symptom Manage. 2006;31:229–241. doi: 10.1016/j.jpainsymman.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12:4–10. doi: 10.1634/theoncologist.12-S1-4. [DOI] [PubMed] [Google Scholar]
- Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13:63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- Hamidou Z, Dabakuyo TS, Bonnetain F. Impact of response shift on longitudinal quality-of-life assessment in cancer clinical trials. Expert Rev Pharmacoecon Outcomes Res. 2011;11:549–559. doi: 10.1586/erp.11.57. [DOI] [PubMed] [Google Scholar]
- Bender R, Lange S. Adjusting for multiple testing–when and how? J Clin Epidemiol. 2001;54:343–349. doi: 10.1016/s0895-4356(00)00314-0. [DOI] [PubMed] [Google Scholar]
- Schmidinger M, Arnold D, Szczylik C, Wagstaff J, Ravaud A. Optimizing the use of sunitinib in metastatic renal cell carcinoma: an update from clinical practice. Cancer Invest. 2010;28:856–864. doi: 10.3109/07357901003631080. [DOI] [PubMed] [Google Scholar]
- Molina AM, Lin X, Korytowsky B, et al. Sunitinib objective response in metastatic renal cell carcinoma: analysis of 1,059 patients treated on clinical trials [abstract] J Clin Oncol. 2012;(30):Page. doi: 10.1016/j.ejca.2013.08.021. Abstract 4542. [DOI] [PubMed] [Google Scholar]
- Davis MP, Figlin RA, Hutson TE, et al. Asthenia and fatigue as potential biomarkers of sunitinib efficacy in metastatic renal cell carcinoma [abstract] Eur J Cancer. 2011;47:S135. Abstract 1139. [Google Scholar]
- Eisen T, Sternberg CN, Robert C, et al. Targeted therapies for renal cell carcinoma: review of adverse event management strategies. J Natl Cancer Inst. 2012;104:93–113. doi: 10.1093/jnci/djr511. [DOI] [PubMed] [Google Scholar]
- Cancer Therapy Evaluation Program. National Cancer Institute Common Terminology Criteria for Adverse Events. 2013. Version 4.0. NIH Pub. No. 09-7473. Bethesda, MD: National Cancer Institute, National Institutes of Health, Department of Health and Human Services; 2009. ctep.cancer.gov . Accessed August 26, 2013.